Abstract
The CD3 antigen is a surface structure associated with the T-cell receptor (TCR) to form a complex involved in antigen recognition and signal transduction. Reports on the structures of the CD3 molecules associated with αβ- and γδ-TCR have been contradictory. To investigate this issue, we raised a panel of monoclonal antibodies (mAb) against purified porcine CD3 molecules. Unlike the conventional anti-CD3, these mAb reacted specifically with peripheral γδ-T cells, but not with αβ-T cells. Immunoprecipitation showed that the antibody recognized a subset of CD3 molecules that were associated with γδ-TCR. Also unlike the conventional anti-CD3, these mAb, though directed at two different epitope groups, failed to induce antigenic modulation, T-cell proliferation and CD3-redirected cytotoxicity. Taken together, these results suggest that there are differences in the antigenicity, signal transduction potentials and probably structural differences between the CD3 molecules expressed at the surface of αβ- and γδ-T cells.
Keywords: CD3, γδ T cells, pig
Introduction
The CD3–T cell receptor (TCR) complex plays a central role in the T-cell-mediated immunoresponse as it is involved in the recognition of antigens and subsequent signal transduction and activation of immunocompetent T lymphocytes. For this reason, the CD3 and TCR molecules are among the surface structures of lymphocytes which have been most extensively studied. There are two types of TCR differentiated by their heterodimers, namely αβ- and γδ-TCR. Recent studies have shown that γδ-TCR-bearing cells (γδ-T cells) are quite different from αβ-TCR-bearing cells (αβ-T cells) in their early appearance in ontogeny, limited V gene usage but extensive junctional diversity, distinct pathway of developmental maturation,1–3 direct recognition of the antigens without antigen processing,2 unique repertoire of antigen specificities,3 unique trafficking and tissue distribution,1,3 unique capacity to provide primary protection against specific pathogens3 and functions not directly related to antigen recognition.2
The structure of CD3 associated with γδ-TCR also seems to be different from that of CD3 associated with αβ-TCR. In earlier studies, no difference, other than certain differential glycosylations of the CD3 δ-chain,4,5 were found in the composition, primary structure and interactions of subunits, in the antigenicity and in the function of the CD3 complex associated with αβ- or with γδ-TCR.6–12 Based on these studies, the CD3 molecule was proposed to be a complex consisting of at least six peptides that form three dimers, i.e. εγ, εδ and ζζ.13,14 However, recent discoveries suggest that this model may only apply to αβ-T cells, as most γδ-TCR complexes expressed on ex vivoγδ-T cells lack the CD3 δ-chain.15,16 The discrepancy between the earlier and more recent discoveries has been interpreted as resulting from the cells being analysed by different laboratories. Whist the earlier studies employed activated T-cell clones or T-cell hybridomas, recent studies analysed unstimulated ex vivo cells.15 Apparently, monoclonal antibodies (mAb) that can differentiate the CD3 molecule expressed on γδ-T cells from that on αβ-T cells may help address this issue.
The pig is an important immunological model as it has a highly complex lymphocyte pool which includes cell subpopulations, such as peripheral CD4+ CD8+ T cells, that are rare or absent in other species.17,18 Furthermore, in contrast to the blood of humans and rodents, porcine peripheral blood has a large proportion of γδ T lymphocytes,19–21 and thus provides a good model with which to explore possible structural differences between CD3 complex expressed on αβ- and γδ-T cells. For this purpose, using purified porcine CD3 molecule as immunogen, we raised a panel of mAbs that reacted specifically with the CD3 molecule expressed on γδ-T cells. The results reveal the differences in antigenicity and signal transduction potentials of the CD3 molecules expressed on γδ-T versus αβ-cells.
Materials and methods
Animals and antibodies
The animals used in this study were adult inbred or outbred Large White pigs of either sex.
The following anti-porcine lymphocyte mAbs have been documented: anti-CD2: MSA4 [immunoglobulin G2a (IgG2a)],22 anti-CD3: PPT3 (IgG1),23 anti-CD4: 74-12-4 (IgG2b),24 anti-CD8: PPT21 and PPT22 (IgG1),25 anti-pig γδ-TCR: PPT2726 and anti-sheep γδ-TCR: 86D (IgG1).27 The mAb MAC320 (IgG2a), directed to a structure on porcine null γδ-T cells,20 was a gift from Dr R. M. Binns. Fluorescein isothiocyanate (FITC)-conjugated goat anti-porcine immunoglobulin and FITC- or phycoerythrin (PE)-conjugated goat anti-murine subclass immunoglobulin antibodies were purchased from Southern Biotechnology Association, Inc, Birmingham, AL.
