Abstract
Neutrophil chemokine receptor expression can be altered by exposure to Toll-like receptor (TLR) agonists, a process that is thought to have the potential to localize neutrophils to sites of infection. In order to investigate this process in more detail, we examined the regulation of highly pure neutrophil CXCR1 and CXCR2 expression and function by selective agonists of TLR2 (Pam3CSK4) and TLR4 (lipopolysaccharide, LPS). CXCR1 and CXCR2 were down-regulated by TLR engagement. CXCR2 loss was more rapid and showed a dependence upon soluble helper molecules (LPS binding protein and CD14) that was not evident for CXCR1, suggesting differential coupling of LPS signalling to CXCR1 and CXCR2 loss. However, TLR engagement in highly pure neutrophils did not result in complete loss of chemokine receptors, and LPS-treated neutrophils remained able to mount a respiratory burst to CXCL8 and CXCL1, and were able to migrate towards CXCL8 in assays of under-agarose chemotaxis. Thus, although treatment of purified human neutrophils with TLR2 and TLR4 agonists modifies chemokine receptor expression, remaining receptors remain functionally competent.
Keywords: inflammation, neutrophil, chemokine receptors, Toll-like receptors
Introduction
Neutrophils are crucial to defence against bacterial infection, but their inappropriate activation is thought to be a major contributor to inflammatory disease.1 Options for therapeutic targeting of their function in disease include inhibiting their recruitment2,3 or preventing their activation.1 The regulation of these processes is likely to be linked, which provides further options to control neutrophilic inflammation in vivo.
Neutrophil recruitment to sites of infection is heavily dependent upon chemokines such as CXCL8.4 The peripheral blood neutrophil expresses two main chemokine receptors on the cell surface, CXCR1 and CXCR2.5,6 CXCR2 is thought to be predominantly responsible for neutrophil recruitment in response to its many ligands, including interleukin-8 (IL-8)/CXCL8 and melanoma growth stimulatory activity (MGSA)/CXCL17 and antagonists of CXCR2 are showing promise in the treatment of inflammatory disease.3 CXCR1, a selective receptor binding only CXCL8 and CXCL6 at high affinity7 is thought to perhaps have a greater role in regulating neutrophil activation.8
At sites of inflammation, neutrophils encounter many molecules for which they have specific receptors and that can regulate chemokine function. Activated complement fragments such as C5a cause some down-regulation of chemokine receptor expression, and temporary reductions in signalling through CXCR1 and CXCR2.6 Bacterial products, such as lippolysaccharide (LPS; activating Toll-like receptor-4 (TLR4)), and lipopeptides (activating TLR2), also cause variable down-regulation of neutrophil chemokine receptors. Some investigators have shown that engagement of neutrophil TLRs causes marked down-regulation of mRNA and protein levels of CXCR1 and 2.9,10 In a more complicated mouse system, LPS pretreatment of neutrophils could also enhance chemokine responses by altering expression of the G protein receptor kinases (GRKs) responsible for chemokine receptor (CKR) desensitization.11 Others have shown that high concentrations of LPS may actually cause human neutrophils to up-regulate CKR expression,12 though such up-regulation may be temporary and followed by a later phase of receptor loss.13
We have recently begun to investigate neutrophil TLR responses using cells depleted of peripheral blood mononuclear cells (PBMC) by negative magnetic selection, as we have found that PBMC contaminating standard neutrophil preparations are important in regulating many neutrophil responses to LPS.14–16 We showed previously that CXCR1 was more resistant to TLR-induced down-regulation than was CXCR214 and set out in this work to study in detail the consequences of TLR2 and TLR4 engagement on neutrophil chemokine receptor expression and function.
Materials and methods
Reagents
General laboratory and cell culture reagents, buffers, low-endotoxin bovine serum albumin (BSA), commercial LPS (cLPS) from Escherichia coli serotype 0111:B4, and granulocyte—macrophage colony-stimulating factor (GM-CSF) were from Sigma (Poole, UK) or Invitrogen (Paisley, UK). fluoroscein isothiocyanate (FITC)-anti-CXCR1 and phycoerythrin (PE)-anti-CXCR2 monoclonal antibodies (mAbs) and controls, and recombinant human soluble CD14 (the extracellular domain) and LPS binding protein (LBP) were from R & D Systems (Abingdon, UK). Pam3CysSerLys4 (Pam3CSK4) was from EMC Microcollections (Tübingen, Germany). Purified LPS (pLPS) from E. coli strain K235 was a generous gift of Dr Stefanie Vogel (University of Maryland), and was prepared as described.17 Cytokines and were from Peprotech EC (London, UK). C5a was a generous gift of Dr Peter Monk (Sheffield, UK). The CXCR2 antagonist SB225002 was purchased from Calbiochem (Merck Biosciences Ltd, Nottingham, UK), and was dissolved in dimethylsulphoxide. Fetal calf serum (FCS) was from Cambrex BioScience, (Wokingham, UK), and contained <0·5 EU/ml endotoxin.
Cell preparation
Peripheral venous blood was taken with informed consent from healthy volunteers in accordance with a protocol approved by the South Sheffield Local Research Ethics Committee. Blood was anticoagulated with trisodium citrate, plasma and platelets removed by centrifugation, and following dextran sedimentation, PBMC were separated from granulocytes by density either over plasma/Percoll or sterile, endotoxin-free Histopaque 1077 gradients.14,15 Neutrophils were further purified by negative magnetic selection as described14,15 using a custom cocktail from StemCell Technologies (Vancouver, Canada), containing antibodies to CD36, CD2, CD3, CD19, CD56 and glycophorin A. This protocol depleted almost all contaminating cells (except eosinophils, which do not express functional TLR2 and TLR415 and would be extremely unlikely to modify the neutrophil responses studied here).
