Abstract
Multidrug resistance proteins [MRPs and P-glycoprotein (Pgp)] are members of the family of ATP-binding cassette (ABC) transport proteins, originally described as being involved in the resistance against anti-cancer agents in tumour cells. These proteins act as ATP-dependent efflux pumps and have now been described in normal cells where they exert physiological roles. The aim of this work was to investigate the expression and activity of MRP and Pgp in the thymoma cell line, EL4. It was observed that EL4 cells expressed mRNA for MRP1, but not for MRP2, MRP3 or Pgp. The activity of ABC transport proteins was evaluated by using the efflux of the fluorescent probes carboxy-2′-7′-dichlorofluorescein diacetate (CFDA) and rhodamine 123 (Rho 123). EL4 cells did not retain CFDA intracellularly, and MRP inhibitors (probenecid, indomethacin and MK 571) decreased MRP1 activity in a concentration-dependent manner. As expected, EL4 cells accumulated Rho 123, and the presence of cyclosporin A and verapamil did not modify this accumulation. Most importantly, when EL4 cells were incubated in the presence of the MRP1 inhibitors indomethacin and MK 571 for 6 days, they started to express CD4 and CD8 molecules on their surface, producing double-positive cells and CD8 single-positive cells. Our results suggest that MRP activity is important for the maintenance of the undifferentiated state in this cell type. This finding might have implications in the physiological process of normal thymocyte maturation.
Keywords: indomethacin, MK 571, MRP inhibitors, MRP, thymoma cell line
Introduction
The thymus is a central lymphoid organ in which bone marrow-derived T-cell precursors undergo maturation and differentiation. During maturation in the thymus, immature T cells express several genes that induce proliferation, generation of a diverse repertoire, changes in phenotype and acquisition of functional competence.1–4 The developmental stages are characterized by variable expression of the co-receptor molecules CD4, CD8 and of the T-cell receptor (TCR)/CD3 complex.1 The maturation process is dependent on numerous factors produced by the thymic microenvironment or exogenous to it, such as cytokines, hormones, chemicals or biological compounds. Such agents are capable of influencing thymocyte development and proliferation, and are also capable of inducing or inhibiting cell death.2, 5 It has been described that inflammatory lipid mediators, such as leukotrienes (LT) and prostaglandins (PG), which result from the enzymatic oxidation of arachidonic acid, modulate immature thymocyte proliferation.6 Furthermore, these mediators alter the expression of surface molecules (CD3, CD4 and CD8) by acting on immature thymocytes.7 More than 90% of thymocytes die by apoptosis,1 in a death process that involves the production of oxygen radicals (ROS).8–11 The oxidation of glutathione (GSH) by ROS is an important mechanism of cellular detoxification, and depletion of GSH induces or increases the sensitivity to apoptosis in thymocytes.8–11
Leukotrienes (LTC4, LTD4, LTE4), or the secretion of prostaglandin (PGA2) and efflux of glutathione disulfide, represent part of the physiological function of multidrug resistance protein 1 (MRP1 or ABCC1) that may transport organic anions and other substances conjugated to glutathione, glucuronide, or sulphate.12, 13 The members of the MRP family are ATP-dependent efflux pumps, belonging to the ABC family of transport proteins, and are also involved in the resistance to anti-cancer drugs.13, 14 MRP1 is expressed in tumour cells14 and in normal tissues, such as brain,15 liver,16 intestine,17 kidney,17 dendritic cells18 and mature lymphocytes.19
Although no protein member of the MRP family has been described in the thymus, another member of the ATP-binding cassette (ABC) family of transport proteins, P-glycoprotein (Pgp), has been identified in this organ. In 1995, MacDonald and collaborators showed that thymocytes in the late stages of maturation present Pgp activity.20 In rodents, Pgp is encoded by a family of three multidrug resistance genes (mdr1a, mdr1b and mdr2) and in humans by two genes (mdr1 and mdr2) (reviewed in ref. 21). Initially, Pgp has been described in tumour cells where it was related to the multidrug resistance (MDR) phenomenon.22 Similarly to MRP1, Pgp acts as an ATP-dependent efflux pump, transporting many substances, such as ions, peptides, hormones and chemotherapics, protecting the cells from toxic compounds (reviewed in ref. 23). The physiological function of Pgp remains unknown. However, some authors suggest that Pgp is involved in the secretion of steroid hormones24 and in the release of cytokines.25 The blockade of Pgp inhibits lymphocyte proliferation and induces apoptosis.26
In the present investigation, we studied the expression and activity of MRP proteins and Pgp in a thymoma cell line (EL4). The EL4 thymoma was first isolated from a C57BL/6 mouse by Gorer.27 These cells do not express CD4 or CD8 surface molecules,28 but express CD3,29 CD9029 and H-2d,30 exhibiting an immature phenotype.