Preparation of mAbs
Isolation of porcine CD3 molecules and production of mAbs was carried out as described elsewhere.23 Hybridoma supernatants were tested for antibodies binding to porcine thymocytes and peripheral blood lymphocytes (PBL) by flow cytometry analysis (FACS) and candidates for anti-CD3 mAbs were selected and cloned twice by limiting dilution and subjected to further characterization.
FACS
For two-colour staining, PBL were treated with a mixture of mAb PPT16 (IgG2b) and anti-CD2 (IgG2a), CD3 (IgG1), anti-pan-CD8 mAb PPT21 (IgG1), anti-CD8hi mAb PPT22 (IgG1) or FITC-conjugated anti-pig immunoglobulin, followed by incubation with a mixture of PE-conjugated anti-mouse IgG2b and either FITC-anti-mouse IgG2a or FITC-anti-mouse IgG1. For costaining with anti-CD4(IgG2b) and PPT27 (IgG2b), the cells were first incubated with PPT16, followed by PE-anti-mouse IgG2b, blocked with 10% normal mouse serum and finally stained with biotinylated anti-CD4 or PPT27 followed by FITC-streptavidin. Cold phosphate-buffered saline containing fetal calf serum (2% v/v) and NaN3 (0·1% w/v) was used for all of the washing and staining operations. For each sample, 5000 or 10 000 cells were acquired and analysed using a FACScan cytometer (Becton Dickenson, San Jose, CA).
Immunoprecipitation
Iodination of cells with 125I and immunoprecipitation were performed as described elsewhere.23
Lymphocyte preparation, proliferation and CD3-redirected cotoxicity
Porcine PBL were prepared as reported earlier.25 Cell subsets were selectively depleted from purified PBL using the mini MACS system (Miltenyi Biotec GmbH, 51429 Bergisch Glabach, Germany) as described previously.25 The CD3-redirected cytotoxicity assay was conducted as described.23
Results
Preparation of anti-CD3 mAbs
To detect possible antigenic differences between the CD3 molecules expressed on αβ- and γδ-T cells, mAbs were prepared using mice immunized with affinity-purified porcine CD3. Among 15 anti-CD3 mAbs selected from one fusion, one (PPT16) showed a unique reactivity in reacting only with γδ-T cells. The PPT16 antigen was then affinity-purified and used as immunogen for four more fusions. These fusions yielded 46 conventional anti-CD3 mAbs as well as seven mAbs with specificity similar to PPT16. This total of eight mAbs uniquely reactive with γδ-T cells were code named PPT15 (IgG2b), PPT16 (IgG2b), PPT17 (IgG1), PPT18 (IgG1), PPT19 (IgG1), PPT24 (IgG1), PPT25 (IgG2b), and PPT26 (IgG1).
Cross-inhibition experiments classified these eight mAbs into two epitope groups. Group 1 included all the three IgG2b mAbs, i.e. PPT15, PPT16 and PPT25, whilst the remaining five mAbs fell within Group 2 which contained all the IgG1 antibodies. The mAbs within the same group blocked each other's binding completely, whereas they only partially inhibited the binding of the mAbs of the other group. Even within the same group, the epitope recognized by the mAbs may not be identical. For example, although PPT26 cross-blocked the binding of the other mAbs of Group 2, this mAb seemed more effective in synergizing with phorbol ester than the others (data not shown). Thus, the epitope groups determined by the cross-inhibition experiments do not necessarily run parallel with the functional domains of the antigen. None of these mAbs, of either group, showed cross-inhibitory effect on conventional anti-CD3-ε or anti-γδ-TCR mAb PPT27 (data not shown).
All these mAbs had strong affinity and worked well in immunoprecipitation, but none of them were effective in Western blotting. In the following characterization, similar results were obtained with all of the eight mAbs. In the results presented below, the biochemical data obtained with mAb PPT16 are presented and the functional experiments reported were carried out with mAb PPT26.
