Abstract
Abdominal aortic aneurysms (AAA) exhibit features of a chronic inflammatory disorder. The functional attributes of the T cells in AAA tissue are unclear, with little quantitative or functional data. Using a novel, non-enzymatic method to isolate viable cells from AAA tissue, functional properties of AAA T cells were investigated for the first time. Composition and phenotype of AAA T cells was determined by flow cytometry and verified by immunohistochemistry. Tissue mononuclear cells (MNCs) were cultured in the presence of T-cell mitogens, and cell cycle analysis and cytokine production assessed. Typical cell yield was 4·5 × 106 cells per gram of AAA tissue. The majority (58·1 ± 5·3%) of haematopoietic (CD45+) cells recovered were CD3+ T cells, B cells comprised 41·1 ± 5·7%, natural killer cells 7·3 ± 2·5%, and macrophages 2%. Freshly isolated T cells were in resting (G1) state, with 25% expressing the activation-associated cell surface antigens major histocompatibility complex II and CD25. When stimulated in vitro, a significant proportion entered S and G2 phase of the cell cycle, up-regulated CD25, and secreted tumour necrosis factor-α, interferon-γ, interleukin (IL)-5 and IL-6. Despite patient differences, the composition of the AAA inflammatory infiltrate was remarkably consistent, and when re-stimulated ex-vivo T cells produced a stereotypical cytokine response, consistent with the hypothesis that AAA T cells can promote tissue inflammation by secretion of proinflammatory cytokines, and in addition provide signals for B-cell help.
Keywords: T cells, abdominal aortic aneurysms
Introduction
Abdominal aortic aneurysms (AAA) exhibit many features of a chronic inflammatory disease, characterized by vascular inflammation and leucocyte infiltration throughout the media and adventitia of the vessel wall.1,2 Inflammation is associated with the breakdown of elastin structure, loss of well-organized smooth muscle cell layers and an increase in the extracellular matrix, causing vessel wall weakness and dilatation that can result in vessel wall rupture and death.
Following the early descriptions of the cellular infiltrate in AAA by Koch3 recent studies of the inflammatory cell infiltrate in AAA using immunohistochemistry (IHC) have provided more detailed descriptions of the cellular components of the infiltrate. The majority of the cells present are T cells and macrophages, with cells such as natural killer (NK) and B cells present in low amounts.1 Double immunostaining of tissue sections has shown the presence of apoptotic macrophages and smooth muscle cells, and it has been suggested that T cells may be responsible for inducing this cell death. The major limitation of this type of analysis, however, is that at best it provides a description of the type and location of cells present within AAA tissue, but is not truly quantitative nor does it enable functional analysis of the cellular infiltrate. It is still unclear what proportion of the cellular infiltrate are T cells, whether this varies widely between patients and what role T cells play in the development of aneurysmal disease.
The presence of memory (CD45RO+) T cells in AAA lesions has focused research into the role of the adaptive immune system in the generation of aneurysmal disease, and raises the question of which antigen(s) the T cells are responding to. Halme et al.4 demonstrated that T-cell lines generated from AAA tissue could proliferate in response to chlamydial antigens. However, this response only occurred in around one-third of the T-cell lines generated, and the cells had been cultured in the presence of interleukin (IL)-2 for a prolonged period, potentially modifying their in vivo properties. Analysis of cytokine production by the cellular infiltrate in AAA disease has been assessed in an attempt to determine the type of T-cell response occurring within the tissue (T helper 1 (Th1) versus Th2 profile), as this may give some insight into the potential triggers of the immune response. As such, studies of whole tissue biopsies using Western blotting has demonstrated the presence of the Th2 cytokines, IL-4, IL-5 and IL-10 in AAA tissue5 whereas analysis of AAA tissue explant culture media by enzyme-linked immunosorbent assay (ELISA) demonstrated production of Th1 cytokines such as interferon-γ (IFN-γ) and IL-66 and tumour necrosis factor-α (TNF-α).7 However, many of the cell types within the aneurysm wall are capable of producing cytokines, making whole tissue analysis less informative when attempting to understand the individual functional roles of T cells within the inflammatory infiltrate, or the stimuli that initially recruited these cells to the tissue.
Further understanding of the pathogenesis of AAA is hampered by a paucity of in vitro models to study the functional attributes of the cellular components within AAA tissue. Previous attempts at functional analysis of the cellular components in AAA tissue have utilized prolonged tissue culture, or the use of collagenases to extract the cellular infiltrate from the vessel wall. Such processing may decrease cell viability and alter cell function. As such, our understanding of how the inflammation in AAA is initiated and sustained is limited.
The availability of a procedure capable of reproducibly isolating viable cells from AAA tissue, which maintain their in vivo properties and functional phenotype would be of great value in elucidating the role different populations of inflammatory cells play in the pathogenesis of AAA. Using a mechanical method to extract the haematopoietic cell infiltrate in AAA tissue, we have characterized T-cell function from AAA tissue in vitro, immediately following isolation. Our results show that the cellular composition of aneurysm tissue is comparable between patients, composed predominately of T and B lymphocytes. Stimulation of T lymphocytes in vitro leads to production of cytokines, which could contribute to the pathology of AAA.
