Abstract
Stromal-derived factor-1 (SDF-1/CXCL12) and its receptor CXCR4 play crucial roles in leukocyte migration and activation, as well as embryogenesis, angiogenesis, cancer and viral pathogenesis. CXCR4 is one of the major human immunodeficiency virus-1 (HIV-1) coreceptors on macrophages. In many tissues macrophages are one of the predominant cell types infected by HIV-1 and act as a reservoir for persistent infection and viral dissemination. In patients infected by HIV-1, blood and tissue levels of transforming growth factor-β1 (TGF-β1) are increased. The purpose of this study was to evaluate the effects of TGF-β1 on CXCR4 expression and function in primary human monocyte-derived macrophages (MDMs) and rat microglia. TGF-β1 up-regulated CXCR4 and enhanced SDF-1α-stimulated ERK1,2 phosphorylation in these cells. The increased CXCR4 expression in human MDMs resulted in increased susceptibility of the cells to entry by dual-tropic CXCR4-using HIV-1 (D-X4). In contrast, TGF-β1 failed to increase CCR5 expression or infection by a CCR5-using virus in MDMs. Our data demonstrate that TGF-β1 enhances macrophage responsiveness to SDF-1α stimulation and susceptibility to HIV-1 by selectively increasing expression of CXCR4. The results suggest that increased expression of CXCR4 on macrophages may contribute to the emergence of dual-tropic X4 viral variants at later stages of HIV-1 infection.
Keywords: HIV-1, CXCR4, CCR5, macrophages, microglia
Introduction
Macrophages are one of the predominant cell types infected with human immunodeficiency virus-1 (HIV-1) in many tissues, including brain, lung and lymph nodes.1–3 Unlike infected CD4+ T-lymphocytes which have a short half life of 1–1·5 days, macrophages are quite resistant to the cytopathic effects of the virus and thus, may provide a reservoir for persistent infection and virus dissemination. The two major coreceptors of HIV-1, CXCR4 and CCR5, are both expressed on macrophages. Macrophages are primarily infected by CCR5 utilizing viruses (R5) in early stages of infection4 but at later stages of the disease they are often infected by dual-tropic CXCR4 using variants (D-X4)5 that are associated with disease progression.6 Several mechanisms have been put forward to explain the tropism evolution in the progress of acquired immune deficiency syndrome (AIDS), including increased expression of R5 variants blocking chemokines at the sites of HIV-1 infection7, 8 and cytokine up-regulation of CXCR4 and down-regulation of CCR5 on CD4+ T-lymphocytes.9 However, the phenomenon is still perplexing and the answers are not yet satisfying.
The susceptibility of macrophages to HIV-1 infection is dependent on multiple factors, including the stage of differentiation of the cells as well as a variety of host factors.10 Previous studies showed that R5 HIV-1 inefficiently infects monocytes. However, as monocytes differentiate into macrophages, mRNA and cell surface expression of CCR5 increases while CXCR4 decreases. Concurrently, the infection level of the cells by R5 viruses also increases.11, 12 Host factors, including cytokines and growth factors, play important roles in regulating pathogenesis of HIV infection. Transforming growth factor-β1 (TGF-β1) is a multifunctional growth factor that is important in development, immune responses, tumour biology and angiogenesis.13, 14 In patients infected with HIV-1, blood levels of TGF-β1 are increased.15 TGF-β1 production can be induced from macrophages, peripheral blood mononuclear cells (PBMCs) and astrocytes infected with HIV-1.16–18 TGF-β1 increases CXCR4 expression, X4-envelope-mediated syncytium and X4-infection levels on human mature dendritic cells.19 Monocyte-derived macrophage (MDM) expression of CXCR4 has also been shown to be regulated by TGF-β1·20 In addition, TGF-β1 can overcome the replicative restriction of T-tropic isolates of HIV-1 in macrophages.21 However, the mechanism of how TGF-β1 regulates macrophage susceptibility to X4 HIV-1 infection is not well characterized.
CXCR4 is the only known receptor for the CXC subfamily chemokine SDF-1α. SDF-1α and CXCR4 are essential in central nervous system (CNS) development, angiogenesis, lymphopoiesis and tumorigenesis.22, 23 SDF-1α activates Ca2+ mobilization, phosphatidylinositol-3 (PI3) kinase and mitogen-activated protein kinase (MAPK) pathways and chemotaxis in CXCR4-expressing cells.24, 25 CXCR4 expression is under extensive regulation in both physiological and pathological states. For example, a previous study showed that the interaction with thymic epithelial cells (TEC) induced an up-regulation of CXCR4 expression on CD4+ mature thymocytes.26 In the CNS, CXCR4 is expressed on astrocytes, microglia and neurons. CXCR4 on microglia can mediate HIV-1 entry into the CNS, although the efficiency of the virus–cell interaction is higher with CCR5.27, 28
In this study, we examined the effects of TGF-β1 on CXCR4 expression, CXCR4-mediated signalling, and HIV-1 infection in human MDMs. We found that TGF-β1 increased CXCR4 expression at both the mRNA and cell surface protein levels. Distribution of CXCR4 in the cells was also changed by TGF-β1 treatment. The increase in CXCR4 expression resulted in enhanced SDF-1α stimulated ERK1,2. In the CNS, TGF-β1 also up-regulated CXCR4 mRNA expression and potentiated SDF-1α-stimulated signalling in primary cultures of rat microglia. More importantly, CXCR4-mediated D-X4 HIV-1 virus entry into human MDMs was enhanced by TGF-β1. In contrast, neither CCR5 expression nor R5 virus entry into MDM was changed by TGF-β1 treatment. Our studies implicate TGF-β1-regulated CXCR4 on macrophages as one possible mechanism responsible for the emergence of X4 HIV-1 variants during later stages of AIDS.
