Abstract
The spirochaete Brachyspira hyodysenteriae causes swine dysentery, a severe colitis characterized by mucosal enlargement as a result of crypt elongation and epithelial necrosis. Most efforts to understand the pathogenesis of this disease have focused on the aetiological agent and its virulence factors. However, the host immune response has been considered an important factor in disease development. Previous research has shown that B. hyodysenteriae induces systemic CD4+ and γδ T-cell responses after intramuscular immunization. Here, we have evaluated changes in the CD4+ and γδ T-cell composition and distribution the different compartments of the colonic mucosa of pigs challenged with B. hyodysenteriae. We report that, in infected pigs, γδ T cells were significantly depleted from the epithelial layer, although their numbers were maintained in the lamina propria. In addition, CD4+ T cells aggregated in clusters located in the lamina propria and submucosa. Ex vivo analyses of CD4+ T-cell responses to B. hyodysenteriae antigens correlated with the changes in the mucosal CD4+ T-cell distribution observed in infected pigs; CD4+ T cells recovered from peripheral blood and colonic lymph nodes of infected pigs proliferated to B. hyodysenteriae antigens, whereas no differences were found in the γδ T-cell responses between challenged and control groups. In addition, colonic lymph node CD4+ T cells had a predominant memory/activated phenotype. These results indicate that infection with B. hyodysenteriae induces a mucosal CD4+ T-cell response and points to CD4+ T cells being important contributors to the immunopathogenesis of swine dysentery.
Keywords: Brachyspira hyodysenteriae, CD4+ T cells, colitis, mucosal immunology
Introduction
Brachyspira hyodysenteriae is a spirochaete that causes swine dysentery, a severe mucohaemorrhagic colitis. The bacteria colonize the intestinal crypts, and occasionally the lamina propria, without spreading systemically.1 The pathogenesis of the disease has not been fully elucidated. A synergistic effect between B. hyodysenteriae and certain members of the normal intestinal flora is necessary for disease development. In this regard, gnotobiotic pigs challenged with B. hyodysenteriae were colonized by the spirochaete, although they did not develop colitis.2,3 The most important virulence factors of B. hyodysenteriae are related to its motility4 and β-haemolytic capacity.5,6 Special emphasis has been given to the latter. Purified B. hyodysenteriae haemolysin preparations were cytotoxic to a number of epithelial cell lines.7 Moreover, similar LESions to those occurring after infection were observed in the intestinal loops of mice and pigs exposed to haemolysin.8,9 Lesion development was accompanied by translocation of luminal bacteria into the lamina propria. Thus, it has been suggested that following the loss of the colonic epithelial cell barrier caused by B. hyodysenteriae haemolysin, exposure of the underlying mucosa to luminal antigens triggers a severe inflammatory response.8
Little attention has been given to the role of the host immune response in the pathogenesis of B. hyodysenteriae-induced colitis. Several experimental models have been developed to investigate the immunopathology of enteric inflammatory diseases of bacterial aetiology.10 It is now well established that CD4+ T cells are involved in the induction of inflammatory bowel diseases.10,11 Inflammatory bowel diseases result from a defective regulation of CD4+ T-cell responses to antigens from the normal intestinal flora by regulatory T cells (i.e. Th3, Tr1 or CD4+ CD25+).11,12 With few exceptions, this leads to excessive polarization towards a T helper type 1 (Th1) response and the consequent cytokine imbalance. In addition, concomitant infections with intestinal pathogens, such as Helicobacter hepaticus, accelerate disease progression and exacerbate lesion severity.13
Peripheral blood and colonic lymph node (CLN) lymphocytes from immunized pigs proliferate to B. hyodysenteriae antigens ex vivo.14 Further dissection of the B. hyodysenteriae-induced response showed that CD4+ and γδ T cells were the responding cells.15,16 In addition, peripheral blood CD4+ T cells from immunized pigs secreted interferon-γ (IFN-γ) upon stimulation with B. hyodysenteriae antigens15 and after infection, exogenous interleukin-10 (IL-10) down-regulated this response.14 Also, IFN-γ expression was increased in the CLN of pigs with swine dysentery.17 These findings suggest that a predominant Th1 response is established during B. hyodysenteriae-induced colitis, which might contribute to the overall inflammatory response and tissue damage. The objective of the present study was to describe the changes in CD4+ and γδ T-cell composition that occur at the colonic mucosa of pigs challenged with B. hyodysenteriae. Here, we report that infection with B. hyodysenteriae induced the loss of colonic γδ intraepithelial lymphocytes (IEL) and multifocal aggregates of CD4+ T cells within the lamina propria and submucosa. In addition, during swine dysentery, CD4+ T cells responding to B. hyodysenteriae antigens were recruited into the CLN. Our data suggest that dysregulation of CD4+ T-cell responses might be an important factor contributing to the immunopathology of swine dysentery.
Materials and methods
Animals, infection and necropsy procedures
Brachyspira hyodysenteriae strain B204 was obtained from the culture collection at the Veterinary Medical Research Institute.18 Three-month old, cross-bred pigs (n = 3) were intragastrically challenged with two doses of 1010 colony forming units of B. hyodysenteriae given on two consecutive days. Pigs were subjected to a 24-hr fasting period starting 5 hr prior to first challenge. Non-infected pigs (n = 3) were used as controls. After challenge, pigs were monitored daily for clinical signs of disease. On day 15 postchallenge, pigs were anaesthetized by administration of 2–3 ml of a combination of Rompun (Bayer; Shawnee, KS) and Telazol (Fort Dodge Laboratories; Fort Dodge, IA) intramuscularly and killed by electrocution. Specimens from the spiral colon were either fixed in 10% buffered formalin for histopathological examination, or embedded in tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC) and snap frozen in an ethanol/dry ice bath. CLN were collected in sterile conditions in cold Hanks' balanced salts solution (Sigma, St Louis, MO) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). Peripheral blood was collected by vena cava puncture in heparinized sterile tubes (Becton Dickinson, Franklin Lakes, NJ). Swabs of spiral colon contents were streaked onto modified BJ blood agar plates containing antibiotics for recovery and growth of B. hyodysenteriae.19 All animal-related procedures were approved by the Iowa State University Animal Care and Use Committee.