The mAb PPT16 recognizes γδ T cells
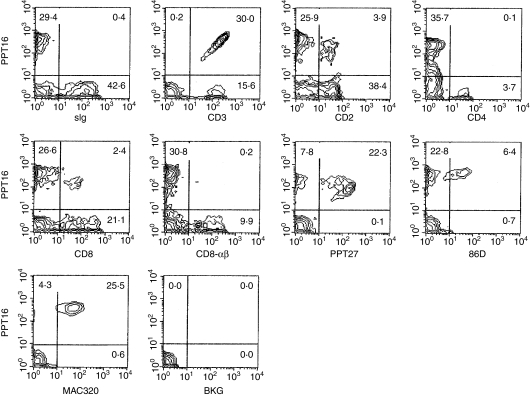
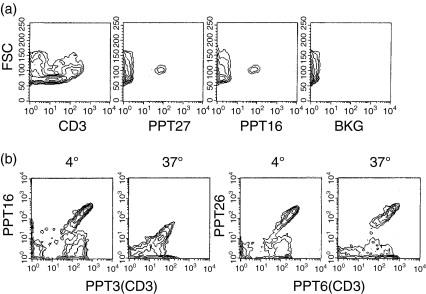
As indicated by FACS analysis, the specificity of mAb PPT16 was different from that of conventional anti-CD3 mAbs, but similar to that of anti-porcine γδ-TCR mAb PPT27 which defines the majority, but not all, of γδ-T cells.26 As shown in Fig. 1, in the blood, PPT16 stained only 65·7% of T cells (CD3+ cells), of which 74·1%, 22·0% and 75·5% were γδ-T cells defined by mAbs PPT27, 86D22 and MAC320,16 respectively. Like γδ-TCR, the PPT16 antigen was mainly expressed on CD2– CD4– CD8– T cells, whilst a small proportion of antigen-positive cells also expressed CD2 and CD8 at a low level (CD8-αα+), but the mAb did not react with CD4+ or CD8hi (CD8-αβ+)T cells (Fig. 1 and Table 1). The staining percentage of T cells in the spleen was similar to that in the blood, though most of the PPT16+ cells bore CD2 and some of them expressed CD8-αα (Table 1). In contrast, the PPT16+ cells in mesenteric and peripheral (not shown) lymph nodes as well as in the tonsil accounted for only a small proportion of total T cells while having a phenotype similar to their counterpart in the spleen (Table 1). In the thymus, the mAb reacted with a small proportion of CD4+ cells, though PPT16+ cells still consisted mainly of CD4– CD8– T cells. In addition, like anti-γδ-TCR mAb, PPT16 reacted at a rather high and uniform level, mainly with the larger thymocytes which contain more mature medullary cells. In contrast, the anti-CD3-ε, as reported elsewhere,19 stained both larger and smaller thymocytes with differential intensity (Fig. 2a). Taken together, the phenotype, tissue distribution and thymocyte parameters of PPT16-positive cells are strikingly similar to those of porcine γδ-T cells defined by an established anti-poricne γδ-TCR mAb.26
Figure 1.
Phenotypes of the PPT16-reactive cells as defined by two-colour FACS. Porcine PBL were stained with a mixture of mAb PPT16 and anti-pig immunoglobulin, CD3-ε, CD2, CD4, CD8, CD8-αβ, PPT27 (anti-porcine γδ-TCR), 86D (anti-sheep γδ-TCR), or MAC320 (anti-CD4– CD8–γδ-T-cell mAb). Figures in the quadrants are percentages. BKG: background staining (cells stained with normal mouse serum solution in place of the first-stage antibody).
Table 1. Staining percentage of T lymphocytes by mAb PPT16 and subset composition of the mAb-reactive cells from blood and lymphoid tissues.
| Subest composition of PPT16+cells (%) | ||||||
|---|---|---|---|---|---|---|
| Tissue | %stained T cell | CD2+ | CD4+ | CD8+ | CD4– CD8– | MAC320+ |
| PBL | 60·7 ± 12·5 | 12·8 ± 3·4 | –1 | 9·1 ± 2·7 | 91·4 ± 4·5 | 79·6 ± 8·7 |
| Spleen | 53·7 ± 7·7 | 85·1 ± 4·4 | – | 25·1 ± 4·3 | 74·3 ± 5·1 | 16·8 ± 2·9 |
| MLN | 6·9 ± 5·6 | 61·4 ± 7·1 | – | 55·9 ± 9·3 | 42·3 ± 6·5 | 37·5 ± 6·1 |
| Tonsil | 9·9 ± 3·0 | 53·3 ± 4·2 | – | 53·0 ± 6·9 | 47·1 ± 6·0 | 44·8 ± 7·4 |
| Large thymocytes | 47·6 ± 6·8 | 87·1 ± 5·0 | 5·4 ± 3·5 | 14·1 ± 4·7 | 85·3 ± 10·4 | 17·4 ± 5·5 |
PBL, peripheral blood monocytes; MLN, mesenteric lymph nodes.
No significant number of cells with this marker found in the mAb-reactive cells.
Values presented are mean ± SD (n = 5). Cells were stained and analysed using FACS as described in Fig. 1.
Figure 2.
(a) The mAb PPT16 reacts mainly with larger mature thymocytes. Porcine thymocytes stained with the mAbs indicated were analysed using FACS for fluorescence intensity versus forward scatter (FSC). Background staining (BKG) was that of cells stained with normal mouse serum solution in place of the first-stage antibody. (b) PPT16 antigen comodulates with CD3-ε. Porcine PBL coated with anti-CD3-ε mAb PPT3 (IgG1) or PPT26 (IgG1, same specificity as PPT16) were cultured at 37° or incubated at 4° on the monolayer of 16·2·CG7 (a mouse L cell line stably transfected with CD32) for 24 hr. The cells were harvested and stained with a mixture of either PPT16 (IgG2b) and PPT3 or PPT6 (IgG2b, anti- CD3-ε) and PPT26, followed by a mixture of PE-anti-mouse IgG2b and FITC anti-mouse IgG1 and analysed by FACS.