Materials and methods
Reagents
The culture medium RPMI 1640 (Sigma) was used for culturing the cell line. Carboxy-2′-7′-dichlorofluorescein diacetate (CFDA) obtained from Molecular Probes (Eugene, OR) was dissolved in dimethylsulfoxide (DMSO; Sigma Chemical Co., St Louis, MO) as a 10 mm stock solution and stored at −20°. Rhodamine 123 (Rho 123) was dissolved in 0·9% (w/v) NaCl as a 100 µg/ml stock solution and stored at −20°. The fluorescent probes, CFDA and Rho 123, were diluted in RPMI 1640 to a final concentration of 500 nm and 200 ng/ml, respectively, just before use. Indomethacin (INDO) was dissolved as a stock solution in DMSO at a concentration of 30 mm and stored at −20°. Probenecid (PRB) and verapamil (VP) were dissolved in 0·9% (w/v) NaCl as stock solutions of 25 mm and 5 mm, respectively, and stored at −20°. The reagents Rho 123, INDO, PRB and VP were all obtained from Sigma. Cyclosporine A (CsA), obtained from Sigma-Pharma (SP Brazil), was dissolved in 0·9% (w/v) NaCl at a concentration of 1 mm and stored at −20°. MK 571 was kindly donated by Merk (Merk, Sharp, Dohme, West Point, PA), and was dissolved in distilled water as a 50 mm stock solution and stored at −20°. The drugs INDO, PRB, CSA and MK 571 were diluted in RPMI 1640 just before use. MTT [(3-[4,5-dithylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)], also obtained from Sigma, was dissolved in saline at 5 mg/ml, and stored at 4°.
Cell line
The murine thymoma cell line EL4 (a gift from the Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brazil) was cultured (at 37° in a humidified atmosphere of 5% CO2 in air) in RPMI 1640 supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco Life Technologies, Rockville, MD), 50 µmβ-mercaptoethanol (Sigma), 60 mg/l penicillin (Sigma) and 100 mg/l streptomycin (Sigma), pH 7·4. The cell line was passaged twice a week at a concentration of 105 cells/ml.
MRP- and Pgp-related transport activities
MRP- and Pgp-related transport activities were investigated by using the fluorescent probes CFDA and Rho 123 in an efflux assay.31, 32 Cells (106 cells/ml) were loaded with 500 nm CFDA or 200 ng/ml Rho 123 in the presence or absence of MRP inhibitors or Pgp inhibitors. To reverse the MRP activity, we used PRB (100, 500 or 1000 µm); INDO (37·5, 75 or 150 µm) or MK 571 (6·25, 12·5 or 25 µm). To reverse Pgp activity, CSA (0·1, 0·5 or 1 µm) and VP (5, 10 or 50 µm) were used. Cells were incubated in RPMI 1640 for 30 min at 37°, in the presence of the fluorescent probes. Then, CFDA- or Rho 123-loaded cells were washed with cold medium. Cells were reincubated in medium, with or without MRP or Pgp inhibitors, for 30 min at 37°. The fluorescence retained by the cells was immediately examined by flow cytometry using a FACScalibur (Becton Dickinson, San Jose, CA). The samples were excited at 488 nm by using an argon laser, and fluorescent emission was detected at 530 nm (FL-1). Ten-thousand cells were acquired based on forward and side scatter. All flow cytometry analyses were accomplished by using winmdi, version 2·8.
Reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted from EL4 cells by using the Trizol® reagent (Invitrogen, Life Technologies, Grand Island, NY) protocol. To eliminate the passenger DNA, the sample was treated with RNAse-free DNAse (Promega, Madison, WI) using the kit protocol. The RT–PCR assay was performed as described in the one-step protocol of the Retrotools kit (Biotools, Alberta, Canada). Briefly, the reverse transcription was performed by using 2 µg of total RNA, 2 µl of RT buffer, 2 µl of MnCl2, 0·5 µl of dNTP mix, 150 U of Retrotools polymerase, 40 pm antisense primer [mrp1, 5′-CGCAGGTTGTGCAGGCCGAT-3′; mrp2, 5′-TTTGTCCTTTCACTACTTC-3′; mrp3, 5′-GGTCATCCGTCTCCAAGTCA-3′; mdr1a, 5′-CTGATGTTGCTTCGTCCAG-3′; mdr1b, 5′-GCAAACACTGGTTGTATGCAC-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-TGAATACGGCTACAGCAACA-3′] and RNAse-free H2O, in a final volume of 10 µl. The reaction was allowed to proceed at 60° for 1 hr. Then, the product was immediately put on ice. To 5 µl of the RT reaction was added 3 µl of 5× DNA-free buffer, 1 µl of MgCl2, 10 pm of the sense primer (mrp1, 5′-GTAGAGTTCCGGGATTAC-3′; mrp2, 5′-TGGCTGAGATCGGAGAG-3′; mrp3, 5′-CGCTCTCAGCTCACCATCAT-3′; mdr1a, 5′-CACAGCTGGGCATTGTGT-3′; and GAPDH, 5′-GAAAGCTGTGGCGTGATG-3′) and RNase-free H2O, in a final volume of 25 µl. The conditions for the PCR were as follows: 2 min at 94°, 15 seconds at 94°, 30 seconds at 50° and 1 min at 72°, for 35 cycles, in a DNA thermal cycler (GeneAmp PCR system 2400; Applied Biosystems, Foster City, CA). The PCR products were size fractionated in 1·8% agarose gels stained with 10 µg/ml ethidium bromide. The DNA band sizes were confirmed by using DNA Ladder (φX174 RF DNA/HaeIII; Invitrogen, Life Technologies).
