Abstract
Heat-shock protein (hsp)60/chaperonin 60 is a potent immunogen which has recently been claimed to have cell-signalling actions upon myeloid and vascular endothelial cells. The literature is controversial with different chaperonin 60 proteins producing different patterns of cellular activation and the ever-present criticism that activity is the result of bacterial contaminants. To clarify the situation we have cloned, expressed and purified to homogeneity the chaperonin 60 proteins from Chlamydia pneumoniae, Helicobacter pylori and the human mitochondrion. These highly purified proteins were compared for their ability to stimulate human peripheral blood mononuclear cell (PBMC) cytokine synthesis and vascular endothelial cell adhesion protein expression. In spite of their significant sequence homology, the H. pylori protein was the most potent PBMC activator with the human protein the least potent. PBMC activation by C. pneumoniae and human, but not H. pylori, chaperonin 60 was blocked by antibody neutralization of Toll-like receptor-4. The C. pneumoniae chaperonin 60 was the most potent endothelial cell activator, with the human protein being significantly less active than bacterial chaperonin 60 proteins. These results have implications for the role of chaperonin 60 proteins as pathological factors in autoimmune and cardiovascular disease, and raise the possibility that each of these proteins may result in different pathological effects in such diseases.
Keywords: adhesion receptors, chaperonin 60, cytokines, myeloid cells, vascular endothelial cells
Introduction
Chaperonin (Cpn)60, also known as heat-shock protein (hsp) 60, is a tetradecameric protein consisting of subunits with an approximate molecular mass of 60 000.1 Two functions of this protein were identified almost simultaneously. The protein-folding or chaperoning activity of Cpn60 was identified in the late 1980s,2 as was the extreme immunogenicity of this protein.3 The latter is an unusual characteristic given the significant sequence conservation of Cpn60 proteins.4 In the last decade a still controversial hypothesis has been propounded, namely that Cpn60 proteins from bacteria and from eukaryotic cells can act as intercellular signals.5 Such studies have used mainly myeloid cells with a few studies of the response of vascular endothelial cells.5 This hypothesis remains controversial because: (1) Cpn60 proteins, in spite of their sequence conservation, have been reported to activate myeloid cells by a number of different receptor-mediated pathways;6–10 (2) there is no explanation of how the chaperonins are exported from cells; and (3) of the evidence produced by some workers that the activity of Cpn60 preparations is the result of contamination with the potent Gram-negative bacterial inflammogen, lipopolysaccharide (LPS).11–13 It has also been known for some years that purified or recombinant Cpn60 proteins are contaminated with cellular proteins,14,15 presumably the cohort of cytosolic/mitochondrial proteins that normally bind to Cpn60 within cells. All of these problems have clouded the evidence supporting the hypothesis that Cpn60 proteins represent a class of immune-activating proteins. It would be sensible to group Cpn60 proteins into the population of bacterial proteins known as pathogen-associated molecular patterns (PAMPs). In Medzhitov and Janeway's definition, these are highly conserved molecules produced only by pathogens and, because of their importance to cellular functions, unlikely to undergo significant evolutionary change.16 The chaperonins fit into this definition, with the important distinction that they are also produced by the host (albeit by the mitochondrion which was once a bacterium) and it is reported that human Cpn60 is able to activate myeloid cells.5,10
In an attempt to bring some clarity to the study of Cpn60 proteins we have developed methods for purifying recombinant versions of these molecular chaperones which remove both contaminating LPS and also contaminating proteins.17,18 In this study we have investigated the relative capacities of Cpn60 proteins from a eukaryote (human) and from two prokaryotes: Chlamydia pneumoniae (an intracellular bacterium) and Helicobacter pylori (a Gram-negative opportunistic pathogen) to stimulate the production of: (1) pro-inflammatory cytokines by human peripheral blood monocytes; and (2) vascular endothelial cell adhesion proteins by human vascular endothelial cells.
Materials and methods
Cloning and expression of the three Cpn60 homologues
The genes encoding the H. pylori, C. pneumoniae and Homo sapiens Cpn60 proteins were cloned, expressed and purified as described in Maguire et al.18 The H. pylori and C. pneumoniae cpn60 genes were amplified by polymerase chain reaction and inserted into the pBAD expression vector (Invitrogen, Paisley, UK). The cpn60 gene from Homo sapiens was inserted into the pQE60 expression vector (Qiagen, Crawley, UK). The proteins were expressed in Escherichia coli and purified using nickel affinity (Ni-NTA) chromatography followed by reactive-red dye affinity chromatography to remove proteins associated with the chaperonin. Contaminating LPS and other hydrophobic material was removed by washing proteins bound to the Ni-NTA column with polymyxin B.17,18 The purity of the proteins was determined by sdoium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) with the proteins being stained with colloidal Coomassie blue (Sigma, Poole, UK). Levels of LPS were determined by the Limulus amoebocyte lysate assay (Associates of Cape Cod; Liverpool, UK). The cell-based experiments described below were repeated at least three times and showed consistent patterns of cell activation.