Histopathology
Haematoxylin & eosin-stained sections from colon were labelled with accession numbers lacking any reference to infection status (i.e. blinded to the evaluator). Tissue sections were examined on the basis of gland depth, metaplasia/hyperplasia (i.e. increased cell turnover and basal cell basofilia, or goblet cell metaplasia), lamina propria vascular change (i.e. capillary dilation and lamina propria haemorrhage), inflammation and submucosal change (i.e. inflammatory cell infiltrates at the lamina propria or submucosa), and epithelial erosions, as previously described.20
Immunohistochemistry
Frozen colonic tissues were sectioned at 5–7 μm, mounted on poly l-lysine-coated slides and fixed in 95% methanol for 2 min. Endogenous peroxidase activity was blocked by incubating with 0·3% hydrogen peroxide for 10 min. Non-specific antibody binding was blocked by adding immunohistochemistry buffer solution (5% normal goat serum, 3% bovine serum albumin, Tris–HCl buffer) at room temperature for 2 hr. Sections were stained with anti-porcine CD4 (74-12-1 hybridoma supernatant), anti-porcine T-cell receptor δ (TCR-δ; PGBL22A, VMRD, Pullman, WA), or anti-porcine CD3 (8E6, VMRD). Slides were incubated with primary antibodies at 4° overnight. After two washes in Tris-HCl buffer, tissues were incubated with peroxidase-conjugated goat anti-mouse immunoglobulin G (H + L) (Jackson ImmunoResearch, West Grove, PA) for 2 hr at room temperature. Antibody binding was visualized with diaminobenzidene (Biomeda Corporation, Foster City, CA). Tissue sections were counterstained with Instant Haematoxylin (Thermo Shandon, Pittsburg, PA) and coverslipped with Immu-mount (Thermo Shandon). The numbers of CD4+, CD3+ and TCR-γδ+ lymphocytes in either epithelium (i.e. IEL) or lamina propria (i.e. LPL) were enumerated for each pig and antibody staining. Data are presented as the sum of positively stained cells in five randomly chosen fields of 0·375-mm2 area, observed at ×400 magnification.
Isolation of peripheral blood mononuclear cells (PBMC) and CLN lymphocytes
Peripheral blood was diluted 1 : 4 in phosphate-buffered saline (PBS), overlaid onto lymphocyte separation media (Mediatech, Herndon, VA) and centrifuged at 400 g for 40 min. PBMC were recovered from the plasma/media interface, washed three times in PBS and resuspended in complete RPMI [RPMI-1640 supplemented with 10% fetal bovine serum (Hyclone), 25 mm HEPES buffer (Sigma), 100 units/ml penicillin (Sigma), 0·1 mg/ml streptomycin (Sigma), 1 mm sodium pyruvate (Sigma), 1 mm non-essential amino acids (Sigma), and 2 mm essential amino acids (Mediatech)]. To prepare single-cell suspensions of CLN cells, CLN were crushed between the frosted ends of two microscope slides in a Petri dish containing 5 ml of RPMI, and cell debris were removed by settling. CLN cells were washed once and resuspended at 2 × 106/ml in complete RPMI.
Flow cytometry
The following antibodies were used for flow cytometric analysis: phycoerythrin (PE) -labelled anti-porcine CD4 (clone 74-12-1), biotinylated anti-porcine CD8α (clone 76-2-11), unlabelled anti-porcine CD45RC (clone MIL15, Srotec, Raleigh, NC), and unlabelled anti-porcine TCR-δ (clone PGBL22A, VMRD, Pullman, WA).
Cell surface staining was conducted by incubating cells in 150 μl of combined primary antibodies (CD4/CD8α/CD45RC, or TCR-δ/CD8α) in FACS buffer [PBS containing 5% fetal bovine serum (Hyclone) and 0·01% sodium azide (Sigma)]. Following two washes, cells were incubated with fluorescein isothiocyanate-labelled secondary antibodies (Southern Biotechnology, Birmingham, AL) for anti-porcine CD45RC and anti-porcine TCR-δ, or streptavidin-PECy5 (Pharmingen, San Diego, CA) for the biotinylated CD8α antibody. Cells were analysed using a FACScan (Becton Dickinson). Between 5000 and 10 000 events were acquired, and data were analysed using the cell quest™ software (Becton Dickinson).
