Abstract
Recent studies employing reverse transription—polymerase chain reaction (RT—PCR) have demonstrated the intrathymic presence of mRNA for various autoantigens, including thyroglobulin (Tg). Deliberations on the mechanisms of central tolerance usually assume that this approach detects intact mRNA transcripts that can be translated to express the whole autoantigen in the thymus. In the present study, we tested this assumption using mRNA transcripts of mouse Tg which encode at least 13 pathogenic peptides, scattered over a large (8·5 kb) sequence. We found that mRNA encoding 11 out of these 13 Tg peptides was present in both the thyroid and the thymus of CBA/J mice, with no apparent temporal fluctuations in expression from birth to 12 weeks of age. Interestingly, detection of these sequences was also demonstrable in the liver and kidney, but not in muscle. However, mRNA encoding two pathogenic peptides (amino acids 1–12 and amino acids 1579–1591) was detected intrathyroidally but not in the other tissues. Further analysis by RT—PCR showed that Tg mRNA transcripts in the thymus, liver and kidney lack segments within the 1–915 bp and 961–5013 bp regions, spanning exons 1–7 and 9–22, respectively. These data strongly suggest that certain known and perhaps other, as yet unmapped, pathogenic T-cell epitopes of Tg cannot be encoded by the truncated isoform(s) of intrathymic Tg mRNA. These findings also imply that central tolerance to endogenous Tg produced by thymic epithelial cells may be incomplete.
Keywords: thymic expression, thyroglobulin, thyroiditis
Introduction
The physiological expression of peripheral tissue-specific antigens in the thymus has been well documented in recent years (reviewed in refs 1 and 2). This phenomenon of ‘promiscuous gene expression’2,3 has been viewed to play a pivotal role in the shaping of the autoreactive T-cell repertoire because the levels of intrathymic expression of several tissue antigens inversely correlate with susceptibility to organ-specific autoimmunity.4–7 Deletion of high-affinity autoreactive T cells by the self-antigen expressed in the thymus is one mechanism that could account for these observations.8 In some instances, however, self-reactive cells may escape thymic deletion because the isoform of the antigen expressed in the thymus differs from that expressed in the target organ. This has been best illustrated in studies of experimental autoimmune encephalomyelitis (EAE) and the target antigen proteolipid protein (PLP), the main protein of the myelin sheath. Intrathymic PLP mRNA exists predominantly as a splice variant, DM20, which lacks a specific loop of 35 amino acids.9 It has been shown that T-cell tolerance to PLP is restricted to those epitopes included in DM20.10,11
Thyroglobulin (Tg) is the largest autoantigen known — with a molecular mass of 660 000 in its homodimeric form — and the most abundant glycoprotein of the thyroid gland. Mapping studies have, to date, identified 13 Tg peptides encompassing T-cell epitopes that elicit experimental autoimmune thyroiditis (EAT) when administered with adjuvant into mice.12,13 However, none of these epitopes has been classified as dominant by proliferative assays in vitro, and the conditions that might promote their participation in the disease process remain speculative.12,14 Several studies, using reverse transcription—polymerase chain reaction (RT—PCR) and other detection methods, have demonstrated promiscuous intrathymic Tg gene expression in rats,15 mice3 and humans.16 In contrast, Mor et al.17 could not find evidence for intrathymic expression of Tg in rats by using RT—PCR and primers that amplified the 532–832 bp fragment. These apparently contrasting results, the large size of the Tg molecule (2748 amino acids), and the expression of autoantigen isoforms in the thymus in other animal models, prompted us to examine whether intrathymic Tg gene detection by RT—PCR could be significantly influenced by the choice of the gene region used for primer design. In this effort, we used thymi of CBA/J mice from birth to 12 weeks of age and the cDNA sequences encoding the known pathogenic Tg peptides as landmarks, because they are scattered over the whole length of the Tg molecule. Tg gene detection in other extrathyroidal tissues, such as liver, kidney and muscle, was also investigated by using the same approach.
Materials and methods
Animals
Male CBA/J (H-2k) mice (at 6 or 12 weeks of age) or breeding CBA/J pairs were purchased from the Jackson Laboratories (Bar Harbor, ME). All animals were maintained under standard (non-specific pathogen-free) conditions.