Materials and methods
Patients and samples
Patients with ‘atherosclerotic’ (that is, non-inflammatory) AAA awaiting open surgical repair were used in this study. Inflammatory or rapidly expanding aneurysms were excluded. Inflammatory AAA were defined by their clinical presentation, biochemical and haematological profile and appearance on computerized tomography scan. Age-matched patients with no evidence of vascular disease were used as healthy controls. The majority of these patients were either undergoing surgery for uncomplicated varicose veins, or hernia repair, and as such had undergone abdominal ultrasound scanning as part of their previous investigations. AAA patient demographics are shown in Table 1. Informed consent was obtained from all patients and local ethical committee approval was given. No patients or controls were receiving any immune modulating therapy. From all AAA patients, preoperative peripheral blood samples, and a section of the anterior aortic wall was sampled immediately after clamping the infrarenal abdominal aorta and common iliac arteries, and placed in cold ‘transport buffer’8 prior to processing. Mononuclear cells (MNCs) were isolated from the vessel wall within 1 hr of cross-clamping the aorta. Samples of peripheral blood were taken from all control subjects.
Table 1. AAA and control patient demographics.
| Parameter | AAA | Control |
|---|---|---|
| Number | 23 | 19 |
| Median age, years (range) | 78 (61–85) | 68 (49–93) |
| Male sex, n (%) | 21 (91) | 12 (63) |
| Median AAA diam, cm (range) | 6·5 (4·9–7·8) | |
| Mean lymphocyte no × 106/ml (±95% CI) | 1·69 (±0·28) | 1·97 (±0·4) |
| Current or ex smoker, n (%) | 21 (91)* | 7 (39) |
| Hypertensive, n (%) | 10 (44) | 4 (22) |
| Diabetes, n (%) | 1 (4) | 1 (6) |
| Previous tumour (Ca bladder) | 2 (9) | 0 (0) |
P = 0·001, Mann–Whitney, compared to controls.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficol-Isopaque (‘Lymphoprep’, Axis-Shield, Oslo, Norway). For the isolation of tissue MNCs, sections of aorta was cleaned and excess connective tissue removed. Thrombus was removed by gentle agitation of the samples in phosphate-buffered saline (PBS). Tissue was then diced using a 35 µm tissue homogenizer (Medimachine, Dako, High Wycombe, UK), filtered through a 70 µm filter (Becton Dickinson, Oxford, UK), washed with PBS and MNCs isolated using density gradient centrifugation as described for PBMCs. The viability of isolated tissue MNCs as assessed by trypan blue dye exclusion was routinely greater than 90%.
Antibody staining and flow cytometry
PBMCs and tissue MNCs were suspended in staining buffer (PBS, 0·1% (v/v) sodium azide (Sigma), 10 µg/ml mouse immunoglobulin G (IgG; Sigma, Poole, UK) and 1% (v/v) fetal calf serum (FCS, LabTech International Ltd, Ringmer, UK)) and incubated at 4° for 20 min with saturating amounts of commercial fluorochrome-conjugated monoclonal antibodies (Caltag; Medsystems Ltd, Towcester, UK; Dako, Cambridgeshire, UK; and Becton Dickinson, San Jose, CA); anti-CD3 (clone S4.1), CD56 (C5.9), CD4 (S3.5), CD8 (DK25), T-cell receptor (TCR)αβ (BMA031), TCRγδ (B1), CD19 (HD37), CD23 (M-L233), CD25 (ACT-1), CD27 (M-T271), CD64 (10.1), CD86 (2331 (FUN-1)), HLA-DP, DQ, DR (CR3/43) and CD45 (HI30). Cells were washed and analysed on a FACS Calibur flow cytometer, using Cell Quest Software (Becton Dickinson). Electronic gating of CD45+ haematopoietic cells was used to identify leucocytes and exclude tissue debris, and 5000 electronic events within the CD45+ lymphocyte gate were acquired for analysis.
