Abstract
Chronic lymphocytic leukaemia (CLL) is the most common B-cell malignancy in the Western world and exists as subtypes with very different clinical courses. CLL is generally described as a disease of failed apoptosis. Apoptosis resistance may stem from a combination of microenvironmental survival signals as well as from intrinsic alterations in the apoptotic machinery within the CLL cell. The molecular mechanism involved in controlling apoptosis in CLL is complex and is influenced by many factors, including Bcl-2 family proteins, which are critical regulators of cell death. Here we review the significance of apoptosis dysregulation in CLL, focusing on the role of Bcl-2 and related Bcl-2 family proteins, such as Bax and Mcl-1. The differential properties of the newly described subsets of CLL are also highlighted.
Keywords: apoptosis, Bax, Bcl-2, chronic lymphocytic leukaemia, Mcl-1
Introduction
Chronic lymphocytic leukaemia (CLL) is the most common B-cell malignancy in the Western world and is characterized by the accumulation of CD5-positive monoclonal B cells in the blood, bone marrow and peripheral lymphoid organs.1 Although long considered as a single disease, the clinical course of CLL is heterogeneous. Some patients have aggressive disease with relatively rapid increases in the number of malignant cells in the blood, and require treatment, most commonly with cytotoxic therapy. Relapses are common and CLL remains incurable. By contrast, other patients have stable, non-progressive disease that often requires no treatment other than careful monitoring.
Studies of the B-cell receptor (BCR) have provided important clues to the mechanisms that underlie the diverse clinical behaviour of CLL.2, 3 Most notably, the presence of somatic mutations in the variable region of heavy (VH) and light (VL) immunoglobulin chains is a powerful prognostic marker for CLL.4, 5 Patients with mutated immunoglobulin genes (M-CLL) have a greatly improved prognosis compared to patients with unmutated germline sequences (U-CLL).
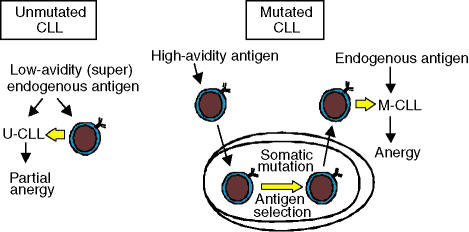
The accumulation of variable-region somatic mutations is a hallmark of antigen-driven affinity maturation within germinal centres, suggesting that these clinically important subsets of disease differ with respect to the stage of maturation reached by the B cell of origin. The usage of selected VH genes in CLL provides evidence for antigenic pressure during the development of both U-CLL and M-CLL.4, 6 However, the nature and outcome of this antigenic stimulation appears to vary between the subsets. Although gene expression profiling has provided evidence to suggest that all CLLs may arise from (or most closely resemble) memory B cells,7 the relatively small differences in the gene-expression profiles of naïve and memory B cells, and evidence for continued activation, limits the conclusions that can be drawn from these analyses.3 An alternative proposal is that U-CLL could arise from B cells undergoing a T-independent stimulation by low-avidity (super)antigen, outside germinal centres.2, 3 By contrast, high-avidity antigen is more likely to contribute to the development of M-CLL via normal T-cell-dependent events within germinal centres (Fig. 1). The expression of activation markers in both subsets of CLL suggests that environmental antigen continues to play a role in the maintenance of the CLL clones.2 Expression of the cell-surface activation marker CD38 has also been linked to disease heterogeneity in CLL.5, 8, 9 Relatively high levels of CD38 are associated with poor prognosis and, although controversial, CD38 expression appears to be largely independent of VH mutational status.
Figure 1.
A model for the development of chronic lymphocytic leukaemia (CLL) subtypes. CLL with unmutated, germline immunoglobulin sequences (U-CLL) may develop in response to low-avidity antigens (potentially superantigens) via T-cell-independent events outside the germinal centre. CLL with mutated, germline immunoglobulin sequences (M-CLL) may develop in response to high-avidity antigens that drive T-cell-dependent germinal centre formation and are therefore associated with the accumulation of immunoglobulin mutations. There is evidence for continued antigen interaction in both subsets of CLL. Whereas M-CLL cells appear to be relatively anergic, U-CLL cells are generally more responsive to B-cell receptor signalling. This may be a result of the nature of the antigen (i.e. low avidity) and differences between B cells of differing maturation in the propensity to become anergic. Resistance to apoptosis will probably contribute to both types of CLL. Interpatient heterogeneity in the susceptibility to apoptosis/expression of apoptosis regulators may impact most significantly on the response to therapy, rather than disease progression. Adapted from Stevenson and Caligaris-Cappio.3
The ability of CLL cells from different patients to transmit signals via the BCR varies between the subsets.10–12 Whereas U-CLL cells are generally able to transmit signals following cross-linking of the BCR with antibodies to immunoglobulin M (anti-IgM), M-CLL cells tend to be unresponsive. This might relate to the presence of zeta-associated protein 70 (ZAP-70), a critical T-cell receptor signalling molecule that has been shown to be overexpressed in U-CLL/signal-responsive CLL.12–14 We have hypothesized that the differential response to BCR ligation reflects variable levels of anergy in CLL, with the extent of anergy being influenced by the stage of B-cell maturation and possibly by the nature of the antigen (Fig. 1).3 Anergy may be associated with improved clinical outcome because anergic cells are less responsive to proliferative/survival signals driven by antigen stimulation of the BCR in specific microenvironments.
