Abstract
Ageing is associated with increased susceptibility to lung infections and delayed resolution of pulmonary infiltrates. The purpose of this study was to investigate the effect of age on the onset of carrageenan-induced lung inflammation. When compared with carrageenan-treated young rats (3 months old), old rats (> 18 months old) exhibited a preponderance of pleural exudation and polymorphonuclear cell infiltration. Lung myeloperoxidase activity, an index of neutrophil infiltration and activation, was significantly increased in old rats in comparison with young rats. Consistent with the biochemical markers of inflammation, increased lung damage, as assessed by nitrosative stress and lipid peroxidation, was observed in carrageenan-treated old rats. In the lung exudate obtained from old rats, a significant reduction in interleukin-10 (IL-10) was observed, while similar expression of monocyte chemotactic protein-1 was induced, suggesting that a decrease in IL-10 rather than increased chemotaxis may account for the preponderance of the inflammatory cellular infiltrate in old rats. Similar to the in vivo situation, freshly isolated alveolar macrophages obtained from old rats produced less IL-10. This defective IL-10 production could be explained by a reduction in the cAMP-dependent signalling pathway, which mediates IL-10 production. Indeed, we found decreased cAMP-responsive element binding protein (CREB) and phosphorous-CREB (P-CREB) expression in old rats, which may account for reduced IL-10 production in old rats.
Keywords: cytokines, lung oedema, signal transduction, monocytes/macrophages, polymorphonuclear cells
Introduction
Carrageenan is a high-molecular-weight sulphated polysaccharide that is used in pharmacology to induce local inflammation (paw oedema and pleurisy). It is a proinflammatory polysaccharide useful to assess the contribution of mediators involved in vascular changes associated with acute inflammation. Acute lung inflammation is an important component of a number of pulmonary diseases.1 Injection of carrageenan into the pleural space leads to pleurisy, characterized by an immediate neutrophil infiltration followed by replacement of neutrophils by carrageenan-containing macrophages, and lung injury. In carrageenan-induced pleurisy, the initial phase of inflammation (oedema, 0–1 hr) has been attributed to the release of histamine, 5-hydroxytryptamine and bradykinin, followed by a late phase (1–6 hr) mainly sustained by prostaglandin and proinflammatory cytokine release.2 It appears that the onset of the carrageenan local inflammation is linked to neutrophil infiltration and the production of neutrophil-derived free radicals [reactive oxygen species (ROS)], such as hydrogen peroxide, superoxide and hydroxyl radicals, as well as to the release of other neutrophil-derived mediators.3 Neutrophil recruitment and activation result in parenchymal lung damage and subsequent lung dysfunctions.
An appropriate balance between pro- and anti-inflammatory cytokines during an immune response is critical in the resolution of local inflammation. In this context, interleukin-10 (IL-10) is of special interest because of its anti-inflammatory, antioxidant and immunosuppressive properties.4,5 In the normal resolution of an inflammatory response, apoptosis of neutrophils is essential to limit inappropriate host tissue damage by decreasing neutrophil tissue load, and release of phlogistic ROS and proteases, and IL-10 is indeed a strong regulator of neutrophil apoptosis during sepsis. In this context, we have recently demonstrated a higher rate of pleural exudation and polymorphonuclear cell migration following carrageenan treatment in IL-10 knock-out mice when compared to carrageenan-treated IL-10 wild-type mice.6 In this study we clearly demonstrated that endogenous IL-10 exerts an anti-inflammatory role during acute inflammation and tissue damage associated with carrageenan-induced pleurisy.
Ageing is associated with a progressive decline in both immune and pulmonary functions, which contribute to increased impact and severity of infections among the elderly.7,8
The purpose of the present study was to investigate the impact of age on the onset of carrageenan-induced lung inflammation. This was assessed by evaluating neutrophil infiltrate in the extravascular space, nitrotyrosine and lipid peroxidation, as signatures of lung damage, and the equilibrium between pro- and counter-inflammatory mediators in the pleural space. When compared with carrageenan-treated young rats, old rats exhibited a preponderance of pleural exudation and inflammatory cell infiltrate, which could be explained by a significant reduction in IL-10 production in old rats. We also demonstrated that this reduced IL-10 production was linked to a defective cAMP-dependent signalling pathway in old rats. Recent reports have demonstrated that IL-10 transcription may involve SV40 promoter 1 (Sp1) and signal transducer and activator of transcription 3 (Stat3) activation9, 10 but also cAMP-responsive element binding protein (CREB)/activating transcription factor (ATF) phosphorylation and CCAAT/enhancer-binding proteins (C/EBP),11 which mediate cAMP responsiveness by indirect mechanisms, indicating the crucial role of the cAMP-dependent signal transduction pathway in IL-10 synthesis. Reduced IL-10 production may account for the delayed resolution of pulmonary infiltrates and the increased lung damage in old rats following carrageenan treatment.
Materials and methods
Chemicals
Unless otherwise stated, all compounds were obtained from Sigma-Aldrich Company Ltd (Poole, UK).
Animals
Young (3-month-old) and old (> 18-month-old) male Sprague-Dawley rats (Charles River, Calco, Italy) were housed in a controlled environment and provided with standard rodent chow and water. Male rats were chosen because old female rats are not commercially available. Animal care was in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with EEC regulations (O.J. of E.C. L 358/1 12/18/1986).