Assessment of T-cell function
Intracellular staining and flow cytometry was used to identify and quantitate IFN-γ- and TNF-α-producing tissue MNC directly ex vivo. Freshly isolated tissue MNCs (1 × 106/ml) obtained from AAA biopsies were cultured with phorbol 12-myristate 13-acetate (PMA, 20 ng/ml), ionomycin (1 µm) and brefeldin A (Sigma, 10 µg/ml) for 5 hr. Cells were then stained with saturating amounts of fluorochrome-conjugated anti-CD3 antibodies, fixed in PBS with 1% paraformaldehyde and 1% FCS, prior to permeabilization using PBS-S (PBS, 1% FCS, 0·1% saponin, 1 mm calcium chloride, 1 mm magnesium sulphate, 10 mm HEPES, 0·05% sodium azide) containing 5% milk protein to block non-specific antibody binding. Following permeabilization, cells were stained with fluoroscein isothiocyanate (FITC) labelled-anti-IFN-γ (4S.B3), -anti-TNF-α (Mab11), -anti-IL-5 (JES1-39D10) -anti-IL-4 (MP4-25C2, all Becton Dickinson) or an isotype-control antibody for 30 min at 20°. Cells were then washed and resuspended in staining buffer and immediately analysed. Alternatively, cytokine secretion by tissue-derived T cells was assessed following culture of freshly isolated tissue MNCs (2 × 106/ml) with anti-CD3 (UCHT1, 1 µg/ml) alone, or in combination with anti-CD28 (MOPC-21, 1 µg/ml, both Becton Dickinson) for 48 hr. Supernatants were harvested and assayed for the presence of IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α and IFN-γ using a cytokine bead array kit (CBA, Dako) and flow cytometry. The CBA kit was also used to assess the presence of cytokines in serum of AAA patients prior to and following AAA repair, and healthy aged matched control subjects. For cell cycle analysis and the expression of activation associated marker CD25 following culture, cells were stained for surface markers CD3 and CD19, permeabilized with PBS-S and incubated with propidium iodide (PI, 50 µl/ml) at 20° for 20 min, or surface stained for CD25 in combination with CD3 and CD19. Flow cytometric analysis was then performed immediately.
Immunohistochemistry
Sections of normal human aorta (trauma victims age >40, Peterborough Tissue Bank, Peterborough, UK), and aorta from AAA patients undergoing surgical repair of aneurysm were fixed for 24 hr in 10% buffered formalin (Sigma) and paraffin embedded. Sections were deparaffinized, rehydrated and incubated with 3% H2O2 (Sigma) to quench endogenous peroxidase activity. Autoclaving the paraffin embedded sections for 30 s to unmask cell antigens was performed, to optimize immunohistochemical staining for each antigen. An antigen-unmasking step was performed by autoclaving for 30 seconds. Endogenous biotin was then blocked using an avidin–biotin reagent (Dako) and non-specific antibody binding prevented using appropriate normal animal serum (Dako). Sections were incubated with the primary antibodies (CD45, CD3, CD20, CD68, HLA-DR, DP, DQ, bcl-2, bcl-6, mib-1, CD79α (Dako), CD138 (Serotec, Oxford, UK), CD10 and CD21 (Novocastra; supplied by Vision Biosystems, Newcastle-upon-Tyne, UK) for 1 hr at 20°, washed, and then incubated with biotin-conjugated anti-mouse IgG antibody for 30 min at room temperature. Antibody labelling was visualized using streptavidin–horseradish peroxidase (Dako), followed by incubation with diaminobenzamine (Sigma). Sections were analysed using AxioVision software and Zeiss microscope.
Statistics
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software. All results are shown as the mean value and 95% confidence interval unless otherwise stated.
Results
The presence, phenotype and function of T-cells in aortic tissue of AAA patients was investigated using immunohistochemistry and flow cytometry. AAA patients PBMCs were assessed in parallel with tissue MNCs. Control patients were healthy outpatients awaiting hernia or varicose vein repair. As the majority of the control group were over 65, the risk of subsequent aneurysm formation is extremely low.9 Twenty-three patients with atherosclerotic AAA were studied. As expected, the group was predominately composed of men, with a median age of 78, the majority being ex or current smokers. Two patients had previously had carcinoma of the bladder resected, were currently well, with no evidence of metastatic disease (Table 1). There were no significant differences between AAA patients and controls with respect to age or gender.
Lymphocyte populations in AAA disease
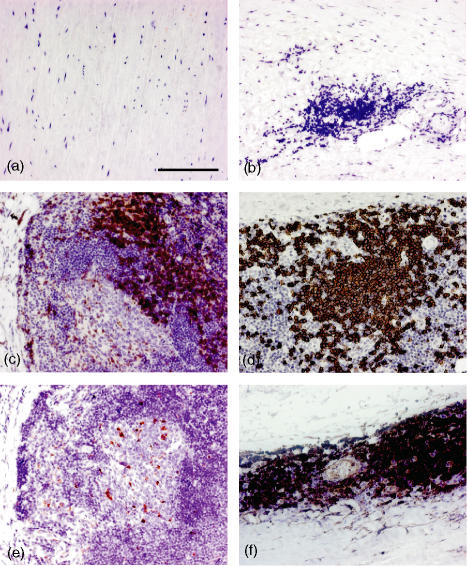
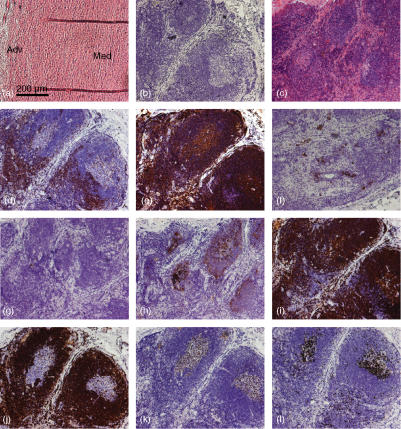
IHC was used to define and localize lymphocyte populations in AAA tissue. Normal, non-aneurysmal aortic tissue was used as a control, which contained no haematopoietic (CD45+) cells (Fig. 1a). By contrast, in aneurysmal aorta, virtually all of the cellular infiltrate was CD45+ (data not shown). The size of the tissue infiltrate varied among different tissue samples, consistent with the variable yield of MNCs from different patients. The inflammatory infiltrate was localized to the media and adventitia of the aortic wall in all patients, although the amount of inflammation present varied from patient to patient. Despite the spectrum of inflammatory change observed between patients, the cellular composition of the infiltrate was similar. The predominant cell types within the infiltrate were CD3+ T-cells (Fig. 1c), and CD20+ B-cells (Fig. 1d). Very few CD68+ macrophages were identified (Fig. 1e) and were mostly found in the more severely inflamed tissue sections, localized to the periphery of the central zone of follicular aggregates formed by inflammatory cells. Structures, resembling lymphoid follicles with a central zone of B-cells and a surrounding rim of T-cells, in the adventitia of one patient are shown in Fig. 2. The central cells within the follicles expressed CD138 (Fig. 2f), a plasma cell marker and CD10 and bcl-6, germinal centre markers (Fig. 2g,k, respectively). Central cells were also found to express mib-1 (ki-67), expressed on all proliferating cells (Fig. 2l). High levels of anti-CD21 staining (Fig. 2h) which is found on mature and follicular mantle B cells, was found in the follicular structures surrounding the germinal centre cells, which also expressed the anti-apoptosis marker bcl-2 (Fig. 2j). The majority of the inflammatory cells also expressed major histocompatibility complex (MHC) II (Fig. 1f).