Alteration in susceptibility to apoptosis is an important feature of many human cancers. Decreased apoptosis contributes not only to tumour development, but also to resistance to conventional anti-cancer therapies, such as radiation and cytotoxic agents. CLL is often considered to be a disease of failed apoptosis.1, 15, 16 Genetic alterations and changes in the expression of many apoptosis regulators have been described, at least in a subset of patients. For example, the loss of p53 and ATM are relatively common genetic alterations and contribute to a reduced response to DNA-damaging agents.17 The nuclear factor-kappa B (NF-κB) transcription factor and phosphatidylinositol-3 kinase signalling pathways are activated in CLL and are important for cell survival.18–21 Many cytokines, including B-cell activation factor (BAFF), a proliferation inducing ligand (APRIL), CD40 ligand and interleukin-4 (IL-4) promote the survival of CLL cells,22–24 and CLL cells appear to be resistant to the effects of signalling via cell-surface death receptors.25 Antigen stimulation via the BCR is also likely to influence apoptotic pathways, although the outcome of signalling via surface IgM and immunoglobulin D (IgD) remains controversial.10, 26 Thus, the molecular control of apoptosis in CLL is complex and, owing to the influence of stromal cells on survival of tumour cells,27, 28 is difficult to assess in vitro.
Bcl-2 family proteins are critical regulators of apoptosis and have also been implicated in CLL. This review will discuss the significance of dysregulation of apoptosis in CLL, with a particular emphasis on disease heterogeneity and the role of Bcl-2 family proteins.
Apoptosis pathways and Bcl-2 family proteins
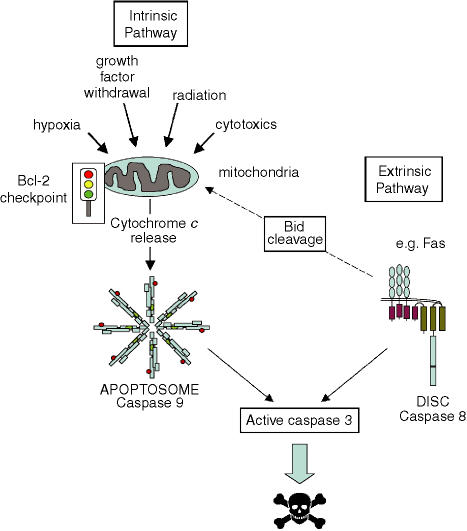
The key players in the execution of the apoptotic cascade are caspases (cysteine proteases with aspartate specificity), which are activated by cleavage during apoptosis. Two major pathways of apoptosis converge on caspases (Fig. 2).29–31 The ‘intrinsic’ cell death pathway is activated by a very wide range of signals, including radiation, cytotoxic drugs, cellular stress and growth factor withdrawal, and involves the release of proteins, including cytochrome c, from the mitochondrial intermembrane space. Cytoplasmic cytochrome c combines with an adaptor molecule, Apaf-1, and an inactive ‘initiator’ caspase, procaspase 9, within a multiprotein complex called the apoptosome. This leads to the activation of caspase 9 which then triggers a cascade of caspase activation, resulting in the morphological and biochemical changes associated with apoptosis (e.g. mediated by the ‘effector’ caspase, caspase 3). There is a second cell-death pathway called the ‘extrinsic’ cell-death pathway, which can function independently of mitochondria. This is activated by cell-surface death receptors, such as Fas, and directly activates the caspase cascade via the ‘initiator’ caspase, caspase 8, within a death-inducing signalling complex (DISC) (Fig. 2).
Figure 2.
Pathways of apoptosis. The ‘intrinsic’ and ‘extrinsic’ apoptosis pathways converge on the effector caspases, such as caspase 3, which promote the biochemical and morphological characteristics of apoptosis. The ‘extrinsic’ pathway is activated by cell-surface death receptors, such as Fas, and is mediated by activation of the initiator caspase, caspase 8, within a death-inducing signalling complex (DISC). The ‘intrinsic’ pathway is activated by a wide range of stimuli which trigger the release of cytochrome c from mitochondria, leading to the activation of a distinct initiator caspase, caspase 9, within the apoptosome. In some situations, the activation of caspase 8 can lead to the cleavage of Bid, which is also able to promote cytochrome c release. Bcl-2 family proteins play a key role in controlling mitochondrial function associated with the ‘intrinsic’ cell-death pathway, either preventing or promoting the release of cytochrome c.
Bcl-2 is the prototypical member of a family of structurally related apoptosis control molecules, discovered by virtue of its activation by the t(14:18) translocation in the majority of follicular B-cell lymphomas (FL).30,31 The translocation fuses the Bcl-2 open reading frame with immunoglobulin regulatory sequences, causing overexpression of the intact Bcl-2 protein. Many cell-based studies have demonstrated that overexpression of Bcl-2 enhances resistance to apoptosis, and the oncogenic potential of Bcl-2 has been confirmed in mouse models. Transgenic mice overexpressing Bcl-2 in the B-cell compartment develop B-lymphoid tumours.32
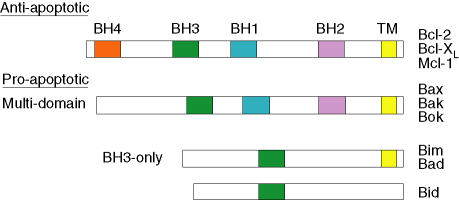
Bcl-2-related proteins can act as cellular bodyguards or assassins to positively or negatively control apoptosis.29–31 Bcl-2 family proteins are characterized by the presence of up to four relatively short sequence motifs (less than 20 amino acid residues in length) termed Bcl-2 homology (BH) domains. More than 20 family members have been identified and these can be divided into three subfamilies, based on structural and functional features (Fig. 3). The anti-apoptotic subfamily contains the Bcl-2 and Bcl-XL proteins, which suppress apoptosis and contain all four BH domains, designated BH1–4. Mcl-1 is another Bcl-2-related survival protein, but is somewhat structurally distinct and probably lacks a ‘classical’ BH4 domain. Pro-apoptotic proteins, such as Bax, Bak and Bok, contain BH1–3 domains and are termed ‘multidomains’, whereas other pro-apoptotic proteins, such as Bim, Bad and Bid, contain only the BH3 domain and are termed ‘BH3-only’. Some Bcl-2 family proteins also contain a carboxy-terminal transmembrane domain. The targeting of Bcl-2 family proteins to mitochondrial membranes is thought to play an important role in controlling the function of mitochondria, key participants in apoptotic cell death. Bcl-2 proteins also target other organelles: this is probably important for apoptosis control but is less well understood.