Viability assay
EL4 cells, in RPMI 1640, were seeded in 96-well plates at a concentration of 5 × 105 cells/well. The cells were incubated in the presence or absence of MK 571 (6·25, 12·5, 25 or 50 µm), INDO (37·5, 75 or 150 µm) or PRB (100, 500 or 1000 µm) for 24 hr at 37° in a humidified atmosphere and 5% CO2. Cell viability was assayed by the addition, to each well, of 20 µl of MTT at a concentration of 5 mg/ml. The method is based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases present in viable cells.33 After incubation for 3 hr at 37°, the plates were centrifuged at 400 g for 5 min, the supernatant was removed and 200 µl of DMSO was added to each well to dissolve the formazan crystals. The plates were read on a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA) at a wavelength of 490 nm. Results represent the mean ± standard deviation (SD) of triplicate determinations. In some experiments the Trypan blue exclusion assay was used to evaluate viability.
Cellular differentiation
EL4 cells were seeded, in RPMI 1640, in a 24-well plate at a concentration of 5 × 105 cells/well. The cells were incubated in the presence or absence of MK 571 (12·5 µm) or INDO (75 µm) for 6 days at 37° in a humidified atmosphere of 5% CO2 in air. Then, the EL4 cells were washed with phosphate-buffered saline (PBS) and stained for 30 min at 4° with anti-CD4 (fluorescein isothiocyanate) and anti-CD8 (phycoerythrin) (R & D Systems, Minneapolis, MN) monoclonal antibodies (mAbs). The cells were then washed with PBS, and analysed by flow cytometry. The samples were excited at 488 nm by using an argon laser, and fluorescence emission was detected at 530 nm (FL-1) and at 585 nm (FL-2). Ten-thousand cells were acquired based on forward and side scatter. All flow cytometry analyses were accomplished by using winmdi, version 2·8.
Statistical analysis
Values are given as mean ± SD. Statistical significance was calculated by one-way analysis of variance followed by Bonferroni's t-test, and P-values of less than 0·05 were considered significant. For the differentiation experiments, Kolmogorov-Smirnov statistics were used.
Results
Expression of mRNAs for MRP1 and Pgp in EL4 cells
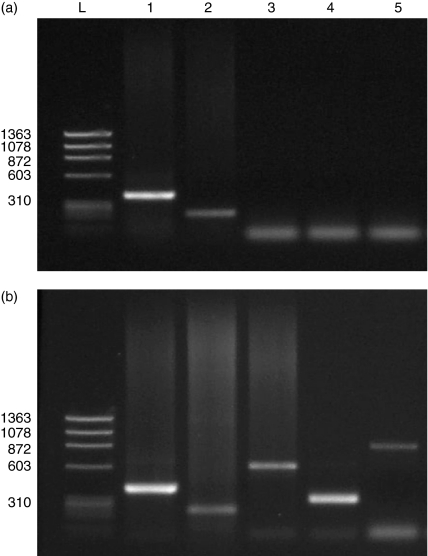
The levels of expression of MRP and Pgp mRNAs were determined by RT–PCR in EL4 cells. It was observed that EL4 cells expressed mRNA for mrp1, but not for mrp2, mrp3 or mdr1a(Fig. 1).
Figure 1.
Expression of the mRNA of mrp1 (224 kb), mrp2 (596 kb), mrp3 (310 kb) and mdr1b (823 kb), as analysed by agarose-gel electrophoresis (1·8% gel) and staining with ethidium bromide (10 µg/ml). Reverse transcription–polymerase chain reaction (RT–PCR)-amplified fragments were obtained from EL4 cells. (a) An RNA ladder from EL4 cells (lane L), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (lane 1) mrp1 (lane 2), mrp2 (lane 3), mrp3 (lane 4) and mdr1b (lane 5). (b) An RNA sample from a murine kidney ladder (lane L), GAPDH (lane 1) mrp1 (lane 2), mrp2 (lane 3), mrp3 (lane 4) and mdr1b (lane 5).
