Abstract
Since it was previously shown that protein antigens applied epicutaneously in mice induce allergic dermatitis mediated by production of T helper 2 (Th2) cytokines we postulated that this might induce suppression of Th1 immunity. Here we show that epicutaneous immunization of normal mice with a different protein antigen applied on the skin in the form of a patch induces a state of subsequent antigen-non-specific unresponsiveness caused by suppressor T cells (Ts) that inhibit sensitization and elicitation of effector T-cell responses. Suppression is transferable in vivo by αβ-T-cell receptor CD4+ CD8+ double positive lymphocytes harvested from lymphoid organs of skin patched animals and are not major histocompatibility complex-restricted nor antigen specific. Both CD25+ and CD25− CD4+ CD8+ T cells are able to suppress adoptive transfer of Th1 effector cells mediating cutaneous contact sensitivity. In vivo treatment with monoclonal antibodies showed that the cytokines interleukin (IL)-4, IL-10 and transforming growth factor-β (TGF-β) are involved in the induction of the Ts cells. Additionally, using IL-10−/− mice we found that IL-10 is involved in skin induced tolerance. Further in vitro experiments showed that lymph node cells of skin tolerized mice non-specifically suppress [3H]thymidine incorporation by antigen-stimulated immune cells and this effect can be abolished by adding anti-TGF-β, but not anti-IL-4 nor anti-IL-10 antibodies. These studies indicate the crucial role of TGF-β in skin induced tolerance due to non-antigen-specific Ts cells and also show that IL-4, IL-10 and TGF-β play an important role in the induction of epicutaneously induced Ts cell suppression.
Keywords: epicutaneous immunization, non-specific unresponsiveness, αβ-TCR suppressor, T cells, TGF-β
Introduction
Surfaces of both the skin and mucosa are constantly exposed to many antigens that play a crucial role in body protection from different pathogens present in the external world. While development of the immune response to pathogens is of vital importance, responses to innocuous antigens are not required and sometimes can be harmful and lead to allergies. It is well known that immunization with antigen via the digestive tract or nasal mucosa, leads to a state of profound immunosuppression1–3 which seems to play an important role in avoiding development of immune responses to non-pathogenic environmental antigen. One can speculate that similar to mucosal tolerance, skin exposure to irrelevant non-pathogen-associated protein antigens also may lead to the development of immunological tolerance. It is worthy to underline that protein antigens or haptens applied in mice epicutaneously (e.c.) by patch method could induce allergic dermatitis4,5 and T helper 2 (Th2)-dependent immunoglobulin E (IgE) production.6 In our previous work we showed that e.c. deposition of protein antigens resulted in the induction of T suppressor cells (Ts) that were able to inhibit both Th1-mediated contact sensitivity to trinitrophenyl (TNP)-coupled protein and also delayed-type hypersensitivity (DTH) to the powerful protein antigen keyhole limpet haemocyanin (KLH).7
In our current work we tried to characterize the skin induced Ts cells more precisely and to determine their mechanism of action and conditions required for their induction. We have found that e.c. immunization of mice with hapten (TNP)-coupled antigen prior to active contact immunization with TNP or a different hapten (OX), or with e.c. immunization with protein antigen (KLH and ovalbumin, OVA), suppressed T effector cell mediated contact sensitivity and in vitro T-cell proliferation immune responses in antigen non-specific manner. Further, we show that the presence of interleukin (IL)-4, IL-10 and transforming growth factor-β (TGF-β) are required during the induction phase of e.c. exposure. In contrast, TGF-β and not IL-4 nor IL-10 mediated the effector phase of the Ts response. These results show that e.c. immunization with protein antigen induces tolerance and show that different mechanisms are involved in the induction and the effector function of Ts that act in an antigen non-specific and major histocompatibility complex (MHC) unrestricted manner on both hapten contact sensitivity (CS) and protein-induced DTH. The ease of induction and potent non-antigen-specific effect of skin induced Ts cells suggests that this may be a procedure applicable to treatment of autoimmune diseases.
Materials and methods
Mice
Male CBA/J and BALB/c mice 6–8 weeks old were from the breeding unit of the Department of Immunology, Jagiellonian University, College of Medicine. Mice were fed autoclaved food, and water. In one of experiments Jα18−/− mice on BALB/c background (formerly Jα281−/−) obtained from Masaru Taniguchi, Chiba University, Japan were used. In some experiments IL-10−/− mice on BALB/c background were used and were kindly provided by Diane McMahon-Pratt of Yale University School of Medicine. All experiments were conducted according to guidelines of the Animal Use and Care Committee of both the Jagiellonian University College of Medicine and Yale Medical School.
Reagents
Trinitrophenyl chloride (TNP-Cl; Chemica Alta, Edmonton, Canada), oxazolone ((OX, 4-ethoxy-methylene-2-phenyloxazolone); British Drug Houses, Poole, UK), KLH (Calbiochem, San Diego, CA), mitomycin C (Sigma, St. Louis, MO), OVA (Grade V; Sigma), RPMI-1640, HEPES buffer (1 m), sodium pyruvate, fetal calf serum (FCS; Life Technologies, Grand Island, NY), [3H]thymidine (Lacomed, Rez, RC), low-tox rabbit complement (RC; Pel-Freeze Biologicals, Brown Deer, WI), were all obtained from the manufacturers. Mouse immunoglobulins were prepared from CBA/J mouse sera and conjugated with TNP hapten.8,9 A single preparation with the level of substitution of 40 TNP per immunoglobulin molecule (TNP40-Ig) was used throughout.
Monoclonal antibodies (mAbs) and hybridoma
Purified anti-mouse cytokine mAbs: anti-IL-4 (Clone 11B11), anti-IL-10 (clone SXC1) and anti-TGF-β (clone HB 9849) were gifts of Dr Charles Janeway, Jr (Yale University, New Haven, CT). As an isotype control rat or mouse IgG were used (Sigma). In some experiments culture supernatants containing mAb were used: anti-T-cell receptor (TCR)-β clone H57-597) from Dr R. Kubo, Cytel Inc. (La Jolla, CA); anti-TCRδ (clone UC7-13D5) from Dr J. Bluestone (University of California, San Francisco, CA); anti-CD4 (clone TIB 207) and anti-CD8 (clone TIB 105.3) were obtained from Dr C.A. Janeway, Jr. Rat anti-mouse TGF-β1 mAb, biotinylated anti-mouse, -human, -pig TGF-β1 antibodies, IL-4 OptEIA™ ELISA Set, IL-10 OptEIA™ ELISA Set (all from BD PharMingen, San Diego, CA).
Active sensitization and measurement of CS in vivo
Mice were actively sensitized by topical application of 0·15 ml of 5% TNP-Cl or 3% OX in an acetone—ethanol mixture (1 : 3) to the shaved abdomen, and hind feet. Control mice were shaved and painted with acetone—ethanol mixture alone as a sham immunization. Four days later, mice were challenged on both sides of the ears with 20 µl of 0·4% TNP-Cl or OX in olive oil—acetone mixture 1 : .1. The subsequent increase in ear thickness was measured 24 hr later with an engineer's micrometer (Mitutoyo, Tokyo, Japan) and expressed in units of 10−2 mm ± SD.10 Background increase in ear thickness (∼2 units at 24 hr), of litter-mate non-immunized animals that were similarly challenged, was subtracted from each experimental group, to yield the net ear swelling expressed in units of 10−2 mm ± SD. Each experimental and control group consisted of five to six mice.
Active immunization and skin testing to elicit DTH
In some experiments, mice were sensitized by intradermal (i.d.) injection of 100 µg KLH diluted in 200 µl of freshly opened sterile pyrogen-free 0·9% saline for injection, into four divided sites in the alcohol cleaned and unshaved abdominal skin without added adjuvants.11,12 On day 4, after immunization, DTH ear swelling responses were elicited via i.d. injection in both ears under halothane anaesthesia with 10 µl containing 5 µg KLH in sterilized pyrogen-free saline.7,11,12 Controls were mock i.d. ‘immunized’ with saline alone, and similarly ear challenged with antigen to obtain background responses (∼2 units at 24 hr), that were subtracted from experimental groups. Resulting thickness of the antigen-challenged ears was measured with a micrometer, before challenge, and 24 hr after challenge. The net increased ear thickness is expressed as mean 10−2 mm ± SD.