Carrageenan-induced pleurisy
Carrageenan-induced pleurisy was induced as previously described.12 Rats were anaesthetized with isoflurane and submitted to a skin incision at the level of the left sixth intercostal space. The underlying muscle was dissected and saline (0·2 ml) or saline containing 1% (w/v) λ-carrageenan (0·2 ml) was injected into the pleural cavity. The skin incision was closed with a suture and the animals were allowed to recover. At 4 and 24 hr after the injection of carrageenan, the animals were killed by inhalation of CO2. The chest was carefully opened and the pleural cavity rinsed with 2 ml of saline solution containing heparin (5 U/ml) and indomethacin (10 µg/ml). The exudate and washing solution were removed by aspiration and the total volume was measured. The amount of exudate was calculated by subtracting the volume injected (2 ml) from the total volume recovered. The leucocytes in the exudate were suspended in phosphate-buffered saline (PBS, 0·01 m, pH 7·4) and counted with an optical microscope in a Burker's chamber after vital trypan blue staining.
Histological examination
Lung biopsies were taken 4 and 24 hr after injection of carrageenan. After lung inflation with 10% (w/v) PBS-buffered formaldehyde solution, lung biopsies were fixed for 1 week at room temperature, dehydrated using graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ). Sections were then deparaffinized with xylene, and stained with hematoxylin and eosin. All sections were studied using light microscopy (Dialux 22; Leitz, Wetzlar, Germany).
Measurement of cytokines
IL-10 and monocyte chemotactic protein-1 (MCP-1) levels were evaluated in the exudate 4 and 24 hr after the induction of pleurisy by carrageenan injection or in culture supernatants using a colorimetric commercial enzyme-linked immunosorbent assay (ELISA) (Amersham, Little Chalfont, UK), with a lower detection limit of 16 pg/ml for IL-10 and 38 pg/ml for MCP-1. Tumour necrosis factor (TNF)-α content was assayed by determining the cytotoxicity of TNF-α against sensitive L929 cells, as previously described.13 The results are expressed in pg/ml. TNF-α concentration was calculated against a standard curve with known amounts of recombinant murine TNF-α.
Measurement of nitrite-nitrate concentration
The total nitrite concentration in exudates, an indicator of nitric oxide (NO) synthesis, was measured as previously described.14 Briefly, the nitrate in the sample was first reduced to nitrite by incubation with nitrate reductase (670 mU/ml) and α-nicotinamide adenine dinucleotide 3′-phosphate (NADPH) (160 µm) at room temperature for 3 hr. The total nitrite concentration in the samples was then measured using the Griess reaction, by adding 100 µl of Griess reagent [0·1% weight/volume (w/v) naphthylethylendiamide dihydrochloride in H2O and 1% (w/v) sulphanilamide in 5% volume/volume (v/v) concentrated H3PO4; vol. 1:1] to the 100-µl sample. The optical density at 550 nm (OD550) was measured using an ELISA microplate reader (SLT-Laboratory Instruments, Salzburg, Austria). Nitrite concentrations were calculated by comparison with OD550 of standard solutions of sodium nitrite prepared in H2O.
Immunohistochemical localization of nitrotyrosine and poly ADP ribose polymerase (PARP)
Tyrosine nitration, an index of the nitrosylation of proteins by peroxynitrite and/or ROS, was determined by immunohistochemistry as previously described.13 At the end of the experiment (4 and 24 hr), the tissues were fixed in 10% (w/v) PBS-buffered formaldehyde and 8-µm sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0·3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 min. The sections were permeated with 0·1% (w/v) Triton X-100 in PBS for 20 min. Non-specific adsorption was minimized by incubating the section in 2% (v/v) normal goat serum in PBS for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with biotin and avidin (Diagnostic Brokers Associated, Milan, Italy), respectively. Sections were incubated overnight with antinitrotyrosine rabbit polyclonal antibody (1:500 in PBS, v/v) or with antipoly (ADP-ribose) goat polyclonal antibody rat (1:500 in PBS, v/v). Sections were washed with PBS, and incubated with secondary antibody. Specific labelling was detected with biotin-conjugated goat antirabbit immunoglobulin G (IgG) and avidin-biotin peroxidase complex (DBA).
Myeloperoxidase (MPO) activity
Myeloperoxidase activity, an indicator of polymorphonuclear leucocyte (PMN) accumulation, was determined as previously described.15 Four and 24 hr following injection of carrageenan, lung tissues were obtained and weighed, and each piece was homogenized in a solution containing 0·5% (w/v) hexadecyltrimethyl-ammonium bromide dissolved in 10 mm potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20 000 g at 4°. An aliquot of the supernatant was then allowed to react with a solution of tetramethylbenzidine (1·6 mm) and 0·1 mm hydrogen peroxide. The rate of change in absorbance was measured spectrophotometrically at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 µmol of peroxide/min at 37° and was expressed in milliunits per gram of wet tissue.
Malondialdehyde (MDA) measurement
Malondialdehyde levels in the lung tissue were determined as an indicator of lipid peroxidation as previously described.16 Lung tissue collected at the specified time was homogenized in 1·15% (w/v) KCl solution. A 100-µl aliquot of the homogenate was added to a reaction mixture containing 200 µl of 8·1% (w/v) sodium dodecyl sulphate (SDS), 1·5 ml of 20% (v/v) acetic acid (pH 3·5), 1·5 ml of 0·8% (w/v) thiobarbituric acid and 700 µl of distilled water. Samples were then boiled for 1 hr at 95° and centrifuged at 3000 g for 10 min. The absorbance of the supernatant was measured using spectrophotometry at 650 nm.