MRP-related transport activity
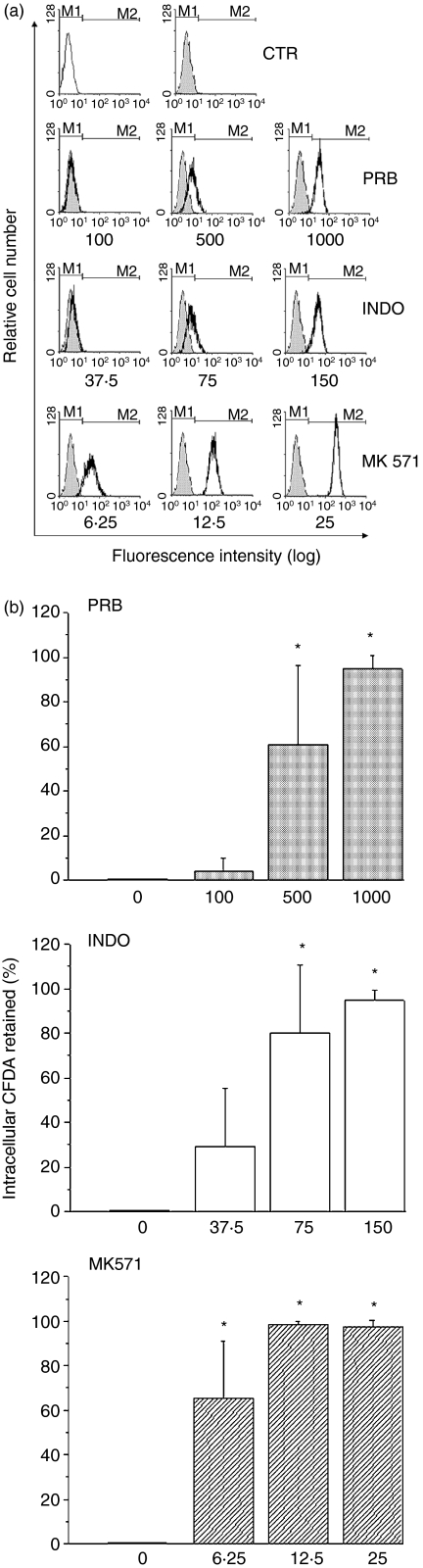
It has been reported that the efflux of the fluorescent dye CFDA in peripheral blood leucocytes and tumour cells34 can be attributed to MRP activity. CFDA is a non-polar, non-fluorescent compound that diffuses freely into cells where it is cleaved by esterases, resulting in the fluorescent carboxy-2′-7′-dichlorofluorescein (CF), which is a substrate for MRP proteins. We observed that EL4 cells did not retain CF intracellularly (Fig. 2). To evaluate if this effect was related to MRP activity, EL4 cells were incubated with CFDA in the presence of MRP inhibitors (Fig. 2). The MRP-related transport activity was inhibited by PRB (500 or 1000 µm); INDO (75 or 150 µm) and MK 571 (6·25, 12·5 or 25 µm). The inhibition was concentration dependent (Fig. 2a,2b). It was observed that in the presence of inhibitors, more than 95% of the EL4 cells retained high levels of CF, and that MK 571 was the best inhibitor of MRP1 activity (Fig. 2b). Thus, we showed that EL4 cells expressed MRP1 mRNA and that the resulting protein was active in these cells.
Figure 2.
Multidrug resistance protein1 (MRP1)-related activity. EL4 cells were loaded with the fluorescent probe carboxy-2′-7′-dichlorofluorescein diacetate (CFDA; 500 nm) for 30 min at 37°. Then, the cells were washed and incubated for a further 30 min at 37° to determine dye extrusion. The fluorescence was measured by flow cytometry. (a) The open histogram represents control (CTR) cells that were incubated without CFDA (autofluorescence). The filled histograms represent control (CTR) cells that were incubated with CFDA. The black line histograms represent cells incubated with CFDA in the presence of different concentrations (lm) of the MRP1 inhibitors probenecid (PRB), indomethacin (INDO) and MK 571. M1 indicates the autofluorescence region and M2 indicates the CFDA fluorescence. This figure is representative of five different experiments. (b) The columns represent the percentage of cells that retain CFDA intracellularly. The results are expressed as the mean ± standard deviation (SD) of five different experiments. *P-value of < 0 05 was considered significant.
Pgp-related transport activity
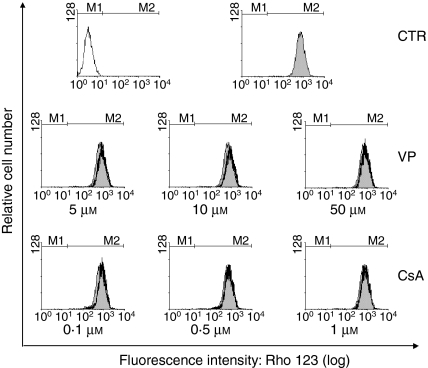
The presence of Pgp activity has been described in normal mature thymocytes.20 Despite finding no expression of mdr1a by EL4 cells, the function of Pgp was investigated by using the efflux of the fluorescent substrate Rho 123. The efflux of Rho 123 in mature and immature lymphocytes has previously been described and attributed to Pgp.20 We observed that EL4 cells retained Rho 123 intracellularly (Fig. 3). However, to evaluate whether there was some dye extrusion and if EL4 cells could accumulate more Rho 123 intracellularly, EL4 cells were incubated with Rho 123 in the presence of Pgp inhibitors; no difference was found (Fig. 3).