Furthermore, mAb PPT16 only reacted with CD3-ε-bearing cells, and expression of the antigen was linearly correlated with that of porcine CD3-ε (Fig. 1). This correlation was observed in the lymphoid cells from all the tissues tested in Table 1 (data not shown). Moreover, when the CD3 antigen was modulated by anti-CD3-ε cross-linked by a monolayer of 16·2·CG7 (an L cell line transfected with IgG FcR cDNA), the expression of the PPT16 antigen decreased accordingly, maintaining its linear correlation with CD3-ε (Fig. 2b). This suggests that the PPT16 antigen, as a γδ T-cell marker, is not only coordinately expressed but is associated with a subset of the CD3 complex.
As the isotype of PPT16 is IgG2b and thus does not bind to the FcR transfectant used to cross-link the mAb, its ability to induce antigen modulation could not be tested. However, all the five IgG1 mAbs with the same specificity as PPT16, though able to bind to FcR transfectants, failed to induce antigenic modulation (Fig. 2b). This suggests that the PPT16 antigen is different from the conventional CD3 antigen in that complementary mAbs do not induce antigenic modulation.
The mAb PPT16 recognizes CD3 associated with γδ-TCR
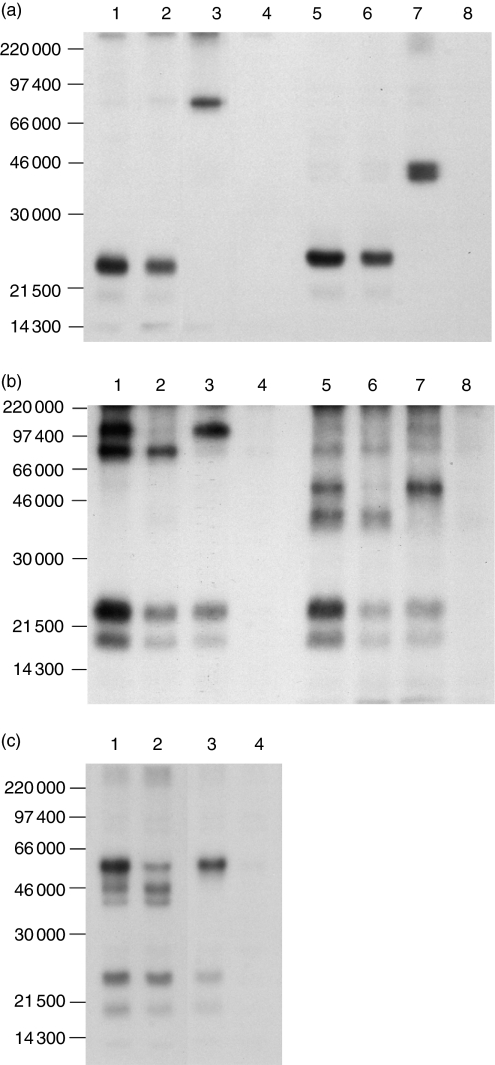
To identify the PPT16 antigen, 125I iodinated porcine PBL were lysed in nonidet P-40 lysis buffer and precipitated with mAb-coated Protein G beads. Both anti-CD3-ε(Fig. 3a, lanes 1, 5) and mAb PPT16 (lanes 2, 6) precipitated a 23 000 MW band. In contrast, anti-γδ-TCR mAb PPT27 (lanes 3, 7) precipitated the γδ-TCR as reported elsewhere.26 This demonstrates that mAb PPT16 is not directed against γδ-TCR, but against the CD3 molecules.
Figure 3.
(a) The mAb PPT16 recognizes CD3, not γδ-TCR. Porcine PBL labelled with 125I were lysed in nonidet P-40 (1%) lysis buffer and precipitated with protein G–Sepharose 4B beads coated with anti-CD3-ε mAb PPT3 (lanes 1, 5), PPT16 (lanes 2, 6) PPT27 (lanes 3, 7) or normal mouse serum (lanes 4,8). The 10% sodium dodecyl sulphate–polyacrylamide gel electrophoreses (SDS–PAGE) gel was run under non-reducing (lanes 1–4) and reducing (lanes 5–8) conditions. (b) PPT16 antigen is associated with γδ-TCR. 125Iodinated PBL were lysed in digitonin (1%) lysis buffer. The lysates were precleared with protein G-Sepharose 4B coated with either normal mouse IgG (lanes 1, 2, 5, 6) or with PPT16 (lanes 3, 4, 7, 8) twice before being precipitated with beads coated with either anti-CD3-ε mAb PPT3 (lanes 1, 3, 5, 7) or mAb PPT16 (lanes 2, 4, 6, 8). The 10% SDS-PAGE gel was run under nonreducing (lanes 1–4) and reducing (lanes 5–8) conditions. (c) PPT16 antigen is associated with a minor part of 55 000 MW dimer in some animals. Lysates of 125I iodinated PBL were precleared with normal mouse serum (lanes 1,2) or PPT16 (lanes 3, 4) and then precipitated with anti-CD3-ε (lanes 1, 3) or PPT16 (lanes 2, 4). The 10% SDS-PAGE gel was run under reducing conditions.