Modulation of chemokine receptor expression
Neutrophils were stimulated with agonists in the indicated buffer for the relevant time period, and cell surface expression of chemokine receptors measured by flow cytometry. Unless indicated, experiments used Assay Buffer (AB), which was comprised of Dulbecco's modified phosphate-buffered saline (PBS) containing Ca2+/Mg2+ + 2% FCS + 10 mm HEPES + 0·18% glucose, pH 7·3–7·4. In some experiments, FCS was substituted with LBP and sCD14, in the presence of a low amount of low-endotoxin BSA (based on pilot experiments determining amounts of LBP and sCD14 required to support pLPS-mediated l-selectin shedding). After stimulation, cells were washed with ice-cold fluorescence-activated cell sorting (FACS) buffer (PBS without Ca2+/Mg2+ + 10 mm HEPES + 0·25% BSA, pH 7·3–7·4), and dual-stained with anti-CXCR1 (10 µg/ml) and anti-CXCR2 (10 µg/ml). Isotype-matched controls and single-stained samples were used to set baselines and compensation. Binding of mAbs was detected using a FACSCalibur flow cytometer (Becton Dickinson, Cowley, UK). Specific binding was calculated by obtaining mean fluorescence intensity values (MFIs) and subtracting the MFI of the isotype control. For the majority of experiments, data were expressed as the percentage specific MFI of buffer-treated cells, in keeping with previous work.6
Detection of respiratory burst
Respiratory burst was detected by changes in DCF (dichlorodihydrofluorescein diacetate) fluorescence as described.14,18 Neutrophils were loaded with 5 µm DCF for 30 min at 37°, 5% CO2, then treated with the indicated agonists. Cells were then washed in cold PBS and analysed immediately by flow cytometry.
Chemotaxis
Neutrophil chemotaxis was measured using an under-agarose chemotaxis system in accordance with published techniques.19,20 Gels consisting of 3 ml of 0·6% low-endotoxin agarose (Promega, Southampton, UK) in 50% RPMI/50% Hank's balanced salt solution (HBSS) were cast in 3 cm tissue culture plates. Holes (3 mm in diameter) were cut in the gel, 2 mm apart, and neutrophils (10 µl, 10 × 106/ml) placed in one well, and chemoattractant placed in the adjacent well. Neutrophils were pretreated with medium alone, or medium containing pLPS. Chemotaxis was subsequently allowed to proceed during which the pLPS remained present in the cell wells. The plates were incubated at 37° in a 5% CO2 incubator for 2·5 hr, and then fixed by incubation overnight in 3 ml methanol. The gel was removed, and cells fixed to the culture dish were visualized by staining with DiffQuick (Gamidor Ltd, Oxford, UK) and photographed under 10× magnification. Data capture was in black and white; colour was applied to the images using Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) to enhance picture clarity.
Statistics
Comparisons of more than two data sets were performed by anova and a posthoc test as indicated, using the Prism 4.0 program (GraphPad Software Inc, San Diego, CA).
Results
Different regulation of CXCR1 and CXCR2 expression by TLR agonists
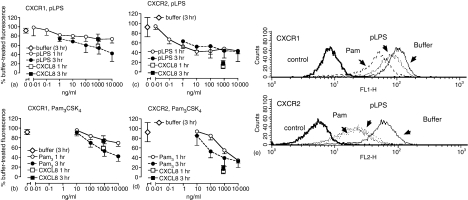
Initial studies determined the ability of selective agonists of TLR2 and TLR4 to regulate expression of neutrophil CXCR1 and CXCR2. Neutrophils were prepared by negative magnetic selection to effectively deplete contaminating PBMC, and stimulated with highly purified LPS (pLPS), Pam3CSK4, or CXCL8. Figure 1 shows that CXCR1 was relatively resistant to loss induced by its homologous ligand (CXCL8), and that incubation (up to 3 hr) with TLR agonists resulted in a slow loss of expression. Purified LPS and Pam3CSK4 treatment reduced CXCR1 expression to 74 ± 9% and 70 ± 7% at 1 hr, respectively, and to 42·5 ± 17% and 42 ± 10% at 3 hr (mean ± SEM, each P < 0·05 for 1 hr versus 3 hr treatment effects, determined by two-way anova). In contrast, CXCR2 expression was more rapidly lost after stimulation with TLR agonists. Stimulation with pLPS exerted its maximal effects on CXCR2 expression at the earliest time point studied (1 hr). Activation of TLR2 caused a concentration-dependent loss of CXCR2 expression similar to that previously described14 but its maximal effect took longer to become apparent and was significantly different at 1 versus 3 hr (P < 0·05 measured by two-way anova, particularly evident at the 100 ng/ml Pam3CSK4 concentration).
Figure 1.
TLR agonists cause down-regulation of CXCR1 and CXCR2. Neutrophils were highly purified by negative magnetic selection and treated with pLPS (a and c) or Pam3CSK4 (b and d) for 1 or 3 hr, then washed and stained with anti-CXCR1 (a and b) and anti-CXCR2 (c and d) mAbs prior to analysis of cell surface receptor expression by flow cytometry. Control buffer or CXCL8-treated samples generated data that is displayed in both the pLPS and Pam3CSK4 graphs for comparison. Data in all graphs are expressed as the percentage of specific mean fluorescence of the sample treated with buffer alone at the one hr time point, and are mean ± SEM of 3–15 experiments, each from a separate donor. (e) The effects of a one hr treatment with buffer (solid line) pLPS (10 µg/ml; fine dashed line) or Pam3CSK4 (10 µg/ml; coarse dashed line) on expression of CXCR1 and CXCR2 as detected by flow cytometry.
