Abstract
Mice on the CBA inbred strain background expressing the well characterized mutation designated xid in the cytoplasmic signalling enzyme Bruton's protein kinase have been previously noted to illustrate shifts in T helper type 1 (Th1)/Th2 immunity which is underlined by an apparent failure to produce the regulatory cytokine interleukin-10. In the current study we examined if this extended to infection with Mycobacterium tuberculosis, which also depends on Th1 immunity. Contrary to expectations, xid mice showed evidence of a transient early susceptibility to pulmonary infection, changes in macrophage morphology, and decreased activation of lung natural killer cells, while showing evidence of substantial IL-10 production and accumulation in lung lesions macrophages, but paradoxically this did not influence the course of the chronic disease. In addition, macrophages from the lungs of xid mice also expressed high levels of CD14. These observations suggest that the xid mutation in cellular signalling has much wider effects on the immune system than previously thought.
Keywords: interleukin-10, macrophages, natural killer cells, T helper type 1 immunity, tuberculosis, xid mutation
Introduction
It is well established that mice need to mount a T helper type 1 (Th1) response, mediated by the cytokines interleukin-12 (IL-12), IL-23, and interferon-γ (IFN-γ), to successfully express host resistance to infection with Mycobacterium tuberculosis.1–5 In contrast, a Th2 response is not protective, although it remains unclear to what extent it might interfere with the overall course of the disease. Most evidence in the mouse model favours a lack of involvement in the expression of initial protective immunity,6,7 whereas much later during the disease process lung macrophages accumulate large amounts of IL-10 in mouse strains prone to reactivation disease.8
This includes mice on the CBA background of inbred strains. In this mouse, a well characterized mutation designated xid in the cytoplasmic signalling enzyme Bruton's protein kinase is the mouse counterpart to the human immunodeficiency X-linked agammaglobulinemia.9,10 Although immunological deficiencies in this mouse mutant are primarily at the B-cell level, their lack of B-1 cells precludes the ability of the animal to make IL-10 through this cellular source. It is thought to date that this inability to make this immunosuppressive cytokine is the basis of the increased resistance of the animal to certain intracellular parasitic infections, including leishmaniasis11 mediated by an enhanced Th1 response in the xid mouse.
We show here however, that these observations do not extend to infection with M. tuberculosis. While the xid mice made a strong Th1 response in terms of cytokines and nitric oxide production, they were in fact much more susceptible over the initial stage of a pulmonary infection delivered by aerosol, although this was quickly compensated for by a strong pulmonary CD4 T-cell influx. Interestingly, differences in overall lung macrophage morphology could be seen in the xid mice, including the presence of increased amounts of IL-10. These data indicate that xid mice can still produce considerable amounts of IL-10, and this ability is retained throughout the disease process.
Materials and methods
Animals
Specific pathogen-free female, 6–8-week-old, CBA/J and CBA/CaHN/Btk-xid/J mice were purchased from Jackson Laboratories, Bar Harbor, ME. All mice were maintained under barrier conditions with sterile mouse chow and water ad libitum.
Aerosol infection with M. tuberculosis
Mycobacterium tuberculosis strain Erdman, originally obtained from the Trudeau Institute (Saranac Lake, NY), was grown in Proskauer-Beck liquid medium containing 0·05% Tween-80 to mid-log phase and then frozen in aliquots at −70° until needed. Mice were infected by procedures described previously.1 Briefly, bacterial stocks were diluted in 5 ml of sterile distilled water to 2 × 106 colony-forming units/ml and placed in a nebulizer attached to an airborne infection system (Glass-Col, Terre Haute, IN). Mice were exposed to 40 min of aerosol where approximately 100 bacteria were deposited in the lungs of each animal. Bacterial load was determined by plating whole organ homogenates onto nutrient 7H11 agar supplemented with OADC. Colonies were used after 21 days incubation at 37°.