Figure 3.
Schematic diagram of representative Bcl-2 family proteins. Examples of anti-apoptotic (Bcl-2, Bcl-XL and Mcl-1), pro-apoptotic multidomain (Bax, Bak and Bok) and pro-apoptotic BH3-only (Bid, Bim, Bad) proteins are shown. Bcl-2 homology (BH) and transmembrane (TM) domains are indicated. Note that Mcl-1 may not contain a classical BH4 domain.
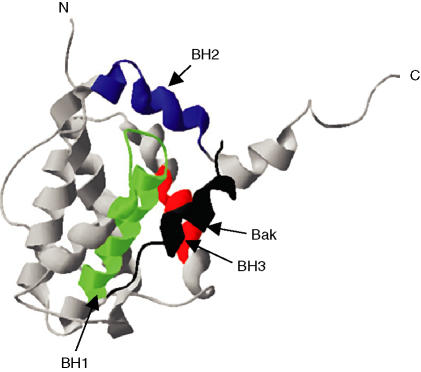
The relative expression (or activity) of various anti-apoptotic and pro-apoptotic Bcl-2 family proteins is a critical determinant of apoptosis sensitivity. Precisely how the functions of the separate subfamilies and individual Bcl-2 proteins are coordinated to control apoptosis is far from clear, but one key target appears to be the release of cytochrome c from mitochondria and, thereby, downstream caspase activation. Bcl-2 prevents mitochondrial cytochrome c release, whereas the addition of Bax or BH3 peptides to isolated mitochondria is sufficient to promote cytochrome c release. Bcl-2 family proteins act in a coordinated manner to control apoptosis, and various homotypic and heterotypic interactions, mediated via the BH domains of these proteins, have been described. This is considered key for their biological function, as mutation of BH domains prevents dimerization and inactivates Bcl-2 family proteins. The basic mode of interaction of Bcl-2 family proteins is the insertion of an alpha-helical BH3 domain into a shallow binding groove formed by the BH1, 2 and 3 domains of a second Bcl-2 family protein (Fig. 4).33 Through the control of cytochrome release, Bcl-2 proteins modulate the ‘intrinsic’ cell-death pathway, but direct regulation of caspases, for example, by physical sequestration, may also play a role.34 Under some conditions, death receptors which can activate the ‘extrinsic’ pathway can also cross-talk to the ‘intrinsic’ pathway, by caspase-mediated cleavage of a BH3-only Bcl-2 family molecule, Bid, which targets the mitochondria (Fig. 2). Therefore, in some cell settings, Bcl-2 proteins can also control susceptibility to death receptor-induced apoptosis.
Figure 4.
Three-dimensional structure of Bcl-2 family proteins. Bcl-XL structure showing insertion of the Bak BH3 alpha-helix (shown in black) into a binding groove formed by domains BH1–3 of Bcl-XL.33 N, amino-terminus; C, carboxy-terminus.
Apoptosis resistance in CLL: bad influences or the devil within?
CLL is commonly considered as a paradigm for a malignancy of failed apoptosis, as CLL cells circulating in the blood are largely non-proliferating and arrested in the G0/G1 phase of the cell cycle. Cell division must occur, presumably in ‘proliferation centres’ in tissue microenvironments, accounting for the inexorable rise in white blood cell counts in some patients and evidenced by the shortening of telomeres.35 However, lack of apoptosis is considered a major component of the dysregulation of normal B-cell homeostasis in all subsets of this malignancy.
A critical question is to what extent the apoptotic resistance mechanism of CLL cells are autonomous, for example, owing to genetic or epigenetic alterations in the expression of apoptosis regulators within the CLL cell, or are driven by environmental signals that are received by the CLL in vivo. The role of the microenvironment is clear from the well-recognized propensity of CLL cells to undergo apoptosis when placed in ex vivo culture,36 but intrinsic cellular alterations could also contribute.