Loss of CXCR2 is not dependent upon generation of its ligands
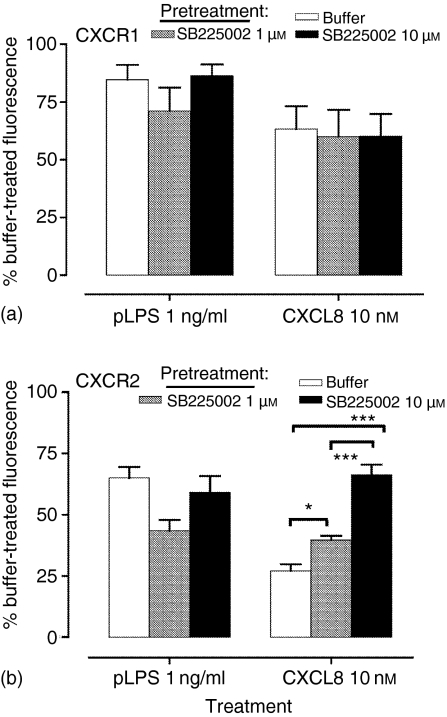
Monocytic cells down-regulate CCR1 after activation by pLPS and Pam3CSK4, via a mechanism involving generation of CCR1 ligands acting in an autocrine manner, and TLR-mediated CCR1 loss is inhibited by a CCR1 antagonist.21 Because neutrophils secrete CXCL822,23 we investigated whether similar mechanisms might be involved in the regulation of neutrophil CXCR2 by TLR agonists. Figure 2 shows that pretreatment of neutrophils with high concentrations of a potent and selective CXCR2 antagonist (IC50 22 nm for binding of 125I-CXCL8 to CXCR224) failed to prevent loss of CXCR2 induced by TLR2 or TLR4 agonists, but inhibited down-regulation of CXCR2 caused by CXCL8.
Figure 2.
CXCR loss is not inhibited by a CXCR2 antagonist. Neutrophils were highly purified by negative magnetic selection, and pretreated with buffer or the CXCR2 antagonist, SB225002, prior to stimulation with pLPS or CXCL8. Following 1 hr of stimulation with these agonists, receptor expression of CXCR1 (a) and CXCR2 (b) was measured by flow cytometry as described. Data in all graphs are expressed as the percentage of specific mean fluorescence of the samples treated with buffer alone in the absence of SB225002, and are the mean ± SEM of five experiments, each from a separate donor. Significant inhibition of CXCL-8-induced down-regulation of CXCR2 after treatment with SB225002 are indicated by *P < 0·05 and ***P < 0·001, analysed by anova and Tukey's post-test.
Loss of CXCRs is enhanced by proinflammatory mediators
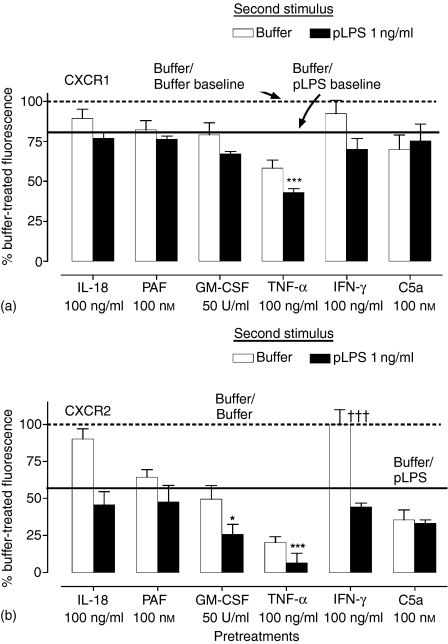
Neutrophils encounter many mediators at sites of inflammation, some of which, such as C5a and tumour necrosis factor-α (TNF-α), can also down-regulate neutrophil chemokine receptor expression.6,25,26 To determine the potential for other inflammatory mediators to enhance TLR-mediated loss of neutrophil CKRs, highly pure neutrophils were pretreated with a panel of neutrophil activators, and then pLPS (1 ng/ml) or medium added to the cells. Figure 3 shows that of the indicated mediators at the concentrations tested, only TNF-α had a marked effect on CXCR1 expression, and this effect was modestly enhanced when pLPS was added to the cells. In contrast, a wide variety of mediators caused loss of CXCR2 from the cell surface. Of the tested mediators, only IL-18 and interferon-γ (IFN-γ) showed no ability to cause CXCR2 loss at the concentrations and times studied. Pretreatment with the cytokines GM-CSF, and in particular TNF-α, resulted in a dramatic loss of CXCR2 expression, with a further additive effect induced by pLPS.
Figure 3.