Lung cell digestion
Mice were killed by CO2 asphyxiation, and the pulmonary cavity was opened. The lung was cleared of blood by perfusion through the pulmonary artery with 10 ml of ice cold phosphate-buffered slaine (PBS) containing 50 U/ml of heparin (Sigma, St Louis, MO). Lungs were aseptically removed, teased apart and treated with a solution of deoxyribonuclease IV (DNAse) (Sigma Chemical, 30 μg/ml) and collagenase XI (Sigma Chemical, 0·7 mg/ml) for 45 min at 37°. To obtain a single-cell suspension, the organs were gently passed through cell strainers (Becton Dickinson, Labware, Lincoln Park, NJ). The remaining erythrocytes were lysed with Gey's solution (0·15 m NH4Cl, 10 mm KHCO3) and the cells were washed with Dulbecco's modified Eagle's minimal essential medium. Total cell numbers were determined by using a Neubauer chamber.
Flow cytometric analysis of cell surface markers
Cells were labelled with the following specific antibodies; fluorescein isothiocyanate (FITC) anti-CD44, anti-Ly-6C (Gr-1) or anti-CD86, phycoerythrin (PE) anti-CD69, anti-CD45RB, anti-CD122, anti-CD14 or anti-CD86; peridinin chlorophyll protein (PercP) anti-CD4, anti-CD8, anti-CD11b or anti-CD3, and allophycocyanin (APC) anti-NK1.1, anti-CD11c or anti-CD62L. All antibodies were purchased from BD Pharmingen and were used at 0·2 μg/106 cells in PBS containing 10% mouse serum and 0·1% sodium azide for 30 min at 4°. After washing with PBS containing 0·1% sodium azide, the cells were analysed on a Becton Dickinson FACScalibur and data were analysed using cellquest software (Becton Dickinson Immmunocytometry Systems, San Jose, CA). Cells were gated on lymphocytes or macrophages based on characteristic forward and side scatter profiles. Individual cell populations were identified according to their presence of specific fluorescent-labelled antibody. All the analyses were performed with an acquisition of at least 100 000 events.
Intracellular cytokine staining
Measurement of intracellular IFN-γ was conducted by preincubating lung cells with monensin (3 μm), anti-CD3 and anti-CD28 (both at 0·2 μg/106 cells) for 4 h at 37°, 5% CO2. The cells were then stained with APC anti-NK1.1, PerCP anti-CD4 and PE anti-CD8 for 30 min, washed with PBS containing 0·1% sodium azide, fixed, and permeabilized with Perm Fix/Perm Wash (BD Pharmingen), and stained for intracellular FITC IFN-γ or FITC rat immunoglobulin G1 (IgG1) isotype control (BD Pharmingen) for a further 30 min. The antibodies were diluted in PBS containing 0·1% sodium azide and 10% normal mouse serum (Serotec, Raleigh, NC). To measure intracellular tumour necrosis factor-α (TNF-α) and IL-10, lung cells were preincubated with lipopolysaccharide (20 ng/ml) and monensin (3 μm) for 6 hr. After that the cells were surface stained for CD11c (APC) and CD11b (PerCP). Following the same protocol described above, the cells were then stained for TNF-α (FITC) and IL-10 (PE).
Real-time polymerase chain reaction (PCR) analysis of mRNA
The right apical lobe of the lung was suspended in Ultraspec (Cinna/Biotecx, Friendswood, TX), homogenized, and frozen rapidly for storage at −70°. Total cellular RNA was extracted and reverse-transcribed using murine Moloney leukaemia virus reverse transcriptase (Life Technologies). PCR was performed with specific primers and probes for IFN-γ, IL-10, IL-12p40 or TNF-α. The detection of the cytokines' mRNA was conducted using a TaqMan 7700 real-time PCR machine (Applied Biosystems, Foster City, CA). For quantification purposes, ribosomal DNA for each sample was also assayed as an endogenous normalizer. Quantification of message was conducted using the Delta Delta Ct method.