Total RNA isolation, cDNA synthesis and RT—PCR
Freshly obtained thymus, thyroid, liver, kidney and muscle tissues were homogenized using disposable RNase-free homogenizers in Trizol Reagent (Life Technologies, Invitrogen, Paisley, UK) and total RNA was isolated according to the manufacturer's instructions. First-strand cDNA was generated from 5 µg of total RNA in a 33-µl reaction volume by using NotI-d(T)18 as primer and  murine reverse transcriptase (Amersham Bioscience, Little Chalfont, Buckinghamshire, UK). PCR was performed in 50-µl reaction mixtures comprising 2 µl of cDNA, 5 µl of 10× PCR buffer (Life Technologies), 2 µl of 50 mm MgCl2 (Life Technologies), 0·4 µl of 25 mm dNTPs (Gibco, Rockville, MA), 1·5 µl of forward primer (10 pmol/µl), 1·5 µl of reverse primer (10 pmol/µl), 0·2 µl of PlatinumTaq DNA polymerase (Life Technologies) and 37·4 µl of nuclease-free water. The cycling conditions were as follows: initial denaturation at 94° for 5 min; followed by 35 cycles of denaturation at 94° for 1 min, ANNealing at 55° for 1 min and extension at 72° for 1 min; and a final extension at 72° for 10 min. The reactions were run on a Perkin-Elmer thermocycler (Cetus, Norwalk, CT). All Tg primers (Table 1) were designed to contain the KpnI and XbaI restriction sites at the 5′ and 3′ ends, respectively, and were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). PCR products were separated on 3% or 1·5% agarose gels, and were visualized by staining with ethidium bromide. The relative intensity of the cDNA was quantified by chemiimager 4000 software (Alpha Innotech, Corp., San Leandro, CA). Relative expression was calculated as the ratio of the relative optical density of the Tg fragment to that of the β-actin in the same sample and under similar conditions of amplification.
murine reverse transcriptase (Amersham Bioscience, Little Chalfont, Buckinghamshire, UK). PCR was performed in 50-µl reaction mixtures comprising 2 µl of cDNA, 5 µl of 10× PCR buffer (Life Technologies), 2 µl of 50 mm MgCl2 (Life Technologies), 0·4 µl of 25 mm dNTPs (Gibco, Rockville, MA), 1·5 µl of forward primer (10 pmol/µl), 1·5 µl of reverse primer (10 pmol/µl), 0·2 µl of PlatinumTaq DNA polymerase (Life Technologies) and 37·4 µl of nuclease-free water. The cycling conditions were as follows: initial denaturation at 94° for 5 min; followed by 35 cycles of denaturation at 94° for 1 min, ANNealing at 55° for 1 min and extension at 72° for 1 min; and a final extension at 72° for 10 min. The reactions were run on a Perkin-Elmer thermocycler (Cetus, Norwalk, CT). All Tg primers (Table 1) were designed to contain the KpnI and XbaI restriction sites at the 5′ and 3′ ends, respectively, and were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). PCR products were separated on 3% or 1·5% agarose gels, and were visualized by staining with ethidium bromide. The relative intensity of the cDNA was quantified by chemiimager 4000 software (Alpha Innotech, Corp., San Leandro, CA). Relative expression was calculated as the ratio of the relative optical density of the Tg fragment to that of the β-actin in the same sample and under similar conditions of amplification.
Table 1. The sequences of polymerase chain reaction (PCR) primers used in this study.