Inflammatory mediators and pLPS can cooperate in the induction of CXCR loss. Neutrophils were highly purified by negative magnetic selection and pretreated with medium, IL-18 (100 ng/ml), PAF (100 nm), GM-CSF (50 U/ml), TNF-α (100 ng/ml), IFN-γ (100 ng/ml), or C5a (10 nm) for 1 hr, after which pLPS (1 ng/ml) or medium was added for a further hour. Expression of CXCR1 (a) and CXCR2 (b) was measured by flow cytometry as described. Data are expressed as the percentage of specific mean fluorescence of the samples treated with buffer alone, and are the mean ± SEM of three experiments, each from a separate donor. The dashed line indicates the 100% expression level, i.e. that of cells treated with buffer alone. The solid line shows the expression level in those samples treated with buffer for the first hour, followed by addition of pLPS (these values are mean ± SEM: 80·9 ± 8% for CXCR1; 57 ± 2·3% for CXCR2). ***Indicates that addition of pLPS to cells pretreated with TNF-α resulted in a significant additive down-regulation (P < 0·001) of CXCR1 and CXCR2 expression, whilst *indicates that addition of pLPS to cells pretreated with GM-CSF resulted in a significant down-regulation (P < 0·05) of CXCR2 expression alone. †††Indicates a significant difference (P < 0·001) in the CXCR2 expression levels between buffer and pLPS treatment in IFN-γ pretreated cells (data analysed by anova and Bonferroni's post-test).
LPS-driven loss of CXCR2, but not CXCR1, is dependent upon CD14 and LBP
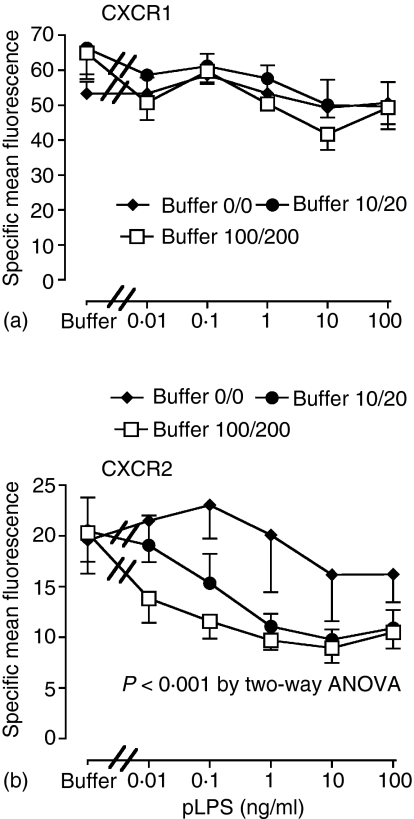
We typically use a low concentration of FCS (2%) in our buffers, which we have previously shown to effectively support LPS-induced l-selectin shedding in neutrophils.15 Serum may contain other neutrophil activators that are poorly characterized, and we therefore investigated pLPS responses in serum-free systems reconstituted with sCD14 and LBP. Responses were studied in two media containing LBP and sCD14 at concentrations of 10 and 20 ng/ml or 100 and 200 ng/ml, respectively, compared to media containing low-endotoxin BSA alone. Human serum from healthy subjects contains a median of 7·9 µg/ml of LBP and 3·2 µg/ml of sCD1427; thus the maximal concentrations we used were approximately equivalent to 1·2% (LBP) and 6% (sCD14) serum, consistent with other in vitro experiments. Figure 4 shows that increasing amounts of available sCD14 and LBP within the tested ranges had no effect on pLPS-induced loss of CXCR1. In contrast, CXCR2 loss induced by pLPS was markedly dependent upon the amounts of available sCD14 and LBP. There were no differences in the down-regulation of CXCR1 and CXCR2 induced by Pam3CSK4 in any medium (data not shown).
Figure 4.
LBP and sCD14 can enhance TLR4-mediated loss of CXCR2 but not CXCR1. Highly purified neutrophils were incubated in low endotoxin BSA containing LBP and sCD14 (0/0, 10/20, or 100/200 ng/ml of LBP/sCD14), with buffer or varying concentrations of pLPS as indicated. One hr later, expression of CXCR1 (a) and CXCR2 (b) was measured by flow cytometry as described. Data shown are mean ± SEM from four to six experiments, each from a separate donor. The LBP and sCD14 concentrations exerted a significant effect in (b) (P < 0·001) as determined by two-way anova.
Despite receptor down-regulation, functional responses can remain
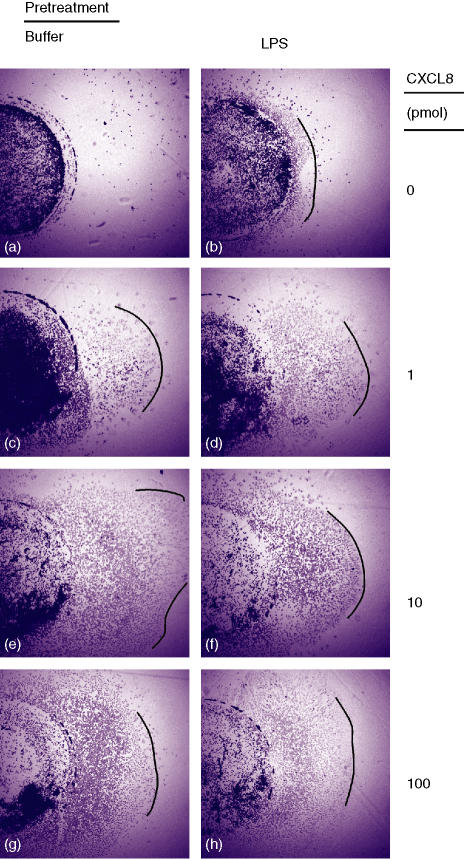
Treatment of neutrophils with cLPS for 1 hr abolishes CXCL8-induced calcium flux28 potentially mediated at least in part by loss of receptor expression. To investigate the effects of pLPS pretreatment on neutrophil chemotaxis, we utilized the under-agarose chemotaxis system. Because CXCR2 has been thought to be primarily responsible for neutrophil chemotaxis, neutrophils were initially treated with pLPS for 1 hr, the first measured time point at which maximal CXCR2 down-regulation was reached. CXCL8 induced a dose-dependent migration of neutrophils that was not abolished by pretreatment of the neutrophils with pLPS (Fig. 5). Cells that were pretreated with pLPS for 3 hr (corresponding with maximal measured CXCR1 down-regulation), also still showed a chemotactic response to CXCL8 that was similar to that seen in cells pretreated with pLPS for 1 hr (n = 3, data not shown). We were unable to observe chemotaxis of neutrophils to CXCL1 at any dose tested (up to 100 pmol/well), whether cells were pretreated with medium alone or pLPS.