PKH67 proliferation assay
A total of 20 × 106 PBMC or CLN cells were resuspended in 1 ml of Diluent C (Sigma). The cell suspension was immediately transferred to a 15-ml conical tube containing 1 ml of a 2 μm solution of PKH67 dye in Diluent C (Sigma). Cells were incubated with the dye for 5 min and the reaction was stopped by the addition of 2 ml of fetal bovine serum (Hyclone) and, after 1 min, 4 ml of RPMI-1640. To eliminate excess fluorescent dye, cells were washed three times in RPMI-1640 and resuspended in complete RPMI to a final concentration of 2 × 106cells/ml. Cells were seeded at 1 × 106/ml in 96-well, flat-bottomed microtitre plates and incubated with complete RPMI only, or B. hyodysenteriae B204 antigen at 5 μg/ml. Cultures stimulated with Concanavalin A (Sigma) at 5 μg/ml were used as positive controls for proliferation. After 5 days in culture, cells were harvested and stained with anti-porcine CD4 (PE) and anti-porcine CD45RC (PeCy5), or with anti-porcine TCR-δ (PE). Cells were analysed by two- or three-colour flow cytometry. Ten thousand events were acquired, and data were analysed using the cell quest™ software (Becton Dickinson). Proliferation was assessed by the decrease in fluorescence intensity of PKH67 staining resulting from cell division. A relative proliferation index was calculated by dividing the percentage of CD4+ or γδ T cells that proliferated in antigen-stimulated cultures by the percentage of CD4+ or γδ T cells that proliferated in cultures maintained with media (i.e. unstimulated), as previously described.21,22
Statistical analysis
Analysis of variance (anova) was used to investigate the main effect of infection. anova was performed using the general linear model procedure of sas. Differences with P < 0·05 were considered statistically significant.
Results
Severe colitis in B. hyodysenteriae-infected pigs
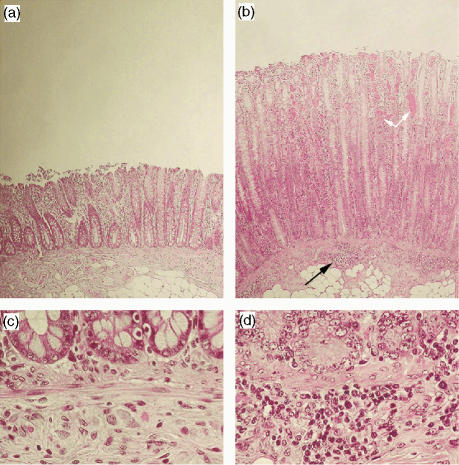
Previous studies have shown that intragastric inoculation of B. hyodysenteriae 2 days in a row combined with fasting provides the highest success of infection with rates close to 100% disease occurrence.17 In this study, all challenged pigs developed signs of colitis within the first week postchallenge and presented with gross and microscopic LESions consistent with swine dysentery, and B. hyodysenteriae was isolated from swabs of spiral colon taken at necropsy. Colonic sections recovered on day 15 postchallenge were stained with haematoxylin & eosin and evaluated for histopathological changes. Inflammation manifested as increased mucosal thickness as a result of pronounced crypt elongation and hyperplasia of crypt epithelial cells (Fig. 1b,d). This LESion was accompanied by increased cellular basophilia and mitotic figures, and depletion of goblet cells. There was severe infiltration of inflammatory cells into the lamina propria, which had also extensive capillary dilation. In addition, multifocal inflammatory cellular infiltrates were present across the interface between the lamina muscularis mucosae and the submucosa (Fig. 1d). Erosion of the epithelial layer, a hallmark of the disease, also occurred in challenged pigs (Fig. 1b).
Figure 1.
Representative photomicrographs of haematoxylin & eosin-stained colonic sections recovered from a healthy pig (a,c) and a pig challenged with Brachyspira hyodysenteriae at day 15 postinfection (b,d). Challenged pigs showed increased mucosal thickness with crypt elongation and hyperplasia. Multifocal cellular aggregates infiltrated the basal mucosa and submucosa (black arrows). Epithelial erosion and capillary dilation (white arrows) were extensive in the distal mucosa of infected pigs. Original magnification: ×200 (a,b) and ×400 (c,d).
Changes in the distribution of lymphocytes at the colonic mucosa of infected pigs
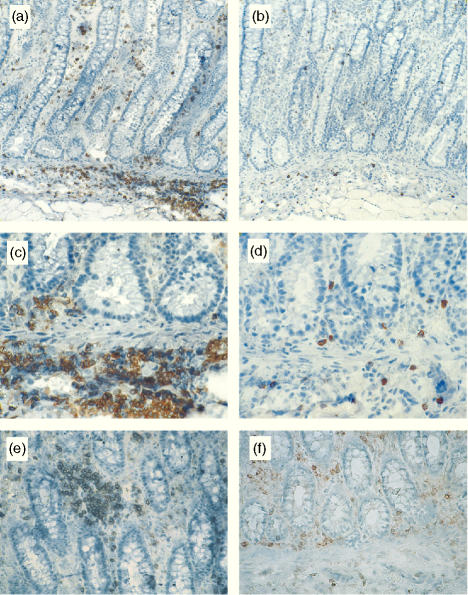
Immunohistochemical analysis of colonic sections obtained from control pigs showed that CD4+ T cells were restricted to the lamina propria (Fig. 2a). In contrast, γδ T cells were present in both compartments (Fig. 2b). γδ LPL were randomly dispersed throughout the lamina propria, whereas CD4+ LPL were distributed either as isolated cells or aggregated in small clusters. Evaluation of colonic tissues from B. hyodysenteriae-infected pigs revealed lower numbers of epithelial T cells (i.e. CD3+), consistent with the loss of γδ IEL (Fig. 3). Although no differences were found in the numbers of CD4+ or γδ LPL between groups (Fig. 3), CD4+ T cells formed large clusters in the lamina propria of infected pigs that were not observed in the colonic mucosa of control pigs (Fig. 4e,f). In addition, analysis of longitudinal colonic sections showed that the multifocal cellular infiltrates found in the submucosa (Fig. 1d) were predominantly composed of CD4+ T cells (Fig. 4a,c). γδ T cells were also detected in these LESions, although in much lower numbers (Fig. 4b,d).