Induction of suppressor cells by epicutaneous immunization
Mice were shaved on their backs with a razor BLAde before immunization. On day 0 100 µl of either TNP-Ig, KLH or OVA (all at 1 mg/ml) in phosphate-buffered saline (PBS), or PBS alone was applied to gauze in the centre of an occlusive patch (DuoDerm Extra Thin, ConvaTec, Princeton, NJ) which was then affixed to their backs.5 Patches were left intact for 4 days and then replaced with new antigen containing patches and kept on until day 7. In one of the experiments, TNP-Ig was applied to the skin together with anti-IL-4, anti-IL-10, anti-TGF-β or isotype control. On day 7 the patches were removed and mice were actively immunized and tested for CS or DTH, or lymphoid cells were harvested for in vitro proliferation assay, or were killed and used as a source of suppressor cells.
Adoptive cell transfer of CS and cell mixing assay to evaluate T suppressor cells (transfer out protocol)
Donors of CS-immune effector cells were contact sensitized with 5% TNP-Cl. Immune axillary and inguinal lymph nodes (LN) and spleens were harvested on day 4 and 7 × 107 immune cells were incubated for 30 min at 37° in RPMI-1640 medium alone, washed and then injected intravenously (i.v.) into normal syngeneic recipients (positive transfer). For the cell mixing assay, 7 × 107 of the CS-effector immune cells from TNP-Cl contact sensitized donors were incubated for 30 min at 37° with 5 × 107 lymphoid cells from mice tolerized by e.c. immunization with TNP-Ig and harvested on day 7.7 After incubation the cell mixture was transferred i.v. into naïve recipients. Mice were challenged with antigen within 30 min after cell transfer and tested for CS at 24 hr as described above.
Phenotype of suppressor cells
To determine the phenotype of patch immunization induced suppressor cells in vivo, LN cells isolated from mice tolerized by epicutaneous immunization with TNP-Ig were incubated in PBS on ice with culture supernatants containing anti-TCR-β, anti-TCRδ, anti-CD4 or anti-CD8 mAbs (1 ml of culture supernatant containing approximately 10 µg antibody/107 cells) or medium alone for 45 min. Then, the cells were washed and incubated with a predetermined dilution of RC for 60 min at 37°, and then washed and resuspended in PBS. After that, 4 day TNP-Cl immune CS-effector cells (7 × 107) were incubated for 30 min at 37° with medium alone (positive control), or 5 × 107 tolerized cells treated with RC alone (suppression control), or with 5 × 107 cell aliquots of suppressor cells treated with each appropriate mAb and RC, and finally transferred i.v. into naïve recipients that were challenged with TNP-Cl and their CS reactions measured 24 hr later.
FACS sorting of lymphoid CD4+ CD8+ double-positive T cells
Lymph node and spleen cells from mice that were tolerized by e.c. application of TNP-Ig (i.e. Ts cells) were stained with anti-CD4—fluoroscein isothiocyanate (FITC) and anti-CD8—phycoerythrin (PE; Pharmingen, San Diego, CA), for 30 min on ice, and then were washed at 4° with PBS. Stained cells were sorted with fluorescence-activated cell sorting (FACS; Dako Cytomation, Fort Collins, CO) to obtain CD4+ CD8+ lymphocytes. Then, 2 × 103 sorted CD4+ CD8+ lymphocytes or 2·5 × 107 unsorted Ts cells were mixed with 7 × 107 of 4-day TNP-Cl immune cells and incubated for 30 min at 37°. After incubation the cell mixture was washed and transferred i.v. into naïve recipients that were tested for CS.
In a second set of experiments, lymph node and spleen cells from mice e.c.-tolerized with TNP-Ig were stained with anti CD4—PE, anti-CD8—Cy and additionally with anti-CD25—FITC mAb. All fluorochrome labelled antibodies were obtained from Pharmingen. After staining with mAbs, the Ts cells were sorted with FACS Vantage SE (Becton Dickinson, San Jose, CA) to obtain CD4+ CD8+ CD25+ and CD4+ CD8+ CD25− subpopulations that, respectively, were 68% and 32% of the total CD4+ CD8+ double-positive cells (each ±98% enriched). Then 6 × 104 CD4+ CD8+ CD25−, or 3 × 104 CD4+ CD8+ CD25+, or 2·5 × 107 unsorted Ts cells were mixed and incubated with separate aliquots of 7 × 107 of 4 day PCl immune cells, and the mixtures then were transferred into naïve recipients that were tested for CS.
Transfer into tolerized mice to confirm active tolerance (transfer in protocol)
To confirm data found in cell mixing assay (‘transfer out’), 4 day TNP-Cl immune CS-effector cells (7 × 107) were transferred i.v. into syngeneic recipients that had been tolerized by skin patching with TNP-Ig antigen 7 days earlier, or into naïve mice (positive transfer). Then mice were challenged and tested for CS.
The influence of e.c. tolerization on the in vitro proliferation of TNP-Cl immune LN cells
CBA/J mice were e.c. tolerized with TNP-Ig or OVA as described above. Then on day 7 mice were actively contact immunized by topical application of 5% TNP-Cl. As positive controls we used mice that were e.c.-treated with PBS alone before active TNP-Cl sensitization. Four days after TNP-Cl immunization, a single cell suspension of axillary and inguinal LNC were prepared under aseptic conditions and 3 × 105 LN cells were incubated in U-bottom 96-well micro plates (Falcon, Oxnard, CA) in triplicate with threefold decreasing dilutions of TNP-Ig starting with 300 µg/ml in 200 µl of RPMI-1640 containing 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mm l-glutamine, 25 mm HEPES, 5 × 10−5 m 2-mercaptoethanol and 10% fetal calf serum for 48 hr. Then 0·5 µCi/well of [3H]thymidine were added and cells incubated for an additional 18 hr. Then cells were harvested and [3H]thymidine incorporation was determined by β liquid scintillation counting.7 Results are presented as mean c.p.m. ± SD. Background scintillation (cells cultured without any antigen) was substracted from all tested groups.
Proliferation assay in the presence of suppressor cells and neutralizing anti-cytokine mAbs
To determine mechanisms of inhibition mediated by the suppressor cells induced by e.c. immunization, 3 × 105 TNP-Cl immune LN cells were cultured alone or were cocultured with 6 × 104 mitomycin C-treated LN cells from mice e.c.-immunized with TNP-Ig and cultured in triplicate with threefold decreasing dilutions of TNP-Ig starting with 300 µg/ml under the conditions described above. In some experimental groups, the mixture of immune and suppressor cells were cultured in the presence of 10 µg/ml of neutralizing anti-IL-4, anti-IL-10 or anti-TGF-β. Then, [3H]thymidine incorporation was tested by β liquid scintillation counting. Results are presented as mean c.p.m. ± SD. Background scintillation (cells cultured without any antigen) was subtracted from all tested groups.
Cytokines (TGF-β, IL-4 and IL-10) immunoassays
Lymph node or spleen cells (3 × 106) from mice e.c.-treated with PBS (control group) or TNP-Ig were cultured in 1 ml RPMI-1640 medium supplemented with 5% FCS in the presence of 100 µg/ml TNP40-Ig were distributed in triplicate wells in flat 24-well Falcon plates and after 48 hr culture supernatants were collected.
For estimating TGF-β supernatants were at first acidified with 1 N HCl in dilution 1 : 25 and then neutralized with 1 N NaOH to pH 7·0 in the same proportion. Cytokine concentrations in culture supernatants were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using monoclonal rat anti-mouse TGF-β antibodies as capture antibody and biotinylated monoclonal rat anti-mouse, -human, -pig TGF-β antibodies as the secondary antibody. The reaction was developed with horseradish peroxidase streptavidin, followed by o-phenylenediamine and H2O2 as substrate and was stopped with 3 m H2SO4. The optical density of each well was measured in a 96-well plate reader at 492 nm. All determinations were done in triplicate. A standard curve was generated by recombinant mouse TGF-β and the lower density limit was 30 pg/ml TGF-β.
IL-4 and IL-10 were determined by using IL-4 OptEIA™ ELISA and IL-10 OptEIA™ ELISA sets as recommended by the manufacturer.