Preparation of human peripheral blood mononuclear cells
Human peripheral blood mononuclear cells (PBMCs) were prepared from buffy coat blood from healthy donors by density gradient centrifugation and differential adherence as previously described.17 PBMCs were seeded at 2 × 106 cells/ml and exposed to a range of concentrations of the recombinant Cpn60 proteins. LPS from E. coli (Sigma) was used as a positive control. Polymyxin B was added at a concentration of 20 μg/ml to neutralize any residual LPS remaining from the purification of recombinant proteins. As further controls for LPS contamination, recombinant proteins were either boiled for 15 min or subjected to proteinase K digestion before addition to the PBMCs. Following a 16-hr exposure to the recombinant Cpn60 proteins, cell culture media were collected and cytokine concentrations were determined by two-site enzyme-linked immunosorbent assays (ELISAs).
To determine if the Cpn60 proteins interacted via Toll-like receptor-2 (TLR2) or TLR4, commercially available neutralizing antibodies to these bacterial recognition receptors [anti-TLR2 antibody clone TL2.1 or anti-TLR4 antibody clone HTA125 (Serotec, Oxford, UK)] or an irrelevant isotype control (Sigma) were incubated at a concentration of 20 μg/ml with the PBMCs for 2 hr prior to addition of the recombinant Cpn60 proteins.
EAhy926 vascular endothelial cell culture
EAhy926, a human vascular endothelial cell line, was routinely cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM; Sigma) supplemented with glutamine, penicillin/streptomycin (Sigma), 10% foetal calf serum (FCS; Sigma) and hypoxanthine–aminopterin–thymidine (HAT; Invitrogen). The cells were grown as monolayers and the medium was changed three times per week. Cells were seeded at a density of 2 × 106/ml and stimulated with the various agonists for 18 hr.
ELISAs for vascular adhesion proteins
The EAhy926 cells were washed with FCS-free DMEM (Sigma) and fixed using 1% paraformaldehyde (Sigma) in phosphate-buffered saline (PBS; Sigma) for 30 min. The cells were washed three times with PBS then incubated with Hanks' balanced salt solution supplemented with Ca/Mg, 1% bovine serum albumin (BSA), 100 mm glycine, 0·05% sodium azide (Sigma) solution for 30 min. Monoclonal antibodies diluted in PBS/1% BSA solution were added at the following concentrations: anti-E-selectin at 10 μg/ml; anti-intracellular adhesion molecule-1 (ICAM-1) at 10 μg/ml and anti-vascular cell adhesion molecule-1 (VCAM-1) at 2 μg/ml. Antibodies were a kind gift from Celltech Therapeutics (Slough, UK). The ELISA used was as described previously.17
Cytokine assays
Interleukin-1β (IL-1β), IL-6 and IL-8 cytokine standards were prepared at the National Institute for Biological Standards and Control (NIBSC, Potters Bar, UK). The cytokine ELISA methods used were as described elsewhere.17
Results
Purification of Cpn60 proteins
Purification of each of the three Cpn60 proteins is seen in Fig. 1 with the elution from the first affinity column and the final purified protein being shown. The material eluted from the Ni-NTA column contained a number of proteins in addition to the 60 000 molecular weight Cpn60 protein. These contaminating proteins were removed by the reactive-red column. The levels of LPS in the starting and final materials are shown in Table 1.
Figure 1.
Purification of recombinant Cpn60 proteins shown on SDS–PAGE stained with Brilliant G colloidal Coomassie blue showing first elution from the Ni-NTA column and the final ultrapure protein free from contaminating proteins and having negligible LPS contamination. (a) Purification of Helicobacter pylori Cpn60: lane 1, eluate from Ni-NTA column; lane 2, purified H. pylori Cpn60 protein after final elution from PD-10 column. (b) Purification of Chlamydia pneumoniae Cpn60: lane 3, eluate from Ni-NTA column; lane 4, purified C. pneumoniae Cpn60 protein after final elution from PD-10 column. (c) Purification of human Cpn60: lane 5, eluate from Ni-NTA column; lane 6,purified human Cpn60 protein after final elution from PD-10 column.
