Abstract
Transforming growth factor (TGF)-β, a pleiotropic cytokine that has multiple effects on immune responses, has been shown to inhibit interleukin (IL)-4/GATA-3 expression as well as T helper 2 (Th2) differentiation. Consistent with these reports, we found that priming T cells from DO11.10 transgenic mice with antigen in the presence of TGF-β inhibited GATA-3 expression and the development of IL-4-producing T cells. Unexpectedly, the inhibition of Th2 development was accompanied by a substantial increase in the number of interferon-γ (IFN-γ)-producing cells. T cells primed with TGF-β secreted IFN-γ in response to both T-cell receptor ligation and IL-12/IL-18 stimulation, and expressed high levels of T-bet and low levels of GATA-3. The TGF-β-mediated enhancement of T helper 1 (Th1) priming was independent of IL-12 and signal transducer and activator of transcription (STAT)-4, but required endogenous IFN-γ. TGF-β-mediated enhancement of the IFN-γ-dependent, IL-12-independent pathway of Th1 priming was mediated primarily by the inhibition of IL-4 produced by memory/activated T cells in the unfractionated CD4+ responder population. Nevertheless, TGF-β did not inhibit this pathway of Th1 differentiation when purified naive CD4+ T cells were used as responders. These data have important implications for strategies being considered for the use of TGF-β-producing T cells for the treatment of autoimmune disorders.
Keywords: IFN-γ, T-cell differentiation, TGF-β1, Th1/Th2
Introduction
The factors that drive T-cell differentiation have been studied extensively, and among T helper cells two primary subsets have been extensively characterized based on their cytokine-secretion patterns. T helper 1 (Th1) cells develop in the presence of interleukin (IL)-12/interferon (IFN)-αβ and signal transducer and activator of transcription (STAT)-4 signalling, and secrete mainly IFN-γ.1 Other factors, such as IL-23/IL-27, have recently been shown to be cofactors in IL-12-driven Th1 differentiation/expansion.2, 3 T helper 2 (Th2) cells develop in the presence of IL-4 and STAT-6 signalling, and secrete mainly IL-4, but also IL-5, IL-13 and IL-10.4 We have previously shown that IFN-γ can also prime Th1 cells independent of IL-12, but only in the absence of IL-4.5 At the molecular level, Th1 cells have been shown to express the transcription factor, T-bet, 6 while Th2 cells have been shown to express GATA-3, c-maf and others.7, 8 While Th2 cells secrete IL-4 in response to T-cell receptor (TCR) stimulation, Th1 cells can secrete IFN-γ via two biochemically distinct pathways: in the first, they secrete IFN-γ after stimulation through their TCR; in the second, they secrete high levels of IFN-γ after stimulation with the combination of IL-12 and IL-18, two cytokines that are strongly associated with the generation of Th1 responses. This second pathway is antigen-independent and resistant to cyclosporin treatment, 9 which has important implications for inflammatory diseases where large numbers of activated, antigen-non-specific T cells are recruited to inflammatory sites. The ability of IL-12 plus IL-18 to stimulate high levels of IFN-γ production correlates with high levels of IL-12 receptor (IL-12R)-β2 and IL-18Rα expression.5 We have previously shown that IL-4 plays a major role in regulating both IL-12 and IL-18 responsiveness during T-cell differentiation.5, 10 In the presence of IL-4, both IL-12 and IFN-γ were found to be required for effective Th1 development, as neither IL-12 nor IFN-γ alone was sufficient for Th1 cell priming. In the absence of IL-4, however, either IL-12 or IFN-γ alone was sufficient to elicit IFN-γ-producing cells and supported the existence of an IL-12/STAT-4-independent pathway of Th1 cell development.
Transforming growth factor-β (TGF-β) is a pleiotropic cytokine produced by various cells, with multiple effects on the immune response depending on the local environment in which it is produced, the cell types present, and the relative concentration of TGF-β.11 The effects of TGF-β on the immune response have mostly been described as inhibitory. In addition to interfering with antigen-presenting cell (APC) functions, TGF-β can inhibit T-cell proliferation by directly inhibiting IL-2 production, IL-2R expression and IL-2R responsiveness.12 Cytotoxic T-lymphocyte antigen-4 (CTLA-4)-mediated inhibition of T-cell responses may be mediated, in part, via the induction of TGF-β, 13 although this remains controversial.14 Regulation of T-cell responses by TGF-β produced by regulatory T cells has also not been resolved.15, 16 Furthermore, TGF-β can inhibit Th1 and Th2 differentiation in vitro. In particular, TGF-β can inhibit Th2 development via inhibition of IL-4 and/or GATA-3 expression.17, 18 The effects of TGF-β on Th1 development are less clear. While some reports have described an inhibitory effect of TGF-β on Th1 development, 17, 18 others have described a Th1-promoting effect.19–22
The goal of the present study was to clarify the role of TGF-β in the development of Th1 responses induced via the IL-12-dependent pathway or by the IFN-γ-dependent, IL-12-independent pathway. As reported previously, 17, 18 the priming of T cells in the presence of TGF-β inhibited both GATA-3 and IL-4 expression. Surprisingly, we found that these T cells developed into Th1 cells that secreted high levels of IFN-γ after both TCR and IL-12/IL-18 stimulation. The enhancing effect of TGF-β on the development of IFN-γ-producing cells was independent of both IL-12 and STAT-4, but required IFN-γ and was primarily mediated by inhibiting IL-4 production by endogenous memory T cells. Collectively, these results demonstrate that TGF-β may actually exacerbate Th1/inflammatory responses and warrant caution when considering TGF-β or TGF-β-producing T cells as a therapy for complex autoimmune diseases.