Statistics
The paired two-tailed Student's t-test was used with P < 0·05 taken as the level of significance.
Results
Dose dependence of skin induced suppression of contact sensitivity
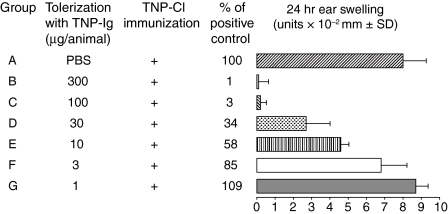
CBA/J mice were e.c. immunized twice with graded doses of TNP-Ig (300, 100, 30, 10, 3 or 1 µg/animal) or exposed to PBS alone as described in Materials and methods. On day 7 patches were removed, then these mice were actively immunized with 5% TNP-Cl on another site and then tested 4 days later for elicitation of CS. Figure 1 shows that mice exposed to doses of TNP-Ig between 300 to only 10 µg/animal prior to TNP-Cl immunization develop significantly decreased CS in a dose—response manner, ranging from 0 to 58% compared to positive controls (Fig. 1 groups B–E versus group A). The lowest doses of TNP-Ig at 3 and 1 µg/animal did not suppress CS.
Figure 1.
Immunization (e.c) with protein antigen induces suppression of CS, which is dose dependent. CBA/J mice were e.c. immunized via patches with graded doses of TNP-Ig (300, 100, 30, 10, 3 or 1 µg/animal) (groups B–G) or exposed to PBS alone (group A). On day 7 patches were removed, mice were actively immunized with 5% TNP-Cl and then tested for CS. Results were expressed in units of 10−2 mm ± SD. Each experimental group consisted of five or six mice. Statistical significance: groups B, C, D and E versus group A; P < 0·001.
These data strongly suggest that e.c. immunization with related antigen TNP-Ig prior to skin sensitization with TNP-Cl significantly reduces CS responses and that the strength of suppression is dependent on the tolerizing dose of antigen applied to the skin.
Skin-induced tolerance is caused by suppressor cells (transfer out protocol)
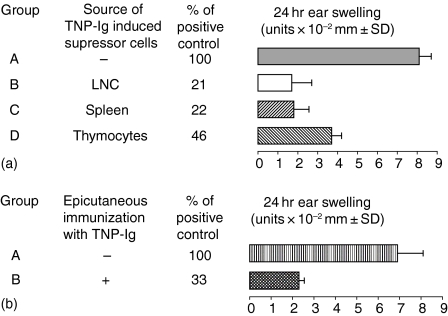
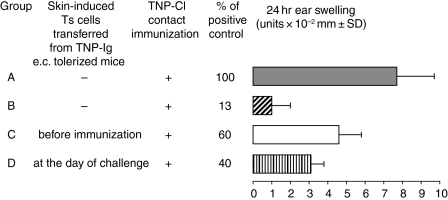
We determined if skin-induced tolerance can be transferred with cells of the immune system, in a classical cell mixing experiment together with effector T cells. Thus, 7 × 107 TNP-Cl immune CS-effector cells harvested on day 4 postsensitization were incubated for 30 min at 37° in medium alone as positive control, or with 5 × 107 peripheral LNC, spleen cells, or thymocytes isolated from mice that were skin tolerized with TNP-Ig 7 days previously. Then resultant cell mixtures were transferred into naive syngeneic recipients that were tested for CS. Figure 2(a) shows that thymocytes, peripheral LN and spleen cells from tolerized donors were able to suppress adoptive transfer of CS (groups B–D versus group A).
Figure 2.
Skin-induced tolerance can be transferred into naïve recipients. (a) Skin induced tolerance can be transferred with cells of the immune system (‘transfer out’ protocol). Four day TNP-Cl immune cells (7 × 107) were incubated for 30 min at 37° in medium alone (group A) or with 5 × 107 peripheral LNC, spleen cells, or thymocytes isolated from mice that were tolerized with TNP-Ig (groups B–D). Then resultant cell mixtures were transferred into naive syngeneic recipients that were tested for CS. Each experimental group consisted of five to six mice. Statistical significance: groups B, C and D versus group A; P < 0·001.(b) Immunization of (e.c.) cell transfer recipients with protein antigen induces a suppressor environment that inhibits CS (transfer in protocol). To confirm data found in the cell mixing assay (‘transfer out’), 4 day TNP-Cl immune cells (7 × 107) were transferred i.v. into syngeneic recipients that were tolerized by skin patching (group B) or into naïve mice (positive transfer) (group A). Then mice were challenged and tested for CS. Each experimental group consisted of five to six mice. Statistical significance: group B versus group A; P < 0·001.
Effector function of CS cells can be suppressed when transferred into skin tolerized recipients (transfer in protocol)
The experiment above (‘transfer out’ protocol) showed that cotransfer of immune cells with CS-effector cells isolated from TNP-Ig patched mice resulted in decreased CS. To show that the observed suppression is not an effect of in vitro cell manipulations, we employed a different ‘transfer in’ protocol where CS-immune effector cells were transferred either into naive recipients (positive transfer), or into mice that were tolerized by skin patch 7 days earlier as a test of suppressor cells in the actively tolerized mice. Figure 2(b) shows that suppression previously shown via the ‘transfer in’ protocol (Fig. 2a) was similar to that observed via the ‘transfer out’ protocol, indicating that the observed suppression was not an artefact of in vitro cell manipulations Fig. 2(b) (group B versus group A).
Dose—response activity of the skin immunization induced suppressor cells
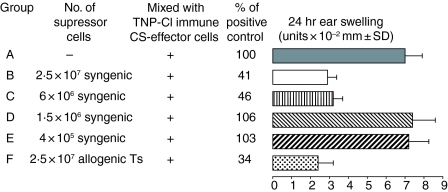
We attempted to find the minimal number of skin induced Ts cells that are able to suppress the activity of CS-effector T cells. Thus 4 day TNP-Cl immune cells (7 × 107) were incubated with decreasing numbers of suppressor cells from e.c.-immunized donors before transfer into naive recipients (‘transfer out’ protocol). Figure 3 shows that suppressor activity of LNC from TNP-Ig patched mice is dose dependent, and that 6 × 106 is the lowest number of cells that can effectively inhibit adoptive transfer of CS (group C versus group A). In the same experiment we also tested if Ts cells are MHC restricted. Day 4 PCl immune cells (7 × 107) were incubated with 2 × 107 suppressor cells isolated from syngeneic CBA/J (H-2k) or BALB/c (H-2d) mice before cell transfer. Data presented in Fig. 3 show that allogenic suppressor cells are as effective as syngeneic suppressor cells (groups F and B versus group A).
Figure 3.
A low number of suppressor cells is required to inhibit CS; 7 × 107 4 day TNP-Cl immune cells were incubated with decreasing numbers of syngeneic suppressor cells [CBA/J (H-2k)] (groups B–E) or with 2 × 107 of allogenic Ts [BALB/c (H-2d)] or alone (group A) before cell transfer into naive recipients that were tested for CS. Statistical significance: groups B, C and F versus group A; P < 0·001.