Table 1. LPS contamination of chaperonin 60 preparations at beginning and end of purification.
| Chaperonin 60 | Initial LPS level (EU/ml) | Final LPS level (EU/ml) |
|---|---|---|
| Human | > 0·5 | 0·03 |
| C. pneumoniae | > 0·5 | 0·025 |
| H. pylori | > 0·5 | 0·01 |
Comparison of PBMC cytokine-inducing activity
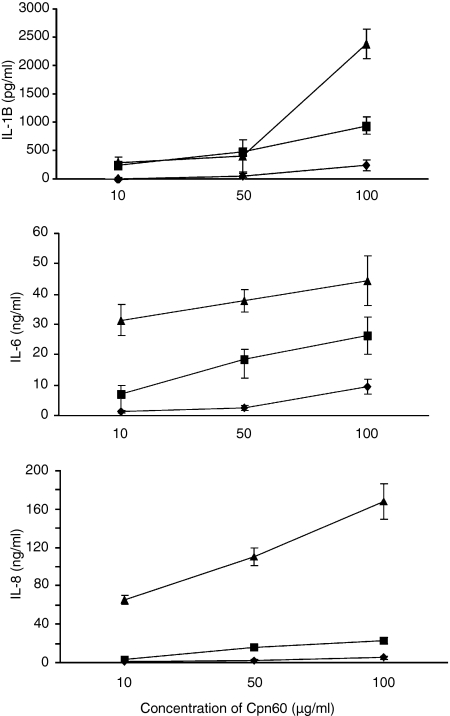
Initial experiments revealed significant differences in the potencies and efficacies of these three recombinant Cpn60 proteins and so the dose–responses presented range from 10 to 100 μg/ml to show the activity of the least active protein. Over this dose range, the human protein was a very weak inducer of IL-1β, IL-6 and IL-8 synthesis. The C. pneumoniae Cpn60 protein had some weak agonist activity at the lowest concentration tested and, with the exception of IL-8 synthesis, showed a more-or-less dose-dependent activation of cytokine synthesis. In contrast, the H. pylori Cpn60 protein was a much stronger cytokine-inducing molecule. This protein was a potent inducer of IL-8 synthesis while the other two proteins were weak agonists in this respect. Helicobacter pylori Cpn60 was also a potent IL-6 inducer with activity being recorded at concentrations as low as 100 ng/ml. This contrasts with the dose-dependent activation of IL-1β synthesis where at low concentrations the H. pylori protein is no more active than the other two Cpn60 proteins. However, at 100 μg/ml (approximately 1 μm– assuming activity is the result of the tetradecameric protein) it is clear that the H. pylori protein exhibits significantly greater efficacy than the other two chaperonins (Fig. 2).
Figure 2.
Relative production of IL-1β, IL-6 and IL-8 by human PBMCs incubated with various concentrations of either Helicobacter pylori Cpn60 (▴), Chlamydia pneumoniae Cpn60 (▪), or human Cpn60 (♦). Each data point represents the mean ± standard error for three replicates from a representative experiment. These experiments were performed four times from different donors.
The activity of all three Cpn60 proteins was significantly inhibited by boiling for 15 min and activity was completely eliminated after the proteins had been proteolysed by proteinase K. The same treatment had no effect on E. coli LPS (results not shown).
Influence of TLR-neutralizing antibodies
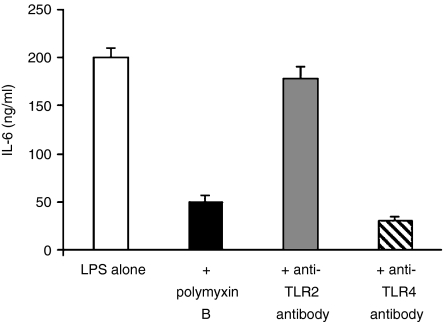
To determine if the purified preparation of Cpn60 proteins are interacting with TLR2 or TLR4 on the surface of human monocytes commercially available blocking antibodies to these bacterial recognition receptors were used. To confirm the specificity of these antibodies (with respect to their capacity to neutralize LPS) we tested their ability to block LPS activation of monocytes and have compared this effect with that of polymyxin B. As can be seen, both the polymyxin B and anti-TLR4 antibody significantly inhibited IL-6 production. In contrast, and as expected, the anti-TLR2 antibody was ineffective, revealing that it had no specificity for the TLR4 receptor (Fig. 3).
Figure 3.
The effect of adding polymxyin B, anti-TLR4, or anti-TLR2 antibodies to PBMCs activated by 100 ng/ml LPS. The addition of polymyxin B and TLR4 each strongly reduces the stimulation of the PBMCs by LPS as expected, the anti-TLR2 antibody has no effect. Each data point represents the mean ± standard errors for three replicates from a representative experiment. The experiments were performed three times from different donors.