Figure 5.
Neutrophils treated with pLPS still show directed migration to CXCL8. Highly purified neutrophils were treated with pLPS (10 ng/ml) or buffer for 1 hr, and then placed in 3 mm diameter wells (still in the medium containing the pLPS) in agarose-filled plates, and allowed to migrate to buffer (a, b) or CXCL8 1 pmol (c, d), 10 pmol (e, f), or 100 pmol (g, h), placed in a separate well 2 mm to the right. Following chemotaxis, the plates were fixed, the cells stained, and photographed at 10× magnification. Lines drawn on the pictures indicate the approximate leading edge of cell migration. Data shown are a representative experiment from six experiments, each from a separate donor. The left hand column (a, c, e, g) shows migration of cells pretreated with buffer, the right hand column (b, d, f, h) shows migration of cells pretreated with pLPS.
In further experiments, we investigated whether pretreatment of neutrophils with pLPS for 1 hr would reduce the respiratory burst seen in response to chemokines acting on CXCR1 and CXCR2 (CXCL8) or CXCR2 alone (CXCL1). CXCL1 and CXCL8 were still able to induce a modest increase in respiratory burst even after pretreatment with 10 ng/ml pLPS for 1 hr in the 100 ng/ml LBP + 200 ng/ml CD14 medium. However, the effect of these chemokines was weak compared to that induced by N-formylmethylleucylphenylanaline (fMLP) 100 nm (100 nm CXCL8 induced 17 ± 3·7% and CXCL1 induced 20 ± 8·4% (mean ± SEM, n = 4) of the fMLP response in pLPS-pretreated cells, data not shown).
Discussion
We and others have shown that TLR engagement can down-regulate neutrophil chemokine receptor expression,9,14 a potentially important mechanism regulating cell positioning and activation.28–31 However, our previous work has shown that neutrophil responses to TLR agonists can be significantly modified by contaminating PBMC in routine cell preparations14–16 and the detailed effects of individual TLR engagement on neutrophil responses still remains to be fully determined. Here, we describe the specific effects of selective TLR2 and TLR4 agonists on the regulation of neutrophil CXCR1 and CXCR2 expression. We show that loss of CXCR2 in response to TLR agonists is more rapid than loss of CXCR1; and furthermore that TLR4-induced loss of CXCR2, but not CXCR1, can be modified by enhanced efficiency of LPS presentation. We also show that despite treatment with TLR agonists, neutrophils can remain at least partially functionally competent with respect to chemokine activation.
In highly purified neutrophils, we found that TLR-induced down-regulation of CXCR1 was slower than that of CXCR2. The levels of neutrophil CXCR1 and CXCR2 down-regulation observed at 1 hr are consistent with previous results using a different pair of specific anti-CXCR1 and anti-CXCR2 mAbs reported by our group.14 Stimulation of neutrophils with TLR agonists over these concentration ranges causes marked leucocyte activation measured by up-regulation of CD11b or cytokine generation, and in the case of TLR4 agonists, some prolongation of life span.14,15 Our data are therefore extremely unlikely to be confounded by decreased cell viability or non-specific down-regulation of multiple cell surface markers. In whole blood, neutrophil CXCR2 is again more sensitive to down-regulation by LPS than CXCR1.32 Other stimuli that cause heterologous desensitization of neutrophil chemokine responses, such as C5a, fMLP, and TNF-α are also relatively poor stimulators of CXCR1 loss,6,26 forming a picture of CXCR1 as a receptor whose expression on the cell surface is relatively protected.
CXCR2 expression was rapidly lost from the neutrophil surface after activation of either TLR2 or TLR4. CXCR2 shows greater homologous down-regulation in response to CXCL8 than does CXCR16 and shows loss of cell surface expression following stimulation with ligands such as C5a (these data and ref 6), fMLP6 TNF-α (these data and ref 26), platelet-activating factor (PAF) and GM-CSF (these data). CXCR2 is overall more likely to be internalized than CXCR1, consistent with data showing that the cytoplasmic regions of these closely homologous receptors are different and subject to specific regulation.33–37 CXCR2 is vulnerable to degradation after internalization38 and is less likely than CXCR1 to be re-expressed following internalization, providing further mechanisms to reinforce its down-regulation.6,8 These data reinforce the lability of CXCR2 expression compared to the relative stability of CXCR1. Consistent with our data, neutrophils derived from synovial fluid of arthritic patients showed down-regulation but not complete loss of CXCR1 and CXCR2 (and of these, down-regulation of CXCR2 was greater).39 Interestingly though, not all studies have seen this slower down-regulation of CXCR1, and some found that activation of neutrophils with LPS caused gradient-purified neutrophils to rapidly lose both CXCR1 and CXCR2.28,30
LPS signalling is dependent upon helper molecules such as CD14 and LBP (reviewed in 40), providing a sensitive and complex regulation of neutrophil responses to LPS.41 TLR4-mediated loss of CXCR2, but not CXCR1, was sensitive to changes in the concentrations of these accessory molecules. These data raise the possibility that LPS-mediated alterations in CXCR1 expression may be mediated by other signalling pathways additional to, or other than, the classic CD14/MD-2/TLR4 system. Such signalling could result from other ‘helper’ or LPS-binding molecules involved in LPS responses such as CD11b.40,42 The concentrations of pLPS required to induce down-regulation of CXCR1 were also relatively high, again suggesting that in vivo, microbial-derived stimuli may be poor stimulators of CXCR1 loss, potentially favouring retention of biologically active receptors on neutrophils in inflammatory sites. TLR2-mediated down-regulation of chemokine receptor expression was not altered by changes in LBP concentration, in keeping with the lack of a role for LBP in responses to lipoproteins. Though TLR2 can use CD14 as an accessory molecule43 responses to Pam3CSK4 were not enhanced by increasing the supply of sCD14.