Skin-induced suppressor cells belong to the population of αβ TCR+ lymphocytes that express CD4+ and CD8+ coreceptors and are both CD25+ or CD25−
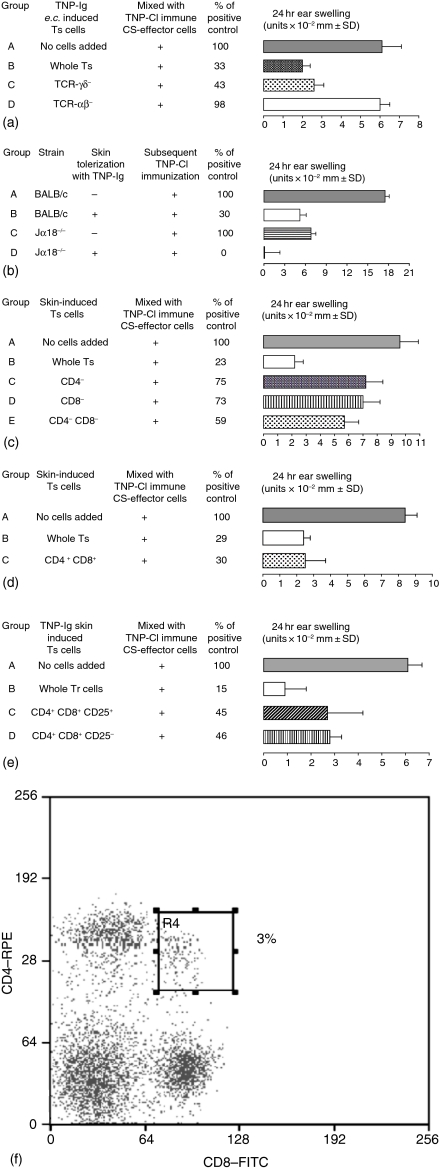
To in vivo phenotype the suppressor cells, LN cells from mice e.c. immunized with TNP-Ig were treated with following mAb: anti-TCR-α, anti-TCRδ, anti-CD4 or anti-CD8 with RC, or with RC alone as a suppression control. Then, resultant cells were washed and incubated with 4 day TNP-Cl immune cells before transfer into syngeneic naive recipients that were tested for CS. Data presented in Fig. 4(a) show that skin-induced suppressor cells are αβ TCR+, but are not γδ T cells (group C versus groups B and D).
Figure 4.
Phenotype of Ts cells induced via e.c. immunization with protein antigen. (a) αβ-TCR+ lymphocytes are involved in skin induced tolerance. LN cells from mice e.c. immunized with TNP-Ig were treated with the following mAb: anti-TCR αβ, anti-TCR γδ (a); anti-CD4 or anti-CD8 (c) and RC or complement alone as a suppression control. Then 5 × 107 suppressor cells treated with RC alone or cell equivalents of suppressor cells treated with appropriate mAb and RC, resultant cells were washed and incubated with 4 day TNP-Cl immune cells before transfer into syngeneic naive recipients that were tested for CS. Presented data show that skin induced suppressor cells belong to the population of TCR αβ+ (group C versus group B). Statistical significance: groups C and B versus group A; not significant; group D versus group A; not significant.(b) CD1d-dependent Vα14+ Jα18+ NKT cells are not required for skin induced tolerance. Additionally, experiments employing Jα18−/− mice e.c. immunized with TNP-Ig show that NKT cells are not involved in skin induced tolerance (group C versus D). Statistical significance: group C versus group D; P < 0·001. (c) Depletion of T cells that express CD4 and CD8 coreceptors abrogates the activity of skin induced suppressor cells. Presented data show that depletion of both CD4 and CD8 cells resulted in abrogation of suppressor activity induced via e.c. immunization (groups C and D versus group B). Additionally, reconstitution of CD4 depleted cells with the CD8 depleted cell population did not restore suppressor activity of skin induced Ts (group E versus group B). Statistical significance: group B versus group A; P < 0·001 and group D versus group C; not significant. (d) Epicutaneous immunization induces CD4+ CD8+ double-positive Ts cells.To determine if skin induced Ts cells coexpress CD4 and CD8, we incubated 2 × 103 of sorted CD4+ CD8+ double-positive cells isolated from skin-tolerized mice with 4 day TNP-Cl immune cells before transfer into syngeneic naive recipients that were tested for CS. The data show that only 2 × 103 CD4+ CD8+ double positive cells were able to efficiently suppress CS at the same level as 5 × 107 unseparated regulatory cells (groups C and B versus group A). Statistical significance: groups C and B versus group A; P < 0·001. (e) Both CD25+ and CD25− CD4+ CD8+ cells from e.c.-immunized mice are able to inhibit adoptive transfer of CS. Presented data show that FACS-sorted CD4+ CD8+ cells that either CD25+ or CD25− can suppress adoptive transfer of CS (groups C and D versus group A). Statistical significance: group B versus group A; P < 0·001; group C versus group A; P < 0·02 and group D versus group A; P < 0·001. (f) Epicutaneous immunization with protein antigen causes increase of CD4+ CD8+ DP cells in the periphery. Spleen cells from mice epicutaneously immunized with TNP-Ig double-stained with FITC anti-CD8 and RPE anti-CD4 and analysed by flow cytometry. Lymphocytes were gated according to size and granularity on FSC versus SSC dotplot and the percentage of double positive CD4+ CD8+ cells within lymphocytes gate is presented. Results are representative of three independent experiments.
To examine if a subpopulation of non-MHC restricted, but CD1d restricted Vα14+ Jα18+ invariant NKT cells were involved, we actively tolerized Jα18−/− BALB/c mice. As shown before, CS responses were reduced in Jα18−/− mice compared to wild type controls.13 In addition, e.c. immunization with TNP-Ig causes reduction of CS in the absence of NKT cells to a similar extent as in wild type BALB/c mice (Fig. 4b, group D versus group C, and group B versus group A), indicating that Vα14+ NKT cells are not involved in skin tolerance similarly to oral tolerance.14
Moreover, data presented in Fig. 4(c) show that depletion of either CD4 or CD8 cells resulted in abrogation of suppressor activity induced via e.c. immunization (groups C and D versus group B). Importantly, mixing CD4 depleted cells with CD8 depleted cell population did not restore suppressor activity of skin induced Ts (group E versus group B). These data suggest that both CD4 and CD8 coreceptors are expressed on the same suppressor T cell. To determine if skin induced Ts cells coexpress CD4 and CD8 receptors, we incubated 2 × 103 of sorted CD4+ CD8+ double-positive cells isolated from skin tolerized mice with 4 day TNP-Cl immune cells before transfer into syngeneic naive recipients that were tested for CS. Figure 4(d) shows that only 2 × 103 CD4+ CD8+ double-positive cells were able to suppress CS as efficiently as 5 × 107 unseparated suppressor cells (groups C and B versus group A). Finally, because many Ts are CD25+ we determined whether skin tolerized cells are CD25+ or CD25−. The experiment presented in Fig. 4(e) shows that both CD25+ and CD25− CD4+ CD8+ T cells isolated from e.c.-tolerized mice are able to inhibit adoptive transfer of CS (groups C and D versus group A). Therefore, we determined that skin-induced Ts cells have both CD4 and CD8 coreceptors and are either CD25+ or CD25−.
Additionally, Fig. 4(f) shows that spleens isolated from e.c.-tolerized mice have increased percentage of CD4+ CD8+ DP cells reaching up to 3% of all T cells.
Transfer of suppressor cells both at the day of immunization or challenge can suppress CS
To determine at what stage of CS the suppressor cells act, regulatory cells isolated from TNP-Ig patch immunized mice were transferred into syngeneic mice on the day of TNP-Cl contact immunization, or just prior to ear challenge (Fig. 5, groups C and D, respectively). There were two additional groups: positive control (mice just actively immunized with TNP-Cl), and a group of mice that were patched with TNP-Ig and then sensitized with TNP-Cl (suppression control; Fig. 5, groups A and B, respectively). Results show that suppressor cells induced by e.c. immunization inhibited CS at either the afferent or the efferent phase of CS (groups C and D versus group A).
Figure 5.
Transfer of regulatory cells both at the day of immunization or challenge can suppress CS. Regulatory cells isolated from TNP-Ig patched mice were transferred into syngeneic mice at the day of TNP-Cl immunization (day 0) or at the day of challenge (day 4) (groups C and D). There were two additional groups: positive control (mice actively immunized with TNP-Cl) (group A) and a group of mice that were patched with TNP-Ig and then actively sensitized with TNP-Cl (suppression control) (group B). Statistical significance: group B versus group A; P < 0·001; group C versus group A; P < 0·05 and group D versus group A; P < 0·01.
Skin induced suppression declines with time
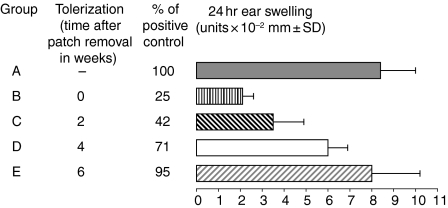
To determine the duration of skin-induced tolerance, CBA/J mice were e.c. immunized with 100 µg/animal of TNP-Ig or exposed to PBS alone as control. On day 7 patches were removed and then mice were actively immunized with 5% TNP-Cl immediately after patch removal or 2, 4 or 6 weeks later. Mice from all experimental groups were challenged and tested for CS at the same time. Figure 6 shows that skin induced suppression of CS declines with time (groups B–E versus group A). Maximal suppression was observed when mice were immunized immediately after patch removal (CS suppressed by 75%; group B), inhibition of CS persisted up to 4 weeks after patch removal (group D versus group A), but can be effective up to 2 weeks after patching.