Cells
Alveolar macrophages were collected by bronchoalveolar lavage as previously described.17 Recovery was 10–15 × 106 cells per animal, of which > 98% were macrophages, as assessed by Giemsa stain. Once washed and resuspended to 106 viable alveolar macrophages/ml, for functional assays cells were allowed to adhere to plastic plates in RPMI 1640 (Sigma-Aldrich Company Ltd) containing 2 mm l-glutamine, 0·1 mg/ml streptomycin, 100 IU/ml penicillin, and 50 ng/ml gentamicin (media) for 1 hr at 37° in 5% CO2. For IL-10 release, 0·3–0·5 × 106 cells were plated in 24-well plates, while for Western blot analysis 4–5 × 106 cells were used. Cells were then exposed to medium with 10% fetal calf serum (Sigma-Aldrich Company Ltd) and incubated with or without lipopolysaccharide (LPS) for 24 hr.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
For determination of IL-10 mRNA levels, semiquantitative RT-PCR was performed as previously described.18 Briefly, 4 × 106 alveolar macrophages were lysed in 1 ml of TriazolTM (Invitrogen, Carlsbad, CA). Total RNA was extracted following the supplier's instructions. PCR primers for IL-10 and β-actin were synthesized by Primm (Milan, Italy) and contained the following sequences:
IL-10 sense: 5′-ACCTGGTAGAAGTGAT-3′
IL-10 antisense: 5′-GGAGAGGTACAAACG-3′
β-actin sense: 5′-CTCTTTGATGTCACGCACGATTTC-3′
β-actin antisense: 5′-GTGGGCCGCTCTAGGCACCAA-3′
The amplified PCR products are 380 bp for IL-10 and 540 bp for β-actin. In preliminary experiments, RNA concentrations and PCR cycles were titrated to establish curves to document linearity and to permit semiquantitative analysis of signal strength (200 ng for IL-10 and 80 ng for β-actin). The PCR was conducted using a Perkin Elmer (Monza, Italy) thermocycler, with the following conditions: 28 cycles of denaturation at 95° for 30 s, annealing at 55° for 30 s and extension at 72° for 30 s, followed by a final 7-min extension at 72°. Gels were photographed with type 55 film (Polaroid, Cambridge, MA).
Western blot analysis
For protein kinase A (PKA), CREB, P-CREB and β-actin, ∼4–10 × 106 cells were lysed in homogenation buffer [50 mm TRIS, 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid (EDTA), pH 7·5, 0·5% Triton X-100, 50 µm phenylmethylsulphonyl fluoride (PMSF), 2 µg/ml aprotinin, 1 µg/ml pepstatin and 1 µg/ml leupeptin] and denatured for 10 min at 100°.19 The protein content of the cell lysate was measured using a commercial kit (Bio-Rad, Hercules, CA). Cell proteins (10 µg) were electrophoresed into a 12% SDS-polyacrylamide gel under reducing conditions. The proteins were then transferred to polyvinylidene difluoride (PVDF) membrane (Amersham). The different proteins were visualized using a PKA antiserum (1 : 200), CREB (1:2500), P-CREB (1:5000) and β-actin (1 : 5000) as the primary antibodies and developed using enhanced chemiluminescence (ECL; Amersham). The image of the immunoblotting was acquired with a Nikon CCD video camera module (Nikon, Torino, Italy). The optical density of the bands was calculated and analysed using the Image 1·47 program for digital image processing (Wayne Rasband, Research Service Branch, NIMH, NIH, Bethesda, MD).
Intracellular cAMP measurement
Intracellular cAMP concentrations were evaluated in cell homogenate using a colorimetric commercial enzymatic immune assay (EZA) (Amersham) following the manufacturer's instructions. A total of 2 × 106 alveolar macrophages were used. The limit of detection was 12·5 fmol. Results are expressed in pmol/mg protein. The protein content of the cell lysate was measured using a commercial kit (Bio-Rad).
Statistical evaluation
All experiments were performed at least twice; representative results are shown. Statistical analysis was performed using GraphPad InStat version 3·0a for Macintosh (GraphPad Software, San Diego, CA). The results were analysed by one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test for multiple comparison. Effects were designated significant if P-values were ≤ 0·05.
Results
The development of carrageenan-induced pleurisy is enhanced in old rats
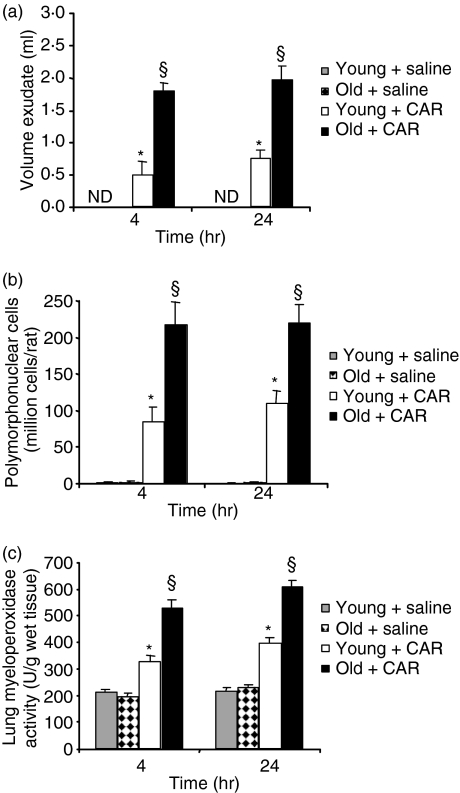
All rats that received carrageenan developed an acute pleurisy, as assessed by pleural exudate and histological examination (Figs 1 and 2). Four and 24 hr post-intrapleural injection of carrageenan, the amplitude of the inflammatory process was higher in the pleural cavity and the lung parenchyma of old rats than in those of young rats. When compared with the volume of exudate or with the number of cells collected from the pleural space of young rats, injection of carrageenan in old rats induced a significant increase (P < 0·01) in the exudate volume and in the number of neutrophils (Figs 1a and b). Histological examination of lung sections of old rats treated with carrageenan showed a significant increase in lung injury and in the level of inflammatory cell infiltrate (Fig. 2). No histological alterations (data not shown), inflammatory cell infiltration or exudate formation was found in sham-operated rats.