Immunohistochemistry
Formalin-fixed (10% formalin in PBS) lung tissue was embedded in paraffin and 7-μm sections were cut. Paraffin was removed using EZ-DeWax solution (BioGenex, CA, HK584–5K) and an antigen retrieval procedure was performed using the Antigen Retrieval Citra solution according to manufacturer protocol (BioGenex, San Ramon, CA, HK086-5K). Tissue endogenous peroxidase was inactivated using a peroxidase block reagent (Immunogenex, San Ramon, CA); after 5 min at room temperature the non-specific binding was blocked by incubating the sections for 30 min with 5% mouse serum (Sigma). Sections were incubated overnight at 4° with goat polyclonal antibody against murine IL-10 or goat IgG as control at 1 : 200 dilutions in PBS-bovine serum albumin (BSA; 3%). (Santa Cruz Biotechnology, Santa Cruz, CA). The sections were washed with PBS/0·5% Tween 20.
The secondary antibody, a polyclonal donkey anti-goat conjugate with horseradish peroxidase (Serotec, at 1 : 200 PBS-BSA) was incubated for 40 min at room temperature. The sections were washed with PBS/0·5% Tween 20. Specific binding of the antibodies was developed using a substrate AEC (BioGenex, San Ramon, CA) which gives a reddish colour. Sections were counterstained with haematoxylin (BioGenex, San Ramon, CA).
Statistics
Statistical significance was determined using the Student's t-test.
Results
CBA/J xid mice exhibit transient increased resistance to aerosol infection
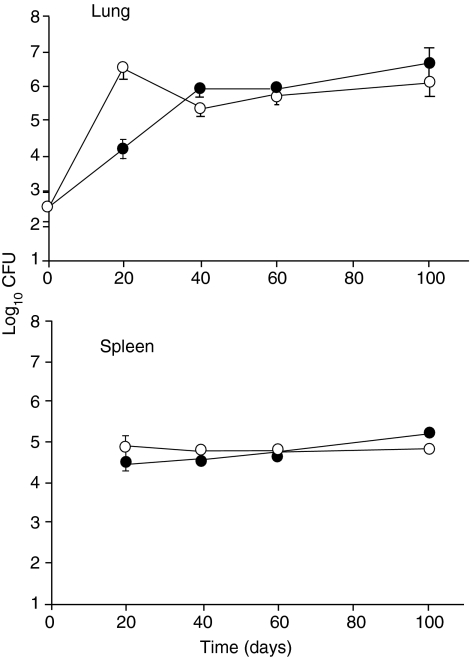
CBA/J and CBA/J xid mice were infected with M. tuberculosis and the course of the infection was followed against time. It was observed (Fig. 1) that the initial infectious load in the xid mice grew to over 100-fold higher in the lungs by day 20 compared to the CBA/J control mice. By day 40 however, the xid mice had compensated for this and the bacterial numbers in the lungs were no different to those in controls. As shown by the spleen data, the rapid early increase of bacterial numbers in the lungs of the xid mice was not associated with increased dissemination to the spleen.
Figure 1.
Course of Mycobacterium tuberculosis infection in control (•) and CBA/J xid mice (○) following low-dose aerosol exposure. Data are expressed as the mean log10 colony-forming units (CFU) per lung or spleen from five mice per group (± SD).
Histological aspects
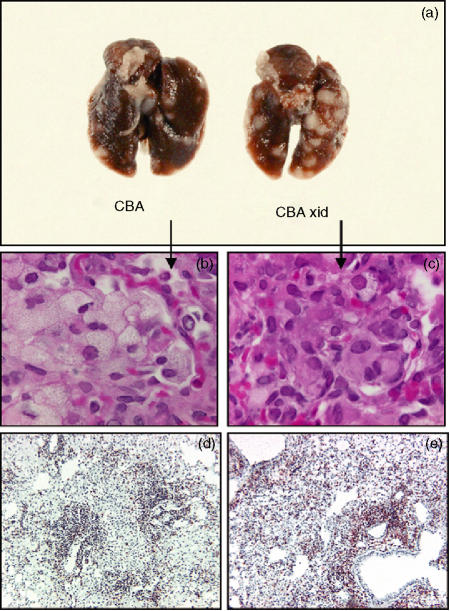
The gross appearance of the lungs of the two groups of mice is shown in Fig. 2(a) and illustrates the increased sizes and pleural aspects of the disease in the xid mice. Other aspects of the histological analysis of these tissues are also shown in this figure. One notable feature was the appearance of macrophages accumulating in lung granulomas. In the CBA/J mice many of the macrophages had a foamy or vacuolated appearance which is commonly seen in such lesions.12 In the xid mice however, this morphological appearance was rare (Fig. 2b, c). In these latter mice the lesions were further characterized by large numbers of CD4 T cells, presumably as compensation for the initial higher bacterial load (Fig. 2d, e).