| Peptide/ fragment | Amino acid co-ordinates | Base pair co-ordinates | Forward primer1 | Reverse primer1 | Product size (bp) |
|---|---|---|---|---|---|
| p1 | 1–12 | 1–36 | AGGGTACCAACATCTTTGAG | TGTCTAGAGGGGCGGAGTGG | 58 |
| p306 | 306–320 | 916–960 | AGGGTACCGATGGTCACTACCAA | TGTCTAGACTGGGCATCCACACA | 79 |
| p1579 | 1579–1591 | 4735–4773 | AGGGTACCGACTCCCCGCTGGTG | TGTCTAGAGAAGCTGCAGGCCTC | 72 |
| p1672 | 1672–1711 | 5014–5133 | AGGGTACCCAGAAGAGCTTCGAA | TGTCTAGAACAGCAGGAATCATT | 154 |
| p1826 | 1826–1836 | 5476–5508 | AGGGTACCGACTTTCCAGGAGAT | TGTCTAGAGGTAATGTCCACAGG | 67 |
| p2102 | 2102–2116 | 6304–6348 | AGGGTACCAGTAACTTCTCCATG | TGTCTAGAAAGGCAGTCCTGGTG | 79 |
| p2340 | 2340–2359 | 7018–7077 | AGGGTACCCTGCTGGACCAAGTG | TGTCTAGATGTCACACGCTGAGG | 94 |
| p2494 | 2494–2510 | 7483–7533 | CGGGTACCATGGGGCTTATCAATAG | CGTCTAGATCAGCCTTGGCTCTCTT | 73 |
| p2549 | 2549–2560 | 7645–7680 | AGGGTACCTTGGAGCACTCCACA | TGTCTAGAGGCATTCTCCAGTGC | 70 |
| p2596 | 2596–2608 | 7786–7824 | AGGGTACCCCCGAAAGCTATGGC | TGTCTAGAAAAAGCATATTGAAC | 73 |
| p2695 | 2695–2713 | 8083–8136 | CGGGTACCATGTGCTCCTTCTGGT | CGTCTAGATCATGCATCCTTGGCTC | 82 |
| p2730 | 2730–2743 | 8188–8229 | AGGGTACCGTTGGACCTGGATTA | TGTCTAGATTTGCTGTAGCTCTT | 82 |
| A | 306–520 | 916–1560 | AGGGTACCAACATCTTTGAG | TGTCTAGACTTCTCAGACACACG | 645 |
| B | 516–745 | 1546–2235 | ATGGTACCCGTGTGTCTGAGAAG | TGTCTAGAAGAGGCACTGCACTGAGG | 690 |
| C | 741–980 | 2221–2940 | AGGGTACCCCTCAGTGCAGTGCC | TGTCTAGACTGAGCAGCCAAGCG | 720 |
| D | 976–1225 | 2926–3675 | AGGGTACCCGCTTGGCTGCTCAG | TGTCTAGACTGCTGAACAGTCGT | 750 |
| E | 1221–1435 | 3661–4305 | AGGGTACCACGACTGTTCAGCAG | TGTCTAGAAGCATCCTGTCTGGT | 645 |
| F | 1436–1711 | 4306–5133 | AGGGTACCCTGGGCTGTGTGAAA | TGTCTAGAACAGCAGGAATCAT | 828 |
| β-actin | – | – | GCTCTTTTCCAGCCTTCCTT | CTTCTGCATCCTGTCAGCAA | 177 |
All primers are given in the 5′ to 3′ direction; the underlined sequence is the KpnI site in the forward primer and the XbaI site in the reverse primer.
Results
mRNA encoding two out of 13 pathogenic Tg peptides is undetectable in the thymus
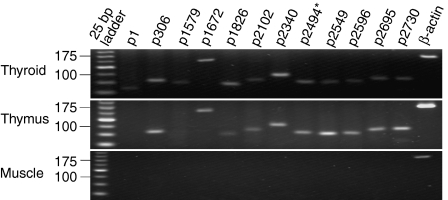
The expression of mRNA encoding each known pathogenic peptide of Tg was first determined by RT—PCR in the thyroid, thymus and muscle tissues of 12-week-old CBA/J mice. As expected, mRNA encoding all 13 Tg peptides was abundantly expressed in the thyroid. However, the mRNA transcripts for two peptides, p1 (amino acids 1–12) and p1579 (amino acids 1579–1591), were undetectable in the thymus (Fig. 1). An identical mRNA expression profile for all Tg peptides under investigation was obtained with thyroid and thymus tissues from newborn, and from 2-, 6- and 12-week-old CBA/J mice (results not shown), suggesting that these data did not reflect any temporal influences on expression. None of the 13 mRNA transcripts was detected in muscle (Fig. 1) and this was also confirmed at all the above time-points tested (results not shown).
Figure 1.