Figure 2.
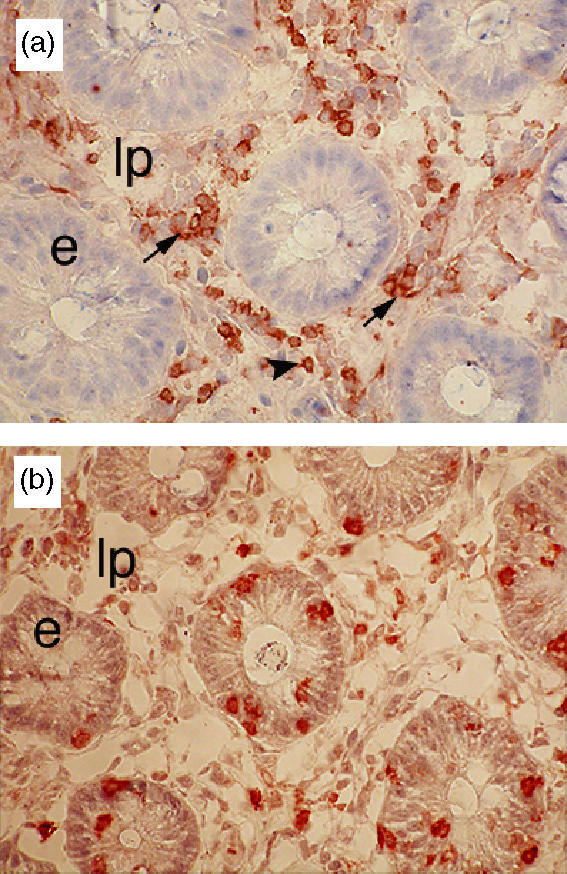
Immunohistochemical staining of colonic sections obtained from a healthy pig with anti-CD4 (a) and anti-TCR-δ (b). Colonic CD4+ T cells were restricted to the lamina propria (lp) and distributed as either small clusters (black arrow) or isolated cells (arrowhead). γδ T cells were scattered throughout the lamina propria and epithelium (e). Original magnification: ×400.
Figure 3.
Modification of the lymphocyte composition induced by Brachyspira hyodysenteriae infection at day 15 postchallenge. Lamina propria and epithelial CD3+, CD4+, and γδ T cells were enumerated in colonic sections recovered from control pigs (open bars) or pigs with swine dysentery (closed bars). Infection induced a significant loss of γδ IEL (*P < 0·05). LPL, lamina propria lymphocytes; IEL, intraepithelial lymphocytes.
Figure 4.
Representative micrographs of CD4+ and γδ T-cell distribution at the colonic mucosa of pigs with swine dysentery (a–e). CD4+ T cells accumulated in large aggregates in the lamina propria (e) and across the submucosa (a,c). Small numbers of γδ T cells were also found in these LESions (b,d). CD4+ T cells were more evenly distributed in the colonic mucosa of control pigs (f).
CLN CD4+ T cells from infected pigs had memory/activated phenotype and responded ex vivo to B. hyodysenteriae antigens
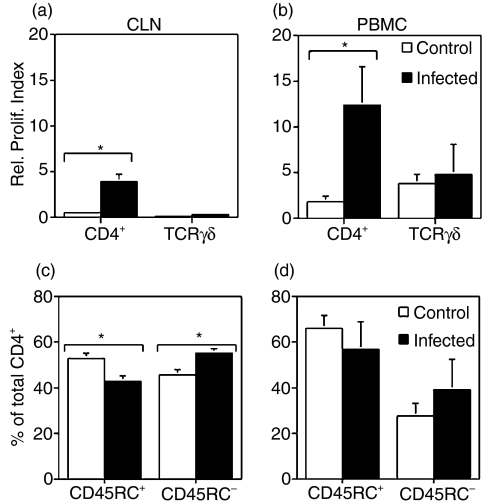
To assess possible correlations between changes at the colonic mucosa and local and systemic antigen-specific responses, proliferation of peripheral blood and CLN CD4+ and γδ T cells to B. hyodysenteriae antigens were evaluated on day 15 postchallenge. γδ T cells were present in CLN in very low numbers (data not shown), and did not respond to stimulation with B. hyodysenteriae antigens (Fig. 5a). No differences were found between control and infected groups in the proliferative response of circulating γδ T cells (Fig. 5b). Peripheral blood and CLN CD4+ T cells from challenged pigs proliferated to B. hyodysenteriae antigens (Fig. 5a,b).
Figure 5.
Antigen-specific proliferation (a,b) and phenotype (c,d) of lymphocytes recovered from peripheral blood and colonic lymph nodes (CLN). PBMC and CLN were isolated on day 15 postchallenge and stained with PKH67 (a,b). Cells were cultured for 5 days with medium or Brachyspira hyodysenteriae antigens. After harvesting, cells were labelled with anti-CD4 or anti-TCR-γδ and analysed by flow cytometry. As cells divided, the PKH67 dye was split between daughter cells, and proliferation was measured by the decrease in PKH67 fluorescence intensity. A relative proliferation index was calculated by dividing the percentage of CD4+ or γδ T cells that proliferated in antigen-stimulated cultures by the percentage of CD4+ or γδ T cells that proliferated in cultures maintained with media alone (i.e. unstimulated). In addition, freshly isolated PBMC and CLN were double-stained with anti-CD4 and anti-CD45RC (c,d). The percentage of CLN CD4+ T cells with a memory/activated phenotype (i.e. CD4+ CD45RC−) was significantly increased in pigs that were challenged (c,d) (*P < 0·05). Flow cytometric analysis of CD45RC expression (c,d) was performed by gating only on CD4+ T cells.