Figure 2.
(a) Gross appearance of the whole lungs of representatives from the two groups 40 days after aerosol exposure illustrating the extensive lesions in the CBA/J xid mice. (b,c) Photomicrographs (magnification × 100) showing characteristic foamy macrophages in infected CBA/J mice, but a lack of this cellular morphology in the CBA/J xid mice. (d,e) Immunohistochemical staining of lung sections from CBA/J and CBA/J xid mice illustrating a substantial influx of CD4 lymphocytes in the latter on day 40. Magnification × 40.
Cellular responses
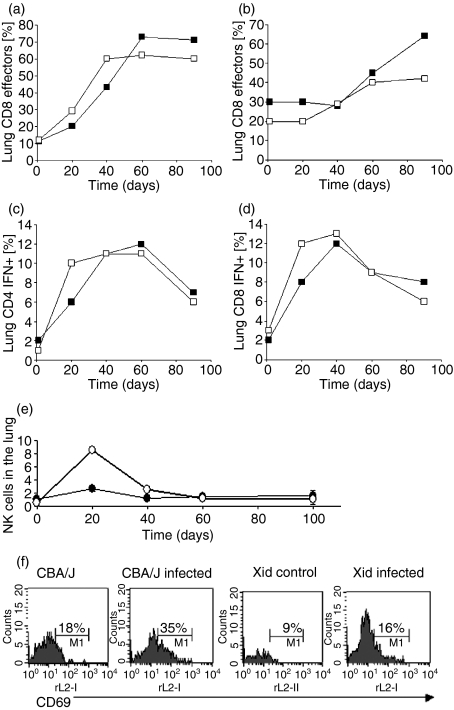
Figure 3 summarizes the results of flow cytometric analysis of cells entering the lungs in response to the aerosol infection. Effector T cells were defined as expressing CD62Llow CD44hi CD45RBmid based upon our previous analyses.13 These data further confirmed our observation of an increased CD4 T-cell influx into the lungs, with more cells producing IFN-γ early during the infection. Also noticeable was a reproducible spike in the numbers of natural killer (NK) cells entering the lungs, as well as the further observation that these cells were poorly activated in terms of their CD69 expression.
Figure 3.
Flow cytometric analysis of T cells and NK cells entering the lungs of infected mice. (a–d) Influx of CD4 and CD8 cells, including the percentages staining positive for IFNγ. CBA/J (▪) CBA/J xid (□). (e) Numbers of NK1.1+ CD3− cells over the same time-course, illustrating a considerable early spike in numbers. CBA/J (•) CBA/J xid (○). (f) Demonstration that these NK cells were far less activated in the xid mice, as determined by numbers of cells expressing the CD69 molecule.
Xid mice exhibit normal Th1 cytokine responses in the infected lungs, but also a substantially raised IL-10 response
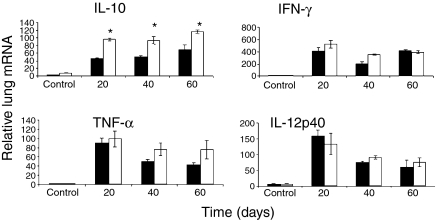
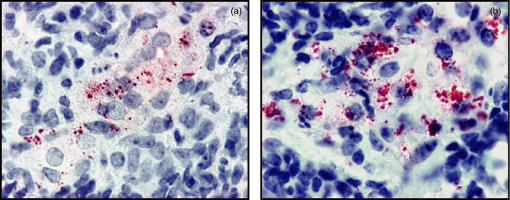
As shown in Fig. 4 the IFN-γ mRNA message in the lungs during the course of the infection was raised as would be expected, and there were similar increases in both groups in terms of TNF and IL-12 message. What was unexpected however, was the very high levels of IL-10 mRNA in the xid mice, in which production of this cytokine is supposedly diminished. To establish if message was translating into protein, we stained macrophages in granulomas for IL-10 protein and found (Fig. 5) that this was indeed the case.