Figure 1.
Distribution and localization of leucocyte populations in AAA tissue. 5 µm sections of normal aorta (a) and aneurysmal aorta (b–f) were stained with antibodies specific for haematopoietic cells (anti-CD45, a), T cells (anti-CD3, c), B cells (anti-CD20, d), macrophages (anti-CD68, e) and MHC class II (HLA DR,DP,DQ, f). The isotype control is shown in (b). Results shown are representative of those obtained from eight patients. Magnification × 200, scale bar 100 µm.
Figure 2.
Immunohistochemical analysis of lymphoid follicle-like structures in AAA tissue. 5 µm sections of normal aorta (a) and AAA tissue (b–l) were stained with haematoxylin and eosin (a and c), isotype control (b), CD3 (d), CD20 (e), CD138 (f), CD10 (g), CD21 (h), CD79α (i), bcl-2 (j), bcl-6 (k) and mib-1 (l). Normal aortic media (Med) and adventitia (Adv) is shown and the staining shows inflammatory aggregates and germinal centre formation in the adventitia of AAA tissue. Results shown are representative of five patients. Magnification × 100, scale bar 200 µm.
Quantitative analysis of inflammatory infiltrate in AAA
Whilst immunohistochemical analysis of AAA tissue described presence and relationships of cellular components in AAA tissue, comparative quantification of such populations is inaccurate using this technique. Therefore, a mechanical disaggregation method was developed to characterize and quantify MNCs from AAA tissue. The cell yield of tissue varied from patient to patient, averaging 4·5 ± 2·4 × 106 leucocytes per gram of AAA tissue, with over 90% viability, of which over 95% were haematopoietic (CD45+) cells (Table 2). The majority of CD45+ cells were CD3+ T lymphocytes (58·1 ± 5·3% CD3+), of which 84·4 ± 4·9% were TCRαβ+, and 69·7 ± 6·0% CD4+. CD19+ B cells (41·1 ± 5·7%) made up the majority of the remainder of the cellular infiltrate. NK cells (7·3 ± 2·5%) and macrophages (2·1 ± 1·4%) were also present within the inflammatory infiltrate in AAA, but in small proportions. Quantitative analysis of activation-associated markers MHC II and CD25 on tissue MNCs demonstrated that approximately 25–30% of the CD45+ cells in AAA tissue were MHC II+ or CD25+ (Table 2). As expected, the majority of tissue B cells were MHC II+ (76·1 ±23·1%), with the majority (48·4 ± 9·4%) of B cells expressing the memory marker CD27 and costimulatory molecules such as CD86 (Table 2).
Table 2. AAA tissue composition and lymphocyte phenotype.
| Leucocyte recovery and phenotype | % positive cells (Mean ± 95% CI) n = 23 |
|---|---|
| Cells/g tissue | 4·5 × 106 ± 2·4 |
| CD3+ | 58·1 ± 5·3 |
| % CD3+ CD4+ | 69·7 ± 6·0 |
| % CD3+ CD8+ | 29·4 ± 6·0 |
| % CD3+ TCRαβ+ | 84·4 ± 4·9 |
| % CD3+ TCRγδ+ | 16·8 ± 3·7 |
| % CD3+ CD25+ | 28·2 ± 15·9 |
| % CD3+ MHC II+ | 25·0 ± 14·2 |
| CD19+ | 41·1 ± 5·7 |
| % CD19+ MHCII+ | 76·1 ± 23·1 |
| % CD19+ CD23+ | 34·0 ± 10·1 |
| % CD19+ CD27+ | 48·4 ± 9·4 |
| % CD19+ CD86+ | 37·8 ± 10·4 |
| CD56+ | 7·3 ± 2·5 |
| CD64+ | 2·1 ± 1·4 |
Leucocytes were identified for phenotype analysis by electronic gating of CD45+, haematopoietic cells. Values in bold are proportion of CD45 cells positive for each marker, with non-bold values representing the subgroups of cells within each population.