Figure 1.
Increased carrageenan (CAR)-induced lung inflammation in old rats. (a) Exudate volume. Old rats showed a significant increase in pleural exudate. Responses in vehicle-treated young and old rats were comparable. (b) Leucocyte accumulation. Old rats showed a significant increase in leucocyte migration. (c) Myeloperoxidase (MPO) activity in the lungs of carrageenan rats killed at 4 and 24 hr. MPO activity was significantly increased in the lungs of carrageenan-treated old rats in comparison with young treated animals. Data are mean ± standard error of mean (SEM) for five rats for each group. The Bonferroni multiple comparison test was performed, with *P < 0·01 vs. vehicle-treated rats and §P < 0·05 vs. young animals treated with carrageenan. ND, not detectable.
Figure 2.
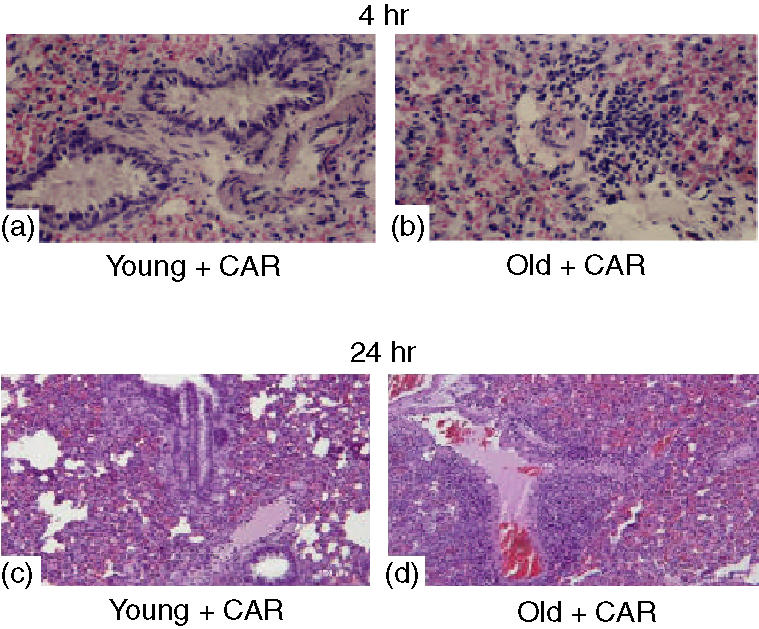
Representative lung sections from carrageenan (CAR)-treated young (a, c) and old (b, d) rats killed at 4 hr (a, b) and 24 hr (c, d) demonstrate increased inflammatory infiltration by neutrophils in old rats. Magnification: × 62.5. The figure is representative of at least two experiments performed on different days.
The histological pattern of lung injury appeared to be correlated with the influx of neutrophils into the lung, as confirmed by measurement of the activity of MPO (Fig. 1c), a biological measurement of neutrophil activation. MPO activity was significantly elevated at 4 and 24 hr after carregeenan administration in both young and old rats. In old rats, however, lung MPO activity was significantly enhanced (P < 0·01) in comparison with young animals.
Increased nitrosative stress and lipid peroxidation in carrageenan-treated old rats
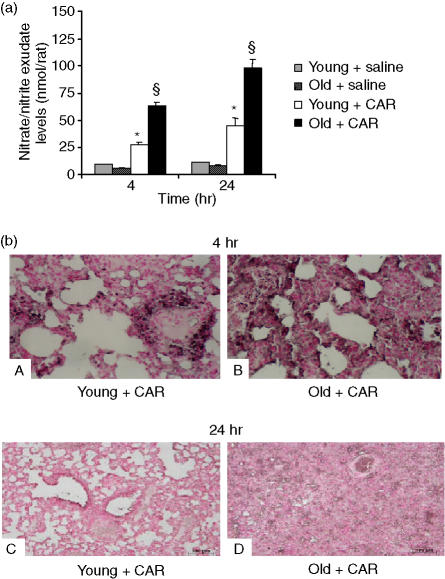
Nitrosative stress and lipid peroxidation were measured as a signature of pleural space and lung damage driven by intrapleural injection of carrageenan. The level of NOx was significantly (P < 0·05) increased in lung exudate from carrageenan-treated rats in comparison with sham-treated rats (Fig. 3a). In old rats, however, the levels of NOx were significantly enhanced (P < 0·05). Immunohistochemical analysis, using a specific antinitrotyrosine antibody, revealed positive staining in lungs (Fig. 3b, parts A and C) from carrageenan-treated young rats. A marked increase in nitrotyrosine staining was found in the lungs (Fig. 3, parts B and D) of carrageenan-treated old rats.
Figure 3.