Materials and methods
Animals
Female C57BL/6, C57BL/6 IL-12 p40 deficient (–/–), and C57BL/6 IFN-γ–/– mice were obtained from Taconic Farms (Germantown, PA) and used at 6–10 weeks of age. Female DO11.10 transgenic mice expressing a TCR transgene specific for ovalbumin (OVA) peptide on the BALB/c background were also obtained from Taconic and used at 6–10 weeks of age. DO11.10×RAG-2–/– mice were obtained from Dr Giles Foucras (NIH, Bethesda, MD). Female STAT-4–/– (C129S2-Stat4) mice on the BALB/c background were obtained from Jackson Laboratories (Bar Harbor, ME). All animals had been backcrossed more than 12 generations prior to use. Age-matched and gender-matched BALB/c mice were obtained from NCI (Frederick, MD) and used at 6–10 weeks of age. All animals were housed under specific pathogen-free conditions and provided with food and water ad libitum. Animals were maintained according to NIH Animal Care Guidelines.
Priming conditions
CD4+ T cells were isolated from the spleens of mice by positive selection using autoMACS magnetic separation (Miltenyi Biotech, Auburn, CA). A total of 2 × 105/ml CD4+ T cells were stimulated in vitro for 5 days with soluble anti-CD3 (2C11, 0·5 mg/ml; Pharmingen, San Diego, CA) and irradiated T-depleted spleen cells as APC (1 × 106/ml); alternatively, CD4+ T cells were stimulated in vitro for 7 days with irradiated T-depleted APC and 1 µm OVA peptide in media supplemented with IL-2 [100 U/ml recombinant human (rhu) IL-2], 10% (v/v) fetal bovine serum, 2 mml-glutamine, 100 U/ml penicillin/100 μg/ml streptomycin, and 50 μmβ-mercaptoethanol. An equal volume of fresh IL-2 media was added on day 4. rhuTGF-β1 (R & D, Minneapolis, MN) was used at final concentrations of 1 or 10 ng/ml. Anti-IFN-γ (XMG 1.2) and anti-IL-12p40 (C17.8) were used at a concentration of 10 µg/ml. The concentrations of cytokines used were based on prior experiments that demonstrated biological potency in vitro.
Intracellular cytokine staining
After 1 week of culture, T cells were harvested, washed and resuspended in fresh IL-2 media. Cells were stimulated (1–2 × 106) for 6–12 hr in 12-well plates with immobilized anti-CD3 (2C11, 5 µg/ml), and 2 µm Monensin was added for the final 4 hr of culture. Alternatively, primed T cells (1–2 × 106) were stimulated for 8–12 hr in 24-well round-bottom plates with IL-2 only (20 U/ml), IL-12 only (10 ng/ml), IL-18 (30 ng/ml) only, or both IL-12 and IL-18. Monensin was added to each well, as described above. Both anti-CD3 and cytokine-stimulated cells were harvested, washed and incubated with anti-CD16/32 to block Fc receptor binding. Cells were then stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 for 10 min, washed, then fixed with 100 ml of fluorescence-activated cell sorter (FACS) fixative [phosphate-buffered saline (PBS) containing 1% (w/v) bovine serum albumin (BSA), 4% paraformaldehyde (PFA) (w/v), and 0·01% (w/v) NaN3] for 5 min at 37°. After washing in PBS, the cells were resuspended in permeabilization buffer [0·1% (w/v) saponin in PBS/BSA] containing rat anti-mouse IFN-γ-APC (Pharmingen) and rat anti-mouse IL-4-conjugated phycoerythrin (PE) (Pharmingen) and incubated for 15–20 min. Cells were then washed in PBS, and analysis was performed by using cellquest software.
Analysis of GATA-3 and T-bet expression in T cells
To perform a quantitative analysis of the expression of both GATA-3 and T-bet, CD4+ T cells were primed, as described above, under neutral conditions (i.e. no antibodies or cytokines were added to cultures), but with TGF-β (10 ng/ml), Th1 (IL-12, anti-IL-4), or Th2 (IL-4, anti-IL-12, anti-IFN-γ) conditions. After 5 days of culture, cells were washed and lysed by using RNAzol. RNA was isolated by chloroform extraction, followed by precipitation with isopropanol, and washed in 75% (v/v) ethanol. The purity of RNA was confirmed by measuring the relative absorbances of the RNA at 260 and 280 nm and calculation of the 260/280 ratio. A 2-µg sample of RNA was converted to cDNA by using first-strand cDNA synthesis and reverse transcriptase. Analysis of GATA-3 and T-bet expression was performed by real-time polymerase chain reaction (PCR) using GATA-3/T-bet specific primers. The sequences of the primers and the MGB probe for GATA-3 are 5′-CAGAACCGGCCCCTTATCA-3′, 5′-CATTAGCGTTCCTCCTCCAGA-3′ and 5′-FAM-cgaaggctgtcggca-3′, respectively. Primer sequences and the MGB probe for T-bet are 5′-GAGTGTCCCCTGCCCCTTT-3′, 5′-TCCCTCTGGGTCCAAAACTCT-3′ and 5′-FAM-ctgcccgaactaca-3′, respectively. TaqManÆ Ribosomal RNA Control Reagents for detecting the 18S ribosomal RNA (VIC MGB Probe) were purchased from PE Applied Biosystems (Foster City, CA), and analysis was performed by using sds2·0 software. The levels of GATA-3 and T-bet are expressed relative to expression of the 18s ribosomal protein.