To verify this interpretation, immunoprecipitation was conducted using digitonin lysis buffer to precipitate the whole CD3–TCR complex. As shown in Fig. 3(b), anti-CD3-ε precipitated four bands (lanes 1, 5). The 55 000 MW dimer and 43 000 MW dimer, as reported earlier, were αβ-TCR and γδ-TCR, respectively,19. The 23 000 and 18 000 MW proteins were the CD3 complex. The mAb PPT16 also precipitated similar components of the CD3 complex, but it only coprecipitated γδ-TCR (Fig. 3b, lanes 2, 6). When the lysate was precleared with PPT16, anti-CD3-ε only precipitated the 55 000 MW αβ-TCR (Fig. 3b, lanes 3, 7) whereas preclearance of the lysate with anti-CD3-ε completely removed the PPT16 antigen (data not shown). These results indicate that mAb PPT16 is directed against the CD3 molecules that are associated only with the γδ-TCR. However, in some animals (four out of 11 tested pigs), in addition to the 43 000 MW dimer, PPT16 also coprecipitated a small amount of 55 000 MW dimer (Fig. 3c, lane 2). Preclearance with PPT16 completely removed the 43 000 MW dimer but not most of the 55 000 MW dimer (Fig. 3c, lane 3). This suggests that even in these animals, the PPT16 antigen was only associated with a small proportion of 55 000 MW dimer, but was associated with all of the 43 000 MW dimer. The reason for this discrepancy in immunoprecipitation pattern between animals is unknown. Nonetheless, there was no significant difference in the phenotype and tissue distribution of the PPT16-positive cells between all the animals tested (more than 60 pigs in total). In particular, CD4+ cells and CD8αβ+ T cells, which account for most of the αβ T cells, were never stained by the mAb in any animal tested, suggesting that the PPT16 antigen is essentially a surface marker of γδ-T cells. Therefore, it seems possible that the 55 kDa-dimer identified in some animals by mAb PPT16 is an uncommon form of γδ-TCR.
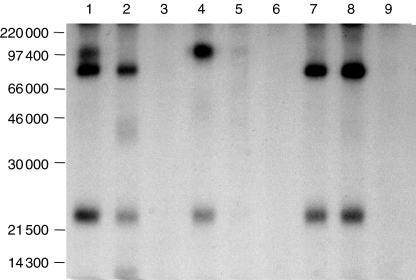
To address this issue further, porcine PBL were selectively depleted of certain cell subsets and subjected to immunoprecipitation. As shown in Fig. 4, anti-CD3-ε coprecipitated αβ-TCR, but not γδ-TCR, from PPT16– cells (lane 4). In contrast, both anti-CD3-ε and PPT16 coprecipitated only γδ-TCR from CD4– CD8– cells (lanes 7, 8). Taken together, these results demonstrate that mAb PPT16 recognizes an epitope of the CD3 molecule which is expressed only at the surface of γδ T cells.
Figure 4.
PPT16 antigen is expressed at the surface of γδ-T cells. PBL (lanes 1–3), PPT16– cells (lanes 4–6) and CD4– CD8– cells (lanes 7, 9) were 125I iodinated, lysed in 1% digitonin buffer and precipitated with anti-CD3-ε (lanes 1, 4, 7), PPT16 (lane 2, 5, 8), or normal mouse serum (lanes 3, 6, 9). The 10% SDS–PAGE gel was run under nonreducing conditions.