Increased carrageenan (CAR)-induced lung tissue damage in old rats. (a) Nitrite and nitrate exudate levels. (b) Nitrotyrosine immunohistochemical localization at 4 and 24 hr after carrageenan administration. Nitrite and nitrate exudate levels (a) in carrageenan-treated old rats were significantly increased relative to levels in young treated rats. Data are mean ± standard error of mean (SEM) for five rats for each group. The Bonferroni multiple comparison test was performed, with *P < 0·05 vs. vehicle-treated rats, and §P < 0·05 vs. young animals treated with carrageenan. Representative immunostaining of lung tissue sections obtained from carrageenan-treated young rats with antinitrotyrosine antibody (b, parts A and C) showed intense positive staining in inflammatory cells and around the vessels. Immunostaining for nitrotyrosine (b, parts B and D) was significantly higher in tissue sections obtained from old animals treated with carrageenan. Magnification: × 61.25.
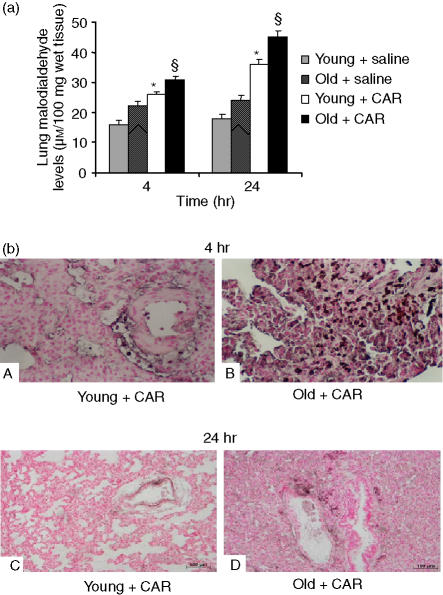
The pulmonary injury in carrageenan-treated animals was also characterized by an increase in the tissue levels of MDA, indicative of lipid peroxidation (Fig. 4a). MDA levels were significantly enhanced in lungs (Fig. 4a) collected from old carrageenan-treated rats when compared to those found in young carrageenan-treated rats. At 4 and 24 hr following carrageenan administration, sections of the lung were also analysed for evidence of PARP activation. Immunohistochemical analysis of lung sections obtained from young carrageenan-treated rats also revealed positive staining for poly ADP ribose (PAR), a product of PARP activation (Fig. 4b, parts A and C). A marked increase in PAR-positive staining was found in the lungs of old carrageenan-treated rats (Fig. 4b, parts B and D). There was no staining for either nitrotyrosine or PAR in lungs obtained from the sham group of rats (data not shown).
Figure 4.
Increased carrageenan (CAR)-induced lung tissue damage in old rats. (a) Malondialdehyde (MDA) levels in the lung and (b) poly ADP ribose (PAR) immunohistochemical localization at 4 and 24 hr after carrageenan administration. MDA levels in the lung (a) in carrageenan-treated old rats were significantly increased relative to young treated rats. Data are mean ± standard error of mean (SEM) for five rats for each group. The Bonferroni multiple comparison test was performed, with *P < 0·05 vs. vehicle-treated rats, and §P < 0·05 vs. young animals treated with carrageenan. Representative immunostaining of lung tissue sections obtained from carrageenan-treated young rats with anti-PAR antibody (b, parts A and C) showed intense positive staining in inflammatory cells and around the vessels. Immunostaining for PAR (b, parts B and D) was significantly higher in tissue sections obtained from old animals treated with carrageenan. Magnification: × 62.5.
Cytokine production
To further characterize the increased carrageenan-induced acute lung inflammation in old rats, the proinflammatory cytokine TNF-α, the C-C chemokine MCP-1 and the anti-inflammatory cytokine IL-10 were measured in the lung exudates. The levels of all three cytokines were significantly increased (P < 0·01) in the exudates from carrageenan-treated young and old rats in comparison to sham-operated rats (Table 1). In old rats, however, significant decreases (P < 0·05) in the levels of TNF-α and IL-10, in comparison to values obtained in treated young rats, were detected, while age did not affect the increase in MCP-1. A decrease in all measured cytokines was observed 24 hr following carrageenan administration in both young and old rats. Because of the pronounced anti-inflammatory impact on neutrophils and monocytes, the decreased IL-10 production in old rats, rather than increased chemotaxis, is likely to contribute to persistent inflammation following carrageenan administration.
Table 1. Cytokine content in the pleural cavity 4 hr after intrapleural injection of carrageenan.
| Treatment | IL-10 (pg/ml) | TNF-α (pg/ml) | MCP-1 (ng/ml) |
|---|---|---|---|
| 4 hr | |||
| Vehicle-treated young rats | 37·5 ± 22·1 | 1·2 ± 0·1 | 0·3 ± 0·1 |
| Carrageenan-treated young rats | 388·1 ± 31·2** | 3093·1 ± 356·2** | 329·1 ± 34·3** |
| Vehicle-treated old rats | 75·2 ± 19·3 | 1·1 ± 0·2 | 0·5 ± 0·1 |
| Carrageenan-treated old rats | 116·5 ± 11·6**§ | 1745·2 ± 251·1**§ | 304·1 ± 9·6** |
| 24 hr | |||
| Vehicle-treated young rats | 10·0 ± 7·0 | 2·0 ± 0·5 | 0·2 ± 0·1 |
| Carrageenan-treated young rats | 135·7 ± 24·2* | 5·3 ± 1·0* | 27·0 ± 2·7* |
| Vehicle-treated old rats | 12·0 ± 10·1 | 1·9 ± 0·6 | 0·2 ± 0·1 |
| Carrageenan-treated old rats | 41·9 ± 24·2§ | 2·3 ± 0·5§ | 21·0 ± 4·7* |
IL-10, interleukin-10; TNF-α, tumour necrosis factor-α; MCP-1, monocyte chemotactic protein-1.