Results
TGF-β inhibits Th2 development and enhances IFN-γ production
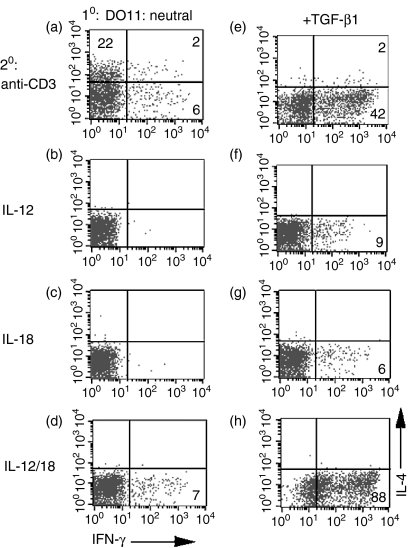
To determine the effects of TGF-β on T-cell differentiation in vitro, we utilized a culture system in which DO11.10 TCR transgenic CD4+ T cells were stimulated with splenic APC and OVA peptide under neutral conditions in the presence of exogenous IL-2, but in the absence of exogenous polarizing cytokines (IL-4 or IL-12). After 1 week of culture, the cells were harvested and their cytokine production determined by intracellular staining. T cells primed under neutral conditions primarily produced IL-4 after restimulation with anti-CD3 (Fig. 1a, 24% IL-4+ versus 8% IFN-γ+), which is consistent with our previous observations. After priming under neutral conditions, very few IFN-γ-producing cells were detected in response to IL-12 (Fig. 1b, < 1%), IL-18 (Fig. 1c, < 1%), or IL-12/IL-18 (Fig. 1d, 7%). When TGF-β was added to the priming cultures, a markedly different cytokine production profile was observed upon secondary challenge. After anti-CD3 stimulation, very few (2%) IL-4-producing cells were detected and a significant increase in the number of IFN-γ-producing cells (Fig. 1e, 44%) was observed. When cells were primed in the presence of TGF-β, only low numbers of IFN-γ-producing cells were detected in response to stimulation with either IL-12 (Fig. 1f, 9%) or IL-18 (Fig. 1g, 6%), but restimulation with the combination of IL-12 and IL-18 led to a very high number of IFN-γ-producing cells (Fig. 1h, 88%). In addition to an increase in the number of IFN-γ-producing cells, we observed a similar increase of IFN-γ in culture supernatants [as detected by enzyme-linked immunosorbent assay (ELISA)] after restimulation with IL-12 and IL-18 (data not shown). In titration experiments, relatively high concentrations of TGF-β (i.e. 1–10 ng/ml range) were most effective at inducing IFN-γ production (data not shown). These results demonstrate that TGF-β not only inhibits the development of IL-4-producing T cells, but also enhances the development of Th1 cells that produce IFN-γ after restimulation by TCR triggering or by IL-12/IL-18 stimulation.
Figure 1.
Transforming growth factor-β (TGF-β) inhibits T helper 2 (Th2) development and enhances interferon-γ (IFN-γ) production. Purified CD4+ T cells from DO11.10 transgenic mice were cultured with ovalbumin (OVA) peptide, antigen-presenting cells (APC), interleukin (IL)-2 (a–h), and TGF-β (e–h) for 1 week. Cells were subsequently restimulated with immobilized anti-CD3 (a, e), IL-12 (b, f), IL-18 (c, g), or IL-12 + IL-18 (d, h). Cells were then analysed by fluorescence-activated cell sorting (FACS) for intracellular IL-4 and IFN-γ. The quadrants were set with respect to non-activated cells. The numbers reflect the percentage of CD4+ T cells secreting cytokine.
The enhancement of IFN-γ production by TGF-β is independent of IL-12
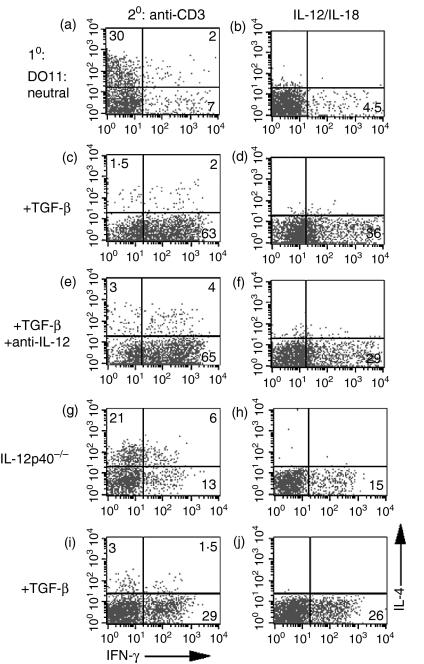
The significant increase in the number of IFN-γ-producing cells observed after priming with TGF-β raised the possibility that TGF-β was increasing IL-12 production, as IL-12 plays a pivotal role in Th1 development. We therefore added neutralizing antibody to IL-12 during the 1-week priming culture period. Anti-IL-12 enhanced the numbers of IL-4-producing cells and inhibited the number of IFN-γ producers when DO11.10 T cells were primed under neutral conditions (compare Fig. 2a, 2b with Fig. 1a, 1b). The addition of anti-IL-12 had very little effect on the enhancement of the numbers of IFN-γ-producing cells seen in the presence of TGF-β (compare Fig. 2c, d with Fig. 2e, f). To rule out the possibility that we had not completely neutralized endogenous IL-12 production with the antibody, we confirmed these results in C57BL/6 IL-12 p40–/– mice. The addition of TGF-β resulted in a marked reduction in the number of IL-4-producing cells (compare Fig. 2i with Fig. 2g) and a substantial increase in the number of IFN-γ-producing cells (compare Fig. 2i,2j with Fig. 2g,2h). Thus, the ability of TGF-β to prime Th1 cells is independent of IL-12. A similar enhancement of IFN-γ-producing cells was seen when CD4+ T cells were primed in the presence of TGF-β in an APC-free system, indicating that the target of TGF-β was the T cell rather than the APC (data not shown).