CLL cells generally undergo rapid ‘spontaneous’ apoptosis following ex vivo culture.36, 37 Clearly the CLL cell does not have autonomous survival capability and must depend, to some extent, on environmental signals. However, does this preclude a role for autonomous genetic/epigenetic alterations that confer further apoptosis resistance? Put another way, without these changes perhaps a CLL cell could have died even more rapidly following removal from the body. Follicular lymphoma cells also undergo apoptosis when placed in culture,38 even though they carry the t(14:18) translocation, the hallmark genetic alteration conferring cellular resistance to apoptosis. Thus, even cells which have genetically deranged apoptosis control mechanisms can readily undergo cell death ex vivo. There is also considerable patient-to-patient heterogeneity in the rate of CLL cell apoptosis ex vivo, suggesting that intrinsic susceptibility to apoptosis may differ. It will be difficult to establish the relative contribution of cell autonomous and environment-derived survival signals for apoptosis dysregulation in the CLL cell, without identifying the elusive normal B-cell counterpart. Even then, comparisons would be difficult, as the CLL cell may be nudged towards apoptosis by oncogene activation/genomic damage. Although environment-derived signals are important, it may be a combination of cell autonomous and exogenous survival signals that underlie defective apoptosis in CLL.
Bcl-2 and Bax in CLL
Essentially all CLL cells express high levels of Bcl-2. Expression generally appears to be equivalent to or to exceed that in normal peripheral blood lymphocytes, or even that in cells containing the t(14:18) translocation.39 The mechanisms that mediate Bcl-2 expression in CLL remain unclear. It has been suggested that a small proportion of patients contain the t(14:18) translocation that is commonly found in FL, although it remains controversial as to whether these cases are truly CLL.40 In the majority of individuals, the promoter region for Bcl-2 is hypomethylated, which may contribute to increased transcription and Bcl-2 protein expression in CLL.39 In FL, the t(14:18) translocation provides strong evidence that activation of Bcl-2 is a tumour-associated event. However, the absence of an analogous genetic alteration in the majority of CLL means that it is unclear whether the abundant expression of Bcl-2 in CLL is acquired during leukaemogenesis or reflects the origin of CLL from B cells that normally express Bcl-2.
Despite this unanswered question, the expression of Bcl-2 and Bax and, perhaps more significantly, the relative expression of these functional antagonists, is an important variable in CLL. CLL cells can have increased Bcl-2/Bax ratios (favouring cell survival) compared to normal controls in at least some individuals.41, 42 Individual variation in the expression of Bcl-2/Bax correlates with apoptosis and clinical outcome. For example, decreased Bcl-2/Bax ratios are associated with increased sensitivity to cytotoxic drugs in vitro and improved responses to chemotherapy in patients.41–43 The relevance of these differences for apoptosis control is demonstrated by experiments using antisense oligodeoxynucleotides (AS-ODN). Those aimed specifically to interfere with the expression of Bcl-2, enhanced spontaneous apoptosis and sensitivity to chlorambucil.44, 45 By contrast, Bax AS-ODN delayed spontaneous apoptosis.46 However, other proteins are also likely to contribute to apoptosis control in CLL. For example, the expression of other Bcl-2 family proteins, such as Mcl-1 (see below) and unrelated survival proteins (Bag-1), may also correlate with response to cytotoxic therapy in patients.42, 47 Notably, variation in susceptibility to apoptosis and in the expression of the regulatory proteins, Bcl-2, Bax and Mcl-1, do not appear to correlate consistently with stage.42, 47 Correlations with disease subtypes identified by the presence of VH mutations have not been reported.
Do tumour-associated events also underlie variation in the expression of Bax in CLL? Bax mutations have been described in malignant B cells, leading to a lack of expression or functional inactivation of the protein.48–50 In some cases, these are clearly associated with microsatellite instability [where single base insertions or deletions in a G(8) tract within the Bax open-reading frame result in a frameshift mutation and premature termination of translation], but the absence of significant microsatellite instability in CLL and direct sequence analysis does not support the idea that mutations of the coding sequence alter the function/expression of Bax in CLL.51
Sequence alterations in the Bax promoter may contribute to decreased expression in some cases of CLL. In a study of 34 patients, Saxena et al. described a G-to-A polymorphism in the Bax 5′ untranslated region, at position −248 relative to the translation start site and 146 nucleotides downstream from the TATA box.51 A heterozygous polymorphism was present in 69% of stage I–IV CLL, but in only 6% of stage 0 CLL and 4% of controls. The polymorphism was homozygous in RL lymphoma cells, which did not express Bax protein. In CLL, the presence of the polymorphism was associated with decreased Bax expression (P= 0·05), progression beyond stage 0 (P = 0·0002) and failure to achieve complete remission (P = 0·038).51 Although of significant interest, further work is required to determine the frequency of the polymorphism in larger cohorts of CLL patients and normal individuals and to determine directly whether the G-to-A change directly modulates Bax expression, e.g. via modulating RNA stability or promoter activity. Given the familial component to CLL it would be interesting to determine the frequency of the polymorphism in CLL twins and families.