Figure 3.
P-glycoprotein (Pgp)-related activity. EL4 cells were loaded with the fluorescent probe rhodamine 123 (Rho 123) (200 ng/ml) for 30 min at 37°, after which the cells were washed and incubated for a further 30 min at 37° to determine dye extrusion. The fluorescence was measured by flow cytometry. The open histogram represents control (CTR) cells that were incubated without Rho 123 (autofluorescence). The filled histograms represent control (CTR) cells that were incubated with Rho 123. The black line histograms represent cells incubated with Rho 123 in the presence of different concentrations of the Pgp inhibitors cyclosporine A (CsA) and verapamil (VP). M1 indicates the autofluorescence region and M2 indicates the Rho 123 fluorescence. This figure is representative of four different experiments.
MRP activity and cell viability
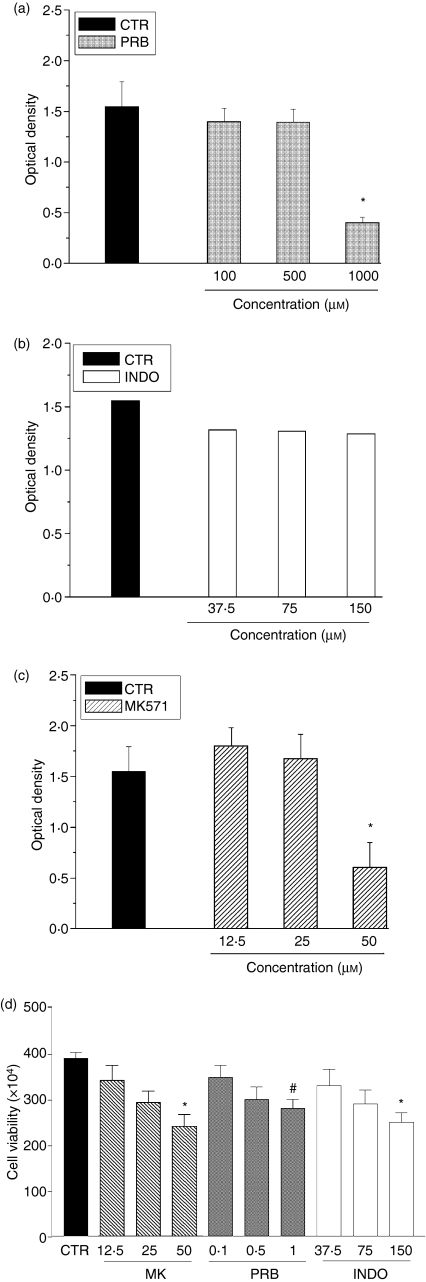
To evaluate the influence of MRP activity on cellular viability, EL4 cells were incubated with the MRP inhibitors PRB (100, 500 or 1000 µm), INDO (37·5, 75 or 150 µm) and MK 571 (6·25, 12, 25 or 50 µm) for 2, 4 or 24 hr. The cells were still alive after 2 or 4 hr in the presence of MRP inhibitors. However, after 24 hr, EL4 cells incubated with PRB (1000 µm), INDO (150 µm) or MK 571 (50 µm) showed, respectively, a 70%, 20% and 50% loss of viability (Fig. 4). The fact that EL4 cells are still viable in the presence of 500 µm PRB and 75 µm INDO (which partially inhibits MRP activity) and 12·5 µm and 25 µm MK 571 (that totally inhibits the efflux activity) suggests that this inhibition does not represent an indiscriminate cytotoxicity. Similar results were obtained by using MTT and Trypan blue dye exclusion assays.
Figure 4.
Effect of multidrug resistance protein (MRP) inhibitors on EL4 cell viability. EL4 cells were incubated in RPMI medium, containing 10% (v/v) fetal bovine serum (FBS), in the presence or absence of the MRP1 inhibitors probenecid (PRB; 100, 500 or 1000 µm), indomethacin (INDO; 37·5, 75 or 150 µm) or MK 571 (12·5, 25 or 50 µm). After 24 hr, cellular viability was measured by using the 3-[4,5-dithylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay (a, b and c) and the Trypan blue dye-exclusion assay (d). The figure shows the mean value ± standard deviation (SD) of the results from three different experiments. A P-value of < 0·05 was considered significant. *P < 0·01; #P < 0·05. CTR, control.