Materials and methods
Primary cultures of human MDMs and rat microglia
Human MDMs were prepared as previously described.11 Two types of media were used in the preparation of human MDM culture. Serum free medium: RPMI-1640 medium (Life Technologies, Rockville, MD), 100 000 U/l penicillin, 100 mg/l streptomycin, pH 7·4. Complete medium: RPMI-1640 medium, 15% human serum (Sigma, St. Louis, MO), 100 000 U/l penicillin, 100 mg/l streptomycin, pH 7·4. Uninfected PBMCs from fresh HIV-1-, cytomegalovirus-, and hepatitis B virus-negative leukapheresis residues were isolated from adult donors by Ficoll gradient centrifugation. Briefly, Leukapheresis residues were washed once with wash buffer (Hank's balance salt solution, 2·5 mm EDTA, 2% fetal bovine serum [FBS; Life Technologies, Rockville, MD], 100 000 U/l penicillin, 100 mg/l streptomycin, pH 7·4) and then incubated with RosetteSep Human Monocyte Enrichment Cocktail (StemCell Technologies, Vancouver, BC, Canada) for 20 min at room temperature before Ficoll gradient centrifugation. The enriched monocytes were washed twice with wash buffer and plated with complete medium into 75 cm2 flasks (Sarstedt, Newton, NC). After 1–2 hr incubation at room temperature, cultures were rinsed twice with serum free medium. Monocytes were incubated in complete medium supplemented with 1 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (Sigma) at 37°, 5% CO2 for a week. After cultures matured into macrophages, the medium and unattached cells were aspirated off. Cell monolayers were washed twice with phosphate-buffered saline (PBS), scraped gently off the flasks, and replated for experiments. One day after plating, the cells were used for experiments. The cultures contain >95% macrophages.11 Primary cultures of rat microglia were prepared from neonatal rat brain as described previously.29
Northern blot analysis
MDMs were plated at a density of 1–1·5 million/well into 6-well plates (Corning Incorporated, Corning, NY). Microglia were seeded at a density of 4 million cells per 100 mm dish. Serum free medium containing various concentrations of human TGF-β1 (Chinese hamster ovary cell expressed, R & D Systems, Minneapolis, MN) was applied to the cells. At various times after addition of TGF-β1, as indicated in Results, total RNA was extracted with TriZol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions and subjected to Northern blot analysis.30[32P]-labelled cDNAs encoding human CXCR4, CCR5 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), or rat CXCR4, CCR5, and cyclophilin were used as hybridization probes. The washed membranes were exposed to film and detected by autoradiography.
Flow cytometry and immunofluorescence microscopy
For flow cytometry analysis, MDMs seeded into 6-well plates at a density of 1–1·5 million/well were treated with 20 ng/ml TGF-β1 for 48 hr. Cells were collected by gentle scraping, washed once with fluorescence-activated cell sorter (FACS) washing buffer (PBS, 0·02% sodium azide, 10% FBS and 2% human serum) and incubated subsequently with either allophycocyanin (APC)-conjugated anti-human CXCR4 monoclonal antibody (mAb 12G5, BD Biosciences Pharmingen, San Diego, CA), APC-conjugated anti-human CD4 (mAb Leu-3a, BD Biosciences Pharmingen), APC-conjugated anti-human IgG2α antibody (BD Biosciences Pharmingen), or APC-conjugated anti-human immunoglobulin G1 (IgG1) antibody (BD Biosciences Pharmingen) for 15 min at 4°. Cells were washed twice with FACS washing buffer, then fixed with fixing buffer (PBS, 1% paraformaldehyde, 0·02% sodium azide) and analysed with a FACScan machine (Becton-Dickinson, San Jose, CA). For immunofluorescence microscopy, 200 000–300 000 cells were seeded onto cover slips in 24-well plates. After 48 hr treatment with TGF-β1, cells were fixed in 4% paraformaldehyde for 30 min at room temperature, permeabilized with PBS−1% Tween for 15 min and then blocked with blocking buffer (PBS, 1% bovine serum albumin) for 1 hr. The cells were incubated in 1 : 100 goat anti-human CXCR4 (C-20) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in blocking buffer at 4° overnight and further with fluorescein isothiocyanate (FITC)-conjugated bovine anti-goat secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 1 hr. The cells were analysed using confocal microscopy (Solamere Technology Group, Salt Lake City, UT) with a Stanford Photonics XR-Mega 10 camera (Palo Alto, CA).
SDF-1α stimulation of ERK1,2 phosphorylation
MDMs (500 000/well) in 12-well plates were treated for 48 hr with serum free medium containing TGF-β1, incubated in serum free medium for an additional 2 hr, and stimulated subsequently with 50 nm human SDF-1α (R & D Systems) for 2 or 5 min. The cells were collected in lysis buffer with proteinase inhibitor cocktail (PBS, 1% NP-40, 0·5% Na-deoxycholate, 0·1% SDS, 100 µg/ml phenylmethylsulphonyl fluoride, 20 µg/ml aprotinin, 1 mm sodium orthovanadate, 1 mm dithiothreitol, pH 7·4) and Western blot analysis was carried out as described previously.29 Mouse anti-phospho-ERK1,2 antibody (Cell Signaling Technology, Beverly, MA), rabbit anti-ERK1,2 antibody (Cell Signaling Technology), HRP-conjugated sheep anti-mouse secondary antibody (Sigma), and HRP-conjugated goat anti-rabbit secondary antibody (Cell Signaling Technology) were used.