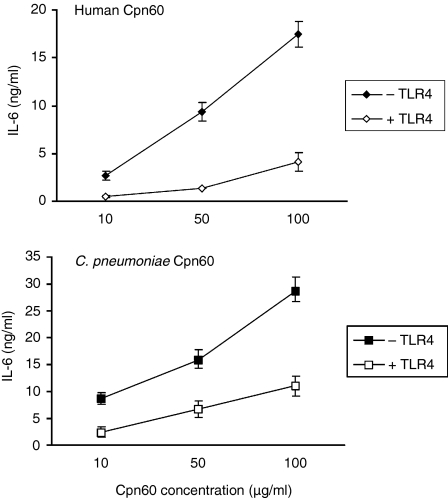
When the anti-TLR2 antibody was added to monocytes stimulated with the individual Cpn60 proteins there was no significant inhibition of IL-6 synthesis, suggesting that none of the three recombinant proteins interacted with TLR2. In contrast, the neutralizing antibody to TLR4 had an almost complete inhibitory effect on the human Cpn60 and significantly inhibited the activity of the C. pneumoniae protein (Fig. 4). Similar results were also seen when IL-1β and IL-8 were measured (results not shown). The effect on the H. pylori protein was minimal (result not shown).
Figure 4.
Inhibition of IL-6 production by the addition of anti-TLR4 antibody. PBMCs were preincubated for 2 hr either with anti-TLR4 antibody HTA125, or an irrelevant isotype control. The cells were then stimulated with either human Cpn60 or Chlamydia pneumoniae Cpn60. The anti-TLR4 antibody reduces the stimulatory effect of both the human and C. pneumoniae Cpn60 proteins. Each data point represents the mean ± standard errors for three replicates from a representative experiment. These experiments were performed three times using blood from different donors.
Induction of vascular endothelial cell adhesion proteins
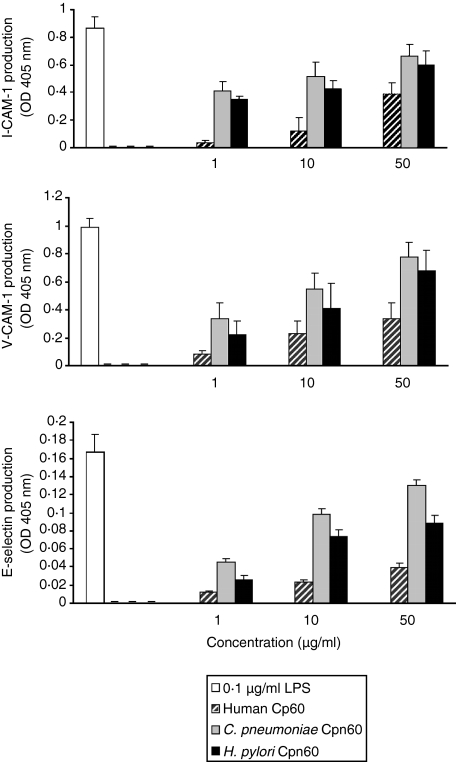
All three Cpn60 proteins had the ability to induce vascular endothelial cells to synthesize E-selectin, ICAM-1 and VCAM-1. Again, the human Cpn60 was the weakest agonist for all three adhesion proteins. However, in contrast to the activation of monocytes, both the C. pneumoniae and H. pylori Cpn60 proteins were equally active and could induce maximum synthesis of all three adhesion proteins (Fig. 5).
Figure 5.
Induction of vascular endothelial cell proteins by the three Cpn60 proteins. EAhy926 cells were incubated for 18 hr either with 0·1 μg/ml LPS (far left column on graph) or with each of the three Cpn60 proteins. Each data point represents the mean ± standard error for three replicates from a representative experiment. This experiment was performed three times.
Discussion
It has long been the dogma one gene–one protein–one function. However, this hypothesis is being disproved by the number of proteins being discovered that have more than one function. Such proteins have been named ‘moonlighting proteins’.19,20 Evidence emerging over the past decade suggests that the group of intracellular proteins known variously as heat-shock proteins, cell-stress proteins, or molecular chaperones (although the terms may not be synonymous) are also capable of moonlighting. Thus, in addition to protein folding within intracellular compartments, these proteins can modify immune responses by chaperoning antigenic peptides and/or they can themselves be processed and presented to T cells.21 In addition, and potentially more controversially, a number of molecular chaperones have been reported to act as intercellular signalling molecules with the capacity to modulate immune and inflammatory responses.21 The early studies in this field were carried out using bacterial preparations of molecular chaperones and suggested that these proteins could act as bacterial virulence factors.22 Much attention has focused on the 60 000 molecular weight tetradecameric protein known as Cpn60.5,23 The chaperonins are highly conserved proteins and the expectations were that all Cpn60 proteins, irrespective of source, should have the same basic biological activity by signalling through a common receptor. This has not proved to be the case.5,23 Moreover, it has been found that the human Cpn60 protein functions as a signalling protein.24–26 Comparisons of bacterial and human Cpn60 proteins by different workers have resulted in a confusing picture in which some proteins interact with myeloid cell CD14, others interact with TLR2 or TLR4 and yet others fail to interact with any of these receptors or, indeed, fail to activate myeloid cells (Table 2). A similar situation exists with hsp 70 where a number of receptors have been implicated in cell binding and activation including CD4027 and lectin-like oxidised low-density lipoprotein receptor (LOX)-1.28
Table 2. Comparison of myeloid modulating actions of chaperonin 60 proteins from diverse sources.