Functional differences between conventional and γδ-T-cell-restricted CD3 molecules
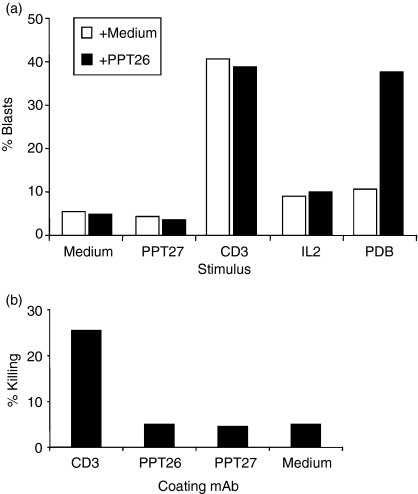
Unlike conventional anti-CD3 mAb, none of the antibodies against the γδ-T-cell-restricted CD3 induced substantial proliferation of porcine T cells, added in soluble form or immobilized on plates or via FcR transfectants. Moreover, these mAbs did not synergize the effect of conventional anti-CD3, anti-γδ-TCR, or IL-2. Exceptionally, these mAbs, especially PPT26, synergized with phorbol ester PPD (Fig. 5a).
Figure 5.
(a) Anti-γδ T-restricted CD3 mAb do not induce lymphocyte proliferation. Porcine peripheral mononuclear cells were cultured for 3 days in the presence of the mAbs indicated (IgG concentration: 50 ng/ml) in 24-well plates coated with goat antimouse IgG (10 lg/ml) and analysed using FACS. Values are mean blast percentage of triplicates. (b) Anti-γδ-T-restricted CD3 mAb do not induce redirected cytotoxicity. PBL coated with the mAbs indicated were cultured for 24 h with 51Cr-labelled 16·2·CG7 before the culture supernatants were taken and measured for isotope release. Values shown are mean of triplicates for percentage specific killing
Similarly, these mAbs failed to induce CD3-redirected cytotoxicity whilst conventional anti-CD3, as reported earlier,19 induced vigorous killing (Fig. 5b).
Discussion
Because of its central role in T-cell-mediated immune response, the CD3–TCR complex has been thoroughly studied. Conflicting evidence has emerged with regard to the structure of the CD3 complex expressed on αβ and γδ T cells. Whist earlier studies showed that there was no fundamental difference in the subunit composition, protein sequence and interactions of subunits, in the antigenicity and in the function of the CD3 complex associated with αβ or with γδ-TCR,6–12 recent studies indicated the CD3 complex on ex vivoγδ-T cells lacked a CD3 δ-chain.15 This discrepancy has been ascribed to the fact that earlier studies involved activated T-cell clones or hybridomas whilst the recent studies analysed unstimulated ex vivo cells.15
The pig, with its high number of circulating γδ-T cells, provides a convenient model for examination of this matter because neither genetic manipulation nor T-cell activation is required for preparation of large numbers of γδ-T cells. Our approach was to raise anti-CD3 mAbs and select them for their capacity to differentiate αβ versus γδ-T cells. As a result, we have obtained a panel of mAb directed at a subset of the CD3 antigen that is only associated with γδ-TCR cells as demonstrated by FACS and biochemical analyses.
Until recently the CD3 molecules on αβ- vs. γδ-T cells were regarded to be fundamentally identical. However, differences in the affinity of some anti-CD3 mAbs for αβ- versus γδ-T cells have been observed. A well-known example is anti-human CD3 mAb WT31. Under normal conditions, this mAb binds only to αβ-T cells and was thus regarded for a time to be directed at αβ-TCR.28 Only later was it found that WT31 also reacted with γδ-T cells at higher concentrations and the reactivity was significantly increased upon removal of sialic acid residues by neuraminidase.29 Further studies showed that WT31 recognized a conformational epitope present on CD3 ε/γ- or ε/δ-dimer.30 Another anti-human-CD3 mAb T3 lost its reactivity with γδ-T cells upon fluorescein conjugation, but not its reactivity with αβ-T cells.31 Nonetheless, this is the first time that anti-CD3 mAbs have been shown to react specifically with γδ-T cells. The antigenic difference revealed by these mAbs strongly suggests that the CD3 molecules on αβ- and γδ-T cells are probably different in their structures. Indeed, in follow-up studies, we have revealed that the anti γδ-T-cell-restricted CD3 mAbs recognize conformational epitopes on CD3-ε/δ dimer which are present in the cytoplasm of both αβ- and γδ-T cells, but only at the γδ-T-cell surface (Yang and Wileman, in preparation). As this discovery is inconsistent with the previous report that the CD3 complex on ex vivoγδ-T cells lacked a CD3 δ-chain,15 it seems that the assembly of the CD3 complex on γδ-T cells is probably more complex then currently understood. In this regard, the anti γδ-T-cell-restricted CD3 mAbs have provided a useful tool for further exploration.