MRP activity and cell differentiation
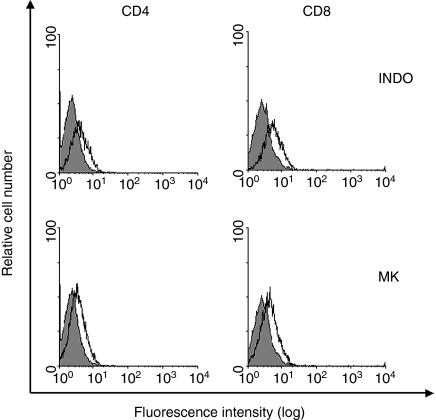
It has been reported that EL4 cells differentiate in the presence of indomethacin.30 To verify if this property could be attributed to MRP1 inhibition, we evaluated whether another inhibitor of MRP1, the drug MK 571, could also induce the differentiation of these cells. We showed that EL4 cells treated with indomethacin (75 µm) or MK 571 (12·5 µm) were capable of up-regulating CD4 and CD8 expression, leading to the appearance of double-positive cells and CD8 single-positive cells (Fig. 5 and Table 1). The differential values given by Kolmogorov–Smirnov statistics (D-value) of EL4 cells treated with indomethacin was 0·41 for CD4+ cells and 0·49 for CD8+ cells (P ≤ 0·001 for both situations). The D-value of the cells treated with MK 571 was 0·33 for CD4+ cells and 0·34 for CD8+ cells (P ≤ 0·001 for both situations). Moreover, cell viability was similar among the three groups after 6 days in culture (36% control, 28% indomethacin, 35% MK 571, compared to day 0). These results suggest that what was being observed was not just a survival selection for CD4 and CD8 positive cells.
Figure 5.
Effect of MK 571 and indomethacin (INDO) on the differentiation of EL4 cells. EL4 cells were incubated in RPMI medium, containing 10% (v/v) fetal bovine serum (FBS), in the presence or absence of the multidrug resistance protein (MRP) inhibitors indomethacin (INDO; 75 µm) or MK 571 (MK; 12·5 µm). After 6 days, the cells were stained with monoclonal antibodies anti-CD4 (left panel) and anti-CD8 (right panel). The grey histograms represent the profile of control cells stained with either antibody. The open histograms represent the profiles of cells treated with INDO or MK 571 and stained with the antibodies 6 days later. The figure shows representative results of two different experiments.
Table 1.
Indomethacin and MK 571 induce cellular differentiation on EL4 cells
| CTR | INDO | MK 571 | |
|---|---|---|---|
| DN | 96·9% | 85·7% | 91·8% |
| DP | 0·7% | 5·6% | 3·2% |
| SP CD4+ | 0·5% | 0·7% | 0·7% |
| SP CD8+ | 1·9% | 7·0% | 4·3% |
CTR, control; DN, double-negative cells; DP, double-positive cells; INDO, indomethacin; SP, single-positive cells.
Discussion
The present work demonstrates, for the first time, that the murine thymoma cell line, EL4, expresses mRNA for mrp1, but not for mrp2 or mrp3. No expression of the gene mdr1a was detected in EL4 cells, and these cells did not show Pgp activity, as determined by using Rho 123 extrusion in the presence or absence of Pgp inhibitors. This result is in agreement with the data of MacDonald et al.20 and Pilarski et al.,35 that reported low or absent Pgp-related activity in immature populations of thymocytes, which increase later during thymus development. The EL4 cells used in this study did not express CD4 or CD8 molecules, but did express CD3 (data not shown), exhibiting an immature phenotype, as described previously.28
mrp1 mRNA has been demonstrated to be present in normal peripheral blood lymphocytes and bone marrow haemopoietic cells.19 The detection of MRP1 mRNA in cells of thymic origin, such as EL4 cells, suggests that the expression of this protein is probably maintained during cell maturation. Furthermore, our data demonstrate that MRP1 is active in EL4 cells, because they were capable of extruding, from the intracellular medium, the fluorescent substrate CF, and this activity was inhibited by MRP reversors. A partial inhibition of MRP activity could be obtained with 500 µm probenecid or 150 µm indomethacin, and complete inhibition of MRP activity was observed with 12·5 µm MK 571. In all of those situations cellular viability was totally preserved, suggesting that the inhibition of MRP activity was not harmful to the cells.
Activated cells are able to produce eicosanoids, such as prostaglandins and leukotrienes, products of cyclooxygenase and 5-lipooxygenase. The known physiological function of MRP1 is to secrete leukotrienes (LTC4, LTD4, LTE4) or prostaglandin (PGA2).13 Previous studies have reported that prostaglandins are present in the thymus,36 and it has been described that leukotrienes and prostaglandins may participate in the intracellular signals involved in proliferation, differentiation and selection of T cells in the thymus.6, 7 Our results indicate that the extrusion of these substrates by MRP1 is not essential, at least for EL4 survival.