HIV-1 infection analysis
Single cycle recombinant viruses were produced by cotransfection of a luciferase-tagged HIV-1 backbone vector and an envelope expression vector containing env sequences, as described previously.31 The vector pNL4-3.Luc.E– encoded the HIV-1NL4-3 provirus with a frameshift in env that prevents expression of the envelope glycoprotein and was tagged with firefly luciferase in the nef reading frame. The envelope expression vector was prepared by inserting the entire env reading frame from either HIV-1JR-FL or HIV-1D-X4 into the cytomegalovirus promoter-based expression vector, pcDNA3·1 + (Stratagene, La Jolla, CA). The two vectors were cotransfected into 293 cells with Superfect transfection reagent (Qiagen, Chatsworth, CA). Viral supernants were harvested two days later, pooled, cleared of cell debris by 0·45-µm pore size filtration, assayed for p24 antigen content and stored in aliquots at −80°.
MDMs were plated into wells of 48-well plates at a density of 250 000 cells/well. After 48 hr treatment with 20 ng/ml TGF-β1, cells were washed once with PBS and fed with complete medium. Cells were inoculated with 60 ng of viral p24 and cell lysates were prepared with cell culture lysis reagent (Promega, Madison, WI) 4 days after infection. The amount of luciferase activity in each lysate was determined by a standard luminometer assay (Monolight 2010; Analytical Luminescence Laboratories, San Diego, CA), which reported in relative light units (RLU).
Statistic analysis
Student's t-test and two-way analysis of variance were done in GraphPad Prism 3.1 (GraphPad Software, San Diego, CA) as indicated in the results. The significance level was set at P < 0·05.
Results
TGF-β1 increased CXCR4 mRNA in MDMs
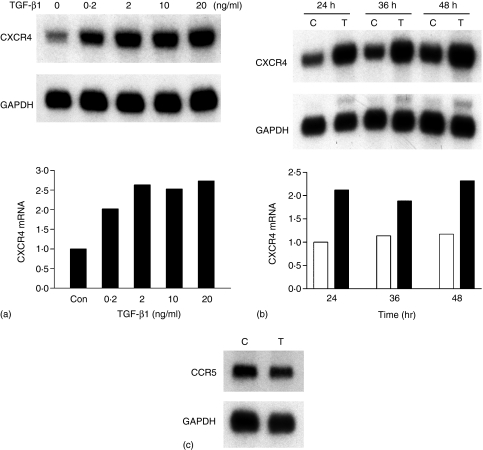
Blood levels of TGF-β1 are increased in individuals infected with HIV-1 and it can be induced from macrophages, PBMCs and astrocytes.15–18 To examine the effects of TGF-β1 on CXCR4 expression in macrophages, human monocytes were differentiated for 1 week in the presence of GM-CSF into mature macrophages. Macrophages were treated with different concentrations (0–20 ng/ml) of TGF-β1 in serum free medium for 24 hr, total RNA was extracted and subjected to Northern blot analysis. The levels of CXCR4 were quantified and normalized to GAPDH hybridization signals. Figure 1(a) shows that TGF-β1 up-regulated CXCR4 mRNA in MDMs in a concentration-dependent manner. Maximal induction of CXCR4 mRNA was about 2·5-fold and occurred at physiologically relevant TGF-β1 concentrations, i.e. 2–20 ng/ml. The kinetics of the TGF-β1 dependent increase in CXCR4 mRNA was also characterized. Induction was evident as early as 8 hr (data not shown), and a maximal increase was seen from 24 to 48 hr (Fig. 1b). CCR5 is the other major coreceptor expressed on MDMs. Macrophages are primarily infected with R5 viruses following transmission and throughout the course of the disease.4,32 To examine if TGF-β1 had any effects on CCR5 mRNA expression, total RNA of control- and TGF-β1-treated MDMs were subjected to Northern blot analysis. Steady state levels of CCR5 mRNA were unaltered after 48 h of 20 ng/ml TGF-β1 treatment (Fig. 1c).
Figure 1.
TGF-β1 increased CXCR4 mRNA in human MDMs. (a) Concentration-dependence of TGF-β1 up-regulation of CXCR4 mRNA in MDMs. Human MDMs were treated with serum free medium alone or in the presence of the indicated concentrations of TGF-β1 for 24 hr and total RNA was extracted and subjected to Northern blot analysis. The intensity of CXCR4 mRNA was quantified by densitometry, normalized to that of GAPDH mRNA, and plotted as relative levels of CXCR4 mRNA. (b) Time dependent effects of TGF-β1 on CXCR4 mRNA. Human MDMs were incubated in serum free medium with (filled bars) or without (open bars) 20 ng/ml TGF-β1. Total RNA was extracted at various time points indicated and subjected to Northern blot analysis. The results are representative of two independent experiments. (c) TGF-β1 had no effect on CCR5 mRNA. Total RNA was isolated from MDMs incubated in serum free medium with or without 20 ng/ml TGF-β1 for 48 hr and subjected to Northern blot analysis.