Figure 6.
Skin induced suppression declines with time. CBA/J mice were e.c. immunized with 100 µg/animal of TNP-Ig or exposed to PBS alone (group A) as described previously. On day 7 patches were removed and then mice were actively immunized with 5% TNP-Cl immediately (group B), or 2, 4 or 6 weeks later (groups C-E correspondingly). Mice from all experimental groups were challenged and tested for CS. Statistical significance: groups B and C versus group A; P < 0·001; group D versus group A; P < 0·02.
Skin-induced suppression of CS is antigen non-specific
To determine if skin-induced tolerance is antigen specific, mice were tolerized with TNP-Ig or KLH or OVA containing patches and subsequently immunized with TNP-Cl, OX or KLH and tested for CS or DTH, respectively. Data presented in Table 1 show that tolerization and subsequent immunization with both corresponding and irrelevant antigen caused decreases of cell mediated CS and DTH responses. These results show that skin patching induces antigen non-specific Ts cells.
Table 1. Epicutaneous immunization induces Ts cells that are antigen-non-specific and are able to inhibit both CS and DTH.
| Immunization with | |||
|---|---|---|---|
| Tolerization with | PCI | OX | KLH |
| – | 6·1 ± 0·4 | 10·9 ± 0·5 | 16·8 ± 1·0 |
| TNP-Ig | 0·9 ± 1·3 | 4·2 ± 1·6 | 7·1 ± 1·2 |
| KLH | 0·6 ± 0·2 | ND | 10·0 ± 0·6 |
| OVA | 3·1 ± 0·4 | 5·4 ± 1·7 | ND |
ND, not done.
To determine if skin-induced tolerance is antigen specific, mice were tolerized with TNP-Ig, KLH, OVA or treated with PBS alone (positive control) and subsequently immunized with TNP-Cl, OX or KLH and tested for CS or DTH respectively.
Statistical significance between groups treated with PBS alone and groups treated before immunization with patches containing protein antigens can vary between P < 0·001 and P < 0·05.
The influence of e.c. tolerization on the in vitro proliferation of PCl immune lymph node cells
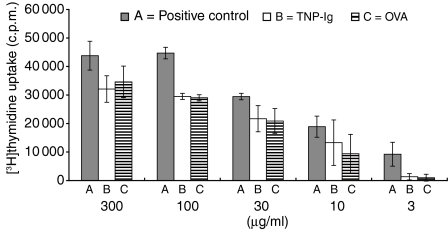
CBA/J mice skin tolerized with TNP-Ig or OVA, were treated with PBS alone as described above. On day 7 patches were removed and mice were actively immunized with 5% TNP-Cl. Four days later axillary and inginual LN were collected and single cell suspensions prepared and tested in a proliferation assay in the presence of TNP-Ig as antigen. Figure 7 shows that skin-induced tolerance with two non-cross-reacting antigens TNP-Ig and OVA resulted in inhibition of proliferation of TNP-specific immune cells compared to lymphoid cells from mice receiving a sham patch with PBS (groups B and C versus group A). We concluded that skin patch with protein antigen inhibits antigen specific proliferation in vitro.
Figure 7.
The influence of e.c. tolerization on the in vitro proliferation of TNP-Cl immune lymph node cells. CBA/J mice were skin tolerized with TNP-Ig (group B), OVA (group C) or treated with PBS alone (group A). On day 7 patches were removed and mice from all groups were actively immunized with 5% TNP-Cl. Four days later axillary and inginual LN were collected and single-cell suspensions prepared and tested in proliferation assay. 3 × 105 LNC per well were cultured in U-bottom 96-well microplates in triplicate in RPMI-1640 plus FCS, l-glutamine, HEPES and 2-mercaptoethanol. Threefold dilutions of TNP-Ig were added to the wells (consecutively 300, 100, 30, 10 and 3 µg/ml). After addition of 0·5 µCi/well of [3H]thymidyne, the cells were incubated for 18 h and then [3H]thymidyne incorporation was measured in a β scintillation counter. Results are expressed as mean ± SD. Statistical significance: 100 µg/ml, groups B and C versus group A; P < 0·05; 30 µg/ml groups B and C versus group A; P < 0·02. Differences in other groups are non-significant.
IL-4, IL-10 and TGF-β are involved in the induction of skin induced suppressor cells
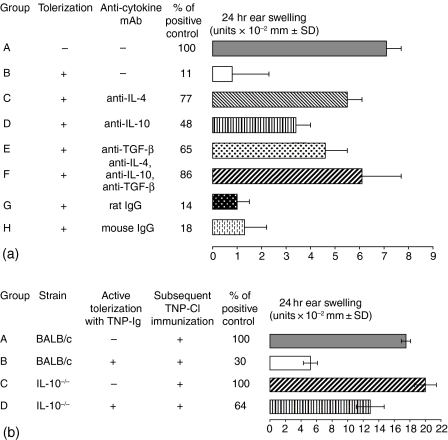
To determine if specific cytokines are required for the induction of suppressor cells via e.c. immunization, mice were tolerized with TNP-Ig alone, or TNP-Ig mixed with 100 µg of anticytokine mAb (anti-IL4, anti-IL-10, anti-TGF-β) or an isotype control. Then mice were actively immunized with 5% TNP-Cl and tested for CS as described before. Figure 8(a) shows that each of these anti-cytokine mAbs significantly diminished suppression induced by skin immunization with TNP-Ig (groups C–E versus group B). The maximal effect was observed when all three cytokines were neutralized (group F versus group B). Thus, all three cytokines — IL-4, IL-10 and TGF-β— seem to be required for successful induction of suppressor cells. Figure 8(b) employing IL-10−/− mice confirmed that IL-10 is involved in the induction of Ts cells.
Figure 8.
Inhibitory cytokines involved in the induction of skin induced suppressor T cells. (a) IL-4, IL-10 and TGF-β are required for induction of Ts cells via e.c. immuization. Mice were e.c. tolerized with TNP-Ig alone (group B) or TNP-Ig plus 100 µg of anticytokine mAb (anti-IL4, anti-IL-10, anti-TGF-β or the mixture of all three mAbs) (groups C-F, respectively). Then mice were actively immunized with 5% TNP-Cl and tested for CS. An additional group of mice were patched with PBS alone and then actively immunized with TNP-Cl (positive control) (group A). Additionally, in groups G and H mice were e.c. tolerized with TNP-Ig plus 100 µg of rat or mouse IgG, respectively, as an isotype control. Statistical significance: group B versus group A; P < 0·001, groups C and F versus group B; P < 0·001, group D versus group B; P < 0·05 and group E versus group B; P < 0·01. groups G and H versus group B; not significant. (b)IL-10 is involved in skin-induced tolerance. To confirm involvement of IL-10 in the induction of Ts cells via e.c. immunization with protein antigen we compared immune response in wild type BALB/c and IL-10-/— mice that were e.c. immunized with TNP-Ig (groups B and D) before active immunization with TNP-Cl. In groups A and C BALB/c and IL-10−/− mice were patched with PBS alone before TNP-Cl sensitization (positive controls). Statistical significance: group B versus group A; P < 0·001 and group D versus group B; P < 0·05.
TGF-β but not IL-4 or IL-10 are involved in the effector phase of skin-induced tolerance
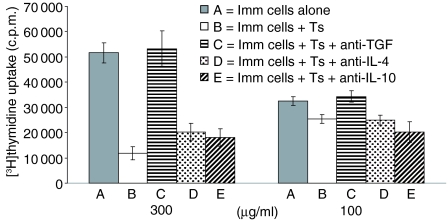
To investigate mechanisms of skin induced tolerance in vitro, mitomycin C-treated LN cells from tolerized mice were cocultured with 4 day TNP-Cl immune cells in the presence of TNP-Ig as antigen with or without the following anti-cytokine mAbs: anti-IL-4, anti-IL-10 or anti-TGF-β. Figure 9 shows that suppression of proliferation could be overcome by addition of neutralizing anti-TGF-β mAb to the culture. Neither anti-IL-4 nor anti-IL-10 had any effect. These data strongly suggest that T suppressor cells induced by e.c. immunization with TNP-Ig operate via release of TGF-β. Additionally, the data presented in Table 2 show increased production of TGF-β by lymph node and spleen cells isolated from TNP-Ig tolerized mice when compared to control mice (animals patched with PBS alone). However there was no significant difference in IL-4 and IL-10 production by lymph node and spleen cells isolated both from TNP-Ig tolerized and control mice.