IL-10 and TNF-α exudate levels in carrageenan-treated old rats were significantly reduced compared with the young group at both time points. Data are mean ± standard error of mean (SEM) for five rats for each group. The Bonferroni multiple comparison test was performed, with
P < 0·01
P < 0·05 vs. vehicle-treated rats
P < 0·05 vs. young animals treated with carrageenan.
The cAMP signal transduction pathway is compromised in old rats
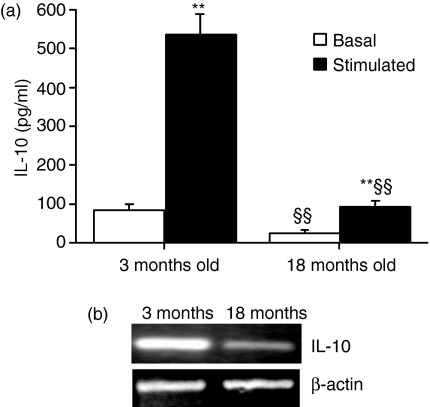
To understand at the molecular level the reduced IL-10 production observed in old rats, IL-10 production was investigated in alveolar macrophages obtained by bronchoalveolar lavage from young (3-month-old) and old (> 18-month-old) rats. As shown in Fig. 5 (a), both basal and LPS-induced IL-10 releases were reduced in old rats, consistent with data obtained in vivo following carrageenan administration. Reduced LPS-induced TNF-α release and equivalent MCP-1 release were also observed in alveolar macrophages (Table 2), reflecting in vivo data.
Figure 5.
Diminished interleukin-10 (IL-10) production in alveolar macrophages obtained from untreated old rats. (a) Alveolar macrophages were treated for 24 hr in the absence (basal) or presence (stimulated) of lipopolysaccharide (100 ng/ml). IL-10 production in conditioned media was evaluated by enzyme-linked immunosorbent assay (ELISA). Data are mean ± standard deviation (SD) of four or five independent samples. **P < 0·01 vs. basal release; §§P < 0·01 vs. alveolar macrophages obtained from young animals. (b) Basal expression of IL-10 mRNA was reduced in alveolar macrophages obtained from old rats. The figure shows results of representative semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) for basal IL-10 mRNA expression in alveolar macrophages obtained from young (3-month-old) and old (> 18-month-old) rats. β-actin expression was used as a control.
Table 2. Alveolar macrophages recovered from old rats synthesize less TNF-α than those recovered from young rats.
| Treatment | TNF-α (pg/ml) | MCP-1 (ng/ml) |
|---|---|---|
| Basal release from young rats | 526 ± 153 | 1·5 ± 0·2 |
| LPS-treated release from young rats | 30542 ± 4661** | 4·3 ± 0·1** |
| Basal release from old rats | 325 ± 49 | 1·6 ± 0·2 |
| LPS-treated release from old rats | 9094 ± 2430**§§ | 4·5 ± 1·0** |
TNF-α, tumour necrosis factor-α; MCP-1, monocyte chemotactic protein-1; LPS, lipopolysaccharide.
TNF-α produced by alveolar macrophages obtained from old rats was significantly reduced compared with alveolar macrophages obtained from young rats. Alveolar macrophages were treated for 24 hr in the absence (basal) or presence of LPS (100 ng/ml). Cytokine levels in conditioned media were assessed by commercially available enzyme-linked immunosorbent assay (ELISA) (MCP-1) and by specific bioassay (TNF-α). Data are mean ± standard error of mean (SEM) for four independent samples. The Bonferroni multiple comparison test was performed, with
P < 0·01 vs. basal release from alveolar macrophages and
P < 0·01 vs. alveolar macrophages obtained from young animals.
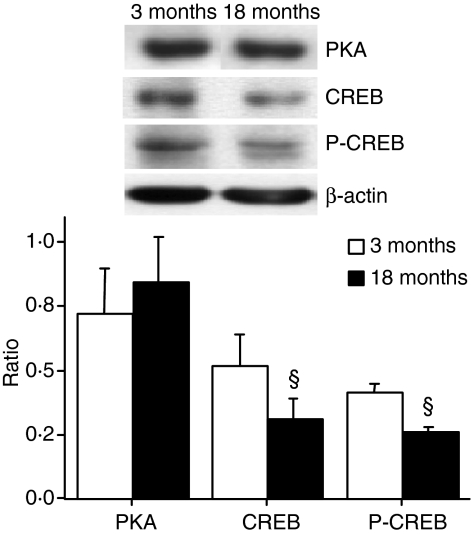
The reduced IL-10 production in old rats was likely to be a result of a transcriptional defect, as shown by semiquantitative RT-PCR analysis (see Fig. 5b), where mRNA for IL-10 was reduced (71 ± 5%) in alveolar macrophages obtained from old rats in comparison to young rats. The critical role of cAMP in the production of IL-10 prompted us to investigate PKA expression, intracellular cAMP levels and CREB expression in alveolar macrophages obtained from young and old rats. As shown in Fig. 6, no change in PKA immunoreactivity was observed in cell homogenates obtained from young and old rats or in intracellular cAMP level (11·2 ± 3·9 and 13·3 ± 4·4 pmol/mg protein in young and old rats, respectively). In contrast, a significant reduction (P < 0·05) in CREB and P-CREB immunoreactivity was found in old rats, which could account for reduced IL-10 transcription. Figure 6 shows a representative Western blot for PKA, CREB and P-CREB and the relative densitometric analysis. β-actin was used to control for protein loading and to normalize protein expression.