There are several observable functional differences when total CD3 and γδ-T-cell-restricted CD3 molecules are ligated by antibodies. Unlike anti-CD3-ε, anti-γδ-T-cell-restricted CD3 mAbs did not induce antigenic modulation, lymphocyte proliferation, or CD3-redirected cytotoxicity. In our previous study, we showed that triggering of different epitopes of CD3 elicits differential cell responses.32 Thus the failure to activate γδ-T cells via the anti-γδ-T-cell-restricted CD3 mAbs may result from a fundamental functional difference between the signalling characteristics of γδ- and αβ-T cells, or, more simply, reflects the fact that the anti-γδ-T-cell-restricted CD3 mAbs recognize non-mitogenic epitopes. In our previous studies, we have shown that lymphoblasts induced by anti-CD3 are mainly αβ-T cells, whilst porcine γδ-T cells, either in bulk culture or isolated, do not proliferate in response to anti-CD3 stimulation even under optimal conditions.32 We have also found that anti-γδ-TCR mAb do not drive lymphocyte to proliferate (ref. 26 and Fig. 5a of this paper). Hence, it appears that mAbs against γδ-T-cell-restricted-CD3 do not induce proliferation of γδ-T cells, not because they are directed to non-stimulating epitopes, but because triggering of CD3–TCR alone is not sufficient to induce porcine γδ-T cells to proliferate and additional signal(s) are needed. Indeed, the fact that anti-γδ-T-cell-restricted CD3 mAbs synergize the effect of phorbol ester supports this interpretation. Therefore, the mechanism of γδ-T-cell activation through the CD3–TCR pathway may be different from that of αβ-T cells. In this respect, our mAb provide a tool for further investigation into this issue.
The inability of the anti-γδ-T-restricted CD3 mAbs to induce antigenic modulation, on the other hand, seems to be the property of the epitope, rather than that of the whole CD3–γδ-TCR complex, because conventional anti-CD3-ε did induce antigenic modulation on γδ-T cells.
With regard to the inability of the mAbs to induce CD3-redirected cytotoxicity, there is not enough evidence at present to determine definitely whether this is ascribed to non-stimulating epitopes or whether it reflects a broader difference in physiological roles played by different CD3 molecules. In our previous studies, we have found that depletion of γδ-T cells from the PBL preparations reduced the killing induced by anti-CD3-ε, indicating that anti-CD3-ε does induce cytotoxicity of γδ-T cells.25 However, anti-CD3-ε failed to induce significant killing by isolated γδ-T cells (Yang, unpublished results), suggesting that γδ-T cells need help from activated αβ-T cells to initiate CD3-redirected cytotoxicity. Therefore, it is possible that the anti-γδ-T-restricted CD3 mAbs failed to induce cytotoxicity because they do not activate αβ-T cells to provide help necessary for initiation of γδ-T-cell cytotoxicity. Whichever the case, the existing evidence suggests that even if the CD3 molecules expressed on γδ-T cells are involved in CD3-redirected cytotoxicity, the requirements for activating the killing mechanism are different from that of CD3 expressed on αβ-T cells.
It is interesting that others have discovered that signal transduction by γδ-TCR is superior to that of αβ-TCR, as measured by its ability to induce calcium mobilization, ERK activation and cellular proliferation.15 The reason for this discrepancy is unknown at the present, though it may reflect the differences between species. Nevertheless, the cells we analysed were ordinary PBL which required no genetic manipulation of animals or long-term cell culture and thus the conditions were more similar to natural circumstances. In this respect, the pig, for the abundance of such cell subsets as γδ-T cells that are rare in circulation in humans and rodents, represents a good model for immunobiological studies. This is particularly true when investigating the physiological processes of lymphocytes because cell lines are already activated in vitro.
Acknowledgments
We thank Dr S. Denham for enthusiastic support, Dr R. M. Binns for providing mAb MAC320, Dr J. Banchereau for cell line 16·2·CG7 and Mr L. Pullen for excellent assistance in animal work.