Figure 9.
Skin induced tolerance operates via αβ-TCR+ cells producing TGF-β. To determine the mechanism of skin induced tolerance, 6 × 104 mitomycin C-treated LNC from tolerized mice (Ts) were cocultured with 3 × 105 4 day TNP-Cl immune cells (Imm. cells) in triplicates with or without following anti-cytokine mAbs: anti-IL4, anti-L-10 or anti-TGF-β. In the positive control immune cells were coultered without Ts cells. Conditions of culture and [3H]thymidyne were described in the legends to Fig. 7. Statistical significance: 300 µg/ml groups B, D and E versus group A; P < 0·05. Differences in other groups are non-significant.
Table 2. Epicutanous immunization results in TGF-β secretion by lymph node and spleen cells.
| Measured cytokine | |||
|---|---|---|---|
| Mice e.c.- treated with | IL-4 (pg/ml) | IL-10 (pg/ml) | TGF-β (pg/ml) |
| LN (PBS) | 0·0 ± 0·0 | 5·0 ± 4·0 | 0·0 ± 0·0 |
| LN (TNP-Ig) | 0·0 ± 0·0 | 8·6 ± 0·5 | 46·0 ± 10·0 |
| Spl. (PBS) | 0·0 ± 0·0 | 104·0 ± 3·0 | 0·0 ± 0·0 |
| Spl. (TNP-Ig) | 0·0 ± 0·0 | 100·0 ± 12·0 | 80·0 ± 32·0 |
To determine production of anti-inflammatory cytokines 3 × 10 6 of lymph node or spleen cells from e.c. PBS (control group) or TNP-Ig treated mice were cultured in 1 ml RPMI-1640 medium supplemented with 5% FCS in the presence of 100 μg/ml TNP40-Ig and were distributed in triplicate wells in flat 24-well Falcon plates. After 48 hr culture supernatants were collected. Then IL-4, IL-10 and TGF-β were measured. Presented data show increased production of TGF-β by lymph node and spleen cells isolated from TNP-Ig-tolerized mice when compared to control mice (animals patched with PBS alone). However there was no significant difference in IL-4 and IL-10 production by lymph node and spleen cells isolated both from TNP-Ig-tolerized and control mice.
Discussion
For many years the skin has been considered an organ where strong T-cell mediated immune responses such as contact sensitivity could easily be induced. However, the skin as a site for induction of tolerance has received limited attention.15
Wang et al. showed that e.c. application of protein antigens like OVA induces the ability to elicit allergic dermatitis accompanied with appearance of IL-4-secreting Th2 cells.6 It is well known that cytokines released by Th2 lymphocytes are able to inhibit Th1-mediated immune responses.16 The data showing that e.c. application of protein antigen results in the induction of T cells secreting Th2 cytokines led us to speculate that skin immunization with protein antigen before induction of Th1-mediated immune response might cause its suppression.
In the current study we determined if e.c. immunization with soluble protein antigen spread over a gauze patch could induce cells that would be able to negatively regulate Th1-mediated immune response. Data presented in Fig. 1 clearly show that e.c. immunization with TNP-mIg results in significant reduction of TNP-specific CS. The optimal dose of antigen that induces this phenomenon is between 30 and 100 µg/animal. Further, the observed suppression lasts for about four weeks (Fig. 6). Additional experiments showed that skin induced tolerance could be transferred by cells of the immune system isolated from subcutaneous lymph nodes, spleens or thymus mixed with CS-effector T cells (‘transfer out’ protocol).
We considered that the observed suppression is not an effect of in vitro cell manipulation or tolerance is not induced by overloading the immune system with large numbers of cells, some of which will be dying or damaged by the preparation process that could be by itself tolerogenic. Therefore, we used another experimental system —‘transfer in’ protocol, where CS-effector cells were transferred into naïve recipients (positive control), or into mice that were previously tolerized by skin patch to test for endogenous suppression. We found that suppression found with this ‘transfer in’ protocol was similar to that observed with the ‘transfer out’ protocol, indicating that inhibition of the Th1-mediated response was not an artefact of in vitro cell manipulation. Additionally, we found that skin-induced suppressor cells were able to significantly inhibit CS effector cells when transferred into naïve recipients at a ratio of 1 : 10.
Negative selection experiments defined the phenotype of the skin-induced suppressor cells showing that they belong to the population of αβ TCR+ lymphocytes. There are some reports that invariant Vα14+ NKT cells can have an immunoregulatory function in many experimental systems.17 However, experiments performed with NKT deficient Jα18−/− mice showed that these predominant NKT cells among Tαβ cells are not involved in skin induced tolerance.
The observed lack of suppression after depletion of CD4 or CD8 cells from lymphoid cells isolated from animals e.c.-immunized with TNP-Ig can suggest that both CD4+ and CD8+ populations have suppressor activity. However, reconstitution of CD4− cells with the CD8 depleted cell population did not restore suppressor activity of skin tolerance induced suppressor cells, suggesting that these cells may coexpress both CD4 and CD8 coreceptors. Dual expression of CD4 and CD8 on the Ts was suggested by showing that skin patching causes the appearance of about 2–3% TCRαβ+ CD4+ CD8+ double-positive T cells in subcutaneous lymph nodes and in the spleen comparing to normal range between 0 and 1%.18 Increased numbers of CD4+ CD8+ double positive T cells began on day 7 (day of removal of the second patch) and remained elevated for a week. Then the proportion of CD4+ CD8+ cells declined to that observed in mice e.c.-treated with PBS alone.
The role of such a peripheral population of CD4+ CD8+ cells is still unclear. It has been reported that peripheral CD4+ CD8+ T lymphocytes increase during the neonatal period in mice and humans, and also in certain infections, autoimmune diseases, tumours, transplantation and also in the aged.19,20 To confirm our hypothesis that e.c. immunization induces Ts cells that belong to the population of CD4+ CD8+ double-positive lymphocytes, we mixed 4-day TNP-Cl immune CS-effector cells with FACS-sorted tolerized CD4+ CD8+ T cells from e.c.-treated mice and transferred the mixture into naïve recipients that were then tested for CS. This cell mixing experiment showed that indeed CD4+ CD8+ T cells isolated from e.c.-immunized mice are responsible for suppression. These data may be related to double positive CD4+ CD8+ intestinal intraepithelial lymphocytes with a greater frequency of cells producing Th2-type cytokines, that perhaps, mediate oral tolerogenic mechanisms in the intestinal environment.21
Other studies show that intraepithelial lymphocytes are CD4+ CD8+ αβ TCR+ cells that can release immunoregulatory cytokines, such as TGF-β and IL-10, suggesting suppressor activity. As noted above, CD4+ CD8+ T cells in naïve mice are less than 1% of peripheral lymphocytes and their origin is unclear. They could represent CD4+ CD8+ T cells that have prematurely escaped from the thymus or CD4+ CD8− T cells that re-express CD8 after activation or exposure to lymphokines such as IL-4, or are present because of prior ‘natural’ suppressive events. In our case we speculate that CD4+ CD8+ T cells with suppressor activity originate from prematurely escaped CD4+ lymphocytes that acquire CD8 coreceptor. Our hypothesis is based on the observed increase of CD4+ CD8+ T cells in lymph nodes 7 days after e.c. immunization, and their decrease to the level observed in naïve mice within a week. These data can also suggest that these immature CD4+ CD8+ suppressor cells then may become single-positive CD4+ T cells
There are many reports showing that Ts cells belong to the population of CD4+ CD25+ lymphocytes. In a FACS sorting experiment employing skin induced suppressor cells we showed that one-third of CD4+ CD8+ T cells expressed CD25 whereas two-thirds of CD4+ CD8+ T cells were CD25−. However, both CD4+ CD8+ CD25+ and CD4+ CD8+ CD25− T cells were able to inhibit adoptive transfer of CS. Therefore, in this system both CD25+ and CD25− CD8+ CD4+ cells have suppressive activity, but we cannot rule out that the CD25− cells become CD25+ after transfer in vivo, and thus also become responsive to IL-2 or possess CD25 below levels detected by FACS.