Figure 6.
Diminished cAMP-responsive element binding protein (CREB) and phosphorous (P)-CREB expression in alveolar macrophages obtained from untreated old rats. Representative Western blot analysis of protein kinase A (PKA), CREB and P-CREB immunoreactivity in alveolar macrophages and relative densitometric analysis are shown. β-actin was used to control for protein loading and to normalize results (ratio). Data are mean ± standard deviation (SD) of four or five independent samples. §P < 0·05 vs. young animals.
Discussion
Understanding of the molecular mechanisms underlying the alteration in immune system functionality with ageing is crucial in defining the progression of infectious diseases in the elderly, and will allow the development of better strategies for prevention and treatment of such diseases. In particular, increased understanding of how elderly individuals deal with acute inflammatory stimuli is likely to facilitate the design of therapeutic strategies that retain the beneficial aspects of the inflammatory response, while avoiding unnecessary tissue damage. The resolution of pulmonary inflammation involves a finely orchestrated balancing act of proinflammatory and anti-inflammatory cytokines. Here, we demonstrated that age is one of the key factors influencing susceptibility to lung inflammatory stimuli and subsequent tissue damage. The reduced IL-10 production observed in old carrageenan-treated rats is likely to be one of the factors contributing to the persistent inflammatory cellular infiltrate and subsequent increased lung damage, as assessed by increased nitrosative stress and lipid peroxidation.
Perturbation of the host inflammatory cycle can have deleterious effects, causing a systemic inflammatory response (sepsis) that can lead to multiorgan failure and death. In elderly individuals with pneumonia, a common problem is slow recovery and delayed resolution of radiographic infiltrates.20 Radiographic clearance of pneumonia decreases by 20% per decade after the age of 20 years. Decreased IL-10 production can impact negatively on the resolution of inflammation, resulting in increased parenchymal lung damage and dysfunction.
The following scenario may be postulated. On initially encountering carrageenan, resident macrophages become activated and secrete proinflammatory mediators, resulting in recruitment of neutrophils. This is followed in young rats by a second wave of antinflammatory cytokine (i.e. IL-10) production to localize the inflammatory response to within the lung microenvironment and to downmodulate this response. In old rats, the decreased IL-10 production results in persistent neutrophil infiltrate, as assessed by measurement of levels of the specific granulocyte enzyme MPO, and in more severe tissue damage, as evaluated by histological examination and measurement of nitrotyrosine formation.
Cytokine dysregulation is likely to compromise lung functions in elderly individuals, impairing the ability to cope with pulmonary insults and infections. The decrease in TNF-α production in old rats is consistent with the results of our previous study,21 where we demonstrated that an age-associated decline in alveolar macrophage TNF-α production reflects an impaired protein kinase C (PKC) signal-transduction pathway, and in particular is correlated with a defective PKC-anchoring system. Decreased TNF-α production can compromise the ability to develop a protective response to bacterial and viral patogens.22,23 In contrast, similar chemokine production was found in young and old rats, suggesting that the ability to recall inflammatory cells is preserved, while the decreased IL-10 production could account for persistent inflammation. The latter could also explain the low-grade inflammation in the respiratory tract observed in elderly individuals.24,25
Overall, the present data are consistent with the enhanced acute lung injury we previously observed in IL-10 knock-out mice following treatment with carrageenan.6 Similarly, pre-treatment of wild-type mice with an antibody against IL-10 also resulted in enhanced inflammatory response caused by subsequent injection of carrageenan,6 supporting the view that endogenous IL-10 contributes to the regulation of the inflammatory response in the model of carrageenan-induced pleurisy.
Our data indicate that critical mediators involved in the regulation of inflammation are defective in the old rats, making them more sensitive to lung oedema and inflammation. At the molecular level, this defective IL-10 production can be explained by a defect in the cAMP signal-transduction pathway and, in particular, by decreased CREB expression. The transcription factor CREB, which is phosphorylated by cAMP-dependent kinase (PKA) via an increase in cAMP, regulates gene transcription by binding to the cAMP responsive element on target genes (i.e. IL-10). In alveolar macrophages obtained from old rats, we demonstrated that ageing was associated with decreased expression of CREB and P-CREB, while PKA expression and cAMP level were similar in young and old rats. Decreases in CREB activity and immunoreactivity with ageing are not limited to immune cells, but have also been described in the brain26,27 and in senescent fibroblasts.28 Decreased CREB expression with advancing age may result in alteration of gene expression, contributing to immunosenescence and age-related neurodegenerative diseases and depression.
Considering the demographic trend of the world population towards an increased incidence of ageing-related conditions, we believe that this study further contributes to our understanding of immunosenescence and its consequences in terms of age-dependent susceptibility to lung complications.
Acknowledgments
This work was partially supported by FIRST funds from the University of Milan and by the Center for Risk Assessment of the University of Milan, Milan, Italy.