TGF-β1 increased MDM cell surface CXCR4 and altered its cellular localization
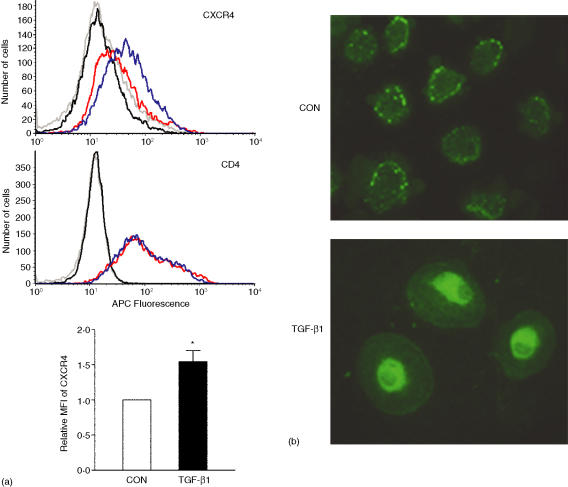
A direct relationship between levels of mRNA and surface expression of CXCR4 and CCR5 has been established.11 Because CXCR4 mRNA was up-regulated by TGF-β1, it was probable that CXCR4 on the cell surface of MDMs would be increased also. MDMs were treated with 20 ng/ml of TGF-β1 for 48 hr and subjected to flow cytometry analysis in order to evaluate cell surface expression of CXCR4. TGF-β1 treatment increased mAB 12G5 immunoreactivity indicating that CXCR4 expression on the cell surface was elevated; isotype control staining of control and TGF-β1 treated cells were less than staining by 12G5 (Fig. 2a, upper panel). A summary of eight independent donors indicated that TGF-β1 increased cell surface CXCR4 protein expression by 50 ± 16% (Fig. 2a, lower bar graph). On the other hand, there was no change in CD4 expression (Fig. 2a, middle panel). The localization of CXCR4 in TGF-β1-treated and untreated MDMs was determined by staining permeabilized cells with an anti-CXCR4 antibody directed against the C-terminus of human CXCR4. Specificity of the immunoreactivity was established by two methods. First, no staining was observed when the primary antibody was omitted and second, HEK293T cells engineered to overexpress human CXCR4 displayed higher immunoreactivity, as compared to mock-transfected cells (data not shown). TGF-β1-treated cells displayed a more rounded shape and, in the confocal plane, appeared somewhat larger than control treated cells (Fig. 2b). This TGF-β1 effect was similar to what has been observed with other TGF-β1 responsive cells.33 CXCR4 immunoreactivity appeared in a punctate pattern in control treated cells, a cellular distribution similar to previous reports.20, 34 On the other hand, CXCR4 was more evenly distributed throughout the TGF-β1-treated MDMs, although more intense perinuclear immunoreactivity was observed. Consistent with the flow cytometry analysis, the fluorescence intensity of the cell surface was increased by TGF-β1.
Figure 2.
TGF-β1 increased cell surface expression of CXCR4 on MDMs and changed its cellular localization. (a) FACS analysis of CXCR4 and CD4 expression in control and TGF-β1-treated cells. MDMs were treated with medium with or without 20 ng/ml TGF-β1 for 48 hr and then examined for cell surface CXCR4 expression with anti-CXCR4 or isotype control (IgG2α) antibody staining and flow cytometry (upper panel). Grey line, isotype control staining of control cells; black line, isotype-control staining of TGF-β1 treated cells; red line, mAb 12G5 staining of control cells; blue line, mAb 12G5 staining of TGF-β1 treated cells. The middle panel depicts CD4 levels in the control and TGF-treated cells. The data depicted are representative of results from three donors. The colour scheme of the lines on the histogram is identical to the upper panel; an IgG1 isotype control antibody. The lower bar graph summarizes results of experiments on eight different donors; the data were analysed by Student's t-test, *P < 0·05. (b) Confocal immunofluorescence microscopy analysis of CXCR4 expression in control and TGF-β1-treated cells. Human MDMs incubated in medium with or without TGF-β1 were immunostained with anti-CXCR4 antibody and visualized by confocal microscopy. The data are representative of five independent experiments. The microscope magnification used was 40×.
SDF-1α-stimulated ERK1,2 phosphorylation was enhanced by TGF-β1 pretreatment
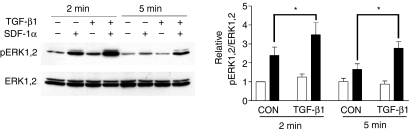
Binding of SDF-1α to CXCR4-expressing cells stimulates intracellular Ca2+ mobilization, as well as PI3 kinase and MAPK pathways. We examined if the increased CXCR4 on MDMs enhanced SDF-1α stimulation of ERK1,2. MDMs were treated with TGF-β1 for 48 hr and then stimulated with 50 nm SDF-1α for 2 and 5 min. At both time points, TGF-β1 pretreatment enhanced SDF-1α-stimulated ERK1,2 phosphorylation (Fig. 3). At 2 and 5 min time points, SDF-1α increased pERK1,2 levels 3·5- and 2·7-fold, respectively, in TGF-β1-treated cells. In control cells, SDF-1α only increased pERK1,2 2·3- and 1·5-fold (2 and 5 min, respectively).
Figure 3.
SDF-1α-stimulated ERK1,2 phosphorylation in control and TGF-β1 treated MDMs. Human MDMs were treated with or without 20 ng/ml TGF-β1 for 48 hr, washed twice and incubated with serum free medium for an additional 2 hr. Cells stimulated with medium alone (open bars) or medium containing 50 nm SDF-1α (filled bars) for 2 min and 5 min were then lysed and subjected to Western blot analysis. The levels of Phospho-ERK1,2 were normalized to those of total ERK1,2. The data was analysed by two-way anova. *, P < 0·05, data represent means ± SEM of four experiments on four different donors.