Mcl-1: new kid on the block
More recent analyses have revealed an important role for another Bcl-2 family protein, Mcl-1, in CLL. Like Bcl-2, Mcl-1 is a survival protein, but one with distinct features. It was first discovered in differentiating myeloid cells where it is thought to play a transient role in promoting cell survival (reviewed in refs 52, 53), but has since been shown to be expressed in various tissues and malignant cells, including CLL, where its expression is significantly associated with a failure to achieve complete remission following cytotoxic therapy.42, 47 Overexpression of Mcl-1 in transgenic mice predisposes to lymphomagenesis, and antisense ablation experiments have demonstrated that Mcl-1 is required for the survival of multiple myeloma and lymphoma cells.52–54 Interestingly, the incidence of tumours is higher in mice expressing an Mcl-1 transgene than in those expressing Bcl-2, and the tumours that develop more closely resemble lymphomas. Mcl-1 is an essential gene in the mouse, and deletion results in very early preimplantation lethality.55 Experiments with chimeric mice demonstrate that Mcl-1 is also essential for B- and T-cell development.56
One of the remarkable features of Mcl-1 is its rapid regulation, and Mcl-1 is thought to play a critical role in regulating apoptosis in response to rapidly changing environmental cues.52, 53 For example, Mcl-1 expression is rapidly induced via control of transcription and protein stability by various growth factors and survival signals. Compared to other Bcl-2 family survival proteins, Mcl-1 is structurally distinct, containing PEST sequences in its amino-terminal regions. Mcl-1 protein has a rapid turnover, and is targeted for degradation by the proteasome through ubiquitination. Mcl-1 typically has a half-life of a few hours, a common feature of highly modulated proteins. Although PEST sequences are often important determinants of degradation, the role of the Mcl-1 PEST sequences in determining rapid protein turnover remains unclear.57
Mcl-1 can also be negatively controlled, down-regulated through control of transcription, translation and stability in response to a wide range of pro-apoptotic signals. Early down-regulation of Mcl-1 may be essential for initiation of the apoptotic cascade in response to DNA damage, in at least some systems.58, 59
Although the rapid turnover of Mcl-1 in healthy cells is largely caused by proteasome-mediated degradation, other proteolytic fates await Mcl-1. During apoptosis, Mcl-1 is a very efficient substrate for caspases.53, 60–62 Caspase cleavage of Mcl-1 simultaneously inactivates the survival function of this protein and converts Mcl-1 into a cell death-promoting molecule, activating a positive feedback loop that results in increased caspase activation.53 Therefore, Mcl-1 acts as a molecular switch during apoptosis, converted from a molecular bodyguard to assassin, by proteolytic cleavage. This mechanism has also been described for Bcl-2 and Bcl-XL, but it appears that Mcl-1 is a particularly efficient substrate for caspases. In CLL cells undergoing apoptosis, both caspase cleavage53, 60 and down-regulation of expression, via mechanisms apparently independent of caspase cleavage,63, 64 have been described, possibly influenced by the nature of the apoptotic signal.
As discussed above, microenvironment signals are thought to play a critical role in CLL. How are microenvironment-derived survival signals communicated to the apoptosis control machinery? A great many signals can promote the survival of CLL cells in culture, including cytokines and ‘nurse’ and other stroma-like cells.27, 65 Although it is difficult to know which of these signals might play a predominant role in promoting CLL survival in specific microenvironments within the body, the up-regulation of Mcl-1 appears to play a key role in at least one of these systems. The HK cell line resembles follicular dendritic cells and is able to promote the survival of CLL cells for extended periods of time.28, 66 HK-promoted survival is mediated, in part, by CD44, and is associated with an increased expression of Mcl-1. Antisense ablation of Mcl-1 reverses the survival mediated by HK cells, confirming the importance of Mcl-1 in this system. However, in other systems, alternate apoptosis control molecules may play a predominant role. CLL-associated microenvironments are rich in T cells, and T-cell-derived survival signals mediated by engagement of the CD40 receptor on CLL cells could provide another relevant pathway of CLL survival in vivo. In contrast to HK cells, CD40 up-regulates the expression of Bcl-XL and also of survivin, an inhibitor of caspases.28, 67 In complex tissues, multiple microenvironment-derived signals may act on the CLL cells to co-ordinately modulate cell survival.
Finally, the recent identification of small sequence insertions in the Mcl-1 promoter in a subset of CLL has provided further evidence that Mcl-1 can play an important role in CLL. The study of Saxena et al. demonstrated the presence of 6- or 18-base pair (bp) insertions in the Mcl-1 promoter in 17/58 (29%) patients with CLL, but in none of the controls.68 The insertion site and the sequences of the 6- or 18-bp inserted regions were the same in all affected individuals. Although the effect of the insertions on promoter activity was not tested, the presence of insertions was correlated with elevated Mcl-1 expression at both RNA and protein levels. The presence of insertions was correlated with rapid disease progression (P = 0·012) and, more strongly, with a poor response to chemotherapy (P = 0·001) and shorter disease-specific survival (P < 0·001). The insertions were more commonly found in CD38-negative patients, suggesting that they may present a poor prognostic marker in what is otherwise a relatively favourable group.
Conclusions
Although widely considered as a paradigm for a malignancy of failed apoptosis, the mechanisms and significance of resistance to apoptosis in CLL remain poorly defined. In this review, we have focused on the role of Bcl-2 family proteins in apoptosis control in CLL. The presence of high levels of Bcl-2 in the majority of patients, and the genetic alterations of Mcl-1 in a subset, contribute to an emerging picture that these critical control molecules are dysregulated in CLL. However, it is important to consider that other apoptosis regulators, such as p53 and ATM, are also altered in CLL cells.
The first key question is, ‘To what extent does apoptosis resistance in CLL stem from cell autonomous or exogenous survival signals?’. The well-described propensity of CLL cells to undergo rapid apoptosis when removed from the body clearly shows that survival is not ‘hard wired’ in CLL cells and that these cells require specific signals in the body for survival. The impact of a multitude of potential microenvironment-derived signals on CLL cells has been described in detail, but the precise nature of the signals relevant for CLL survival in the body remain unclear. It is probable that multiple environmental factors and target genes contribute to apoptosis control in CLL cells.