Additionally we found that very low numbers of skin induced Ts cells, such as 2 × 103 per mouse were able to inhibit the effector function of 7 × 107 4 day TNP-Cl immune cells in vivo. We previously also found that small numbers of γδ TCR+ suppressor cells could inhibit Th1 TNP-Cl induced CS effector cells, because only 2·5 × 103 of these cells efficiently suppressed 7 × 107 CS-effector immune cells.22
In further experiments we transferred skin-induced Ts cells at the day of immunization or challenge to determine at what stage of CS the suppressor cells act. The observed suppression of CS in both cases might suggest that Ts cells induced by e.c. immunization operate both at the afferent and efferent phase of Th1-mediated CS. However, this experiment does not exclude the possibility that Ts cells transferred at the time of immunization do not affect induction of Th1 effector cells, and can persist in the body of the recipient to suppress Th1 cells only in the efferent phase, at the time of challenge.
Further study of the antigen specificity of skin induced tolerance showed suppression of both CS and DTH responses in mice immunized epicutaneously with four different non-cross-reacting antigen like TNP-Cl, OX, OVA and KLH prior to contact sensitization or intradermal immunization with the protein antigen KLH. These results show that the final mediation of suppressor T-cell activity is antigen non-specific. The data of the in vivo experiments were confirmed in vitro and showed that skin induced tolerance with two non-cross-reacting antigen TNP-Ig and OVA resulted in inhibition of proliferation of TNP-specific immune cells.
In classic T-cell mediated suppression both suppressor and effector cells often are specific for the same antigen. The spreading of tolerance observed in our experiments possibly is akin to the phenomenon known as ‘bystander suppression’ or determinant spreading,23,24 in which suppressor cells elicited by a particular antigen secrete antigen non-specific cytokines that down-regulate immune responses of other specific cell populations to their respective antigen. In the well-studied model of oral tolerance, orally administrated antigen appear to interact with gut associated lymphoid tissue and generate Ts cells that produce TGF-β, IL-4 and IL-10,25 and also mediate bystander suppression.24 It was shown both in vivo and in vitro, that orally induced suppression is dependent on antigen-specific triggering of Ts. Once antigen-specific triggering occurs, the effector phase of suppression is mediated by Ts cells producing non-specific suppressive cytokines.23 In our case all tested antigen used to induce skin tolerance before immunization were able to inhibit CS. We made similar findings in experimental autoimmune encephalomyelitis (EAE) showing that e.c. immunization with any tested protein antigen induced Ts cells protecting from the onset of EAE and reducing signs of disease via released TGF-β (Szczepanik M et al. submitted). Thus, the presence of the antigen responsible for inducing Ts cells is not required during elicitation of the Ts effect.26
This might suggest that skin induced suppressor cells work either via different mechanisms than are active in bystander suppression in mucosa oral tolerance, or an original antigen persists for a long period in peripheral lymph organs, or alternatively, anti-inflammatory cytokines released by Ts cells interfere with the induction and/or effector phase of the T effector cell mediated immune response.
In support of our results showing that e.c. immunization is an effective strategy to induce a population of regulatory T cells able to protect from EAE, is a recent paper from the Janeway laboratory, in which a similar level of protection was induced in TCR transgenic mice bearing a TCR specific for myelin basic protein (MBP).27 However, several important differences in the mechanism of tolerance in our study and the report by Janeway and colleagues are indicated. First, in our hands the suppression was antigen-non-specific, while the report from the Janeway laboratory found antigen-specific suppression. Secondly, we found suppression both in vitro and in vivo to be mediated by TGF-β, while the Janeway study found no role for TGF-β. We attribute these differences to the type of antigen used in the e.c. immunization. In our study, we used whole protein antigen for the e.c. immunizations, while the Janeway study used peptides from myelin antigens known to be encephalitogenic. It is possible that differences in the mechanism of tolerance are the result of the requirement for the processing of the whole protein prior to presentation. Thus, it is feasible that different antigen-presenting cells are required to induce the development of regulatory T cells when whole protein versus peptide is used as the tolerizing agent. This possibility is supported by work performed by the Bottomly laboratory, where they showed that peptide antigens were most efficiently presented to CD4 T cells by dendritic cells, while B cells were required for priming to protein antigen.28 In addition, the TCR transgenic mouse specific for MBP has a restricted T-cell repertoire, which may not be sufficient to facilitate the development of antigen-non-specific regulatory T cells.29
The association of non-antigen-specific suppression with inhibitory cytokines led us to determine if a specific cytokine milieu is required for induction of Ts cells via e.c. immunization as shown in some systems.30 In one system both IL-10 and TGF-β play an important role in the differentiation of naïve CD4+ cells into Ts.24 The mechanism by which a special cytokine environment affects induction of regulatory cells is not fully understood. It has been reported that pulmonary dendritic cells (DC) that were isolated after nasal mucosal exposure to an antigen secreted IL-10 and were able to induce CD4+ T regulatory type I (Tr1) cells.31 Another study showed that DCs isolated from mesenteric lymph nodes of mice tolerized by feeding with an antigen showed increased production of TGF-β which facilitates induction of another down-regulatory cell — the Th3 regulatory cell.32 There is also some evidence that IL-4 prevents maturation of DC and subsequent interaction of these immature DCs with T cells results in T-cell tolerization.31
These data do not fully explain how anti-inflammatory cytokines are involved in the induction of T suppressor cells. However, it appears that a unique suppressive cytokine mediated immunological milieu plays crucial role in the induction of immunological tolerance. As noted before, exposure to an antigen via skin induces CD4+ CD8+ regulatory cells similar to mucosal immunization. We speculate that in these two organs exposed to similar and innocuous antigen at the surface of an organism in contact with the environment, are similarly capable of induction of suppressor cells to prevent needless reactivity. To answer the question concerning whether a specific cytokine milieu is required to induce suppressor cells via skin immunization, we tolerized mice with TNP-Ig alone or with TNP-Ig plus anti-cytokine antibodies: anti-IL-4, anti-IL-10 or anti-TGF-β or control antibody on the skin before induction of CS. All of the anti-cytokine mAbs significantly diminished suppression induced by skin immunization with TNP-Ig, suggesting that anti-inflammatory cytokines are involved in the induction of Ts cells. At present, it is not clear how these cytokines regulate the differentiation of T lymphocytes into Ts cells. Further experiments are in progress.
Finally we tried to determine how e.c.-induced Ts cells inhibit Th1 effector cells. To determine the mechanism of skin induced tolerance, mitomycin C-treated tolerized LN cells were cocultured with 4 day TNP-Cl immune cells with or without anti-cytokine mAb: anti-IL-4, anti-IL-10 or anti-TGF-β. This experiment demonstrated that skin induced Ts cells inhibited antigen induced proliferation of T effector cells of CS in vitro via TGF-β, while IL-4 and IL-10 seem not to play a crucial role in the effector stage of suppression.
The above data showing the mechanism of suppression mediated by e.c.-induced regulatory cells were fully confirmed by measurement of cytokine production by lymph node and spleen cells isolated from TNP-Ig-tolerized mice. The data presented in Table 2 show increased production of TGF-β by lymph node and spleen cells isolated from TNP-Ig-tolerized mice when compared to control mice (animals patched with PBS alone). However there was no significant difference in IL-4 and IL-10 production by lymph node and spleen cells isolated both from TNP-Ig tolerized and control mice.
As mentioned, orally administrated antigen appear to interact with gut-associated lymphoid tissues and generate T regulatory cells that produce TGF-β, IL-4 and IL-1030 and also mediate bystander suppression.24 Our data thus suggest that e.c. exposure to an antigen, similar to mucosal immunization, results in the induction of Ts cells that have much the same mechanism of action and therefore emphasize the similarity of immune tolerance mechanisms operating on these two body surfaces. The ease of e.c. generation of non-antigen specific T suppressor cells producing TGF-β may have important implications for designing therapeutic schemes aimed at modulating immune responses to self antigens involved in autoimmune diseases.