mRNA sequences encoding two pathogenic thyroglobulin (Tg) peptides (p1 and p1579) are detectable in the thyroid but not in the thymus of CBA/J mice. Data are representative of individual samples from five mice. mRNA from muscle tissue was used as a control. *The expression of mRNA encoding the 13 known pathogenic Tg peptides was assessed in 12 tracks because the p2494 mRNA sequence codes for two overlapping pathogenic peptides: 2495–2503 and 2498–2506.12
Relative expression of mRNA encoding Tg peptides among various tissues
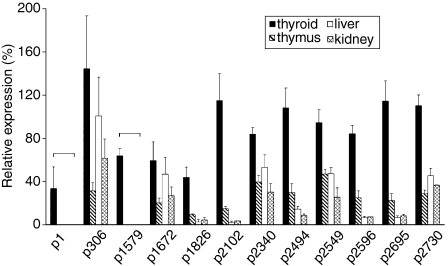
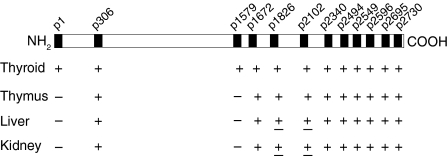
Using the same set of primers, we subsequently determined the relative expression of the Tg peptide mRNA in thyroid, versus thymus, liver and kidney, by using mRNA from β-actin as a reference. As expected, the Tg mRNA transcripts were most prevalent in the thyroid gland, with their relative expression ranging from 33·6 ± 19·8% for p1 to 144·3 ± 49·1% for p306 (Fig. 2), perhaps reflecting the relative efficiency of the selected primer pairs for each region. mRNA transcripts for all tested sequences also showed a lower and variable expression in thymus, liver and kidney, except for the mRNA transcripts for p1 and p1579, which were detected only in the thyroid. These results (summarized in Fig. 3) suggest the presence of Tg isoforms in extrathyroidal tissues, including the thymus, that are truncated at the 5′ end (within the 1–915-bp segment) and carry internal deletion(s) within a large fragment spANNing bp 961–5013.
Figure 2.
Relative expression of mRNA encoding pathogenic thyroglobulin (Tg) peptides in various tissues of CBA/J mice. The results are expressed as the mean value ± standard deviation (SD) obtained from two or three mice.
Figure 3.
Summarized data on the relative expression of mRNA encoding pathogenic thyroglobulin (Tg) peptides in various tissues of CBA/J mice. ±, The relative expression varies from 0 to 10% among samples; −, no detection.
mRNA encoding a large Tg fragment (bp 961–5013) is undetectable in the mouse thymus using RT—PCR
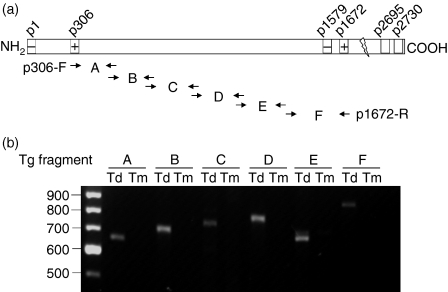
To determine the approximate boundaries of a potential deletion between bp 961–5013 in intrathymic Tg mRNA, we used six pairs of overlapping primers to amplify, by RT—PCR, six 600–800 bp segments (A to F) spANNing the 916–5133 bp region, as shown in Fig. 4(a). It was found that mRNA encoding all six Tg segments was present in the thyroid, but absent in the thymus of the same donor (Fig. 4b). These data strongly support the lack of a potentially large (4 kb) sequence segment within the 961–5013 bp segment of intrathymic Tg mRNA.
Figure 4.
A large fragment (bp 961–5013) of thyroglobulin (Tg) mRNA transcript is undetectable in mouse thymus. (a) A series of overlapping primers were designed and used in reverse transcription—polymerase chain reaction (RT—PCR) to amplify the Tg mRNA fragment encoding the amino acid sequence from p306 to p1672. (b) Visualization of PCR products in a 1·5% agarose gel stained with ethidium bromide. Td, thyroid; Tm, thymus.
Discussion
In this study, we tested the intrathymic expression of mRNA encoding 13 pathogenic peptides scattered throughout the length of the large Tg autoantigen. It was found that mRNA encoding the N-terminal peptide p1 (amino acids 1–12) and the peptide p1579 (amino acids 1579–1591) towards the middle of the molecule, was detectable in the thyroid, but not in the thymus. In contrast, mRNA encoding all other peptides was easily detectable in both organs. Overlapping primer pairs, spANNing the 916–5133 bp region, were subsequently found to similarly amplify RT—PCR products of the expected size in thyroid, but not in thymic tissue. These results suggest that Tg mRNA in the mouse thymus exists as an isoform(s) with a potentially large (4 kb) deletion, but it remains unclear whether this mRNA comprises one or more truncated and/or differentially spliced species.