Tissue T cells are functional
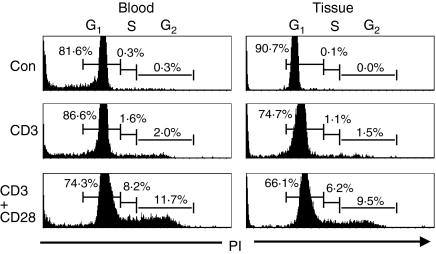
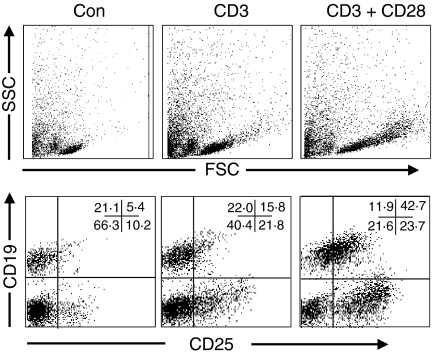
Having established a method to extract viable mononuclear cells from AAA tissue, subsequent functional analysis on individual cell populations was performed. To assess the presence or ability of MNCs to divide, cell cycle analysis was performed on freshly isolated tissue MNCs and PBMCs by PI staining and flow cytometry. As expected from the IHC studies, the majority (80–90%) of freshly isolated PBMCs and tissue MNCs were in the G1, or resting phase of the cell cycle (Fig. 3). Following stimulation in vitro with anti-CD3 and -CD28 antibodies, to mimic stimulation through the TCR/CD3 complex and costimulatory signals for T-cell activation, respectively, a significant proportion of both peripheral blood (19·9%) and tissue T cells (15·7%) progressed to the S and G2, or proliferative phase of the cell cycle (Fig. 3), coincident with increased expression of the activation associated antigen CD25 (Fig. 4), from approximately 25% to 76% positive cells following stimulation. Following anti-CD3 stimulation alone, smaller proportions of cells from both the blood (3·6%) and tissue (2·6%) entered the proliferative phase of the cell cycle, perhaps reflecting the availability of T-cell costimulatory molecules, such as CD28, on cells present in the cocultures, such as B cells.
Figure 3.
Cell cycle analysis of mononuclear cells from the peripheral blood and aortic tissue of patients with AAA. Mononuclear cells were cultured for 48 hr in medium alone (Con), or with anti-CD3 alone (CD3), or with anti-CD3 and CD28 antibodies (CD3+ CD28), prior to surface staining with T- and B-cell antibodies, permeabilization of the cells and intracellular staining with PI, followed by flow cytometric analysis. The percentage values shown on the histograms represent the proportion of cells within each phase of the cell cycle. Results are shown from a single patient, and are representative of four patient samples.
Figure 4.
Activation of tissue-derived T cells following stimulation with anti-CD3 and CD28. AAA tissue mononuclear cells were cultured for 48 hr in medium alone (Con), with anti-CD3 alone (CD3), or with anti-CD3 and -CD28 antibodies (CD3+ CD28), prior to surface staining with T- or B-cell antibodies and anti-CD25. Top panel shows characteristic change in forward (FSC) and side (SSC) scatter profiles on cell stimulation. CD25 expression on B cells (CD19+, right upper quadrant) and T cells (CD19–, right lower quadrant) is shown in the bottom panels, with numbers representing percentage cells positive in each quadrant. Results shown are from a single patient and are representative of four patient samples. Quadrants set using isotype control antibodies.
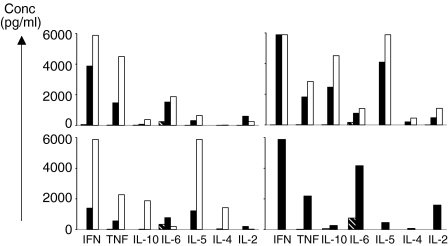
Following stimulation of blood and tissue T cells with anti-CD3 and CD28, culture supernatants were assayed for a variety of inflammatory cytokines (IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α and IFN-γ) using a multiplex bead, flow cytometric assay. In response to TCR/CD3-mediated activation, tissue MNCs cells from all patient samples (n = 5) produced predominantly TNF-α and IFN-γ, with variable amounts of IL-5 and IL-6 (Fig. 5). Secretion of IL-4 and IL-10 by tissue MNCs was not detected. By contrast, PBMCs from AAA patients and healthy controls secreted varying amounts of all cytokines assessed following stimulation (Fig. 5 and data not shown). Systemic serum cytokine levels were evaluated in AAA patients prior to and following AAA repair (n = 10, all groups). We found no difference in the levels of serum IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α and IFN-γ (all less than 10 pg/ml) following AAA repair, and no difference in cytokine levels between patients and healthy controls.
Figure 5.