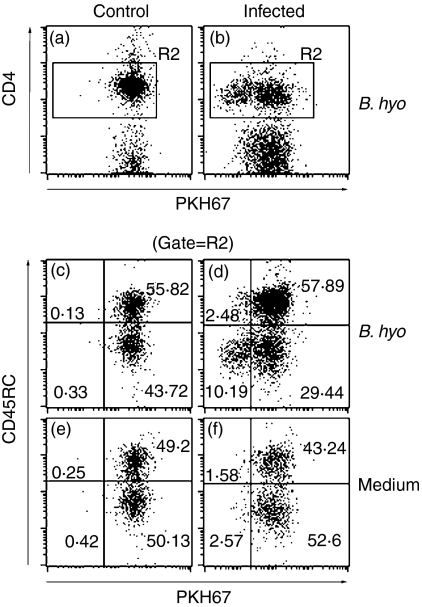
Porcine CD4+ T cells in which CD45RC is absent, or expressed at low levels are considered memory/activated cells.23,24 Phenotypic analyses performed in freshly isolated CLN cells showed that infected pigs had a significantly higher percentage of CD4+ CD45RC− cells than control pigs (Fig. 5c). No statistical differences in the percentage of memory/activated CD4+ T cells were found in PBMC (Fig. 5d). We then evaluated whether a portion of the memory/activated CLN CD4+ T cells were specific for B. hyodysenteriae antigens. Although a small fraction of the CD4+ proliferating cells expressed high levels of CD45RC (Fig. 6d, upper-left quadrant), most of the CD4+ T cells that proliferated to B. hyodysenteriae antigens were CD45RC− (Fig. 6d, lower-left quadrant). In challenged pigs, there was a three-fold increase in the average relative proliferation index of CLN CD4+ CD45RC− (i.e. memory/activated) versus CLN CD4+CD45RC+ (i.e. naïve) following stimulation with B. hyodysenteriae antigens (4·32 ± 1·4 versus 1·47 ± 0·22, respectively). The relative proliferation index of CLN CD4+ CD45RC− from non-infected pigs to B. hyodysenteriae antigens was 0·673 ± 0·32.
Figure 6.
Antigen-specific proliferation of colonic lymph node (CLN) CD4+ T cells from representative control and infected pigs. CLN cells isolated on day 15 postchallenge were stained with PKH67 and cultured with medium only or with Brachyspira hyodysenteriae antigens for 5 days. Cells were harvested and double-stained with anti-CD4 and anti-CD45RC. CD4+ T cells from infected pigs proliferated upon stimulation with B. hyodisenteriae antigens, as determined by the decreased PKH67 fluorescence intensity (b). Further phenotypic analysis showed that proliferating CD4+ T cells had memory/activated phenotype as they were CD45RC− (d). (a) and (b) are gated on CLN cells, and plots (c–f) are gated on CLN CD4+ cells only (i.e. R2).
Discussion
Although it has been proposed that the host immune response contributes to the development of B. hyodysenteriae-induced colitis,8 the role of mucosal CD4+ and γδ T cells in swine dysentery remains unknown. Pigs have large numbers of circulating γδ T cells25–27 which, following immunization with B. hyodysenteriae, proliferate in antigen-recall responses.15,16,28,29 CD4+ T cells from vaccinated pigs also proliferate16,28,29 and secrete IFN-γ15 in response to B. hyodysenteriae antigens. The results from this study show that only CD4+ T cells respond specifically to B. hyodysenteriae antigens. The B. hyodysenteriae-induced CD4+ T-cell proliferation was associated with changes in their distribution at the colonic mucosa. However, in contrast to what has been shown after intramuscular immunization, the ex-vivoγδ T-cell response to B. hyodysenteriae antigens was not different between control and infected pigs. In addition, the numbers of γδ LPL remained unaltered whereas γδ IEL were significantly depleted as a result of the infection.
The lack of γδ T-cell responses to B. hyodysenterie antigens is an intriguing finding of this study. γδ T cells down-regulate inflammatory responses30,31 and constitute a first line of defence at mucosal surfaces, representing a link between innate and adaptive immunity.32,33 On the basis of previous reports on the response of γδ T cells to B. hyodysenteriae following immunization15,16,28,29 and their involvement in inflammation, we predicted that after challenge, γδ T cells would proliferate to B. hyodysenteriae and be recruited to the colonic mucosa. However, our results are not consistent with this hypothesis because no differences were found in the numbers of γδ LPL between control and dysenteric pigs. Also, peripheral blood and CLN γδ T-cell responses to B. hyodysenteriae antigens were not different between infected and control pigs. The low numbers of γδ T cells found in CLN34 is consistent with the homing pattern that has been previously described for this cell type; in contrast to αβ T cells, γδ T cells migrate directly to sites of infection without recirculating through lymph nodes.35 This property explains that γδ T cells from CLN failed to respond to B. hyodysenteriae antigens. However, the lack of differences in the responses of peripheral blood γδ T cells between treatments is inconsistent with previous reports obtained after intramuscular immunization.15,16,28 Circulating porcine γδ T cells express IL-2 receptor α at background levels and respond to IL-2 in the absence of additional stimuli (i.e. TCR activation).15 Thus, the ex-vivoγδ T-cell response to B. hyodysenteriae antigens could have been masked by the non-specific proliferation to IL-2. This hypothesis is supported by the fact that peripheral blood γδ T cells from control pigs proliferated at background levels when stimulated with antigen. Alternatively, because this study only considered one time-point after infection, the γδ T-cell response might have peaked immediately following challenge or during the phase of resolution, as has been demonstrated in other infectious disease models, such as influenza virus or Listeria monocytogenes infections.30,36–38 Finally, the route of antigenic exposure (i.e. intramuscular versus oral in vaccination or infection, respectively) might account for the contradictory results between immunized and challenged pigs.