TGF-β1 increased CXCR4 and enhanced SDF-1α-stimulated ERK1,2 phosphorylation in rat microglia
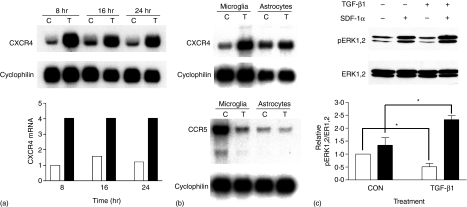
Microglia are immune cells resident in the CNS and can perform such functions of macrophages as antigen presentation, cytokine synthesis and phagocytosis.35 These cells also constitutively express CXCR4, which can be activated by SDF-1α and mediate HIV-1 infection within the CNS. We investigated if TGF-β1 could alter the expression of CXCR4 on rat microglia. Primary cultured neonatal rat microglia were treated with 2 ng/ml TGF-β1 for 8, 16, and 24 hr and total RNA was extracted and subjected to Northern blot analysis. As shown in Fig. 4(a), as early as 8 hr after TGF-β1 treatment, a dramatic increase (about fourfold) of CXCR4 mRNA was evident; the increase was maintained through 24 hr of TGF-β1 treatment. Steady state levels of CXCR4 mRNA in primary cultures of rat astrocytes were unaltered by TGF-β1 (Fig. 4b top panel). In contrast to human MDMs, TGF-β1 reduced steady state levels of CCR5 mRNA (Fig. 4b lower panel) in rat microglia. TGF-β1 did not affect CCR5 mRNA in cultured astrocytes (Fig. 4b lower panel). TGF-β1-treated and untreated microglia were also subjected to analysis of CXCR4 function. As observed in the human MDM, TGF-β1 significantly enhanced the SDF-1α-stimulated ERK1,2 phosphorylation (Fig. 4c). A decreased basal level of ERK1,2 phosphorylation in TGF-β1-treated rat microglia was also observed, consistent with our previously published data.29
Figure 4.
TGF-β1 increased CXCR4 mRNA and enhanced SDF-1α-stimulated ERK1,2 phosphorylation in primary cultures of rat microglia. (a) Rat microglial CXCR4 mRNA was increased by TGF-β1. Rat microglia were treated with (filled bars) or without (open bars) 2 ng/ml TGF-β1 and total RNA was collected at the indicated time points. The levels of CXCR4 mRNA were analysed by Northern blot analysis and normalized to the cyclophilin hybridization signal. (b) Rat microglia and astrocytes were treated with or without 2 ng/ml TGF-β1 for 16 hr. The levels of CXCR4 (top panel) and CCR5 (lower panel) mRNA were analyzed by Northern blot. (c) Rat microglia were treated with or without 2 ng/ml TGF-β1 for 16 hr. Cells stimulated with medium alone (open bars) or medium containing 50 nm SDF-1α (filled bars) for 2 min were then lysed and subjected to Western blot analysis. The data represent means ± SEM of three independent experiments and analysed with Two-way anova. *, P < 0·05.
TGF-β1 increased D-X4 HIV-1 infection of MDMs
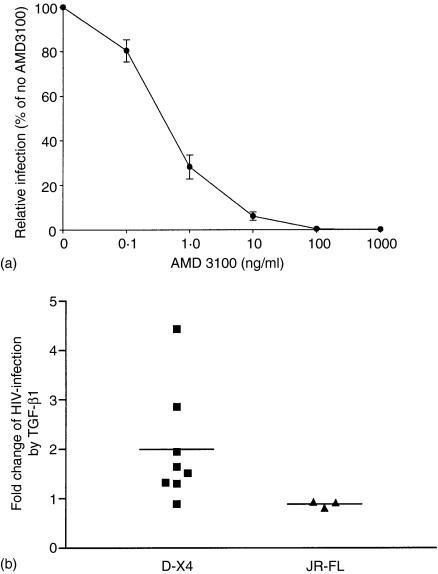
Although macrophages are primarily infected with R5 viruses, variants of HIV-1 can also use CXCR4 to gain entry into these cells.32, 36–39 We evaluated the susceptibility of TGF-β1-treated MDMs to X4 HIV-1 virus infection. To this end we used a dual-tropic (D-X4) virus that can use CXCR4 to infect both macrophages and CD4+ T-lymphocytes. The recombinant HIV-1D-X4 infects MDMs through CXCR4 since AMD3100 completely inhibited entry (Fig. 5a). MDMs treated with TGF-β1 for 48 hr were infected with recombinant virus and 4 days later, viral entry was measured by a luciferase activity assay. TGF-β1 increased viral infection amongst seven of eight donors that were examined (Fig. 5b). Statistical analysis of all eight donors demonstrated a significant twofold enhancement by TGF-β1. While previous data showed that TGF-β1 could overcome the replicative restriction of T-tropic HIV-1 virus in MDMs21 in our system, HIV-1LAI virus was unable to infect either TGF-β1- or control-treated MDMs (data not shown). The effect of TGF-β1 on the infection capacity of R5 variants on MDMs was also assessed using a JR-FL recombinant virus. Consistent with the CCR5 mRNA data, there was no difference in infection levels by this virus between control and TGF-β1 treated cells (Fig. 5b).
Figure 5.
TGF-β1 increased the susceptibility of MDMs to D-X4 HIV-1 entry. (a) DX-4 entry is blocked by the CXCR4 antagonist, AMD3100. The data represent means ± SEM from experiments conducted on four different MDM donors. (b) Cells treated with medium in the presence or absence of 20 ng/ml TGF-β1 for 48 hr were washed once with PBS, incubated with complete medium, and subsequently inoculated with recombinant D-X4 or JR-FL HIV-1. The graph shows the fold change derived from experiments on eight different donors for D-X4 virus and three different donors for JR-FL virus. Each experiment was done on three to six replicates. The means ± SEMs for fold changes in viral entry caused by TGF-β1 treatment were: D-X4, 2·00 ± 0·40*; JR-FL, 0·89 ± 0·04. The data was analysed by Student's t-test. *Significantly different from 1 at P < 0·05.