| Species | Putative receptor | Myeloid cell activation | Ref. |
|---|---|---|---|
| Mycobacterium tuberculosis Cpn60·1 | CD14 | activates | 7 |
| Mycobacterium tuberculosis Cpn60·2 | unknown (not CD14) | activates | 7 |
| Mycobacterium leprae Cpn60.2 | unknown | activates | unpublished |
| Actinobacillus actinomycetemcomitans Cpn60 | not TLR4 | activates | 6 |
| Escherichia coli GroEL | unknown (not CD14) | activates | 18 |
| Chlamydia trachomatis Cpn60.1 | CD14/TLR4/MD2 | activates | 8 |
| Chlamydia pneumoniae | TLR2 | activates | 31 |
| Rhizobium Cpn60.1 | ? | fails to activate | 44 |
| Rhizobium Cpn60.3 | CD14 | activates | 44 |
| Helicobacter pylori Cpn60 | unknown (not TLR2/TLR4) | activates | 10 |
| Human Cpn60 | CD14/TLR2 and/or TLR4 | activates | 24–26 |
The diversity of receptors for these various preparations of cell-stress proteins has resulted in a lack of confidence in the results being presented and has led to the speculation that the findings are the result of contaminants in these protein preparations. It has been known for years that the prototypic Cpn60 protein –E. coli GroEL – is heavily contaminated with other proteins.14,15 These are the proteins which are associating with the Cpn60 protein within the bacterium's cytosol at the time of purification. We have shown that similar protein contamination is found with other bacterial and human Cpn60 proteins expressed as recombinant proteins in E. coli.18 To remove these proteins we have modified a dye-binding purification method for GroEL29 which involves the Ni-NTA-purified Cpn60 protein being applied to a reactive-red column in the presence of ATP. This results in the sequestration of contaminating proteins/peptides and the elution of a homogeneous Cpn60 protein.17,18 This process alone resulted in the removal of LPS. To remove the majority of the LPS we wash the recombinant Cpn60 protein, while it is on the Ni-NTA column, with a high concentration of polymyxin B, an antibiotic that binds strongly to the lipid A component of LPS. This significantly lowers the LPS concentration in the final preparations to very low levels18 and prevents the major losses of protein that occur if the Cpn60 protein is passed over a polymyxin B column to remove LPS. The activity of the final preparations of the Cpn60 proteins was heat labile and proteinase K-sensitive, ruling out non-proteinaceous components, such as LPS, as being responsible for cellular activation. In addition, the fact that all three preparations of Cpn60 proteins have similar levels of LPS contamination but very different potencies also demonstrates that the activity of these proteins is not the result of LPS.