Figure 2.
Transforming growth factor-β (TGF-β)-mediated enhancement of interferon-γ (IFN-γ) production is interleukin (IL)-12-independent. Purified CD4+ T cells from DO11.10 transgenic mice (a–f) and IL-12 p40–/– mice (g–j) were cultured with anti-CD3, antigen-presenting cells (APC), IL-2 (a–j), TGF-β (c–f, i, j), and anti-IL-12 p40 (e, f) for 1 week. The cells were subsequently restimulated with either immobilized anti-CD3 (a, c, e, g, i)or IL-12 + IL-18 (b, d, f, h, j).Cells were then analysed by fluorescence-activated cell sorting (FACS) for intracellular IL-4 and IFN-γ. Numbers reflect the percentage of CD4+ T cells secreting cytokine.
STAT-4 is not required for TGF-β enhancement of IFN-γ production
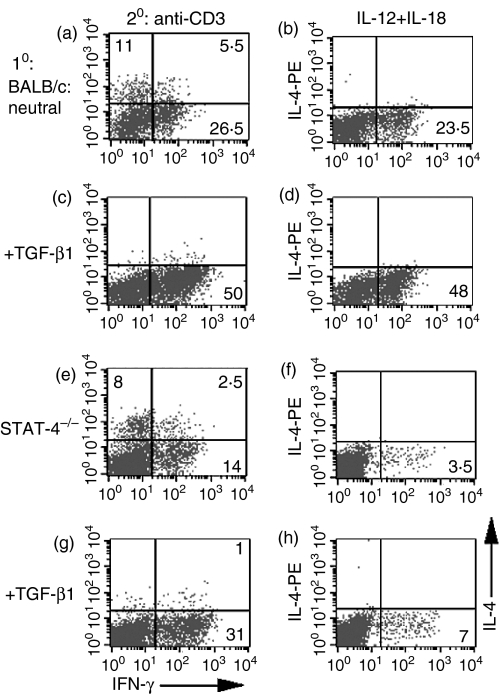
A principal intracellular mediator of IL-12 activity is STAT-4, which translocates to the nucleus and binds to the IFN-γ promoter for induction of IFN-γ production. Although IL-12 has been shown to play no role in the enhancement of IFN-γ production by TGF-β, it is still possible that STAT-4 participates in the process, as IFN-αβ can activate STAT-4 in human T cells.23 However, CD4+ T cells from wild-type BALB/c mice and from BALB/c STAT-4–/– mice demonstrated equivalent enhancement in the number of IFN-γ-producing cells when primed with anti-CD3 and TGF-β and restimulated with anti-CD3 (compare Fig. 3a,3c with Fig. 3e,3f). Although wild-type T cells primed in the presence of TGF-β also demonstrated an increased number of IFN-γ-producing cells when restimulated with IL-12/IL-18 (Fig. 3b, panel d versus b), as expected CD4+ T cells from STAT-4–/– mice, primed in the presence or absence of TGF-β, only produced low numbers of IFN-γ-producing cells (Fig. 3f,3h).
Figure 3.
Transforming growth factor-β (TGF-β)-mediated enhancement of cytokine-induced interferon-γ (IFN-γ) production is STAT-4 independent. Purified CD4+ T cells from BALB/c mice (a–d) and STAT-4–/– mice (e–h) were cultured with anti-CD3, antigen-presenting cells (APC), interleukin (IL)-2 (a–h) or TGF-β (c–d, g–h) for 1 week. The cells were subsequently restimulated with either immobilized anti-CD3 (a, c, e, g) or IL-12 + IL-18 (b, d, f, h). Cells were then analysed by fluorescence-activated cell sorting (FACS) for intracellular IL-4 and IFN-γ. Numbers reflect the percentage of CD4+ T cells secreting cytokine.
The TGF-β enhancement of IFN-γ production is IFN-γ dependent
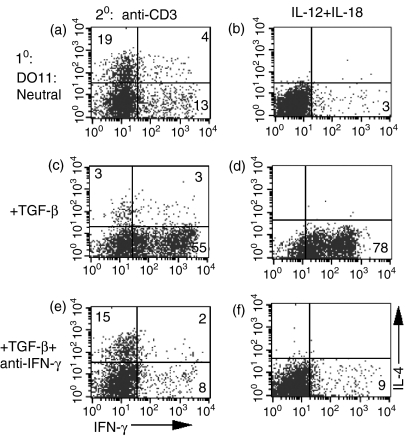
As we have shown previously that IFN-γ is sufficient to prime IFN-γ-producing T cells in the absence of IL-4, 5 and TGF-β had inhibitory effects on IL-4 production (this report), it seemed probable that the enhancement of IFN-γ production by TGF-β was also dependent on IFN-γ. To test this hypothesis, we neutralized endogenous IFN-γ production in cultures primed in the presence of TGF-β. The addition of TGF-β to the priming of DO11.10 CD4+ T cells inhibited IL-4 production and markedly enhanced IFN-γ production (compare Fig. 4c,4d with Fig. 4a,4b). Neutralization of endogenous IFN-γ in the priming cultures completely reversed the enhancement of IFN-γ production seen in the presence of TGF-β (compare Fig. 4e,4f with Fig. 4c,4d). Thus, the ability of TGF-β to enhance IFN-γ production is IL-12/STAT-4 independent, but IFN-γ dependent.
Figure 4.