Discussion
Macrophages express both major HIV-1 coreceptors, CXCR4 and CCR5. In this report, we demonstrated that the pleiotropic growth factor/cytokine, TGF-β1, increased CXCR4 expression in human MDM and rat microglia which resulted in two functional consequences. These included increased activation of ERK1,2 by SDF-1α and, in the case of human MDM, an enhanced susceptibility to D-X4 HIV-1 infection. This latter change was very likely caused by the increase in surface expression of CXCR4 because:1, 2 CD4 expression was unchanged; and the expression of the other major HIV-1 coreceptor, CCR5, was unaltered by TGF-β1 in MDMs and so was the susceptibility of the cells to a R5 HIV-1 variant.
CXCR4 expression and function are under extensive regulation in both physiological and pathological conditions. TGF-β1 can increase CXCR4 expression on naïve T cells, which results in increased homing of these cells to the spleen.40 In the CNS, CXCR4 expression is up-regulated in HIV encephalitis, experimental allergic encephalomyelitis and brain tumours. In these disease scenarios, the increased expression was localized primarily to microglia, astrocytes and infiltrating leucocytes.41–44 In our study, Northern blot, flow cytometry and immunostaining demonstrated that TGF-β1 could increase CXCR4 expression at both mRNA and protein levels in human macrophages and rat microglia.
In addition to increasing steady state levels of CXCR4 mRNA and cell surface protein, TGF-β1 also changed the localization of CXCR4 in human MDMs. CXCR4 immunostaining and confocal microscopy showed that CXCR4 on the plasma membrane was increased by TGF-β1, which was consistent with data from the flow cytometry analysis. More surprisingly, in control cells, CXCR4 immunoreactivity appeared in a punctate pattern in the cytoplasm. However, in TGF-β1-treated cells, a more even cellular distribution was observed, although more intensive staining was seen around the nucleus. CXCR4 and CCR5 have been shown by immunoelectron microscopy to form homogeneous clusters in human macrophages, mostly on microvilli.45 CCR5 microclusters were also found in secretory vesicles of the Golgi apparatus and they were speculated to be transported to the cell membrane.45 The CXCR4 punctate pattern is consistent with this earlier report. TGF-β1 may be increasing the rate of transportation of CXCR4 from these vesicles to the plasma membranes. Concurrently, a TGF-β1-dependent signal to increase de novo synthesis of CXCR4 could explain the enhanced immunofluorescence intensity around the nucleus. Nonetheless, further investigation will be required to verify this hypothesis.
SDF-1α-mediated ERK1,2 phosphorylation was enhanced in TGF-β1 pretreated cells indicating that the increased CXCR4 expression functionally enhanced activation of this MAPK signalling pathway. ERK1,2 phosphorylation is part of an important set of intracellular signalling pathways that are involved in cell survival, proliferation and activation.25, 46, 47 ERK1,2 activation has also been shown to be involved in regulating HIV-1 infectivity and production. Exposure of HIV-1 infected cells to SDF-1α enhanced the production of X4 HIV-1 that was dependent on SDF-1α-stimulated ERK1,2 activation.48 ERK1,2 activated by serum or constitutively active Ras, Raf or MEK also enhanced the infectivity of HIV-1 virions.49, 50 Thus, TGF-β1-enhanced SDF-1α-stimulated ERK1,2 activation may result in greater activation of macrophages in immune responses and increased HIV-1 infectivity. TGF-β1 also increased CXCR4 expression and SDF-1α-stimulated intracellular signalling in the central nervous system macrophage, i.e. microglia. We previously reported that fractalkine-stimulated ERK1,2 phosphorylation in microglia was diminished by TGF-β1 pretreatment although it up-regulated CX3CR1 expression.29 These contrasting effects of TGF-β1 on CX3CR1 and CXCR4 functions suggest that TGF-β1 differentially and specifically regulates the responsiveness of mononuclear cells to the chemokine ligands fractalkine and SDF-1α.
Blood levels of TGF-β1 have been shown to be elevated in individuals infected with HIV-1.15 Increased production of TGF-β1 has been demonstrated from HIV-1-infected monocytes, macrophages and astrocytes.16–18 When HIV-infected individuals progress towards AIDS, variants using CXCR4 emerge compared to predominantly R5 variants in early infection. We demonstrated that TGF-β1 could increase CXCR4 expression and consequently, enhance D-X4 HIV-1 infection of MDMs indicated by luciferase activity. However, this latter observation could be due to other possible mechanisms. TGF-β1 may increase other attachment receptors such as DC-SIGN and mannose receptors which may allow more efficient HIV-1 entry.51, 52 In addition, it is possible that TGF-β1 could enhance events after HIV-1 entry, such as nuclear uptake, reverse transcription of DNA and transcription. Because TGF-β1 failed to change CCR5 expression and JR-FL HIV-1 infection of MDMs, the TGF-β1-enhanced D-X4 infection was more likely a result of the TGF-β1 dependent increase in CXCR4 expression since these other possible mechanisms would not be specific for D-X4 viruses. Our results suggest that one possible mechanism to achieve viral tropism evolution of R5 towards R5 X4 and X4 is to increase CXCR4 expression on macrophages while keeping CCR5 expression unchanged. The entry of D-X4 into human MDMs was up-regulated twofold in TGF-β1 pretreated cells. Our experiments used single cycle viruses that were replication incompetent in order to address the effect of TGF-β1 on levels of D-X4 entry. Thus it is reasonable to predict the increase of viral entry in TGF-β1-treated cells could result in exponential amplification of virus production in subsequent replication cycles. Further amplification of D-X4 virus infection of macrophages, in vivo, could result from increased TGF-β1 from HIV-1-infected macrophages, as well as enhanced number of these cells as a consequence of the potent monocyte chemoattractant property of TGF-β1.53, 54 TGF-β1 also increases CXCR4 expression in primary CD4+ T cells.55 Because D-X4 variants can use CXCR4 to get into both macrophages and CD4+ T cells, the increased production of these viruses by macrophages exposed to TGF-β1 could provide a mechanism to promote further infection of CD4+ T cells.