MK 571 and indomethacin were capable of inhibiting MRP1 activity and of inducing the expression of CD4 and CD8 molecules in EL4 cells, suggesting that these cells underwent maturation, leading to a stage of double-positive cells and CD8 single-positive cells. Similarly, it has been reported by other authors that indomethacin reduced the surface proteins CD90 and the peanut agglutinin receptor in the thymoma cell line EL4,30 changing the phenotype of these cells to a more mature one. Furthermore, immature and mature lymphoid cells that express significant levels of the peanut agglutinin receptor are capable of circulating, but remain stationary when these levels are altered.30 Thus, the use of MRP1 inhibitors could alter the differentiation process and the output of T lymphocytes to the periphery, or the circulation to secondary lymphoid tissues.
In conclusion, identification of the presence and activity of the MRP1 molecule in EL4 cells allows the use of these cells as a model when studying the regulation and function of the MRP1 protein.
Acknowledgments
We would like to thank Daniel Vazquez Figueiredo, Dr Franklin D. Rumjanek and Dr Marcelo R. Fantappie for helpful discussions. This work was supported by PRONEX and CNPq (Brazilian National Research Council) and by the Howard Hughes Medical Institute (departmental sharing of grant no. 55003669). Juliana Echevarria-Lima was the recipient of a PhD Fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). Daniela F. P. Leite was the recipient of a PhD Fellowship from CNPq. We would like to acknowledge Ms Eliane Corrêa de Santana for the generous gift of the thymoma cell line EL4.
References
- 1.Werlen G, Hausmann B, Naeher D, Palmer E. Signalling life and death in the thymus: timing is everything. Science. 2003;299:1859–63. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 3.Sen J. Signal transduction in thymus development. Cell Mol Biol (Noisy-le-Grand) 2001;47:197–215. [PubMed] [Google Scholar]
- 4.Love PE, Chan AC. Regulation of thymocyte development: only the meek survive. Curr Opin Immunol. 2003;15:199–203. doi: 10.1016/s0952-7915(03)00002-5. [DOI] [PubMed] [Google Scholar]
- 5.Savino W, Dardenne M. Neuroendocrine control of thymus physiology. Endocr Rev. 2000;21:412–43. doi: 10.1210/edrv.21.4.0402. [DOI] [PubMed] [Google Scholar]
- 6.Delebassee S, Gualde N. Effect of arachidonic acid metabolites on thymocyte proliferation. Ann Inst Pasteur Immunol. 1988;139:383–99. doi: 10.1016/0769-2625(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 7.Daculsi R, Vaillier D, Carron JC, Gualde N. Effect of PGE2 on the cell surface molecule expression in PMA treated thymocytes. Immunol Lett. 1998;60:81–8. doi: 10.1016/s0165-2478(97)00133-8. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez A, Kiefer J, Fosdick L, McConkey DJ. Oxygen radical production and thiol depletion are required for Ca(2+)-mediated endogenous endonuclease activation in apoptotic thymocytes. J Immunol. 1995;155:5133–9. [PubMed] [Google Scholar]
- 9.Beaver JP, Waring P. A decrease in intracellular glutathione concentration precedes the onset of apoptosis in murine thymocytes. Eur J Cell Biol. 1995;68:47–54. [PubMed] [Google Scholar]
- 10.Bustamante J, Tovar BA, Montero G, Boveris A. Early redox changes during rat thymocyte apoptosis. Arch Biochem Biophys. 1997;337:121–8. doi: 10.1006/abbi.1996.9754. [DOI] [PubMed] [Google Scholar]
- 11.Will Y, Kaetzel RS, Brown MK, Fraley TS, Reed DJ. In vivo reversal of glutathione deficiency and susceptibility to in vivo dexamethasone-induced apoptosis by N-acetylcysteine and L-2-oxothiazolidine-4-carboxylic acid, but not ascorbic acid, in thymocytes from gamma-glutamyltranspeptidase-deficient knockout mice. Arch Biochem Biophys. 2002;397:399–406. doi: 10.1006/abbi.2001.2662. [DOI] [PubMed] [Google Scholar]
- 12.Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988–94. [PubMed] [Google Scholar]
- 13.Renes J, de Vries EG, Jansen PL, Muller M. The (patho)physiological functions of the MRP family. Drug Resist Updat. 2000;3:289–302. doi: 10.1054/drup.2000.0156. [DOI] [PubMed] [Google Scholar]
- 14.Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart Aj Kurtz EU, Duncan AMV, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human cancer cell line. Science. 1992;258:1650–4. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 15.Regina A, Koman A, Piciotti M, Hafny BE, Center MS, Bergmann R, Couraud PO, Roux F. Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem. 1998;71:705–15. doi: 10.1046/j.1471-4159.1998.71020705.x. [DOI] [PubMed] [Google Scholar]