In the present study we have prepared recombinant highly purified Cpn60 proteins from Homo sapiens, C. pneumoniae and H. pylori. These three Cpn60 proteins have been studied in greater detail than other members of this protein family. One significant reason for this is that these proteins have been implicated in the pathogenesis of atherosclerosis.30 There is controversy in the literature about the nature of the receptors used by these various preparations of Cpn60 proteins in cell activation. The human Cpn60 protein has been claimed to activate cells by binding to CD14,24 TLR4,25 TLR2,26 or a combination of TLR2 and TLR4.9 The Cpn60 protein from C. pneumoniae has been claimed to bind to CD14,24 TLR4,8 or TLR2.31 In contrast the receptor for the H. pylori Cpn60 protein has been reported to be distinct from TLR2 and TLR4.10
Using standardized preparations of the three recombinant Cpn60 proteins, and using human PBMCs as a relevant source of activatable cells, we have found substantial differences in the activity of these three proteins. Using a number of preparations of human PBMCs the human Cpn60 protein was always found to be the least active of these three chaperonins. Indeed, in a previous publication, in which we compared a number of commercially available human Cpn60 preparations, we found that the majority of the activity of human Cpn60 was the result of contaminating LPS.32 In the present study, the C. pneumoniae Cpn60 protein was slightly more potent and efficacious than the human protein. However, the H. pylori Cpn60 protein was the most active, being significantly more potent and efficacious than the other two chaperonins. This was most marked with the induction of IL-8 synthesis. Efficacy or intrinsic activity is a pharmacological concept which relates to the magnitude of the biological response to an agonist at a receptor.33 Current views suggest that efficacy is not simply a property of the interaction of a ligand with its receptor but also depends upon the nature of the native receptor ensemble.34
To determine the nature of the receptor for these highly purified Cpn60 proteins use was made of well-established neutralizing antibodies to TLR2 or TLR4 to identify if one or both of these bacterial recognition proteins played a role in cell activation. It was established that only the anti-TLR4 antibody neutralized the activity of LPS. The anti-TLR2 antibody had no inhibitory effect on any of the three recombinant Cpn60 proteins. In contrast, the anti-TLR4 antibody almost completely inhibited the activity of the human Cpn60 protein and significantly inhibited the C. pneumoniae Cpn60. However, and confirming the work of Fererro's group10 who used native purified H. pylori Cpn60, the anti-TLR4 antibody had no effect on the activity of the recombinant H. pylori Cpn60. These findings either support the hypothesis that the receptor for the H. pylori protein is some other cell surface protein or proteins independent of the CD14/TLR2/TLR4 complex or they support the hypothesis that the H. pylori Cpn60 interacts with this receptor in a manner that is unaffected by the antibodies used. The finding that the anti-TLR4 antibody only partially inhibited (around 60% in repeat experiments) the action of the C. pneumoniae hsp 60 suggests that this protein may interact both with TLR4 and with other receptors such as the receptor(s) for the H. pylori hsp 60. There is evidence that H. pylori Cpn60 is used by this organism as an adhesin.35 Indeed, this is emerging as one of the moonlighting functions of bacterial Cpn60 proteins.36,37 Could such adhesion receptors for these bacterial Cpn60 proteins on human cells be transducing the binding signal and causing cytokine synthesis?
As noted above, there is a very plausible hypothesis linking immunity to bacterial (particularly C. pneumoniae and H. pylori) Cpn60 proteins in the autoimmune pathogenesis of atherosclerosis. In the most recent recasting of this hypothesis the direct role played by Cpn60 proteins in activating vascular endothelial cells is emphasized.38 Various preparations of Cpn60 proteins from Mycobacterium tuberculosis,39 E. coli,40 C. pneumoniae41 and Homo sapiens41 have been reported to activate vascular endothelial cells to synthesize and express cell surface adhesion proteins, including E-selectin, ICAM-1 and VCAM-1. Only one comparative study of Cpn60 proteins has been made and this suggested that human and C. pneumoniae Cpn60 proteins were equally active in stimulating endothelial cell adhesion protein synthesis.41 Using our protein preparations for comparative purposes two points quickly became evident. The first was that the human vascular endothelial cell line, EAhy926, was more responsive to Cpn60 proteins than were monocytes. The second was that the human Cpn60 protein was significantly less active as an inducer of adhesion protein synthesis than were the two bacterial proteins. Thus, maximum induction of ICAM-1 synthesis was with 1–10 μg/ml H. pylori or C. pneumoniae Cpn60. In contrast, even at 50 μg/ml, the human equivalent did not stimulate maximum synthesis. It is intriguing that the C. pneumoniae Cpn60 should be so relatively inactive as a monocyte activator yet be such a potent activator of this vascular endothelial cell line. Indeed, this is the first report of the interaction of H. pylori Cpn60 with vascular endothelial cells and reveals that this protein is a potent activator of these cells showing maximum activity (assuming activity is the result of the oligomeric structure) at concentrations of 1–10 nm. This is similar to the potency of pro-inflammatory cytokines such as tumour necrosis factor-α. Again, the differences in the potency of these proteins is not reflected by the (low) levels of LPS contamination.
Having cloned the cpn60 genes from three organisms, and expressed and purified to homogeneity the three recombinant proteins, it is clear that in spite of the significant sequence conservation these Cpn60 molecules exhibit differences in potency, efficacy and apparent receptor binding. In both the PBMC and endothelial cell assays the human protein has the weakest agonist activity. The most active of the three proteins as PBMC activators is the H. pylori protein. With the vascular endothelial cells the two bacterial proteins are equally active. The explanation for this pattern of activity is not immediately clear. The antibody neutralization studies suggest that the H. pylori protein does not interact with TLR4 or with that part of TLR4 that the neutralizing antibody binds to. The most compelling evidence that Cpn60 proteins bind to different receptors is the work of Kolb and co-workers who have shown that the commercially available human, rat, or mouse Cpn60 proteins do not cross-compete with hamster or bacterial Cpn60 proteins for binding to murine monocytes.42 This suggests that there has been a co-evolution of interaction between bacterial and mammalian Cpn60 proteins such that multiple receptors are available to bind to Cpn60 proteins from different species. The advantage to the prokaryotic and eukaryotic partners of this evolutionary diversity of the recognition of Cpn60 proteins is not clear and will require much more precise elucidation of both the panoply of the host receptors for the chaperonin proteins and the structure–function relationships of the Cpn60 proteins. The finding that single residue mutations in GroEL can result in this protein gaining insect neurotoxic activity43 reveals the potential complexity of the structure–function relationships of this family of moonlighting proteins.