We have shown that agonists of TLR2 and TLR4 induce down-regulation of CCR1 expression in monocytic cells via the generation of MIP-1α/CCL3, which acts in an autocrine manner to cause loss of its cognate receptor.21 Neutrophils express CXCL8 in response to activation14,22,23 and accordingly, we investigated whether a similar mechanism might apply in the neutrophil. We observed no inhibition of TLR-mediated CXCR2 loss by a small molecule antagonist of CXCR2, data that are in keeping with work that showed that anti-CXCL8 antibodies did not prevent cLPS-induced loss of neutrophil CXCRs in whole blood.29 Similarly, cycloheximide does not inhibit LPS-induced CXCR1/2 down-regulation.30 However, neutrophil CXCL8 generation is regulated by cell density and autocrine mediators in a complex fashion23 and it would be unsurprising if autocrine CXCL8 generation did not under some circumstances contribute to TLR-mediated neutrophil CXCR1/2 down-regulation.
Neutrophils in the peripheral blood express approximately 2–4 × 104 CXCL8 receptors per cell44 with a ratio of CXCR1:CXCR2 of approximately 1 : 15 to 2 : 1,45,46 and therefore even a 60% reduction in receptor expression would leave a relatively large pool of potentially competent receptors. We previously showed that pretreatment of neutrophils with C5a or fMLP caused rapid heterologous desensitization of CXCL8 and CXCL1-induced calcium flux followed by a phase of loss of CXCR2 expression.6 However, the degree to which desensitization of receptor signalling persisted or recovered was at least in part independently regulated from receptor expression.6 Also, the regulation of internalization, chemotaxis, and calcium flux occurs through different regions of CXCR1 and CXCR2, thus loss of one signal does not mean that all responses will be absent.33 Furthermore, under certain circumstances, TLR engagement may actually enhance neutrophil chemokine signalling by inhibiting expression of GRKs.11 This complicated interplay between desensitization of signalling, priming of signalling, and regulation of responses by control of cell-surface receptor expression renders prediction of the consequences of receptor loss on cell function difficult. Whilst others have shown that LPS pretreatment completely inhibits CXCL8 calcium signalling28,30 and neutrophil chemotaxis in chemotaxis chambers,9 we observed that pLPS pretreatment did not abolish chemotaxis under agarose towards CXCL8, suggesting that remaining receptors remain at least partially functional. Whilst not surprising, given the level of receptor loss we observed, these data using under-agarose chemotaxis are different to results where chemotaxis was studied in Boyden chambers, and suggest that some preservation of chemokine responses at sites of inflammation is likely to be desirable. We cannot exclude that this might in part reflect an alteration in CXCR expression or function in neutrophils interacting with the chemotaxis plate and/or agarose, but in keeping with our observations, neutrophils from patients with sepsis show decreased CXCR2 expression and chemotactic responses, but preserved functional CXCR1 expression.47 Furthermore in our study, LPS treatment did not prevent CXCL8 and CXCL1-induced respiratory burst.
Our studies do not determine whether CXCR1 or CXCR2 is principally responsible for the observed under-agarose chemotaxis to CXCL8. A number of studies have investigated the roles of these receptors in chemotaxis, with sometimes conflicting results.8,48 Experience with small molecule antagonists of CXCR2 favours a dominant role for this receptor in mediating neutrophil recruitment;24 however, there may be a role for both receptors in the regulation of cell recruitment. CXCL1 and CXCL7 are high affinity ligands for CXCR2 and low affinity ligands for CXCR1. Ludwig et al. demonstrated that these chemokines cause neutrophil chemotaxis at low concentrations via CXCR2, and at high concentrations via CXCR149 in a mechanism also postulated to allow recruitment of cells into areas with high local chemokine concentrations.49 Again, relative preservation of CXCR1 expression would aid this mechanism.
In summary, we show that CXCR2 is more sensitive to TLR-induced loss, and that LPS signalling for CXCR1 loss is less dependent upon CD14 and LBP. We also show that neutrophil recruitment to CXCL8 is unlikely to be completely inhibited by TLR-mediated CXCR loss. In combination with other inflammatory mediators present at sites of inflammation, particularly TNF-α, down-regulation of neutrophil chemokine receptors is likely to be more profound. Because concentrations of mediators such as CXCL8 are likely to increase as a cell progresses towards sites of inflammation, we speculate that these processes may alternatively provide a way of regulating the sensitivity of the cell to widely differing concentrations of local agonists. This would allow preservation of chemokine responses even if concentrations change many fold, as has been suggested before in the context of fMLP-mediated cross-desensitization.6
Acknowledgments
The authors are grateful to Dr James E. Pease (Imperial College London) for his critical review of the manuscript. Funding: this work was supported by the Medical Research Council (UK), through a Clinician Scientist Fellowship (G108/388, to IS) within the ‘Mechanisms of Cytokine Action in Chronic Inflammatory Diseases’ Co-operative Group (G9827663).