Transforming growth factor-β (TGF-β)-mediated priming of T helper 1 (Th1) cells is interferon-γ (IFN-γ) dependent. Purified CD4+ T cells from DO11.10 transgenic mice (a–f) were cultured with ovalbumin (OVA), antigen-presenting cells (APC), interleukin (IL)-2 (a–f), TGF-β (c–f), and anti-IFN-γ (e–f) for 1 week. The cells were subsequently restimulated with either immobilized anti-CD3 (a, c, e) or IL-12 + IL-18 (b, d, f). Cells were then analysed by fluorescence-activated cell sorting (FACS) for intracellular IL-4 and IFN-γ. Numbers reflect the percentage of CD4+ T cells secreting cytokine.
IFN-γ-producing cells primed in the presence of TGF-β have a decreased expression of GATA-3, but an increased expression of T-bet
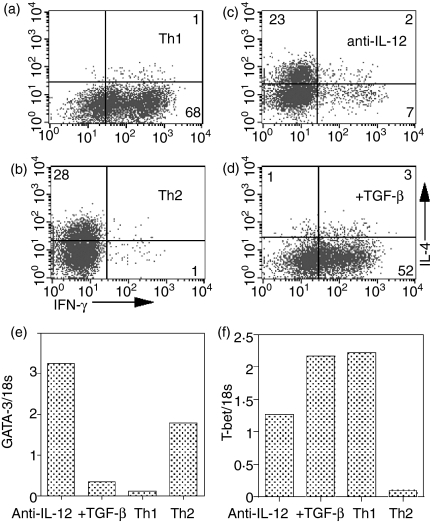
T cells primed in the presence of TGF-β showed enhanced IFN-γ production after restimulation with either anti-CD3 or the combination of IL-12/IL-18. Although cells generated under these culture conditions resembled true Th1 cells, based on cytokine production, we wished to further characterize them to determine whether they express transcription factors typically associated with Th1/Th2 differentiation. CD4+ T cells from DO11.10 mice were primed under Th1 conditions (IL-12 and anti-IL-4), Th2 conditions (IL-4, anti-IFN-γ and anti-IL-12), with anti-IL-12, or with anti-IL-12 + TGF-β. After 5 days of culture, the cells were harvested and split into two groups. The first group was restimulated with anti-CD3 to determine the cytokine profile of the primed cells. The second group was used for real-time PCR analysis of GATA-3 and T-bet gene expression. T cells primed under standard Th1 conditions produced high levels of IFN-γ(Fig. 5a, 69%) and, as expected, expressed low levels of GATA-3 and high levels of T-bet (Fig. 5e,5f). Conversely, T cells primed under Th2 conditons expressed a high number of IL-4-producing cells after restimulation with anti-CD3 (Fig. 5b, 28%), and expressed high levels of GATA-3 (Fig. 5e), but low levels of T-bet (Fig. 5f). T cells primed in the presence of anti-IL-12 were mainly IL-4 producers (Fig. 5c, 25%), but a small number of IFN-γ producers was also present (Fig. 5c, 9%). Both GATA-3 (Fig. 5e) and T-bet (Fig. 5f) were detectable in this population. However, the addition of TGF-β to cultures primed in the presence of anti-IL-12 resulted in a marked increase of the number of IFN-γ-producing cells (compare Fig. 5d with Fig. 5c), low levels of GATA-3 (Fig. 5e) and high levels of T-bet (Fig. 5f). Thus, it appears that Th1 cells primed after culture with TGF-β closely resemble Th1 cells generated in the presence of IL-12.
Figure 5.
Priming with transforming growth factor-β (TGF-β) leads to decreased GATA-3 and increased T-bet expression. Purified CD4+ T cells from DO11.10 transgenic mice (a–f) were cultured with ovalbumin (OVA), antigen-presenting cells (APC), interleukin (IL)-2 (a–d), IL-12 + anti-IL-4 (a), IL-4 + anti-IL-12 + anti-interferon-γ (IFN-γ)(b), anti-IL-12(c), or anti-IL-12 + TGF-β(d)for 1 week. Cells were subsequently restimulated with immobilized anti-CD3 (a–d) and analysed by fluorescence-activated cell sorting (FACS) for intracellular IL-4 and IFN-γ. Numbers reflect the percentage of CD4+ T cells secreting cytokine. cDNA made from cells in (c) was subjected to real-time polymerase chain reaction (PCR) using both GATA-3 and T-bet-specific primers, and the expression of GATA-3(e)and T-bet(f)was determined. Expression is reported as relative units compared to 18s expression. Th1, T helper 1; Th2, T helper 2.
TGF-β-mediated enhancement of IFN-γ production is primarily indirectly mediated by inhibition of IL-4 production by endogenous memory/activated CD4+ T cells
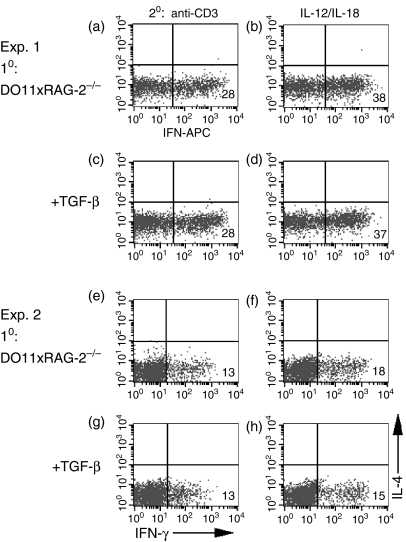
The experiments performed in this study have utilized CD4+ T cells purified from TCR transgenic DO11.10 mice maintained on a conventional background that consists of a mixture of naive and memory T cells. As TGF-β has been reported to have different effects on naive versus memory T cells, 20 we examined the effects of TGF-β on the priming of T cells obtained from transgenic mice on a RAG-2–/– background. T cells from DO11.10×RAG-2–/– mice primed with APC/OVA and anti-IL-12 developed solely into IFN-γ-producing cells (Fig. 6a, 6b). As previously reported, 5 expression of the DO11.10 transgene on a RAG-2–/– background virtually eliminated the development of IL-4-producing cells (Fig. 6a,6b), suggesting that priming of OVA-specific IL-4-producing T cells in conventional DO11.10 mice is linked to IL-4 production by endogenous memory T cells. When T cells from DO11.10×RAG-2–/– mice were primed in the presence of anti-IL-12, substantial numbers of IFN-γ-producing T cells were generated, but no enhancement in the number of IFN-γ-producing cells was observed in the presence of TGF-β (Fig. 6). However, it should also be emphasized that TGF-β did not inhibit the induction of Th1-producing cells in these cultures. These data support the hypothesis that TGF-β enhances the priming of Th1 cells primarily by blocking IL-4 production from previously activated/memory T cells. Similar results were observed when we repeated these studies using CD4+ CD62Lhigh T cells from DO11.10 mice maintained on a conventional background (data not shown).