Abbreviations
- MCP-1
monocyte chemotactic protein-1
- MDA
malondialdehyde
- MPO
myeloperoxidase
- PAR
poly ADP ribose
- PARP
poly ADP ribose polymerase
- PKA
protein kinase A
- ROS
reactive oxygen species
References
- 1.Lentsch AB, Ward PA. Regulation of experimental lung inflammation. Respir Physiol. 2001;128:17–21. doi: 10.1016/s0034-5687(01)00260-2. [DOI] [PubMed] [Google Scholar]
- 2.Nantel F, Denis D, Gordon R, Northey A, Cirino M, Metters KM, Chan CC. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol. 1999;4:853–9. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–20. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 4.Haddad J, Fahlman CS. Redox- and oxidant-mediated regulation of interlukin-10: an anti-inflammatory, antioxidant cytokine? Biochem Biophys Res Comm. 2002;297:163–76. doi: 10.1016/s0006-291x(02)02094-6. [DOI] [PubMed] [Google Scholar]
- 5.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–21. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 6.Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Di Paola R, Genovese T, De Sarro A, Caputi AP. Absence of endogenous interleukin-10 enhances the evolution of acute lung injury. Eur Cytokine Netw. 2002;13:285–97. [PubMed] [Google Scholar]
- 7.Emmerling P, Hof H, Finger H. Age-related defense against infection with intracellular pathogens. Gerontology. 1979;25:327–36. doi: 10.1159/000212361. [DOI] [PubMed] [Google Scholar]
- 8.Meyer KC. The role of immunity in the susceptibility to respiratory infection in aging lung. Respir Physiol. 2001;128:23–31. doi: 10.1016/S0034-5687(01)00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, Diaz-Mitoma F, Kumar A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–74. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 10.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–7. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 11.Brenner S, Prosch S, Schenke-Layland K, Riese U, Gausmann U, Platzer C. cAMP-induced interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J Biol Chem. 2003;278:5597–604. doi: 10.1074/jbc.M207448200. [DOI] [PubMed] [Google Scholar]
- 12.Cuzzocrea S, Sautebin L, De Sarro G, et al. Role of IL-6 in the pleurisity and lung injury caused by carrageenan. J Immunol. 1999;163:5094–104. [PubMed] [Google Scholar]
- 13.Rosenthal GJ, Corsini E. Tumor necrosis factor-α in immunotoxicity assessment. In: Burleson GR, Dean JH, Munson AE, editors. Methods in Immunotoxicology. Vol. 1. New York: Wiley-Liss, Inc.; 1995. pp. 327–43. [Google Scholar]
- 14.Cuzzocrea S, Rossi A, Serraino I, et al. 5-lipoxygenase knockout mice exhibit a resistance to pleurisy and lung injury caused by carrageenan. J Leukoc Biol. 2003;73:739–46. doi: 10.1189/jlb.1002477. [DOI] [PubMed] [Google Scholar]
- 15.Laight DW, Lad N, Woodward B, Waterfall JF. Assessment of myeloperossidase activity in renal tissue after ischemia-reperfusion. Eur J Pharmacol. 1994;292:81–8. doi: 10.1016/0926-6917(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Corsini E, Craig WA, Rosenthal GJ. Modulation of tumor necrosis factor release from alveolar macrophages treated with pentamidine isethionate. Int J Immunopharmacol. 1992;14:121–30. doi: 10.1016/0192-0561(92)90022-d. [DOI] [PubMed] [Google Scholar]
- 18.Corsini E, Limiroli E, Marinovich M, Cohen C, Roguet R, Galli CL. Selective induction of interleukin 12 in reconstituted human epidermis by chemical allergens. Altern Lab Anim. 1999;27:261–9. doi: 10.1177/026119299902700205. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli VK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Cassiere HA, Niederman MS. Community-acquired pneumonia. Dis Mon. 1998;44:613–75. doi: 10.1016/s0011-5029(98)90012-8. [DOI] [PubMed] [Google Scholar]
- 21.Corsini E, Battaini F, Lucchi L, Marinovich M, Racchi M, Govoni S, Galli CL. A defective protein kinase C anchoring system underlying age-associated impairment in TNF-α production in rat macrophages. J Immunol. 1999;163:3468–73. [PubMed] [Google Scholar]
- 22.Flynn JL, Glodstein MM, Chan J, et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 23.Paul NL, Ruddle NH. Lymphotoxin. Ann Rev Immunol. 1988;6:407–38. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- 24.Thompson AB, Scholer SG, Daughton DM, Potter JF, Rennard SI. Altered epithelial lining fluid parameters in old normal individuals. J Gerontol. 1992;47:M171–6. doi: 10.1093/geronj/47.5.m171. [DOI] [PubMed] [Google Scholar]
- 25.Meyer KC, Ershler W, Rosenthal AN, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Resp Crit Care Med. 1996;153:1072–9. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto A. Age-dependent changes in phosphorylated cAMP response element-binding protein immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernous of male rats. Neurosc Lett. 2000;279:117–20. doi: 10.1016/s0304-3940(99)00965-9. [DOI] [PubMed] [Google Scholar]
- 27.Chung YH, Kim EJ, Shin CM, Joo KM, Kim MJ, Woo HW, Cha CI. Age-related changes in CREB binding protein immunoreactivity in the cerebral cortex and hippocampus of rats. Brain Res. 2002;956:312–8. doi: 10.1016/s0006-8993(02)03562-x. [DOI] [PubMed] [Google Scholar]
- 28.Chin JH, Okazaki M, Fraizer JS, Hu ZW, Hoffman BB. Impaired cAMP-mediated gene expression and decreased cAMP response element binding protein in senescent cells. Am J Physiol. 1996;271:C362–71. doi: 10.1152/ajpcell.1996.271.1.C362. [DOI] [PubMed] [Google Scholar]