Our findings caution against using primers from random Tg sites to study extrathyroidal Tg gene expression by RT—PCR. Also, they indicate that apparently contrasting observations of other investigators on this issue may be explained on the basis of the Tg mRNA segment chosen for amplification. Heath et al. have reported intrathymic Tg gene expression in male PVG rats using primers amplifying the 7597–8241 bp fragment,15 whereas Mor et al. have not been able to detect Tg mRNA in thymocytes of Lewis rats17 with primers amplifying the 532–832 bp segment, close to the N terminus of the molecule. Furthermore, differential Tg transcripts between species may preclude RT—PCR-based extrapolations from mice to humans in regard to intrathymic Tg expression. For example, Spitzweg et al.16 detected Tg gene expression in human thymus using primers amplifying the 4464–5146 bp region, whereas we have been unable to detect Tg in mouse thymus via amplification of the 4735–4773 bp segment (encoding p1579), which is localized within the above region.
The presence of intrathymic Tg isoforms may have implications for mechanisms of central tolerance to Tg, as negative selection may not occur against dominant T-cell epitopes if they map within regions not represented in the thymus. Also, the hierarchy of immunodominance, resulting from the processing of intrathymic Tg, may be different from that of the peripheral Tg. To date, all known pathogenic T-cell epitopes in Tg have been classified as non-dominant,12 and some are known to be produced in vitro under certain conditions, e.g. after the processing of Tg—antibody immune complexes18 or the processing of highly iodinated Tg.19 It is not known, however, to what extent non-dominant T-cell epitopes can be generated in the thymus from endogenous Tg produced in thymic epithelial cells3,15 or blood-borne Tg molecules that leak from the thyroid in small amounts.20 One, therefore, cANNot draw correlates between the presence of intrathymic mRNA encoding a non-dominant Tg epitope and the immunogenicity of this epitope, as has been performed with dominant peptides in other systems.11 For example, the Tg peptide (p1) whose mRNA is undetectable in the thymus, has been found to be weakly immunopathogenic.21
Our results confirm those of previous studies reporting Tg gene expression in mouse — as well as human — kidney.22 On the other hand, it is not clear to what extent the detection of Tg mRNA transcripts in the liver is caused by the presence of contaminant leukocytes, because it has been reported that Tg is expressed in blood cells.23 Nevertheless, it is intriguing that the extrathyroidal expression patterns of various Tg transcripts in our study is similar among thymus, liver and kidney, i.e. they indicate a lack of segments within the 1–915 bp and 961–5013 bp regions, spANNing exons 1–7, and 9–22, respectively. This concordant pattern of amplicon expression and the number of amplicons examined also supports the view that they originate from Tg mRNA, although this has not been formally shown by sequencing data. The possibility of an in situ production of Tg protein in the kidney has been raised previously,22 but the functional properties, if any, of extrathyroidal Tg remain unknown. Tg has been reported to possess an intrinsic cAMP-dependent protein kinase activity, and it may autophosphorylate serine residues in vitro.24,25 The catalytic or ligand-binding activity of Tg probably lies with two motifs comprised of amino acid residues 154–160 and 468–475, i.e. sites which, as our data indicate, may be missing in extrathyroidal Tg.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research.
References
- 1.Anderson AC, Kuchroo VK. Expression of self-antigen in the thymus: a little goes a long way. J Exp Med. 2003;198:1627–9. doi: 10.1084/jem.20031803. 10.1084/jem.20031803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyewski B, Derbinski J, Gotter J, Klein L. Promiscuous gene expression and central T-cell tolerance: more than meets the eye. Trends Immunol. 2002;23:364–71. doi: 10.1016/s1471-4906(02)02248-2. 10.1016/S1471-4906(02)02248-2. [DOI] [PubMed] [Google Scholar]
- 3.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–9. doi: 10.1038/ni723. 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 4.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–12. [PubMed] [Google Scholar]