Cytokine production by T cells from AAA patients. Peripheral blood (open bars) and AAA tissue derived mononuclear cells (solid bars) from AAA patients were cultured for 48 hr in medium alone (hatched bars) or in media containing anti-CD3 and CD28 antibodies, after which supernatants were analysed for the presence of cytokines by cytokine bead array and flow cytometry. The negative controls shown represent the highest control value for blood or tissue samples, value often zero. Each graph represents results from four individual patient samples and are representative of a total of five patients.
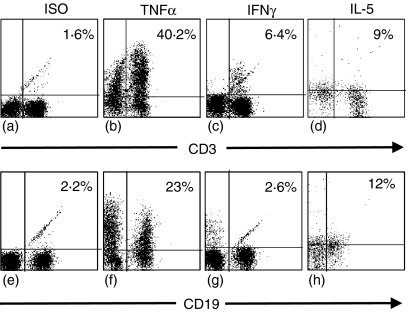
Intracellular staining for IL-4, IL-5, TNF-α and IFN-γ production by tissue MNCs was performed to corroborate flow based cytokine profiles and to identify cellular sources of the major cytokines detected and the contributions of individual cells to total cytokine production. It was not possible to detect any intrinsic cytokine production by the tissue MNCs directly ex vivo. However, following PMA and ionomycin stimulation both T and B cells produced TNF-α and IL-5, whilst only T cells produced IFN-γ(Fig. 6). No IL-4 production was detected. Over 50% of T and B cells present produced TNF-α, with over 60% of the total TNF-α produced coming from T cells. T cells were the sole producers of IFN-γ, with around 10% of T cells producing IFN-γ on stimulation. IL-5 was produced equally by T and B cells following extraction from AAA tissue.
Figure 6.
Intracellular cytokine production by AAA tissue mononuclear cells. T and B cells isolated from AAA tissue were stimulated with PMA and ionomycin in culture for 4 hr, followed by permeabilization and intracellular staining with fluorescein-conjugated antibodies to TNF-α (b and f), IFN-γ (c and g), IL-5 (d and h) or isotype-control antibody (a and e). Values shown are percentage of cells secreting cytokine. Results shown are from two patients and are representative of four patient samples.
Discussion
Previous studies of the cellular infiltrate in aneurysmal disease have relied on immunohistochemical analysis to characterize cellular components in AAA, which have provided a limited amount of information concerning the functional properties of tissue leucocytes. In this study, the phenotypic and functional properties of the inflammatory lymphocyte infiltrate in AAA have been characterized using a novel tissue disaggregation and cell extraction procedure. This study has provided the first direct demonstration that the major cellular component in AAA, T cells, have the potential to produce cytokines such as IL-5, IL-6, TNF-α and IFN-γ, which may influence or contribute to the maintenance of inflammation within the vasculature. As this method does not use any enzymatic treatment of tissue, or prolonged incubation of tissue explants, the subsequent functional studies are, we believe, likely to reflect the phenotype and function of T cells in AAA tissue.
The ability to faithfully recover intact inflammatory cells from AAA tissue as described here has enabled the accurate quantification, by flow cytometry, of the cell types involved in AAA disease. Despite the variation in the amount of inflammation in the aortic tissues of patients with atherosclerotic AAA, the proportions of the cellular components of this infiltrate are similar between patients. Whereas our analysis demonstrates that 50% of the cellular infiltrate in AAA are T cells, other immunohistochemical studies have reported that T cells comprise up to 90% of the infiltrate.2,4 Also, in contrast to previous studies10 we have shown that B cells form a significant proportion of the inflammatory infiltrate in AAA. Such discrepancies may arise from the limitations of immunohistochemical studies. Whilst immunohistochemical analysis is semiquantitative, inaccuracies will arise depending upon which cross sections of the aortic wall have been sampled, the specificity of available antibodies and the preservation of cell antigens during tissue processing. Whilst a recent AAA phenotype study by Ocana et al.11 has produced results similar to our own, our study identifies some important differences in tissue cell phenotype to those of Ocana et al.11 While both studies establish the composition of AAA leucocytes to be predominately T, B and NK cells, our study identifies B cells as comprising a larger proportion of the tissue MNCs (41% compared with 30%). This difference may relate to the greater number of tissue samples processed in our study (n = 23, compared to n = 6), or the more efficient release of lymphocytes from tissue using our mechanical method versus enzymatic digestion of AAA tissue. Interestingly, neither this study, or that of Ocana's group11 found large numbers of macrophages within AAA tissue, despite the widely held belief that macrophages comprise a significant proportion of the inflammatory cells within AAA tissue.1 Our only immunohistochemical section to adequately demonstrate tissue macrophages is shown in Fig. 1(e), and tissue cell extraction and analysis for macrophages in this patient showed that 5% of the tissue MNCs expressed CD64. It is unlikely that this is a result of tissue sampling or processing problems, and probably reflects that fact that only a small proportion of the inflammatory infiltrate in AAA tissue are macrophages, and that cross-sectional sampling using immunohistochemical analysis can be misleading. Of the B cells present, the majority were CD27+ memory cells, although a substantial proportion were naïve CD23+ cells, suggestive of ongoing recruitment and activation of B cells within AAA tissue. These areas may be sites of antigen driven T- and B-cell responses, contributing to the ongoing inflammatory response occurring within the tissue.