Figure 6.
Role of endogenous memory/activated T cells in the transforming growth factor-β (TGF-β)-mediated enhancement of interferon-γ (IFN-γ) production. Purified CD4+ T cells from DO11.10×RAG-2–/– transgenic mice (a–h) were cultured with ovalbumin (OVA), antigen-presenting cells (APC), interleukin (IL)-2 (a–h) and TGF-β (c, d, g, h), for 1 week. The cells were subsequently restimulated with either immobilized anti-CD3 (a, c, e, g) or IL-12 + IL-18 (b, d, f, h). Cells were then analysed by fluorescence-activated cell sorting (FACS) for intracellular IL-4 and interferon-γ (IFN-γ). Numbers reflect the percentage of CD4+ T cells secreting cytokine.
Discussion
TGF-β has been shown to inhibit IL-2 production and IL-2 responsiveness.12 In addition, TGF-β inhibits T-cell differentiation/polarization even under conditions in which exogenous IL-2 is added to the cultures and T-cell proliferation is normalized. In general, almost all studies have demonstrated that TGF-β efficiently inhibits the induction of Th2 responses, even in the presence of exogenous IL-4.17, 18 However, a clear consensus on the effects of TGF-β on Th1 differentiation are lacking. Several early studies showed that the addition of TGF-β to unfractionated CD4+ T cells activated by polyclonal T-cell stimulation led to an enhancement of IFN-γ production.21 More recent studies, using naive CD4+ T cells, have shown that TGF-β is able to down-regulate Th1 development in the presence of IL-12.19, 20 In the present report, we have confirmed that TGF-β is a potent inhibitor of Th2 differentiation. However, we show that the inhibition of Th2 differentiation leads to a default pathway of Th1 differentiation that is IFN-γ dependent, but IL-12 independent. We intentionally used unfractionated CD4+ T cells in these studies in an attempt to mimic the physiological situation where TGF-β might be used therapeutically to inhibit immune responses in vivo. The primary effect of TGF-β in enhancing Th1 differentiation was mediated by inhibiting IL-4 production by memory/activated T cells in our unfractionated T-cell population, as TGF-β failed to augment the induction of Th1 differentiation when naive T cells were used as responders. However, when naive T cells were stimulated in the presence of anti-IL-12 and TGF-β, Th1 differentiation was not inhibited, demonstrating complete resistance of the IFN-γ-dependent, IL-12-independent pathway of Th1 differentiation to inhibition by TGF-β.
The prevailing paradigm for many years has been that IL-4 is the principal cytokine for inducing Th2 responses, while IL-12 plays a similar role in the induction of Th1 responses. However, the identification of the transcription factor, T-bet, has prompted a re-examination of the inductive signals for Th1 differentiation.6 Naive T cells do not express the IL-12R, STAT-4, or T-bet. T-bet appears to be induced by TCR signals, in concert with IFN-γ, by a STAT-1-dependent process. T-bet subsequently regulates IL-12 responsiveness by inducing the IL-12Rβ2 chain and IL-12 signalling via STAT-4, which facilitates Th1 development. It is still unclear whether full Th1 differentiation can occur in the absence of IL-12. IL-12–/– mice exhibit a defect in their ability to mount Th1 responses, but polarized CD4+ Th1 cells, secreting high levels of IFN-γ, can be detected in IL-12–/– mice in response to certain viral infections.24, 25 IL-23 and IL-27 have recently been reported to prime/enhance Th1 differentiation and IFN-γ production.2, 3 IL-23 consists of the IL-12 p40 subunit paired with a p19 subunit. Because IL-12 p40–/– mice were used in these experiments, IL-23 probably does not play a role in the TGF-β-mediated enhancement of IFN-γ. Although STAT 4–/– mice exhibit a defect in their ability to mount a Th1 response as the result of disruption of the major IL-12 signalling pathway, simultaneous disruption of the STAT-6 gene leads to the development of IFN-γ-producing cells.26 The generation of primed, uncommitted T cells secreting IL-2, but not IFN-γ or IL-4, occurs when TGF-β and anti-IFN-γ are added to cultures.27 Thus, both in vivo and in vitro, IFN-γ alone may be sufficient for the activation of some Th1 responses.