In contrast to FL, where the recurrent t(14:18) translocation provides direct evidence for tumour-associated dysregulation of apoptosis, it is unclear whether the high-level expression of Bcl-2 in CLL is acquired during tumorigenesis or whether it reflects abundant Bcl-2 expression in the cell(s) of origin of CLL. However, as there is now evidence for genetic alteration of apoptosis control molecules in at least some CLL (p53, ATM, Bax and Mcl-1 promoter alterations), it seems probable that the intrinsic apoptosis sensitivity is altered in at least some CLL, and this may contribute to patient-to-patient heterogeneity.
Resistance to apoptosis can contribute to the development of cancer and determine the response to therapy. A second key question is therefore, ‘To what extent does variation in apoptosis sensitivity impact on the clinical behaviour of CLL?’. Although CLL exists as at least two distinct subsets of disease (Fig. 1), there is no compelling evidence that consistent differences in apoptosis regulation underlie the variable clinical course associated with these subtypes. By contrast, differences in BCR signalling capacity do appear to correlate. We favour a model where an underlying resistance to apoptosis, potentially driven by both environmental and/or cell autonomous events, contributes to all CLL, providing a foundation on which different (super)antigen-driven events (such as anergy) make a significant contribution to variation in the clinical course. However, interpatient variability in the expression of Bcl-2 family proteins has been demonstrated in CLL. Although identified in some studies, these variables are not consistently associated with disease stage, suggesting that they are not important determinants of clinical progression, and direct correlations with VH status have not been reported. By contrast, these changes are relatively strongly correlated with differences in response to cytotoxic therapy. For example, differences in expression of the Bcl-2/Bax ratio, Mcl-1 expression and the presence of potentially activating insertions in the Mcl-1 promoter, all correlate with response to therapy.41, 42, 47, 68 Therefore, we suggest that a degree of apoptosis resistance provides a stage on which antigen/BCR-mediated events modulate the clinical course of the disease. However, once the disease progresses and therapy is initiated, individual differences in apoptosis susceptibility, mediated in part by Bcl-2 family proteins but also influenced by deficits in p53 and ATM, may play a significant role in determining response to therapy.
The expression and functional role of Bcl-2 and related survival proteins in CLL cells suggests that targeting these molecules has therapeutic potential. Indeed, results of antisense ablation of Bcl-2 in preclinical experiments have provided some encouragement and trials are ongoing. However, delivery and specificity remain problematic. A new generation of small-molecule inhibitors of Bcl-2-related proteins, the so-called BH3-mimetics, which can neutralize survival proteins by mimicking the binding of proapoptotic BH3-only proteins, may offer a new avenue.69 Fine tuning of the structures of these molecules may permit the inhibition of specific Bcl-2 proteins. Their potential activity in CLL must be weighed against the risk of toxicity; for example, deletion of Mcl-1 is lethal in mice. Regardless, they will be exciting agents to assess in clinical trials and valuable chemical tools for using to further investigate the function of Bcl-2 family proteins in CLL.
Acknowledgments
We wish to thank Kathy Potter, Ian Mockridge, Myra Armstrong, Louise Neville, Christian Ottensmeier, Andrew Duncombe, Debbie Richardson and Becky Pickering for invaluable contributions. Work on CLL in the Stevenson and Packham laboratories is funded by Cancer Research UK, Tenovus and the Leukaemia Research Fund. We apologise for not being able to cite all primary literature; this is because of space constraints.
References
- 1.Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999;17:399–408. doi: 10.1200/JCO.1999.17.1.399. 2. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Ferrarini M. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu Rev Immunol. 2003;21:841–94. doi: 10.1146/annurev.immunol.21.120601.141018. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103:4389–95. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 4.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V (H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–54. [PubMed] [Google Scholar]
- 5.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–7. [PubMed] [Google Scholar]
- 6.Fais F, Ghiotto F, Hashimoto S, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–25. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein U, Tu Y, Stolovitzky GA, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194:1625–38. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morabito F, Mangiola M, Oliva B, et al. Peripheral blood CD38 expression predicts survival in B-cell chronic lymphocytic leukemia. Leuk Res. 2001;25:927–32. doi: 10.1016/s0145-2126(01)00049-2. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin TJ, Orchard JA, Ibbotson RE, Davis Z, Thomas PW, Stevenson FK, Oscier DG. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood. 1999;94:1840–7. doi: 10.1182/blood.v99.3.1023. [DOI] [PubMed] [Google Scholar]
- 10.Zupo S, Isnardi L, Megna M, Massara R, Malavasi F, Dono M, Cosulich E, Ferrarini M. CD38 expression distinguishes two groups of B-cell chronic lymphocytic leukemias with different responses to anti-IgM antibodies and propensity to apoptosis. Blood. 1996;88:1365–74. [PubMed] [Google Scholar]