The loss of γδ IEL was most likely the result of epithelial erosion. The γδ IEL participate in the regeneration of the epithelial barrier by secreting cytokines and growth factors, such as keratinocyte growth factor.39 Hence, the decreased numbers of γδ IEL could indirectly contribute to the pathogenesis of the disease by delaying epithelial restitution. It has been reported that high percentages of circulating γδ T cells previous to an experimental challenge with B. hyodysenteriae correlated with increased susceptibility to the disease.40 This study did not evaluate changes in γδ T-cell composition at the colonic mucosa. Thus, the involvement of γδ T cells in the development of swine dysentery is still unclear.
Brachyspira hyodysenteriae-induced colitis has similarities with CD4+ T-cell-induced murine colitis. In both cases the presence of normal intestinal flora is required for disease development.2,10 Both pathologies share similar histological LESions, namely thickening of the epithelium with crypt hyperplasia.41,42 A previous study showed that CD4+ T cells were diminished in the colonic mucosa of pigs with swine dysentery.17 Here, no differences in the numbers of CD4+ LPL were found between treatments. The more even distribution of CD4+ LPL in control pigs as opposed to the large clusters of cells found across the lamina propria and submucosa of challenged pigs might explain the lack of differences in CD4+ T-cell numbers between treatments. In fact, a similar distribution of colonic CD4+ T cells has been described in the CD4+ T-cell-induced murine colitis. These LESions corresponded to areas of co-localization of dendritic cells and proliferating pathogenic CD4+ T cells.43,44 Recognition of bacterial components of the normal flora by Toll-like receptors on dendritic cells induces IL-12 which, during antigen presentation to naïve CD4+ T cells at regional lymph nodes, favours their differentiation into Th1 pro-inflammatory cells.10 CD4+ T cells from both PBMC and CLN proliferated to antigenic stimulation with B. hyodysenteriae ex vivo. Because B. hyodysenteriae does not disseminate beyond the colonic mucosa, priming of naïve CD4+ T cells most likely occurred in the CLN after antigen uptake, processing and presentation by dendritic cells. Activated CD4+ T cells could home into the colonic lamina propria and be further stimulated by macrophages or mucosa-resident dendritic cells. In this regard, an additional consideration is that, as a result of the translocation of luminal antigens into the lamina propria, the CD4+ T-cell repertoire during swine dysentery is most probably polyclonal rather than restricted exclusively to B. hyodysenteriae epitopes. In this context, the mucosal CD4+ T-cell response would be mainly secondary to the translocation of luminal bacteria into the lamina propria and a downstream effect of epithelial damage. In contrast, the loss of γδ IEL would be a direct result of B. hyodysenteriae action on the colonic epithelium.
Both IFN-γ and IL-10 mRNA were up-regulated in CLN of pigs with swine dysentery17 which suggests the induction of both pro-inflammatory and regulatory responses. Thus, B. hyodysenteriae infection could induce two functionally distinct CD4+ T-cell responses: pro-inflammatory Th1, involved in disease development, and regulatory CD4+ T cells which would down-regulate the inflammatory response.45–47 Protection induced by different vaccine preparations against swine dysentery has been associated with decreased LESion development, rather than with prevention of bacterial colonization.14,16,17 In this regard, in a recent experimental vaccine trial with a recombinant cell wall lipoprotein (i.e. BmpB) from B. hyodysenteriae outer membrane, protection did not correlate with decreased bacterial shedding or increased B. hyodysenteriae-specific immunoglobulin A secretion.48 Our results suggest that CD4+ T cells are a component of the host immune response that participates in the inflammatory response triggered by the spirochaete B. hyodysenteriae. Future studies should aim at dissecting the nature of the mucosal CD4+ T-cell response during swine dysentery and its implication for the development of more effective vaccines.
Acknowledgments
This work was supported by USDA formula funds, a grant from the NRI, USDA-CSREES # 2001-035204-10183, an Immunobiology Competitive Research Award from Iowa State University Graduate College, and a grant from the National Pork Board. The authors would like to thank Drs Mark Ackermann and Randy Sacco for helpful discussions, and Jack Gallup for his assistance with the immunohistochemistry assay.