Other cytokines can also contribute to increasing HIV-1 infection of macrophages. Interferon-γ and interleukin (IL)-6 increased the capacity of MDMs to favor CXCR4 using HIV-1 infection while decreasing infection by R5 variants.56 Cytokine regulation of cell susceptibility to HIV-1 infection by changing coreceptor expression levels has also been seen in other cells. IL-2, IL-4, IL-7 and IL-15 induced functional cell surface expression of CXCR4 on CD4+ CCR7+ human memory T cells, and increased the infection by X4 strains of HIV-1 in these cells.16–18 IL-7 has been reported to increase CXCR4 expression on CD4+ mature thymocytes and X4 virus replication in these cells.26 Our results, together with others, demonstrate that the cytokine milieu contributes to the complex regulation of an individual's susceptibility to HIV-1 infection and viral tropism change.10
A previous report showed that TGF-β1 could overcome replicative restriction of X4 HIV-1 (LAI) in macrophages.21 However, in our experiments, MDM cultures derived from multiple donors were resistant to infection by HIV-1LAI with or without TGF-β1 pretreatment (data not shown). While the different donor populations could account for this discrepancy, it may reflect the nature of the experimental paradigm. Our experiments were specifically designed to evaluate the effects of TGF-β1 on viral entry, as the virus-reporter system we utilized was not replication competent. Thus, the difference between the two sets of results suggests that there could be multiple TGF-β1-regulatable steps during the process of viral infection. Nonetheless, a clear correlation between CXCR4 surface expression and viral entry is evident from our study.
In summary, we report that TGF-β1 increased CXCR4 expression and SDF-1α-stimulated intracellular signalling in macrophages in both the peripheral immune and the central nervous systems. Furthermore, the increased CXCR4 expression results in enhanced susceptibility of the cells to entry of dual-tropic X4 HIV-1 variants. Our data demonstrate that TGF-β1 regulates the expression of chemokine receptor on human macrophages and rat microglia with clear functional consequences on viral pathogenesis as well as potential alterations in mechanisms whereby chemokines contribute to host immune responses.
References
- 1.Koenig S, Gendelman HE, Orenstein JM, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–93. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 2.Pantaleo G, Graziosi C, Butini L, Pizzo PA, Schnittman SM, Kotler DP, Fauci AS. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:9838–42. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salahuddin SZ, Rose RM, Groopman JE, Markham PD, Gallo RC. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986;68:281–4. [PubMed] [Google Scholar]
- 4.Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 5.Singh A, Collman RG. Heterogeneous spectrum of coreceptor usage among variants within a dualtropic human immunodeficiency virus type 1 primary-isolate quasispecies. J Virol. 2000;74:10229–35. doi: 10.1128/jvi.74.21.10229-10235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor RI, Mohri H, Cao Y, Ho DD. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–7. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tedla N, Palladinetti P, Kelly M, et al. Chemokines and T lymphocyte recruitment to lymph nodes in HIV infection. Am J Pathol. 1996;148:1367–73. [PMC free article] [PubMed] [Google Scholar]
- 8.Trumpfheller C, Tenner-Racz K, Racz P, Fleischer B, Frosch S. Expression of macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES genes in lymph nodes from HIV+ individuals: correlation with a Th1-type cytokine response. Clin Exp Immunol. 1998;112:92–9. doi: 10.1046/j.1365-2249.1998.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentin A, Lu W, Rosati M, Schneider R, Albert J, Karlsson A, Pavlakis GN. Dual effect of interleukin 4 on HIV-1 expression: implications for viral phenotypic switch and disease progression. Proc Natl Acad Sci USA. 1998;95:8886–91. doi: 10.1073/pnas.95.15.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedzierska K, Crowe SM, Turville S, Cunningham AL. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39–56. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 11.Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72:4962–9. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naif HM, Li S, Alali M, et al. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–6. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–44. [PubMed] [Google Scholar]
- 15.Navikas V, Link J, Wahren B, Persson C, Link H. Increased levels of interferon-gamma (IFN-gamma), IL-4 and transforming growth factor-beta (TGF-beta) mRNA expressing blood mononuclear cells in human HIV infection. Clin Exp Immunol. 1994;96:59–63. doi: 10.1111/j.1365-2249.1994.tb06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kekow J, Wachsman W, McCutchan JA, Cronin M, Carson DA, Lotz M. Transforming growth factor beta and noncytopathic mechanisms of immunodeficiency in human immunodeficiency virus infection. Proc Natl Acad Sci USA. 1990;87:8321–5. doi: 10.1073/pnas.87.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu R, Oyaizu N, Than S, Kalyanaraman VS, Wang XP, Pahwa S. HIV-1 gp160 induces transforming growth factor-beta production in human PBMC. Clin Immunol Immunopathol. 1996;80:283–9. doi: 10.1006/clin.1996.0125. [DOI] [PubMed] [Google Scholar]
- 18.Wahl SM, Allen JB, McCartney-Francis N, et al. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991;173:981–91. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoeteweij JP, Golding H, Mostowski H, Blauvelt A. Cytokines regulate expression and function of the HIV coreceptor CXCR4 on human mature dendritic cells. J Immunol. 1998;161:3219–23. [PubMed] [Google Scholar]
- 20.Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, Norcross MA. Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J Biol Chem. 2001;276:49236–43. doi: 10.1074/jbc.M108523200. [DOI] [PubMed] [Google Scholar]
- 21.Lazdins JK, Klimkait T, Woods-Cook K, et al. The replicative restriction of lymphocytotropic isolates of HIV-1 in macrophages is overcome by TGF-beta. AIDS Res Hum Retroviruses. 1992;8:505–11. doi: 10.1089/aid.1992.8.505. [DOI] [PubMed] [Google Scholar]