- 11.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101:1087–93. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, Kipps TJ. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–14. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 13.Rosenwald A, Alizadeh AA, Widhopf G, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–47. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Apgar J, Huynh L, Dicker F, Giago-McGahan T, Rassenti L, Weiss A, Kipps TJ. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2004 doi: 10.1182/blood-2004-05-1715. Epub. [DOI] [PubMed] [Google Scholar]
- 15.Schimmer AD, Munk-Pedersen I, Minden MD, Reed JC. Bcl-2 and apoptosis in chronic lymphocytic leukemia. Curr Treat Options Oncol. 2003;4:211–8. doi: 10.1007/s11864-003-0022-y. [DOI] [PubMed] [Google Scholar]
- 16.Jewell AP. Role of apoptosis in the pathogenesis of B-cell chronic lymphocytic leukaemia. Br J Biomed Sci. 2002;59:235–8. doi: 10.1080/09674845.2002.11783667. [DOI] [PubMed] [Google Scholar]
- 17.Pettitt AR, Sherrington PD, Stewart G, Cawley JC, Taylor AM, Stankovic T. Dysfunction in B-cell chronic lymphocytic leukemia: inactivation of ATM as an alternative to TP53 mutation. Blood. 2001;98:814–22. doi: 10.1182/blood.v98.3.814. [DOI] [PubMed] [Google Scholar]
- 18.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of the PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 19.Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, Decker T. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 20.Barragan M, Bellosillo B, Campas C, Colomer D, Pons G, Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99:2969–76. doi: 10.1182/blood.v99.8.2969. [DOI] [PubMed] [Google Scholar]
- 21.Romano MF, Lamberti A, Tassone P, et al. Triggering of CD40 antigen inhibits fludarabine-induced apoptosis in B chronic lymphocytic leukemia cells. Blood. 1998;92:990–5. [PubMed] [Google Scholar]
- 22.Kern C, Cornuel JF, Billard C, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–88. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 23.Schattner EJ. CD40 ligand in CLL pathogenesis and therapy. Leuk Lymphoma. 2000;37:461–72. doi: 10.3109/10428190009058499. [DOI] [PubMed] [Google Scholar]
- 24.Pu QQ, Bezwoda WR. Interleukin-4 prevents spontaneous in vitro apoptosis in chronic lymphatic leukaemia but sensitizes B-CLL cells to melphalan cytotoxicity. Br J Haematol. 1997;98:413–7. doi: 10.1046/j.1365-2141.1997.2113028.x. [DOI] [PubMed] [Google Scholar]
- 25.MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, Cohen GM. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002;21:6809–18. doi: 10.1038/sj.onc.1205853. [DOI] [PubMed] [Google Scholar]
- 26.Bernal A, Pastore RD, Asgary Z, Keller SA, Cesarman E, Liou HC, Schattner EJ. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 27.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 28.Pedersen IM, Kitada S, Leoni LM, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–801. [PubMed] [Google Scholar]
- 29.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 30.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 31.Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–49. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Adams JM, Harris AW, Strasser A, Ogilvy S, Cory S. Transgenic models of lymphoid neoplasia and development of a pan-hematopoietic vector. Oncogene. 1999;18:5268–77. doi: 10.1038/sj.onc.1202997. [DOI] [PubMed] [Google Scholar]
- 33.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 34.Marsden VS, O'Connor L, O'Reilly LA, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–7. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 35.Damle RN, Batliwalla FM, Ghiotto F, et al. Telomere length and telomerase activity delineate distinctive replicative features of the B-CLL subgroups defined by immunoglobulin V gene mutations. Blood. 2004;103:375–82. doi: 10.1182/blood-2003-04-1345. [DOI] [PubMed] [Google Scholar]
- 36.Ghia P, Caligaris-Cappio F. The indispensable role of the microenvironment in the natural history of low-grade B-cell neoplasms. Adv Cancer Res. 2000;79:157–73. doi: 10.1016/s0065-230x(00)79005-1. [DOI] [PubMed] [Google Scholar]
- 37.Collins RJ, Verschuer LA, Harmon BV, Prentice RL, Pope JH, Kerr JF. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br J Haematol. 1989;71:343–50. doi: 10.1111/j.1365-2141.1989.tb04290.x. [DOI] [PubMed] [Google Scholar]
- 38.Ghia P, Boussiotis VA, Schultze JL, Cardoso AA, Dorfman DM, Gribben JG, Freedman AS, Nadler LM. Unbalanced expression of bcl-2 family proteins in follicular lymphoma: contribution of CD40 signaling in promoting survival. Blood. 1998;91:244–51. [PubMed] [Google Scholar]
- 39.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–8. [PubMed] [Google Scholar]
- 40.Nelson BP, Variakojis D, Peterson LC. Leukemic phase of B-cell lymphomas mimicking chronic lymphocytic leukemia and variants at presentation. Mod Pathol. 2002;15:1111–20. doi: 10.1097/01.MP.0000031710.32235.24. [DOI] [PubMed] [Google Scholar]
- 41.Pepper C, Hoy T, Bentley DP. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br J Cancer. 1997;76:935–8. doi: 10.1038/bjc.1997.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saxena A, Viswanathan S, Moshynska O, Tandon P, Sankaran K, Sheridan DP. Mcl-1 and Bcl-2/Bax ratio are associated with treatment response but not with Rai stage in B-cell chronic lymphocytic leukemia. Am J Hematol. 2004;75:22–33. doi: 10.1002/ajh.10453. [DOI] [PubMed] [Google Scholar]