Acknowledgments
We are grateful to Dr Martyn Robinson, Celltech Therapeutics for providing monoclonal antibodies to the vascular cell adhesion proteins. M.M. was supported by a grant from the British Heart Foundation (grant PG/1999117) to B.H. and A.R.M.C.
Abbreviations
- Cpn
chaperonin
- LOX
lectin-like oxidised low-density lipoprotein receptor
- TLR
Toll-like receptor
- VCAM
vascular cell adhesion molecule
References
- 1.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–8. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 2.Hemmingsen SM, Woolford C, van der Vies SM, et al. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 2001;333:330–4. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 3.Young DB, Ivanyi J, Cox JH, Lamb JR. The 65 kDa antigen of mycobacteria – a common bacterial protein? Immunol Today. 1987;8:215–19. doi: 10.1016/0167-5699(87)90168-X. [DOI] [PubMed] [Google Scholar]
- 4.Karlin S, Brocchieri L. Heat shock protein 60 sequence comparisons: duplications, lateral transfer, and mitochondrial evolution. Proc Natl Acad Sci U S A. 2000;97:11348–53. doi: 10.1073/pnas.97.21.11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire M, Coates ARM, Henderson B. Chaperonin 60 unfolds its secrets of cellular communication. Cell Stress Chaperones. 2002;7:317–29. doi: 10.1379/1466-1268(2002)007<0317:cuisoc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby AC, Meghji S, Nair SP, et al. The potent bone resorbing mediator of Actinobacillus actinomycetemcomitans is homologous to the molecular chaperone, GroEL. J Clin Invest. 1995;96:1185–94. doi: 10.1172/JCI118150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewthwaite JC, Coates ARM, Tormay P, et al. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (hsp 65) and contains a CD14-binding domain. Infect Immun. 2001;69:7349–56. doi: 10.1128/IAI.69.12.7349-7355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulut Y, Faure E, Thomas L, et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88 dependent pathway. J Immunol. 2002;168:1435–40. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- 9.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 10.Gobert AP, Bambou JC, Werts C, Balloy V, Chignard M, Moran AP, Ferrero RL. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a Toll-like receptor (TLR) -2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J Biol Chem. 2004;279:245–50. doi: 10.1074/jbc.M307858200. [DOI] [PubMed] [Google Scholar]
- 11.Bausinger H, Lipsker D, Ziylan U, et al. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur J Immunol. 2002;32:3708–13. doi: 10.1002/1521-4141(200212)32:12<3708::AID-IMMU3708>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem. 2003;278:22523–39. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- 13.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–44. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 14.Price N, Kelly SM, Wood S, auf de Mauer A. The aromatic amino acid content of the bacterial chaperone protein groEL (cpn60). evidence for the presence of a single tryptophan. FEBS Lett. 1991;293:9–12. doi: 10.1016/0014-5793(91)80821-j. [DOI] [PubMed] [Google Scholar]
- 15.Hayer-Hartl MK, Hartl F-U. A comment on ‘The aromatic amino acid content of the bacterial chaperone protein groEL (cpn60): evidence for the presence of a single tryptophan’ by N.C. Price, S.M. Kelly, S. Wood, and A. aur de Mauer (FEBS Lett 1991;293:9–12) FEBS Lett. 1991;320:139–41. doi: 10.1016/0014-5793(91)80821-j. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 17.Tabona P, Reddi K, Khan S, et al. Homogeneous Escherichia coli chaperonin 60 induces IL-1 and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J Immunol. 1998;161:1414–21. [PubMed] [Google Scholar]
- 18.Maguire M, Coates ARM, Henderson B. Cloning, expression and purification of three chaperonin 60 homologues. J Chromatogr. 2003;786:117–27. doi: 10.1016/s1570-0232(02)00732-8. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 20.Jeffery CJ. Multifunctional proteins: examples of gene sharing. Ann Med. 2003;35:28–35. doi: 10.1080/07853890310004101. [DOI] [PubMed] [Google Scholar]