Figure 4.
RNA message in the lungs determined by real time PCR analysis. Note the increase in IL-10 message throughout the study [*P < 0·01].
Figure 5.
In situ immunohistochemical detection of IL-10 protein in lung lesions of CBA mice (a) and xid mice (b) showing the intense staining in the latter 35 days after aerosol infection. Magnification × 100.
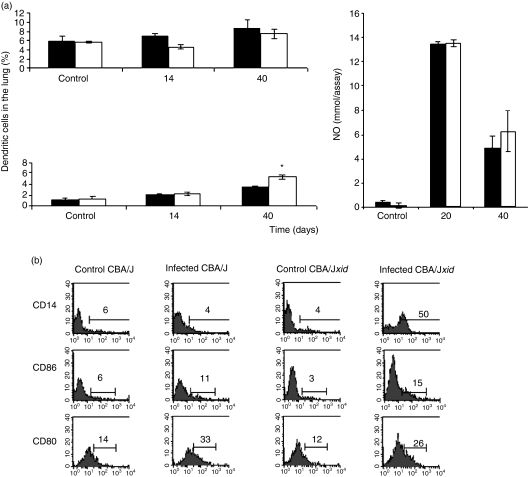
Macrophage responses
In both sets of mice, macrophages accumulated in the lungs of infected animals, and this was accentuated in xid mice at day 40 (P < 0·05). Despite the presence of the very high IL-10 levels described above, macrophages from both sets of mice secreted similar levels of nitric oxide and TNF-α(Fig. 6). Both sets of mice expressed the costimulatory molecules CD80 and CD86 to a similar extent, but interestingly the expression of CD14 was considerably increased (to 50% expression) in the xid mice.
Figure 6.
Macrophage numbers and activity in the lungs of infected animals. (a) Cell subsets were distinguished for counting by differential expression of CD11b and CD11c; alveolar macrophages in the lungs stain as CD11blow D11chigh, whereas dendritic macrophages stain CD11bhigh CD11chigh. Increases in lung macrophages and dendritic cells were noted (*P < 0·01). Nitric oxide production by macrophages from the two groups was similar. Closed bars, CBA/J; open bars CBA/J xid. (b) Flow cytometric analysis showing similar expression of costimulatory molecules, but a substantial increase in CD14 expression in the xid mice.
Discussion
The role of the xid mutation in the immune response in CBA mice to certain pathogens, particularly parasitic infections, is still not completely clear. To date it has been demonstrated14,15 that mice carrying this mutation are more susceptible to filarial worm infections because their immune response becomes skewed away from a necessary Th2 response, and this may be related to a lack of IL-10 production, primarily from B-1 B cells.14 On the other hand, in infections such as leishmaniasis in which the Th1 response is critical16–18 it has been demonstrated that lack of IL-10 in xid mice allows for a substantially enhanced Th1 response mediated by IFN-γ and a better clearance of the organism.11 The question we asked here therefore, given the similar need for Th1 immunity in the host response to M. tuberculosis, was whether xid mice would have increased resistance to this infection that could be attributed to a skewing of immunity to a stronger Th1 response.
As shown here, rather than a diminished response in the xid mice, the IL-10 response to pulmonary infection was considerably increased. This was associated with a transient increase in early susceptibility to the infection, but this was soon controlled by acquired immunity. The influx of CD4 was increased in xid mice, but this was more likely to be the result of a compensatory response to the higher bacterial load present initially in the lungs of these mice. It seems to be much less likely to be the result of a lack of IL-10, because this was in fact increased, as shown by its substantial detection in macrophages in lung lesions.