Abbreviations
- CLN
colonic lymph nodes
- IEL
intraepithelial lymphocytes
- IFN-γ
interferon-γ
- IL-10
interleukin-10
- LPL
lamina propria lymphocytes
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- TCR
T-cell receptor
- Th1
T helper type 1 cells
References
- 1.Jensen TK, Boye M, Moller K, Leser TD, Jorsal SE. Association of Serpulina hyodysenteriae with the colonic mucosa in experimental swine dysentery studied by fluorescent in situ hybridization. Apmis. 1998;106:1061–8. [PubMed] [Google Scholar]
- 2.Neef NA, Lysons RJ, Trott DJ, Hampson DJ, Jones PW, Morgan JH. Pathogenicity of porcine intestinal spirochetes in gnotobiotic pigs. Infect Immun. 1994;62:2395–403. doi: 10.1128/iai.62.6.2395-2403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whipp SC, Robinson IM, Harris DL, Glock RD, Matthews PJ, Alexander TJ. Pathogenic synergism between Treponema hyodysenteriae and other selected anaerobes in gnotobiotic pigs. Infect Immun. 1979;26:1042–7. doi: 10.1128/iai.26.3.1042-1047.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy MJ, Rosey EL, Yancey RJ., Jr Characterization of flaA- and flaB- mutants of Serpulina hyodysenteriae: both flagellin subunits, FlaA and FlaB, are necessary for full motility and intestinal colonization. FEMS Microbiol Lett. 1997;153:119–28. doi: 10.1111/j.1574-6968.1997.tb10472.x. [DOI] [PubMed] [Google Scholar]
- 5.ter Huurne AA, van Houten M, Muir S, Kusters JG, van der Zeijst BA, Gaastra W. Inactivation of a Serpula (Treponema) hyodysenteriae hemolysin gene by homologous recombination: importance of this hemolysin in pathogenesis in mice. FEMS Microbiol Lett. 1992;71:109–13. doi: 10.1016/0378-1097(92)90550-8. [DOI] [PubMed] [Google Scholar]
- 6.ter Huurne AA, Muir S, van Houten M, Koopman MB, Kusters JG, van der Zeijst BA, Gaastra W. The role of hemolysin(s) in the pathogenesis of Serpulina hyodysenteriae. Zentralbl Bakteriol. 1993;278:316–25. doi: 10.1016/s0934-8840(11)80848-0. [DOI] [PubMed] [Google Scholar]
- 7.Muir S, Koopman MB, Libby SJ, Joens LA, Heffron F, Kusters JG. Cloning and expression of a Serpula (Treponema) hyodysenteriae hemolysin gene. Infect Immun. 1992;60:529–35. doi: 10.1128/iai.60.2.529-535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutto DL, Wannemuehler MJ. A comparison of the morphologic effects of Serpulina hyodysenteriae or its beta-hemolysin on the murine cecal mucosa. Vet Pathol. 1999;36:412–22. doi: 10.1354/vp.36-5-412. [DOI] [PubMed] [Google Scholar]
- 9.Lysons RJ, Kent KA, Bland AP, Sellwood R, Robinson WF, Frost AJ. A cytotoxic haemolysin from Treponema hyodysenteriae– a probable virulence determinant in swine dysentery. J Med Microbiol. 1991;34:97–102. doi: 10.1099/00222615-34-2-97. [DOI] [PubMed] [Google Scholar]
- 10.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 11.Cong Y, Weaver CT, Lazenby A, Elson CO. Colitis induced by enteric bacterial antigen-specific CD4+ T cells requires CD40–CD40 ligand interactions for a sustained increase in mucosal IL-12. J Immunol. 2000;165:2173–82. doi: 10.4049/jimmunol.165.4.2173. [DOI] [PubMed] [Google Scholar]
- 12.Annacker O, Powrie F. Homeostasis of intestinal immune regulation. Microbes Infect. 2002;4:567–74. doi: 10.1016/s1286-4579(02)01574-5. [DOI] [PubMed] [Google Scholar]
- 13.Kullberg MC, Andersen JF, Gorelick PL, et al. Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope. Proc Natl Acad Sci USA. 2003;100:15830–5. doi: 10.1073/pnas.2534546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters WR, Sacco RE, Dorn AD, Hontecillas R, Zuckermann FA, Wannemuehler MJ. Systemic and mucosal immune responses of pigs to parenteral immunization with a pepsin-digested Serpulina hyodysenteriae bacterin. Vet Immunol Immunopathol. 1999;69:75–87. doi: 10.1016/s0165-2427(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 15.Hontecillas R, Bassaganya-Riera J. Differential requirements for proliferation of CD4+ and γδ+ T cells to spirochetal antigens. Cell Immunol. 2003;224:38–46. doi: 10.1016/s0008-8749(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 16.Waters WR, Pesch BA, Hontecillas R, Sacco RE, Zuckermann FA, Wannemuehler MJ. Cellular immune responses of pigs induced by vaccination with either a whole cell sonicate or pepsin-digested Brachyspira (Serpulina) hyodysenteriae bacterin. Vaccine. 1999;18:711–19. doi: 10.1016/s0264-410x(99)00266-2. [DOI] [PubMed] [Google Scholar]
- 17.Hontecillas R, Wannemeulher MJ, Zimmerman DR, Hutto DL, Wilson JH, Ahn DU, Bassaganya-Riera J. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J Nutr. 2002;132:2019–27. doi: 10.1093/jn/132.7.2019. [DOI] [PubMed] [Google Scholar]
- 18.Wannemuehler MJ, Hubbard RD, Greer JM. Characterization of the major outer membrane antigens of Treponema hyodysenteriae. Infect Immun. 1988;56:3032–9. doi: 10.1128/iai.56.12.3032-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunkle RA, Kinyon JM. Improved selective medium for the isolation of Treponema hyodysenteriae. J Clin Microbiol. 1988;26:2357–60. doi: 10.1128/jcm.26.11.2357-2360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutto DL, Galvin JE, Wannemuehler MJ. Morphologic and temporal characterisation of LESions in an enhanced murine model of Serpulina hyodysenteriae infection. J Med Microbiol. 1998;47:275–80. doi: 10.1099/00222615-47-3-275. [DOI] [PubMed] [Google Scholar]
- 21.Bassaganya-Riera J, Hontecillas R, Zimmerman DR, Wannemuehler MJ. Long-term influence of lipid nutrition on the induction of CD8(+) responses to viral or bacterial antigens. Vaccine. 2002;20:1435–44. doi: 10.1016/s0264-410x(01)00465-0. [DOI] [PubMed] [Google Scholar]