- 16.Keppler D, Leier I, Jedlitschky G, Mayer R, Buchler M. The function of the multidrug resistance proteins (MRP and cMRP) in drug conjugate transport and hepatobiliary excretion. Adv Enzyme Regul. 1996;36:17–29. doi: 10.1016/0065-2571(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 17.Peng KC, Cluzeaud F, Bens M, Van Huyen JPD, Wioland MA, Lacave R, Vandewalle A. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem. 1999;47:757–67. doi: 10.1177/002215549904700605. [DOI] [PubMed] [Google Scholar]
- 18.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–68. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 19.Legrand O, Perrot JY, Tang RP, Simonin G, Gurbuxani S, Zittoun R, Marie JP. Expression of the multidrug resistance-associated protein (MRP) mRNA and protein in normal peripheral blood and bone marrow haemopoietic cells. Br J Haematol. 1996;94:23–33. doi: 10.1046/j.1365-2141.1996.d01-1776.x. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald HR, Bommhardt U, Cerottini JC. Developmentally regulated expression of P-glycoprotein (multidrug resistance) activity in mouse thymocytes. Eur J Immunol. 1995;25:1457–60. doi: 10.1002/eji.1830250549. [DOI] [PubMed] [Google Scholar]
- 21.Schiengold M, Schwantes L, Schwartsmann G, Chies JA, Nardi NB. Multidrug resistance gene expression during the murine ontogeny. Mech Ageing Dev. 2001;122:255–70. doi: 10.1016/s0047-6374(00)00223-2. [DOI] [PubMed] [Google Scholar]
- 22.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 23.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–83. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 24.Farrell RJ, Menconi MJ, Keates AC, Kelly CP. P-glycoprotein-170 inhibition significantly reduces cortisol and cyclosporin efflux from human intestinal epithelial cells and T lymphocytes. Aliment Pharmacol Ther. 2002;16:1021–31. doi: 10.1046/j.1365-2036.2002.01238.x. [DOI] [PubMed] [Google Scholar]
- 25.Raghu G, Park SW, Roninson IB, Mechetner EB. Monoclonal antibodies against P-glycoprotein, an MDR1 gene product, inhibit interleukin-2 release from PHA-activated lymphocytes. Exp Hematol. 1996;24:1258–64. [PubMed] [Google Scholar]
- 26.Gollapudi S, Gupta S. Anti-P-glycoprotein antibody-induced apoptosis of activated peripheral blood lymphocytes: a possible role of P-glycoprotein in lymphocyte survival. J Clin Immunol. 2001;21:420–30. doi: 10.1023/a:1013177710941. [DOI] [PubMed] [Google Scholar]
- 27.Gorer PA. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950;4:372–9. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skinner MA, Sambhara SR, Benveniste P, Miller RG. Characterization of alpha beta + CD4 – CD8 – CTL lines isolated from mixed lymphocyte cultures of adult mouse spleen cells. Cell Immunol. 1992;139:375–85. doi: 10.1016/0008-8749(92)90079-5. [DOI] [PubMed] [Google Scholar]
- 29.Varga G, Dreikhausen U, Kracht M, Appel A, Resch K, Szamel M. Molecular mechanisms of T lymphocyte activation: convergence of T cell antigen receptor and IL-1 receptor-induced signalling at the level of IL-2 gene transcription. Int Immunol. 1999;11:1851–62. doi: 10.1093/intimm/11.11.1851. [DOI] [PubMed] [Google Scholar]
- 30.Tomooka S, Serushago BA, Koga Y, Taniguchi K, Nomoto K. Indomethacin-induced sialic acid-mediated changes in surface markers from ‘cortical type’ to ‘medullary type’ in murine thymoma line EL4. Immunobiology. 1986;171:345–56. doi: 10.1016/S0171-2985(86)80067-5. [DOI] [PubMed] [Google Scholar]
- 31.Laupezè B, Amiot L, Courtois A, Vernhet L, Drenou B, Fauchet R, Fardel O. Use of the anionic dye carboxy-2′,7′-dichlorofluorescein for sensitive flow cytometric detection of multidrug resistance-associated protein activity. Int J Oncol. 1999;15:571–6. doi: 10.3892/ijo.15.3.571. [DOI] [PubMed] [Google Scholar]
- 32.Neyfakh AA. Use of fluorescent dyes as molecular probes for the study of multidrug resistance. Exp Cell Res. 1988;174:168–76. doi: 10.1016/0014-4827(88)90152-8. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 34.Laupezè B, Amiot L, Payen L, Drenou B, Grosset JM, Lehne G, Fauchet R, Fardel O. Multidrug resistance protein (MRP) activity in normal mature leukocytes and CD34-positive hematopoietic cells from peripheral blood. Life Sci. 2001;68:1323–31. doi: 10.1016/s0024-3205(00)01026-2. [DOI] [PubMed] [Google Scholar]
- 35.Pilarski LM, Paine D, McElhaney JE, Cass CE, Belch AR. Multidrug transporter P-glycoprotein 170 as a differentiation antigen on normal human lymphocytes and thymocytes. Am J Hemotol. 1995;49:323–35. doi: 10.1002/ajh.2830490411. [DOI] [PubMed] [Google Scholar]
- 36.Bergstom S, Samuelson B. Isolation of prostaglandin E1 from calf thymus. Acta Chem Scand. 1963;17:282–7. [Google Scholar]