We used two different stimuli to demonstrate that tissue infiltrating T and B lymphocytes are capable of producing pro-inflammatory cytokines. Whilst PMA and ionomycin stimulation of MNCs is a pharmacological mitogen and potent T- and B-cell mitogen and should elicit maximal responses, anti-CD3 and -CD28 stimulation of MNCs reflects a more physiological activation of leucocytes by directly cross-linking the TCR and CD28 receptors, respectively. Demonstration of production of cytokines such as TNF-α is consistent with the previous detection of TNF-α in proteins extracted from the AAA walls by ELISA.7 Both T and B cells appear to be the cellular source of TNF-α in AAA tissue. We have also demonstrated that T cells produce IFN-γ, which in animal models of AAA has been shown to be essential for dilatational changes in the aorta.12 Production of IFN-γ by T cells was also detected using intracellular staining, to demonstrate which cells of the AAA inflammatory infiltrate were producing individual cytokines. However, the relatively small amount of IFN-γ produced following a 5-hr stimulation with PMA and ionomycin stimulation, may reflect the fact that cells are already active in vivo, and as such relatively refractory to further stimulation. Our findings that tissue T-cells produce pro-inflammatory (Th1) cytokines contrasts with previous studies suggesting that AAA is predominantly a Th2 disorder5 based on detection of IL-4, IL-5 and IL-10 in lysates from AAA biopsies by Western blotting. However, both T and B cells from AAA tissue were capable of producing IL-5, but not IL-4. These contrasting findings may be explained by production of cytokines by non-T-cell populations in AAA, as a possible attempt to control tissue inflammation, or could reflect sampling at different time points in disease progression. Alternatively, our assessment of cytokine production at 48 hr may have been suboptimal for early cytokines such as IL-4. However, no IL-4 was seen by intracellular staining following a 5-hr stimulation of freshly isolated cells with PMA and ionomycin stimulation of tissue MNCs which suggests that this may not be the case. Whilst we have shown that tissue T cells can produce TNF-α, IL-5 and IFN-γ, it is possible that other anti-inflammatory cytokines are produced by other cells present in AAA, such as B cells or macrophages.
Although the majority of the lymphocytes extracted from AAA tissue were in the resting state, T cells readily responded to TCR/CD3-mediated activation in vitro, entering the cell cycle and secreting IL-5, IL-6, TNF-α and IFN-γ. In the event that this mimics activation of T cells in situ, production of these cytokines would be consistent with the destructive, chronic inflammatory tissue changes seen in the aortic wall, and could contribute to potentiating the inflammation within the vascular tissue. Whilst stimulation of PBMCs with anti-CD3 and anti-CD28 provoked secretion of a wide range of cytokines in AAA patients and controls, tissue MNCs predominately produced only IL-5, IL-6, TNF-α and IFN-γ. The total amount of cytokine production did vary considerably between patients, although this did not reflect the degree of tissue inflammation present. Unlike PBMCs, tissue MNCs did not produce significant amounts of any of the anti-inflammatory, Th2 type cytokine, IL-4, although they did produce significant quantities of IL-5. These findings indicate that a distinct set of tissue lymphocytes are found within AAA tissue, primed to produce a stereotypical range of cytokines following activation, common to all patients with AAA. Coupled with the fact that the cellular components of AAA tissue are highly similar between patients, these findings suggest that AAA pathology is the result of a highly regulated tissue response, sustained by interactions occurring in the germinal centre-like structures seen within the AAA tissue. Potential antigens that might trigger this response include proteins within the vessel wall, or microbial pathogens such as Chlamydia, or oxidized low-density lipoprotein shown to activate T cells extracted from AAA4 and atherosclerotic plaques,13 respectively.
The production of IL-5, by tissue T cells is a novel finding, and the role of this cytokine in AAA disease is unclear. However, in view of our finding that over one third of the inflammatory cells in AAA tissue are B cells, IL-5 production by tissue T and B cells may have a role in activation of tissue B cells14 where local B-cell activation could occur within tissue germinal centres. The production of IL-5, as well as IFN-γ and TNF-α, by tissue T cells is also unusual, and characteristic of a Th0 CD4 cell phenotype. Whilst it is possible that multiple subsets of T cells exist within AAA tissue, one set secreting pro-inflammatory (IFN-γ, TNF-α) Th1 cytokines and the other anti-inflammatory (IL-5) Th2 cytokines, a subset of CD30+ T cells has been reported to secrete both IFN-γ and IL-5. IL-5 in combination with IFN-γ has been shown to have potent helper activity for B-cell immunoglobulin production,15 raising the possibility that antibody production by tissue B cells may play a role in AAA formation or progression. We were unable to demonstrate CD30 expression on freshly isolated tissue T cells (data not shown), but it is possible that this surface receptor is up-regulated during activation, similar to CD25 on tissue T cells. As such, the T cells in AAA could represent a single population of Th0 cells, capable of producing Th1 and Th2 type cytokines.