References
- 1.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 2.Chien YH, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–32. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 3.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 4.Krangel MS, Bierer BE, Devlin P, Clabby M, Strominger JL, McLean J, Brenner MB. T3 glycoprotein is functional although structurally distinct on human T-cell receptor γ T lymphocytes. Proc Natl Acad Sci USA. 1987;84:3817–21. doi: 10.1073/pnas.84.11.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alarcon B, De Vries J, Pettey C, Boylston A, Yssel H, Terhorst C, Spits H. The T-cell receptor γ chain-CD3 complex: Implication in the cytotoxic activity of CD3+CD4–CD8– human natural killer clones. Proc Natl Acad Sci USA. 1987;84:3861–5. doi: 10.1073/pnas.84.11.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samelson LE, Harford JB, Klausner RD. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985;43:223–31. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- 7.Oettgen HC, Pettey CL, Maloy WL, Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986;320:272–5. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- 8.Brenner MB, McLean J, Dialynas DP, et al. Identification of a putative second T-cell receptor. Nature. 1986;322:145–9. [PubMed] [Google Scholar]
- 9.Borst J, van de Griend RJ, van Oostveen JW, Ang S-L, Melief CJ, Seidman JG, Bolhuis LH. A T-cell receptor gamma/CD3 complex found on cloned functional lymphocytes. Nature. 1987;325:683–8. doi: 10.1038/325683a0. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM, Fowlkes BJ, Bluestone JA, Kruisbeek A, Maloy WL, Coligan JE, Schwartz RH. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987;326:79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- 11.Littman DR, Newton M, Crommie D, Ang SL, Seidman JG, Gettner SN, Weiss A. Characterization of an expressed CD3-associated Ti γ-chain reveals Cγ domain polymorphism. Nature. 1987;326:85–8. doi: 10.1038/326085a0. [DOI] [PubMed] [Google Scholar]
- 12.Van Neerven J, Coligan JE, Koning F. Structural comparison of α/β and γ/δ T cell receptor-CD3 complexes reveals identical subunit interactions but distinct cross-linking patterns of T cell receptor chains. Eur J Immunol. 1990;20:2105–11. doi: 10.1002/eji.1830200932. [DOI] [PubMed] [Google Scholar]
- 13.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–94. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 14.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–84. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 15.Hayes SM, Love PE. Distinct structure and signaling potential of the gamma delta TCR complex. Immunity. 2002;16:827–38. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 16.Hayes SM, Shores EW, Love PE. An architectural perspective on signaling by the pre-, alphabeta and gammadelta T cell receptors. Immunol Rev. 2003;191:28–37. doi: 10.1034/j.1600-065x.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 17.Pescovitz MD, Lunney JK, Sachs DH. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985;134:37–44. [PubMed] [Google Scholar]
- 18.Saalmuller A, Reddehase MJ, Buhring HJ, Jonjic S, Koszinowski UH. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987;17:1297–301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- 19.Reddehase MJ, Saalmuller A, Hirt W. γ/δ T-lymphocyte subsets in Swine. Curr Top Microbiol Immunol. 1991;173:113–17. doi: 10.1007/978-3-642-76492-9_16. [DOI] [PubMed] [Google Scholar]
- 20.Binns RM, Duncan IA, Powis SJ, Hutchings A, Butcher GW. Subsets of null and γδ T-cell receptor+ T lymphocytes in the blood of young pigs identified by specific monoclonal antibodies. Immunology. 1992;77:219–27. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Parkhouse RME. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76–83. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammerberg C, Schurig GG. Characterization of monoclonal antibodies directed against swine leucocytes. Vet Immunol Immunopathol. 1986;11:107–21. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Oura CAL, Kirkham PA, Parkhouse RME. Preparation of monoclonal anti-porcine CD3 antibodies and preliminary characterization of porcine T lymphocytes. Immunology. 1996;88:577–85. doi: 10.1046/j.1365-2567.1996.d01-682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368–75. [PubMed] [Google Scholar]
- 25.Yang H, Parkhouse RME. Differential expression of CD8 epitopes amongst porcine CD8-positive functional lymphocyte subsets. Immunology. 1997;92:45–52. doi: 10.1046/j.1365-2567.1997.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Parkhouse RME. Characterization of the porcine γδ T-cell receptor structure and cellular distribution by monoclonal antibody PPT27. Immunology. 2000;99:504–9. doi: 10.1046/j.1365-2567.2000.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackay CR, Hein WR. A large proportion of bovine T cells express the γδ T cell receptor and show a distinct tissue distribution and surface phenotype. Int Immunol. 1989;1:540–5. doi: 10.1093/intimm/1.5.540. [DOI] [PubMed] [Google Scholar]
- 28.Spits H, Borst J, Tax W, Capel PJ, Terhorst C, de Vries JE. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. J Immunol. 1985;135:1922–8. [PubMed] [Google Scholar]
- 29.van de Griend RJ, Borst J, Tax WJ, Bolhuis RL. Functional reactivity of WT31 monoclonal antibody with T cell receptor-gamma expressing CD3+4–8– T cells. J Immunol. 1988;140:1107–10. [PubMed] [Google Scholar]
- 30.Salmeron A, Sanchez-Madrid F, Ursa MA, Fresno M, Alarcon B. A conformational epitope expressed upon association of CD3-epsilon with either CD3-delta or CD3-gamma is the main target for recognition by anti-CD3 monoclonal antibodies. J Immunol. 1991;147:3047–52. [PubMed] [Google Scholar]
- 31.Mullersman JE, White G, Tung KS. Differential staining of human alpha beta and gamma delta T cells by the fluorescein conjugate of an anti-CD3 monoclonal antibody. Clin Exp Immunol. 1991 May;84:324–8. doi: 10.1111/j.1365-2249.1991.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Parkhouse RME. Differential activation requirements associated with stimulation of T cells via different epitopes of CD3. Immunology. 1998;93:26–32. doi: 10.1046/j.1365-2567.1998.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]