The inhibitory effects of TGF-β on the induction of IL-4-driven Th2 differentiation and IL-12-driven Th1 differentiation appear to be mediated by inhibiting the induction of GATA-3 and T-bet, respectively.17, 19 T cells ectopically expressing GATA-3 were markedly resistant to the inhibitory effects of TGF-β. Similarly, restoration of T-bet expression alone through retroviral transduction completely reversed the TGF-β-mediated inhibition of IL-12-driven Th1 differentiation. None of the previous studies have examined the effects of TGF-β on T-bet expression induced by IFN-γ in the absence of IL-12. As clearly seen from the data presented in Fig. 5, the level of T-bet induction occurring after priming with TGF-β and anti-IL-12 is highly significant and similar to that of T cells stimulated under conventional Th1 conditions in the presence of IL-12. It remains possible that in the presence of IL-12, TGF-β may inhibit the induction of T-bet by inhibiting IL-12R expression or signalling, 28 but the effects of TGF-β on IL-12R signalling or expression have not been seen in all studies.19 The lack of effect of TGF-β on the IFN-γ-dependent induction of T-bet and, subsequently, Th1 differentiation also appear to conflict with the very recent studies of Chen et al., 29 who demonstrated that TGF-β may globally inhibit both Th1 and Th2 cell polarization by preventing phosphorylation of the Tec kinase, Itk, with subsequent inhibition of the elevation of [Ca2+]i and NFATc activation. However, it should be pointed out that in this latter study the inhibitory effects of TGF-β on T-bet induction and NFATc activation in Th1 differentiation were not complete and were only studied in the presence of IL-12. Residual NFAT activation under nonpolarizing conditions has been shown to favour the development of IFN-γ-producing T cells in vitro.30 Furthermore, T cells from Itk-deficient mice primed under nonpolarizing conditions not only failed to differentiate into Th2 cells, but expressed high levels of T-bet and IFN-γ.31 The roles of Itk and NFATc in the induction of T-bet and IFN-γ production, independent of IL-12, remain to be determined.
While we cannot fully reconcile our observations with those of others in which TGF-β acts as a global inhibitor of both Th1 and Th2 differentiation, 19, 20 it is clear that the TGF-β-mediated inhibition of T-bet is not complete and that, under certain culture conditions, residual T-bet may be sufficient to drive IL-12-independent Th1 differentiation in the absence of IL-4. The effect of TGF-β on Th1 priming is further supported by the fact that in this study T cells were equally capable of IFN-γ secretion following stimulation via their TCR or via the IL-12/IL-18 receptor pathway, thus defining them as true Th1 cells. T-cell stimulation via phorbol 12-myristate 13-acetate (PMA)/ionomycin or other culture conditions may not necessarily reflect the full effects on Th1 cell priming. It is also possible that inclusion of exogenous IL-2 during T-cell priming bypasses a critical inhibitory step.
TGF-β–/– mice develop multiorgan inflammatory immune infiltrates at 2–3 weeks of age.32 Similarly, transgenic mice that express a dominant negative form of the TGF-βRII under control of the CD233 or CD4 promoter, 34 and have blocked TGF-β signalling in T cells, have pronounced lymphocyte infiltrations and spontaneous T-cell activation. Systemic administration of TGF-β has been shown to prevent the induction of experimental allergic encepholomyelitis (EAE) and collagen-induced arthritis, 35 although prolonged treatment with TGF-β may lead to liver fibrosis and glomerulosclerosis.36, 37 Other approaches, such as the administration of T-cell clones that recognize organ-specific antigens and that produce TGF-β38 or have been transduced to express TGF-β, 39 have also been proven to lead to the amelioration of autoimmune disease, and these approaches may have fewer systemic side-effects. TGF-β also contributes to the therapeutic effects of regulatory T cells in preventing the development of inflammatory bowel disease induced by CD45RBhi effector cells, although it has yet to be shown that the TGF-β is produced by the transferred regulatory T cells, rather than by the host cells.40 Collectively, these studies suggest that TGF-β has potential as a therapeutic for Th1-mediated autoimmune diseases. Although these studies appear to conflict with our data that one pathway of Th1 differentiation is enhanced by TGF-β, it should be pointed out that most of the therapeutic effects of TGF-β were observed when it was administered early in the course of the disease in relatively well-defined experimental models. Most spontaneously occurring autoimmune diseases have both Th1 and Th2 components, and the latter may be protective rather than pathogenic. There is little doubt from this and earlier studies that TGF-β is a potent inhibitor of Th2 differentiation, and our present findings raise the possibility that under certain conditions it may simultaneously enhance the activation of pathogenic Th1 cells. Caution should therefore be exercised in the design of therapeutic trials of TGF-β producing T cells for the therapy of both complex systemic and organ-specific autoimmune diseases.
Acknowledgments
The authors wish to thank Dr Jeff Zhu (NIH, NIAID) for technical assistance with the real-time PCR analysis.