- 22.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–48. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 23.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10:179–85. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 24.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 25.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–75. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt N, Chene L, Boutolleau D, Nugeyre MT, et al. Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication. J Virol. 2003;77:5784–93. doi: 10.1128/JVI.77.10.5784-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O'Connor MJ, Doms RW, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–13. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shieh JT, Albright AV, Sharron M, Gartner S, Strizki J, Doms RW, Gonzalez-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–9. doi: 10.1128/jvi.72.5.4243-4249.1998. 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Luo D, Streit WJ, Harrison JK. TGF-beta1 upregulates CX3CR1 expression and inhibits fractalkine-stimulated signaling in rat microglia. J Neuroimmunol. 2002;133:46–55. doi: 10.1016/s0165-5728(02)00354-5. [DOI] [PubMed] [Google Scholar]
- 30.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–5. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuttle DL, Anders CB, Aquino-De Jesus MJ. Increased replication of non-syncytium-inducing HIV type 1 isolates in monocyte-derived macrophages is linked to advanced disease in infected children. AIDS Res Hum Retroviruses. 2002;18:353–62. doi: 10.1089/088922202753519133. [DOI] [PubMed] [Google Scholar]
- 32.Crowe SM. Role of macrophages in the pathogenesis of human immunodeficiency virus (HIV) infection. Aust N Z J Medical. 1995;25:777–83. doi: 10.1111/j.1445-5994.1995.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown KA, Roberts RL, Arteaga CL, Law BK. Transforming growth factor-β induces Cdk2 relocalization to the cytoplasm coincident with dephosphorylation of retinoblastoma tumor suppressor protein. Breast Cancer Res. 2004;6:R130–R139. doi: 10.1186/bcr762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volin MV, Joseph L, Shockley MS, Davies PF. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242:46–53. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 35.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 36.Simmons G, Reeves JD, McKnight A, et al. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol. 1998;72:8453–7. doi: 10.1128/jvi.72.10.8453-8457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, Lusso P, Siccardi AG, Vercelli D. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J Immunol. 1998;161:2084–8. [PubMed] [Google Scholar]
- 38.Yi Y, Rana S, Turner JD, Gaddis N, Collman RG. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–7. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi Y, Isaacs SN, Williams DA, Frank I, Schols D, De Clercq E, Kolson DL, Collman RG. Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism. J Virol. 1999;73:7117–25. doi: 10.1128/jvi.73.9.7117-7125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franitza S, Kollet O, Brill A, Vaday GG, Petit I, Lapidot T, Alon R, Lider O. TGF-beta1 enhances SDF-1alpha-induced chemotaxis and homing of naive T cells by up-regulating CXCR4 expression and downstream cytoskeletal effector molecules. Eur J Immunol. 2002;32:193–202. doi: 10.1002/1521-4141(200201)32:1<193::AID-IMMU193>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Salafranca MN, Adhikari S, et al. Chemokine receptor expression in cultured glia and rat experimental allergic encephalomyelitis. J Neuroimmunol. 1998;86:1–12. doi: 10.1016/s0165-5728(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 42.McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Chemokine and chemokine-receptor expression in human glial elements. induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol. 2000;156:1441–53. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–6. [PubMed] [Google Scholar]
- 44.Sehgal A, Ricks S, Boynton AL, Warrick J, Murphy GP. Molecular characterization of CXCR-4: a potential brain tumor-associated gene. J Surg Oncol. 1998;69:239–48. doi: 10.1002/(sici)1096-9098(199812)69:4<239::aid-jso9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 45.Singer II, Scott S, Kawka DW, et al. CCR5, CXCR4, and CD4 are clustered and closely apposed on microvilli of human macrophages and T cells. J Virol. 2001;75:3779–90. doi: 10.1128/JVI.75.8.3779-3790.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bajetto A, Barbero S, Bonavia R, Piccioli P, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001;77:1226–36. doi: 10.1046/j.1471-4159.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 47.Kayali AG, Van Gunst K, Campbell IL, et al. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859–69. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montes M, Tagieva NE, Heveker N, Nahmias C, Baleux F, Trautmann A. SDF-1-induced activation of ERK enhances HIV-1 expression. Eur Cytokine Netw. 2000;11:470–7. [PubMed] [Google Scholar]
- 49.Yang X, Chen Y, Gabuzda D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB. J Biol Chem. 1999;274:27981–8. doi: 10.1074/jbc.274.39.27981. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J Virol. 1999;73:3460–6. doi: 10.1128/jvi.73.4.3460-3466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee B, Leslie G, Soilleux E, et al. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol. 2001;75:12028–38. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen DG, Hildreth JE. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur J Immunol. 2003;33:483–93. doi: 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- 53.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA. 1987;84:5788–92. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiseman DM, Polverini PJ, Kamp DW, Leibovich SJ. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun. 1988;157:793–800. doi: 10.1016/s0006-291x(88)80319-x. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Guan E, Roderiquez G, Norcross MA. Synergistic induction of apoptosis in primary CD4 (+) T cells by macrophage-tropic HIV-1 and TGF-beta1. J Immunol. 2001;167:3360–6. doi: 10.4049/jimmunol.167.6.3360. [DOI] [PubMed] [Google Scholar]
- 56.Zaitseva M, Lee S, Lapham C, Taffs R, King L, Romantseva T, Manischewitz J, Golding H. Interferon gamma and interleukin 6 modulate the susceptibility of macrophages to human immunodeficiency virus type 1 infection. Blood. 2000;96:3109–17. [PubMed] [Google Scholar]