- 22.Bassaganya-Riera J, Pogranichniy RM, Jobgen SC, Halbur PG, Yoon KJ, O'Shea M, Mohede I, Hontecillas R. Conjugated linoleic acid ameliorates viral infectivity in a pig model of virally induced immunosuppression. J Nutr. 2003;133:3204–14. doi: 10.1093/jn/133.10.3204. [DOI] [PubMed] [Google Scholar]
- 23.Haverson K, Bailey M, Stokes CR. T-cell populations in the pig intestinal lamina propria: memory cells with unusual phenotypic characteristics. Immunology. 1999;96:66–73. doi: 10.1046/j.1365-2567.1999.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saalmuller A, Werner T, Fachinger V. T-helper cells from naive to committed. Vet Immunol Immunopathol. 2002;87:137–45. doi: 10.1016/s0165-2427(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 25.Carr MM, Howard CJ, Sopp P, Manser JM, Parsons KR. Expression on porcine gamma delta lymphocytes of a phylogenetically conserved surface antigen previously restricted in expression to ruminant gamma delta T lymphocytes. Immunology. 1994;81:36–40. [PMC free article] [PubMed] [Google Scholar]
- 26.Saalmuller A, Pauly T, Pfaff E. [Phenotypic and functional characterization of porcine T-lymphocytes] Zentralbl Chir. 1998;123:798–802. [PubMed] [Google Scholar]
- 27.Yang H, Parkhouse RM. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76–83. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassaganya-Riera J, Hontecillas R, Zimmerman DR, Wannemuehler MJ. Dietary conjugated linoleic acid modulates phenotype and effector functions of porcine CD8 (+) lymphocytes. J Nutr. 2001;131:2370–7. doi: 10.1093/jn/131.9.2370. [DOI] [PubMed] [Google Scholar]
- 29.Waters WR, Hontecillas R, Sacco RE, Zuckermann FA, Harkins KR, Bassaganya-Riera J, Wannemuehler MJ. Antigen-specific proliferation of porcine CD8αα cells to an extracellular bacterial pathogen. Immunology. 2000;101:333–41. doi: 10.1046/j.1365-2567.2000.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan PJ, Carding SR. Downmodulation of the inflammatory response to bacterial infection by gammadelta T cells cytotoxic for activated macrophages. J Exp Med. 2000;191:2145–58. doi: 10.1084/jem.191.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994;153:3101–15. [PubMed] [Google Scholar]
- 32.Ferrick DA, King DP, Jackson KA, Braun RK, Tam S, Hyde DM, Beaman BL. Intraepithelial gamma delta T lymphocytes: sentinel cells at mucosal barriers. Springer Semin Immunopathol. 2000;22:283–96. doi: 10.1007/s002810000047. [DOI] [PubMed] [Google Scholar]
- 33.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Parkhouse RM. Characterization of the porcine gammadelta T-cell receptor structure and cellular distribution by monoclonal antibody PPT27.PG. Immunology. 2000;99:504–9. doi: 10.1046/j.1365-2567.2000.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 36.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by gamma/delta + T cells. J Exp Med. 1990;172:1225–31. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carding SR, Egan PJ. The importance of gamma delta T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- 38.Skeen MJ, Rix EP, Freeman MM, Ziegler HK. Exaggerated proinflammatory and Th1 responses in the absence of gamma/delta T cells after infection with Listeria monocytogenes. Infect Immun. 2001;69:7213–23. doi: 10.1128/IAI.69.12.7213-7223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99:14338–43. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonasson R, Johannisson A, Jacobson M, Fellstrom C, Jensen-Waern M. Differences in lymphocyte subpopulations and cell counts before and after experimentally induced swine dysentery. J Med Microbiol. 2004;53:267–72. doi: 10.1099/jmm.0.05359-0. [DOI] [PubMed] [Google Scholar]
- 41.Leach MW, Bean AG, Mauze S, Coffman RL, Powrie F. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am J Pathol. 1996;148:1503–15. [PMC free article] [PubMed] [Google Scholar]
- 42.Bassaganya-Riera J, Reynolds K, Martino-Catt S, et al. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–91. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 43.Leithauser F, Trobonjaca Z, Moller P, Reimann J. Clustering of colonic lamina propria CD4(+) T cells to subepithelial dendritic cell aggregates precedes the development of colitis in a murine adoptive transfer model. Laboratory Invest. 2001;81:1339–49. doi: 10.1038/labinvest.3780348. [DOI] [PubMed] [Google Scholar]
- 44.Leithauser F, Krajina T, Trobonjaca Z, Reimann J. Early events in the pathogenesis of a murine transfer colitis. Pathobiology. 2002;70:156–63. doi: 10.1159/000068148. [DOI] [PubMed] [Google Scholar]
- 45.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–15. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin B, Banz A, Bienvenu B, Cordier C, Dautigny N, Becourt C, Lucas B. Suppression of CD4+ T lymphocyte effector functions by CD4+CD25+ cells in vivo. J Immunol. 2004;172:3391–8. doi: 10.4049/jimmunol.172.6.3391. [DOI] [PubMed] [Google Scholar]
- 47.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice. Control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971–8. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 48.La T, Phillips ND, Reichel MP, Hampson DJ. Protection of pigs from swine dysentery by vaccination with recombinant BmpB, a 29.7 kDa outer-membrane lipoprotein of Brachyspira hyodysenteriae. Vet Microbiol. 2004;102:97–109. doi: 10.1016/j.vetmic.2004.06.004. [DOI] [PubMed] [Google Scholar]