References
- 1.OíShea JJ, Paul WE. Regulation of Th1 differentiation-controlling the controllers. Nat Immunol. 2002;3:506–8. doi: 10.1038/ni0602-506. [DOI] [PubMed] [Google Scholar]
- 2.Cua DJ, Sherlock J, Murphy CA, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 3.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Role of IL-27/WSX-1 signaling for induction of T-bet through activation of Stat-1 during initial Th1 commitment. J Immunol. 2003;170:4886–90. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 4.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–711. [PubMed] [Google Scholar]
- 5.Smeltz RB, Chen J, Ehrhardt R, Shevach EM. Role of IFN-γ in Th1 differentiation: IFN-γ regulates IL-18Rα expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor β2 expression. J Immunol. 2002;168:6165–72. doi: 10.4049/jimmunol.168.12.6165. [DOI] [PubMed] [Google Scholar]
- 6.Szabo SJ, Kim St Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 7.Kim JI, Ho IC, Grusby MJ, Gllimcher LH. The transcription factor c-maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–51. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, OíGarra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105–15. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Murphy TL, Ouyang W, Murphy KM. Induction of interferon- production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur. J Immunol. 1999;29:548–55. doi: 10.1002/(SICI)1521-4141(199902)29:02<548::AID-IMMU548>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Smeltz RB, Chen J, Hu-Li J, Shevach EM. Regulation of interleukin (IL)-18 receptor α chain expression on CD3+ T cells during T helper (TH)1/TH2 differentiation: critical downregulatory role of IL-4. J Exp Med. 2001;194:143–53. doi: 10.1084/jem.194.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letterio JJ. Murine models define the role of TGF-β as a master regulator of immune cell function. Cytokine Growth Factor Rev. 2000;11:81–7. doi: 10.1016/s1359-6101(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 12.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor β by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–50. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WJ, Jin WW, Wahl SM. Engagement of cytotoxic T lymphocyte-associates antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-β) production by murine CD4+ T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan TJ, Letterio JJ, van Elsas A, et al. Lack of a role for transforming growth factor-beta in cytotoxic antigen-4-mediated inhibition of T cell activation. Proc Natl Acad Sci USA. 2001;98:2587–92. doi: 10.1073/pnas.051632398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+ CD25+ regulatory T cells is mediated by a cell surface-bound transforming growth factor β. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4+ CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J Exp Med. 2002;196:237–45. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelik L, Fields PE, Flavell RA. Cutting edge. TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–7. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 18.Heath VL, Murphy EE, Crain C, Tomlinson MG, OíGarra A. TGF-β1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–49. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor-β-induced inhibiton of T helper type 1 differentiation. J Exp Med. 2002;195:1499–505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludviksson BR, Seegers D, Resnick AS, Strober W. The effect of TGF-β1 on immune responses of naive versus memory CD4+ Th1/Th2 cells. Eur J Immunol. 2000;30:2101–11. doi: 10.1002/1521-4141(200007)30:7<2101::AID-IMMU2101>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Lingnau K, Hoehn P, Kerdine S, Koelsch S, Neudoerfl C, Palm N, Ruede E, Schmitt E. IL-4 in combination with TGF-β favors an alternative pathway of Th1 development independent of IL-12. J Immunol. 1998;161:4709–18. [PubMed] [Google Scholar]
- 22.Farrar JD, Smith JD, Murphy TL, Leung S, Stark GR, Murphy KM. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat Immunol. 2000;1:65–9. doi: 10.1038/76932. [DOI] [PubMed] [Google Scholar]
- 23.Swain S, Huston G, Tokonogy S, Weinberg A. Transforming growth factor-β and IL-4 cause helper cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and surface phenotype. J Immunol. 1991;147:2991–3000. [PubMed] [Google Scholar]
- 24.Oxenius A, Karrer U, Zinkernagel R, Hentgartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–73. [PubMed] [Google Scholar]
- 25.Schijns VEC, Haagmans BL, Wierda CMH, Kruithof B, Heijnen IAFM, Alber G, Horzinek MC. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J Immunol. 1998;160:3958–64. [PubMed] [Google Scholar]
- 26.Kaplan MH, Wurster AL, Grusby MJ. A signal transducer and activator of transcription (Stat) 4-independent pathway for the development of T helper type 1 cells. J Exp Med. 1998;188:1191–6. doi: 10.1084/jem.188.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Mossman T. Synthesis of several chemokines but few cytokines by primed uncommitted precursor CD4 T cells suggests that these cells recruit other immune cells without exerting direct effector function. Eur J Immunol. 2004;34:1617–26. doi: 10.1002/eji.200424939. [DOI] [PubMed] [Google Scholar]
- 28.Gorham JD, Guler ML, Fenoglio D, Gubler U, Murphy KM. Low dose TGF-β attentuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161:1664–70. [PubMed] [Google Scholar]
- 29.Chen CH, Seguin-Devaux C, Burke NA, Oriss TB, Watkins SC, Clipstone N, Ray A. Transforming growth factor β blocks Tec kinase phosphorylation Ca2+ influx, an dNFATc translocation causing inhibition of T cell differentiation. J Exp Med. 2003;197:1689–99. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter CM, Clipstone NA. Sustained NFAT signaling promotes a Th1-like pattern of gene expression in primary murine CD4+ T cells. J Immunol. 2002;168:4936–45. doi: 10.4049/jimmunol.168.10.4936. [DOI] [PubMed] [Google Scholar]
- 31.Miller AT, Wilcox HM, Lai Z, Berg L. Signalling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Christ M, McCartney-Francis NL, Kulkarni AB, et al. Immune dysregulation in TGF-β1-deficient mice. J Immunol. 1994;153:1936–46. [PubMed] [Google Scholar]
- 33.Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor β II receptor. J Exp Med. 2000;191:1187–96. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelick L, Flavell RA. Abrogation of TGF-β signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 35.Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Palladino NA, Thorbecke GJ. Protective effect of transforming growth factor-β-1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA. 1991;88:2918–21. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-β 1 transgenic mice. J Clin Invest. 1997;100:2697–713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabresi PA, Fields NS, Maloni HW, et al. Phase 1 trial of transforming growth factor β2 in chronic progressive MS. Neurology. 1998;51:289–92. doi: 10.1212/wnl.51.1.289. [DOI] [PubMed] [Google Scholar]
- 38.Chen YH, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T-cell clones induced by oral tolerance-suppression of autoimmune encephalomyelitis. Science. 1994;265:127–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 39.Chen LZ, Hochwald GM, Huang C, et al. Gene therapy in allergic encephalomyelitis using myelin basic protein-specific T cells engineered to express latent transforming growth factor-β1. Proc Natl Acad Sci USA. 1998;95:12516–21. doi: 10.1073/pnas.95.21.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-β but not interleukin 4 in suppression of T helper type 1-mediated colitis by CD45Rblow CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]