There is much evidence that cytokine production is altered in patients with AAA. Systemically, patients with AAA have increased plasma levels of IL-1β, IL-6 and TNF-α compared to controls.16 In addition, increasing concentrations of IL-6 have been shown to correlate with AAA size.17 It is unclear whether such elevations in circulating cytokines originate from inflammation within the aneurysm wall, or reflect an intrinsic difference in the immune system in patients with AAA. Despite reports of elevations in cytokines in patients with AAA, there are no studies which have re-evaluated cytokine profiles following AAA repair, when patients have returned to preoperative health and activity. We found no difference in the serum cytokine profile AAA patients, prior to, or following AAA repair compared to controls, with respect to IL-2, IL-4, IL-5, IL-6, IL-10, IFN-γ and TNF-α (N.F., unpublished observations), suggesting that the systemic cytokine environment is not influenced by the inflamed vasculature. It may be that no difference in cytokines was demonstrated between patients and controls because of the increased sensitivity of cytokine detection by the cytokine bead array kit used, in comparison to traditional ELISA methods6,7 or the relatively small number of samples analysed. In addition, it is possible that cytokines produced locally in the tissue remain localized to the aortic wall, and do not affect systemic levels of cytokines.
In conclusion, this study provides the first direct evidence that the infiltrate in AAA tissue may actively contribute towards sustaining inflammation within the vasculature, by the ability of the T cells to proliferate and produce a stereotypical set of pro-inflammatory cytokines, which may influence leucocyte recruitment to vascular tissue, destroy tissue components and provide appropriate signals for B-cell antibody production. Furthermore, we have developed and characterized a novel in vitro model with which to further study the aetiology of AAA.
Acknowledgments
This work was supported in part by grants from British Heart Foundation (grant no FS/02/067), Royal College of Surgeons of England, British Vascular Foundation, European Society for Vascular Surgery and NIH (SRC). We would like to thank Andrew Jacks and Andrew Rawstron of the Haematology and Malignancy Diagnostic Service, Leeds, for their help and advice in the B-cell immunohistochemical staining of aneurysm tissue.
References
- 1.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 2.Pearce WH, Koch AE. Cellular components and features of immune response in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:175–85. doi: 10.1111/j.1749-6632.1996.tb33308.x. [DOI] [PubMed] [Google Scholar]
- 3.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199–213. [PMC free article] [PubMed] [Google Scholar]
- 4.Halme S, Juvonen T, Laurila A, Juvonen J, Mosorin M, Saikku P, Surcel HM. Chlamydia pneumoniae reactive T lymphocytes in the walls of abdominal aortic aneurysms. Eur J Clin Invest. 1999;29:546–52. doi: 10.1046/j.1365-2362.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 5.Schonbeck U, Sukhova GK, Gerdes N, Libby P. T (H) 2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994;42:159–62. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 7.Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:224–227. [PubMed] [Google Scholar]
- 8.Cruickshank S, Southgate J, Selby P, Trejdosiewicz L. Inhibition of T cell activation by normal human biliary epithelial cells. J Hepatol. 1996;31:1026–33. doi: 10.1016/s0168-8278(99)80315-8. [DOI] [PubMed] [Google Scholar]
- 9.Crow P, Shaw E, Earnshaw JJ, Poskitt KR, Whyman MR, Heather BP. A single normal ultrasonographic scan at age 65 years rules out significant aneurysm disease for life in men. Br J Surg. 2001;88:941–4. doi: 10.1046/j.0007-1323.2001.01822.x. [DOI] [PubMed] [Google Scholar]
- 10.Walton LJ, Powell JT, Parums DV. Unrestricted usage of immunoglobulin heavy chain genes in B cells infiltrating the wall of atherosclerotic abdominal aortic aneurysms. Atherosclerosis. 1997;135:65–71. doi: 10.1016/s0021-9150(97)00152-4. [DOI] [PubMed] [Google Scholar]
- 11.Ocana E, Bohorquez JC, Perez-Requena J, Brieva JA, Rodriguez C. Characterisation of T and B lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis. 2003;170:39–48. doi: 10.1016/s0021-9150(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 12.Xiong W, Zhao Y, Prall A, et al. Key roles of CD4 (+) T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–12. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 13.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK, Rymo L. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–7. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumann MA, Paul CC. Interleukin-5 and human B lymphocytes. Methods. 1997;11:88–97. doi: 10.1006/meth.1996.0392. [DOI] [PubMed] [Google Scholar]
- 15.Alzona M, Jack HM, Fisher RI, Ellis TM. CD30 defines a subset of activated human T cells that produce IFN-gamma and IL-5 and exhibit enhanced B cell helper activity. J Immunol. 1994;153:2861–7. [PubMed] [Google Scholar]
- 16.Juvonen J, Surcel HM, Satta J, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscl Thromb Vasc Biol. 1997;17:2843–7. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 17.Treska V, Topolcan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med. 2000;38:1161–4. doi: 10.1515/CCLM.2000.178. [DOI] [PubMed] [Google Scholar]