- 21.Panayi GS, Corrigall VM, Henderson B. Stress cytokines: pivotal proteins in immune regulatory networks. Curr Opin Immunol. 2004;16:531–4. doi: 10.1016/j.coi.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Lewthwaite J, Skinner A, Henderson B. Are molecular chaperones microbial virulence factors? Trends Microbiol. 1998;6:426–8. doi: 10.1016/s0966-842x(98)01362-6. [DOI] [PubMed] [Google Scholar]
- 23.Henderson B. Chaperonins: chamelion proteins that influence myeloid cells. In: van Eden W, editor. Heat Shock Proteins and Inflammation. Basle: Birkhauser; 2003. pp. 175–92. [Google Scholar]
- 24.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 26.Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–9. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Kelly CG, Karttunen JT, et al. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity. 2001;15:971–83. doi: 10.1016/s1074-7613(01)00242-4. [DOI] [PubMed] [Google Scholar]
- 28.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 29.Clark AC, Hugo E, Frieden C. Determination of regions in the dihydrofolate reductase structure that interact with the molecular chaperonin GroEL. Biochemistry. 1996;35:5893–901. doi: 10.1021/bi953051v. [DOI] [PubMed] [Google Scholar]
- 30.Mayr M, Kiechl S, Willeit J, Wick G, Xu Q. Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation. 2000;102:833–9. doi: 10.1161/01.cir.102.8.833. [DOI] [PubMed] [Google Scholar]
- 31.Costa CP, Kirschning CJ, Busch D, et al. Role of chlamydial heat shock protein 60 in the stimulation of innate immune cells by Chlamydia pneumoniae. Eur J Immunol. 2002;32:2460–70. doi: 10.1002/1521-4141(200209)32:9<2460::AID-IMMU2460>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Meghji S, Lillicrap M, Maguire M, Tabona P, Gaston JSH, Poole S, Henderson B. Human chaperonin 60 (Hsp60) stimulates bone resorption: structure/function relationships. Bone. 2003;33:419–25. doi: 10.1016/s8756-3282(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 33.Kenakin T. Agonist-receptor efficacy. I. Mechanisms of efficacy and receptor promiscuity. Trends Pharmacol Sci. 1995;16:188–92. doi: 10.1016/s0165-6147(00)89020-3. [DOI] [PubMed] [Google Scholar]
- 34.Weiss JM, Morgan PH, Lutz MW, Kenakin TP. The cubic ternary complex receptor-occupancy model. III. resurrecting efficacy. J Theor Biol. 1996;181:381–97. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi H, Osaki T, Kurihara N, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Heat-shock protein 60 homologue of Helicobacter pylori is associated with adhesion of H. pylori to human gastric epithelial cells. J Med Microbiol. 1997;46:825–31. doi: 10.1099/00222615-46-10-825. [DOI] [PubMed] [Google Scholar]
- 36.Hennequin C, Porcheray F, Waligora-Dupriet A, Collignon A, Barc M, Bourlioux P, Karjalainen T. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology. 2001;147:87–96. doi: 10.1099/00221287-147-1-87. [DOI] [PubMed] [Google Scholar]
- 37.Wampler JL, Kim KP, Jaradat Z, Bhunia AK. Heat shock protein 60 acts as a receptor for the Listeria adhesion protein in Caco-2 cells. Infect Immun. 2004;72:931–6. doi: 10.1128/IAI.72.2.931-936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 39.Verdegaal ME, Zegveld ST, van Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;157:369–76. [PubMed] [Google Scholar]
- 40.Galdiero M, de l'Ero GC, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect Immun. 1997;65:699–707. doi: 10.1128/iai.65.2.699-707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–7. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Habich C, Kempe K, van der Zee R, Burkart V, Kolb H. Different heat shock protein 60 species share pro-inflammatory activity but not binding sites on macrophages. FEBS Lett. 2003;533:105–9. doi: 10.1016/s0014-5793(02)03772-9. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida N, Oeda K, Watanabe E, Mikami T, Fukita Y, Nishimura K, Komai K, Matsuda K. Protein function. Chaperonin turned insect toxin. Nature. 2001;411:44. doi: 10.1038/35075148. [DOI] [PubMed] [Google Scholar]
- 44.Lewthwaite JC, George R, Lund PA, Poole S, Tormay P, Sharp L, Coates ARM, Henderson B. Rhizobium leguminosarum chaperonin 60.3, but not chaperonin 60.1, induces cytokine production by human monocytes: activity is dependent on interaction with cell surface CD14. Cell Stress Chaperones. 2002;7:130–6. doi: 10.1379/1466-1268(2002)007<0130:rlcbnc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]