Acknowledgments
The authors are grateful to Tom Taylor and Dr Juliusz Pryjma for helping us with FACS cell sorting. This work was supported by the grants from the Polish Committee of Scientific Research (KBN, Warsaw) no. 4 PO 5B 04 519 and 3 PO5B 091 25 to M.S. and grants from the NIH AI-59801-01 and AI-07174 to P.W.A.
Abbreviations
- CS
contact sensitivity
- DC
dendritic cell
- DTH
delayed-type hypersensitivity
- e.c.
epicutaneous
- FCS
fetal calf serum
- i.d.
intradermal
- i.v.
intravenous
- KLH
keyhole limpet haemocyanin
- LN
lymph node
- mAb
monoclonal antibody
- OVA
ovalbumin
- OX
oxazolone
- TNP-Cl
trinitrophenyl chloride
- RC
rabbit complement
- TNP
trinitrophenyl
- TNP-Ig
TNP-conjugated immunoglobulins
- Ts
T suppressor cells
References
- 1.Weiner HL, Friedman A, Miller A, et al. Oral tolerance: Immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 2.Xiao B-G, Link H. Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85:119–28. doi: 10.1006/clin.1997.4432. 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 3.Chen YJ, Inobe I, Kuchroo VK, Baron JL, Janeway CA, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor trangenic mice. Suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA. 1996;93:388–99. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of Th1 and Th2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–11. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105:765–75. doi: 10.1172/JCI8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol. 1996;156:4077–82. [PubMed] [Google Scholar]
- 7.Ptak W, Szczepanik M, Bryniarski K, Tutaj M, Ptak M. Epicutaneous application of protein antigens incorporated into cosmetic cream induces antigen-nonspecific unresponsiveness in mice and affects the cell-mediated immune response. Int Arch Allergy Immunol. 2000;128(1):8–14. doi: 10.1159/000057998. 10.1159/000057998. [DOI] [PubMed] [Google Scholar]
- 8.McKinney M, Parkinson A. A single non-chromatographic procedure to purify immunoglobulins from serum and ascites. J Immunol Methods. 1987;96:271–8. doi: 10.1016/0022-1759(87)90324-3. 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- 9.Little JR, Eisen HN. Preparation and characterization of antibodies specific for the 2, 4, 6-trinitro group. Biochemistry. 1966;5:3385–95. doi: 10.1021/bi00875a001. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji RF, Szczepanik M, Kawikova I, et al. B cell-dependent T cell responses: IgM antibodies are required to elicit contact sensitivity. J Exp Med. 2002;196(10):1277–90. doi: 10.1084/jem.20020649. 10.1084/jem.20020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szczepanik M, Akahira-Azuma M, Bryniarski K, et al. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003;171:6225–35. doi: 10.4049/jimmunol.171.11.6225. [DOI] [PubMed] [Google Scholar]
- 12.Akahira-Azuma M, Szczepanik M, Tsuji RF, et al. Early delayed-type hypersensitivity eosinophil infiltrates depend on T helper 2 cytokines and interferon-gamma via CXCR3 chemokines. Immunology. 2004;111:306–17. doi: 10.1111/j.0019-2805.2004.01818.x. 10.1111/j.0019-2805.2004.01818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, Askenase PW. Cutaneous immunization rapidly activates liver invariant Valpha 14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785–96. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishimitsu R, Yajima T, Nishimura H, Kawauchi H, Yoshikai Y. NKT cells are dispensable in the induction of oral tolerance but are indispensable in the abrogation of oral tolerance by prostaglandin E. Eur J Immunol. 2003;33:183–93. doi: 10.1002/immu.200390021. 10.1002/immu.200390021. [DOI] [PubMed] [Google Scholar]
- 15.Shreedhar VK, Pride MW, Sun Y, Kripke ML, Strickland FM. Origin and characteristics of ultraviolet-B radiation-induced suppressor T lymphocytes. J Immunol. 1998;161:1327–35. [PubMed] [Google Scholar]
- 16.Mitchison NA, Schuhbauer D, Muller B. Natural and induced regulation of Th1/Th2 balance. Springer Semin Immunopathol. 1999;21:199–200. doi: 10.1007/BF00812253. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha 14 NKT cells in innate and aquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 18.Bonomo A, Kehn PJ, Shevach EM. Premature escape of double-positive thymocytes to the periphery of young mice. Possible role in autoimmunity. J Immunol. 1994;152:1509–14. [PubMed] [Google Scholar]
- 19.Jimenez E, Sacedon R, Vicente A, Hernandez-Lopez C, Zapata AG, Varas A. Rat peripheral CD4+ CD8+ T lymphocytes are partially immunocompetent thymus-derived cells that undergo post-thymic maturation to become functionally mature CD4+ T lymphocytes. J Immunol. 2002;168:5005–13. doi: 10.4049/jimmunol.168.10.5005. [DOI] [PubMed] [Google Scholar]
- 20.Lee W-H, Nam K-H, Terao K, Akari H, Yoshikawa Y. Age-related increase of peripheral CD4+ CD8+ double-positive T lymphocytes in cynomolgus monkeys: longitudinal study in relation to thymic involution. Immunology. 2003;109:217–25. doi: 10.1046/j.1365-2567.2003.01646.x. 10.1046/j.1365-2567.2003.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujihashi K, Yamamoto M, McGhee JR, Kiyono H. αβ T cell receptor positive intraepithelial lymphocytes with CD4+, CD8+ phenotypes from orally immunized mice provide Th2-like function for B cell responses. J Immunol. 1993;151:6681–91. [PubMed] [Google Scholar]
- 22.Szczepanik M, Anderson LR, Ushio H, Ptak W, Owen MJ, Hayday AC, Askenase PW. γδ T cells from tolerized αβ T cell receptor (TCR)-deficient mice inhibit contact sensitivity-effector T cells in vivo, and their interferon-γ production in vitro. J Exp Med. 1996;184:2129–39. doi: 10.1084/jem.184.6.2129. 10.1084/jem.184.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T-lymphocyte associated antigen 4 (CTLA 4) induces transforming growth factor β (TGF-β) production by murine CD4+ T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoyne GF, Lamb JR. Regulation of T cell function in mucosal tolerance. Immunol Cell Biol. 1997;75:197–201. doi: 10.1038/icb.1997.29. [DOI] [PubMed] [Google Scholar]
- 25.Stingl G, Konig F, Yamada H. Thy1+ dendritic epidermal cells express T3 antigen and the T cell receptor γ chain. Proc Natl Acad Sci USA. 1987;84:4586–90. doi: 10.1073/pnas.84.13.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczepanik M. Regulation of contact hypersensitivity responses by different populations of T suppressor cells. Skin induced tolerance and its clinical implication. In: Pandalai SG, editor. Research Signpost. Vol. 4. Kerala, Trivandrum: 2002. pp. 641–68. (Recent Research in Developmental Immunology). [Google Scholar]
- 27.Bynoe MS, Evans JT, Viret C, Janeway CA., Jr Epicutaneous immunization with autoantigenic peptides induces T suppressor cells that prevent experimental allergic encephalomyelitis. Immunity. 2003;19:317–28. doi: 10.1016/s1074-7613(03)00239-5. [DOI] [PubMed] [Google Scholar]
- 28.Constant S, Sant'Angelo D, Pasqualini T, Taylor T, Levin D, Flavell R, Bottomly K. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol. 1995;154:4915–23. [PubMed] [Google Scholar]
- 29.Dittel BN. Evidence that Fas and FasL contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Arch Immunol Ther Exp. 2000;48:381–8. [PubMed] [Google Scholar]
- 30.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin-4 in the suppression of T helper type 1-mediated colitis by CD45RB (low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature Immunol. 2001;2:725–31. doi: 10.1038/90667. 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 32.Weiner HL. The mucosal milieu creates tolerogenic dendritic cells and TRI and TH3 regulatory cells. Nature Immunol. 2000;2:671–7. doi: 10.1038/90604. 10.1038/90604. [DOI] [PubMed] [Google Scholar]