Abbreviations
- TLR
Toll-like receptor
- CXCL
C-X-C chemokine ligand
- CXCR
C-X-C chemokine receptor
- pLPS
purified LPS
- LBP
LPS-binding protein
References
- 1.Donnelly SC, Haslett C. Cellular mechanisms of acute lung injury: implications for future treatment in the adult respiratory distress syndrome. Thorax. 1992;47:260–3. doi: 10.1136/thx.47.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sue RD, Belperio JA, Burdick MD, et al. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol. 2004;172:3860–8. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- 3.Podolin PL, Bolognese BJ, Foley JJ, et al. A potent and selective nonpeptide antagonist of CXCR2 inhibits acute and chronic models of arthritis in the rabbit. J Immunol. 2002;169:6435–44. doi: 10.4049/jimmunol.169.11.6435. [DOI] [PubMed] [Google Scholar]
- 4.Broaddus VC, Boylan AM, Hoeffel JM, Kim KJ, Sadick M, Chuntharapai A, Hébert CA. Neutralization of IL-8 inhibits neutrophil influx in a rabbit model of endotoxin-induced pleurisy. J Immunol. 1994;152:2960–7. [PubMed] [Google Scholar]
- 5.Chuntharapai A, Lee J, Hébert CA, Kim KJ. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J Immunol. 1994;153:5682–8. [PubMed] [Google Scholar]
- 6.Sabroe I, Williams TJ, Hébert CA, Collins PD. Chemoattractant cross-desensitization of the human neutrophil IL-8 receptor involves receptor internalization and differential receptor subtype regulation. J Immunol. 1997;158:1361–9. [PubMed] [Google Scholar]
- 7.Sabroe I, Lloyd CM, Whyte MK, Dower SK, Williams TJ, Pease JE. Chemokines, innate and adaptive immunity, and respiratory disease. Eur Respir J. 2002;19:350–5. doi: 10.1183/09031936.02.00253602. 10.1183/09031936.02.00253602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–94. [PubMed] [Google Scholar]
- 9.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi SD, Braughton KR, Whitney AR, Voyich JM, Schwan TG, Musser JM, DeLeo FR. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci USA. 2003;100:10948–53. doi: 10.1073/pnas.1833375100. 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–21. doi: 10.1038/nm832. 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 12.Manna SK, Samanta AK. Upregulation of interleukin-8 receptor in human polymorphonuclear neutrophils by formyl peptide and lipopolysaccharide. FEBS Lett. 1995;367:117–21. doi: 10.1016/0014-5793(95)00525-e. 10.1016/0014-5793(95)00525-E. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya C, Samanta S, Gupta S, Samanta AK. A Ca2+-dependent autoregulation of lipopolysaccharide-induced IL-8 receptor expression in human polymorphonuclear neutrophils. J Immunol. 1997;158:1293–301. [PubMed] [Google Scholar]
- 14.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MKB. Selective roles for Toll-like receptor (TLR) 2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 15.Sabroe I, Jones EC, Usher LR, Whyte MKB, Dower SK. Toll-like receptor (TLR) 2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 16.Sabroe I, Prince LR, Dower SK, Walmsley SR, Chilvers ER, Whyte MKB. What can we learn from highly purified neutrophils? Biochem Soc Trans. 2004;32:468–9. doi: 10.1042/BST0320468. 10.1042/BST0320468. [DOI] [PubMed] [Google Scholar]
- 17.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge. repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 18.Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MK. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol. 2002;168:1861–8. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- 19.Foxman EF, Kunkel EJ, Butcher EC. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J Cell Biol. 1999;147:577–88. doi: 10.1083/jcb.147.3.577. 10.1083/jcb.147.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heit B, Kubes P. Measuring chemotaxis and chemokinesis: the under-agarose cell migration assay. Sci STKE. 2003;2003::PL5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- 21.Parker LC, Whyte MKB, Vogel SN, Dower SK, Sabroe I. Toll-like receptor (TLR) 2 and TLR4 agonists regulate CCR expression in human monocytic cells. J Immunol. 2004;172:4977–86. doi: 10.4049/jimmunol.172.8.4977. [DOI] [PubMed] [Google Scholar]
- 22.Strieter RM, Kasahara K, Allen RM, Standiford TJ, Rolfe MW, Becker FS, Chensue SW, Kunkel SL. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol. 1992;141:397–407. [PMC free article] [PubMed] [Google Scholar]
- 23.Hattar K, Fink L, Fietzner K, Himmel B, Grimminger F, Seeger W, Sibelius U. cell density regulates neutrophil IL-8 synthesis. Role of IL-1 receptor antagonist and soluble TNF receptors. J Immunol. 2001;166:6287–93. doi: 10.4049/jimmunol.166.10.6287. [DOI] [PubMed] [Google Scholar]
- 24.White JR, Lee JM, Young PR, et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–8. doi: 10.1074/jbc.273.17.10095. 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 25.Richardson RM, Ali H, Pridgen BC, Haribabu B, Snyderman R. Multiple signaling pathways of human interleukin-8 receptor A. Independent regulation by phosphorylation. J Biol Chem. 1998;273:10690–5. doi: 10.1074/jbc.273.17.10690. 10.1074/jbc.273.17.10690. [DOI] [PubMed] [Google Scholar]
- 26.Asagoe K, Yamamoto K, Takahashi A, et al. Down-regulation of CXCR2 expression on human polymorphonuclear leukocytes by TNF-alpha. J Immunol. 1998;160:4518–25. [PubMed] [Google Scholar]