- 5.Liu H, MacKenzie-Graham AJ, Kim S, Voskuhl RR. Mice resistant to experimental autoimmune encephalomyelitis have increased thymic expression of myelin basic protein and increased MBP specific T cell tolerance. J Neuroimmunol. 2001;115:118–26. doi: 10.1016/s0165-5728(01)00269-7. 10.1016/S0165-5728(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 6.Pugliese A, Zeller M, Fernandez A, Jr, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–7. doi: 10.1038/ng0397-293. 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 7.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–92. doi: 10.1038/ng0397-289. 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 8.Targoni OS, LehmANN PV. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J Exp Med. 1998;187:2055–63. doi: 10.1084/jem.187.12.2055. 10.1084/jem.187.12.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nave KA, Lai C, Bloom FE, Milner RJ. Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proc Natl Acad Sci USA. 1987;84:5665–9. doi: 10.1073/pnas.84.16.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson AC, Nicholson LB, Legge KL, Turchin V, Zaghouani H, Kuchroo VK. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–70. doi: 10.1084/jem.191.5.761. 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein L, KlugmANN M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 12.CarayANNiotis G. The cryptic self in thyroid autoimmunity: the paradigm of thyroglobulin. Autoimmunity. 2003;36:423–8. doi: 10.1080/08916930310001602975. [DOI] [PubMed] [Google Scholar]
- 13.Gentile F, Conte M, Formisano S. Thyroglobulin as an autoantigen: what can we learn about immunopathogenicity from the correlation of antigenic properties with protein structure? Immunology. 2004;112:13–25. doi: 10.1111/j.1365-2567.2004.01861.x. 10.1111/j.1365-2567.2004.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CarayANNiotis G, Kong YC. Pathogenic thyroglobulin peptides as model antigens: insights on the induction and maintenance of autoimmune thyroiditis. Int Rev Immunol. 2000;19:557–72. doi: 10.3109/08830180009088512. [DOI] [PubMed] [Google Scholar]
- 15.Heath VL, Moore NC, Parnell SM, Mason DW. Intrathymic expression of genes involved in organ specific autoimmune disease. J Autoimmun. 1998;11:309–18. doi: 10.1006/jaut.1998.0210. 10.1006/jaut.1998.0210. [DOI] [PubMed] [Google Scholar]
- 16.Spitzweg C, Joba W, Heufelder AE. Expression of thyroid-related genes in human thymus. Thyroid. 1999;9:133–41. doi: 10.1089/thy.1999.9.133. [DOI] [PubMed] [Google Scholar]
- 17.Mor F, Boccaccio GL, Unger T. Expression of autoimmune disease-related antigens by cells of the immune system. J Neurosci Res. 1998;54:254–62. doi: 10.1002/(SICI)1097-4547(19981015)54:2<254::AID-JNR13>3.0.CO;2-4. 10.1002/(SICI)1097-4547(19981015)54:2<254::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y, CarayANNiotis KA, Eliades P, Lymberi P, Shepherd P, Kong Y, CarayANNiotis G. Enhancing or suppressive effects of antibodies on processing of a pathogenic T cell epitope in thyroglobulin. J Immunol. 1999;162:6987–92. [PubMed] [Google Scholar]
- 19.Dai YD, Rao VP, CarayANNiotis G. Enhanced iodination of thyroglobulin facilitates processing and presentation of a cryptic pathogenic peptide. J Immunol. 2002;168:5907–11. doi: 10.4049/jimmunol.168.11.5907. [DOI] [PubMed] [Google Scholar]
- 20.Spencer CA. Thyroglobulin. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's the Thyroid: a Fundamental and Clinical Text. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 402–13. [Google Scholar]
- 21.Wan Q, Motte RW, McCormick DJ, Fuller BE, Giraldo AA, David CS, Kong Y. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis: role of MHC class II. Clin Immunol Immunopathol. 1997;85:187–94. doi: 10.1006/clin.1997.4443. 10.1006/clin.1997.4443. [DOI] [PubMed] [Google Scholar]
- 22.Sellitti DF, Akamizu T, Doi SQ, Kim GH, Kariyil JT, Kopchik JJ, Koshiyama H. Renal expression of two ‘thyroid-specific’ genes: thyrotropin receptor and thyroglobulin. Exp Nephrol. 2000;8:235–43. doi: 10.1159/000020674. 10.1159/000020674. [DOI] [PubMed] [Google Scholar]
- 23.Bugalho MJ, Domingues RS, Pinto AC, et al. Detection of thyroglobulin mRNA transcripts in peripheral blood of individuals with and without thyroid glands: evidence for thyroglobulin expression by blood cells. Eur J Endocrinol. 2001;145:409–13. doi: 10.1530/eje.0.1450409. [DOI] [PubMed] [Google Scholar]
- 24.Alvino CG, Acquaviva AM, Catanzano AM, Tassi V. Evidence that thyroglobulin has an associated protein kinase activity correlated with the presence of an adenosine triphosphate binding site. Endocrinology. 1995;136:3179–85. doi: 10.1210/endo.136.8.7628349. 10.1210/en.136.8.3179. [DOI] [PubMed] [Google Scholar]
- 25.Kohn LD. Thyroglobulin — a new cyclic adenosine monophosphate-dependent protein kinase? Endocrinology. 1995;136:3177–8. doi: 10.1210/endo.136.8.7628348. 10.1210/en.136.8.3177. [DOI] [PubMed] [Google Scholar]