- 43.Thomas A, El Rouby S, Reed JC, Krajewski S, Silber R, Potmesil M, Newcomb EW. Drug-induced apoptosis in B-cell chronic lymphocytic leukemia: relationship between p53 gene mutation and bcl-2/bax proteins in drug resistance. Oncogene. 1996;12:1055–62. [PubMed] [Google Scholar]
- 44.Pepper C, Thomas A, Hoy T, Cotter F, Bentley P. Antisense-mediated suppression of Bcl-2 highlights its pivotal role in failed apoptosis in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;107:611–5. doi: 10.1046/j.1365-2141.1999.01726.x. [DOI] [PubMed] [Google Scholar]
- 45.Pepper C, Hooper K, Thomas A, Hoy T, Bentley P. Bcl-2 antisense oligonucleotides enhance the cytotoxicity of chlorambucil in B-cell chronic lymphocytic leukaemia cells. Leuk Lymphoma. 2001;42:491–8. doi: 10.3109/10428190109064606. [DOI] [PubMed] [Google Scholar]
- 46.Pepper C, Thomas A, Hoy T, Bentley P. Antisense oligonucleotides complementary to Bax transcripts reduce the susceptibility of B-cell chronic lymphocytic leukaemia cells to apoptosis in a bcl-2 independent manner. Leuk Lymphoma. 2002;43:2003–9. doi: 10.1080/1042819021000015961. [DOI] [PubMed] [Google Scholar]
- 47.Kitada S, Andersen J, Akar S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91:3379–89. [PubMed] [Google Scholar]
- 48.Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Sloetjes AW, de Witte T, Waksman G, Korsmeyer SJ. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–7. [PubMed] [Google Scholar]
- 49.Brimmell M, Mendiola R, Mangion J, Packham G. Frameshift mutations in cell lines derived from human haemopoietic malignancies are associated with resistance to apoptosis and microsatellite instability. Oncogene. 1998;16:1803–12. doi: 10.1038/sj.onc.1201704. [DOI] [PubMed] [Google Scholar]
- 50.Peng H, Aiello A, Packham G, Isaacson PG, Pan L. Infrequent bax gene mutations in B-cell lymphomas. J Pathol. 1998;186:378–82. doi: 10.1002/(SICI)1096-9896(199812)186:4<378::AID-PATH203>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Saxena A, Moshynska O, Sankaran K, Viswanathan S, Sheridan DP. Association of a novel single nucleotide polymorphism, G (−248) A, in the 5′-UTR of BAX gene in chronic lymphocytic leukemia with disease progression and treatment resistance. Cancer Lett. 2002;187:199–205. doi: 10.1016/s0304-3835(02)00378-6. [DOI] [PubMed] [Google Scholar]
- 52.Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. 2002;16:444–54. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
- 53.Michels J, Johnson PW, Packham G. Mcl-1. Int J Biochem Cell Biol. 2005;37:267–71. doi: 10.1016/j.biocel.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Michels J, O'Neill JW, Dallman CL, et al. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene. 2004;23:4818–27. doi: 10.1038/sj.onc.1207648. [DOI] [PubMed] [Google Scholar]
- 55.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–7. [PMC free article] [PubMed] [Google Scholar]
- 56.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 57.Akgul C, Moulding DA, White MR, Edwards SW. In vivo localisation and stability of human Mcl-1 using green fluorescent protein (GFP) fusion proteins. FEBS Lett. 2000;478:72–6. doi: 10.1016/s0014-5793(00)01809-3. [DOI] [PubMed] [Google Scholar]
- 58.Nijhawan D, Fang M, Traer E, Zhong Q, Du Gao WF, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–86. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17:2922–32. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snowden RT, Sun XM, Dyer MJ, Cohen GM. Bisindolylmaleimide IX is a potent inducer of apoptosis in chronic lymphocytic leukaemic cells and activates cleavage of Mcl-1. Leukemia. 2003;17:1981–9. doi: 10.1038/sj.leu.2403088. [DOI] [PubMed] [Google Scholar]
- 61.Clohessy JG, Zhuang J, Brady HJ. Characterisation of Mcl-1 cleavage during apoptosis of haematopoietic cells. Br J Haematol. 2004;125:655–65. doi: 10.1111/j.1365-2141.2004.04949.x. [DOI] [PubMed] [Google Scholar]
- 62.Herrant M, Luciano F, Loubat A, Auberger P. The protective effect of phorbol esters on Fas-mediated apoptosis in T cells. Transcriptional and postranscriptional regulation. Oncogene. 2002;21:4957–68. doi: 10.1038/sj.onc.1205689. [DOI] [PubMed] [Google Scholar]
- 63.Bellosillo B, Villamor N, Colomer D, Pons G, Montserrat E, Gil J. In vitro evaluation of fludarabine in combination with cyclophosphamide and/or mitoxantrone in B-cell chronic lymphocytic leukemia. Blood. 1999;94:2836–43. [PubMed] [Google Scholar]
- 64.Pepper C, Thomas A, Hoy T, Fegan C, Bentley P. Flavopiridol circumvents Bcl-2 family mediated inhibition of apoptosis and drug resistance in B-cell chronic lymphocytic leukaemia. Br J Haematol. 2001;114:70–7. doi: 10.1046/j.1365-2141.2001.02895.x. [DOI] [PubMed] [Google Scholar]
- 65.Panayiotidis P, Jones D, Ganeshaguru K, Foroni L, Hoffbrand AV. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1996;92:97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 66.Kim HS, Zhang X, Klyushnenkova E, Choi YS. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol. 1995;155:1101–9. [PubMed] [Google Scholar]
- 67.Granziero L, Ghia P, Circosta P, et al. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2777–83. doi: 10.1182/blood.v97.9.2777. [DOI] [PubMed] [Google Scholar]
- 68.Moshynska O, Sankaran K, Pahwa P, Saxena A. Prognostic significance of a short sequence insertion in the MCL-1 promoter in chronic lymphocytic leukemia. J Natl Cancer Inst. 2004;96:673–82. doi: 10.1093/jnci/djh122. [DOI] [PubMed] [Google Scholar]
- 69.Oxford SM, Dallman CL, Johnson PW, Ganesan A, Packham G. Current strategies to target the anti-apoptotic Bcl-2 protein in cancer cells. Curr Med Chem. 2004;11:1031–9. doi: 10.2174/0929867043455486. [DOI] [PubMed] [Google Scholar]