- 27.Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001;98:3800–8. doi: 10.1182/blood.v98.13.3800. [DOI] [PubMed] [Google Scholar]
- 28.Khandaker MH, Mitchell G, Xu L, et al. Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood. 1999;93:2173–85. [PubMed] [Google Scholar]
- 29.Tikhonov I, Doroshenko T, Chaly Y, Smolnikova V, Pauza CD, Voitenok N. Down-regulation of CXCR1 and CXCR2 expression on human neutrophils upon activation of whole blood by S. aureus is mediated by TNF-alpha. Clin Exp Immunol. 2001;125:414–22. doi: 10.1046/j.1365-2249.2001.01626.x. 10.1046/j.1365-2249.2001.01626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khandaker MH, Xu L, Rahimpour R, et al. CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J Immunol. 1998;161:1930–8. [PubMed] [Google Scholar]
- 31.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–60. doi: 10.1083/jcb.139.5.1349. 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juffermans NP, Dekkers PE, Peppelenbosch MP, Speelman P, van Deventer SJ, van Der Poll T. Expression of the chemokine receptors CXCR1 and CXCR2 on granulocytes in human endotoxemia and tuberculosis: involvement of the p38 mitogen-activated protein kinase pathway. J Infect Dis. 2000;182:888–94. doi: 10.1086/315750. 10.1086/315750. [DOI] [PubMed] [Google Scholar]
- 33.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–11. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 34.Feniger-Barish R, Ran M, Zaslaver A, Ben-Baruch A. Differential modes of regulation of cxc chemokine-induced internalization and recycling of human CXCR1 and CXCR2. Cytokine. 1999;11:996–1009. doi: 10.1006/cyto.1999.0510. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Baruch A, Grimm M, Bengali K, et al. The differential ability of IL-8 and neutrophil-activating peptide-2 to induce attenuation of chemotaxis is mediated by their divergent capabilities to phosphorylate CXCR2 (IL-8 receptor B) J Immunol. 1997;158:5927–33. [PubMed] [Google Scholar]
- 36.Fan GH, Yang W, Sai J, Richmond A. Hsc/Hsp70 interacting protein (hip) associates with CXCR2 and regulates the receptor signaling and trafficking. J Biol Chem. 2002;277:6590–7. doi: 10.1074/jbc.M110588200. 10.1074/jbc.M110588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan GH, Yang W, Wang XJ, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001;40:791–800. doi: 10.1021/bi001661b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller SG, Schraw WP, Richmond A. Activation of protein kinase C enhances the phosphorylation of the type B interleukin-8 receptor and stimulates its degradation in non-haematopoietic cells. J Biol Chem. 1995;270:10439–48. doi: 10.1074/jbc.270.18.10439. 10.1074/jbc.270.18.10439. [DOI] [PubMed] [Google Scholar]
- 39.Bruhl H, Wagner K, Kellner H, Schattenkirchner M, Schlondorff D, Mack M. Surface expression of CC- and CXC-chemokine receptors on leucocyte subsets in inflammatory joint diseases. Clin Exp Immunol. 2001;126:551–9. doi: 10.1046/j.1365-2249.2001.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabroe I, Read RC, Whyte MKB, Dockrell DH, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003;171:1630–5. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- 41.Troelstra A, Giepmans BN, Van Kessel KP, Lichenstein HS, Verhoef J, Van Strijp JA. Dual effects of soluble CD14 on LPS priming of neutrophils. J Leukoc Biol. 1997;61:173–8. doi: 10.1002/jlb.61.2.173. [DOI] [PubMed] [Google Scholar]
- 42.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and Taxol-inducible gene expression. J Immunol. 2001;166:574–81. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 43.Muroi M, Ohnishi T, Tanamoto K-I. Regions of the mouse CD14 molecule required for toll-like receptor 2- and 4-mediated activation of NF-kappa B. J Biol Chem. 2002;277:42372–9. doi: 10.1074/jbc.M205966200. 10.1074/jbc.M205966200. [DOI] [PubMed] [Google Scholar]
- 44.Horuk R. The interleukin-8-receptor family: from chemokines to malaria. Immunol Today. 1994;15:169–74. doi: 10.1016/0167-5699(94)90314-X. 10.1016/0167-5699(94)90314-X. [DOI] [PubMed] [Google Scholar]
- 45.Besemer J, Hujber A, Kuhn B. Specific binding, internalization, and degradation of human neutrophil activating factor by human polymorphonuclear leukocytes. J Biol Chem. 1989;264:17409–15. [PubMed] [Google Scholar]
- 46.Vita N, Lefort S, Brouillaud MJ, Magazin M, Guillemot JC, Ferrara P. Functional linkage of the Gro beta and IL-8 receptors on the surface of human neutrophils. Eur Cytokine Netw. 1993;4:197–204. [PubMed] [Google Scholar]
- 47.Cummings CJ, Martin TR, Frevert CW, et al. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J Immunol. 1999;162:2341–6. [PubMed] [Google Scholar]
- 48.Hammond MEW, Lapointe GR, Feucht PH, et al. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol. 1995;155:1428–33. [PubMed] [Google Scholar]
- 49.Ludwig A, Petersen F, Zahn S, Götze O, Schröder J-M, Flad H-D, Brandt E. The CXC-chemokine neutrophil activating peptide-2 induces two distinct optima of neutrophil chemotaxis by differential interaction with interleukin-8 receptors CXCR-1 and CXCR-2. Blood. 1997;90:4588–97. [PubMed] [Google Scholar]