It seems apparent therefore from these results and those of others that the relative importance of the xid mutation on susceptibility or resistance probably differs depending upon the nature of the infection and the complexity of the host response to it. Infection caused by M. tuberculosis is well known to be a potent inducer of the IL-10 response and is often seen in patients with disease.19,20 In this regard, we have previously shown that mice on the CBA/J background have difficulty in sustaining the chronic disease state and undergo reactivation after about 150 days.8 Foamy macrophages dominate the lesions in the lungs at this time, and these contain high levels of IL-10. Such macrophages in xid mice are known to be more phagocytic21 and it has been suggested that B-1 B cells are a key source of IL-10 dampening this event.22,23 Although they do not contribute to protection, B-cell influx into granulomas has been documented.24 Furthermore, T cells can be a source of IL-10, particularly CD25-positive regulatory T cells, as recently demonstrated in a leishmaniasis model.25
The fact that IL-10 was produced early but had no apparent effect on Th1 immunity is consistent with studies implying that this cytokine is not important in this process during its initial stages,6,7 in turn implying that the effector cells involved either are not expressing IL-10 receptors, or do so but are refractory. Only when IL-10 appears to become stockpiled in large amounts within macrophages in lesions in the chronically infected mouse lung does this cytokine now play a key role in the disease process by promoting lesion reactivation.8 In that study macrophages became highly vacuolated (‘foamy’) through chronic activation and so it was interesting to see that this effect was absent in the xid mice early in the infection. A further disparity was seen in the NK cell response in which cells arrived but failed to activate; it is not known to date if the xid mutation affects signaling in these cells but these data may imply this. These observations, coupled with the unexplained increase in CD14 expression, suggest that the xid mutation in cellular signalling has much wider effects on the immune system than previously thought.
Acknowledgments
This work was supported by National Institutes of Health Public Health Service grants AI-40488 and AI-44072.
References
- 1.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–47. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–27. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–12. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 6.Jung YJ, Ryan L, LaCourse R, North RJ. Increased interleukin-10 expression is not responsible for failure of T helper 1 immunity to resolve airborne Mycobacterium tuberculosis infection in mice. Immunology. 2003;109:295–9. doi: 10.1046/j.1365-2567.2003.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North RJ. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–8. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, Cooper AM. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–51. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 9.Rawlings DJ, Saffran DC, Tsukada S, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–61. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 10.Ridderstad A, Nossal GJ, Tarlinton DM. The xid mutation diminishes memory B cell generation but does not affect somatic hypermutation and selection. J Immunol. 1996;157:3357–65. [PubMed] [Google Scholar]
- 11.Hoerauf A, Solbach W, Lohoff M, Rollinghoff M. The Xid defect determines an improved clinical course of murine leishmaniasis in susceptible mice. Int Immunol. 1994;6:1117–24. doi: 10.1093/intimm/6.8.1117. [DOI] [PubMed] [Google Scholar]
- 12.Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 13.Junqueira-Kipnis AP, Turner J, Gonzalez-Juarrero M, Turner OC, Orme IM. Stable T-cell population expressing an effector cell surface phenotype in the lungs of mice chronically infected with Mycobacterium tuberculosis. Infect Immun. 2004;72:570–5. doi: 10.1128/IAI.72.1.570-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Qaoud KM, Fleischer B, Hoerauf A. The Xid defect imparts susceptibility to experimental murine filariosis – association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int Immunol. 1998;10:17–25. doi: 10.1093/intimm/10.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S, Sahoo PK, George A, Bal V, Rath S, Ravindran B. Delayed clearance of filarial infection and enhanced Th1 immunity due to modulation of macrophage APC functions in xid mice. J Immunol. 1999;163:875–83. [PubMed] [Google Scholar]
- 16.Erb KJ, Blank C, Moll H. Susceptibility to Leishmania major in IL-4 transgenic mice is not correlated with the lack of a Th1 immune response. Immunol Cell Biol. 1996;74:239–44. doi: 10.1038/icb.1996.43. [DOI] [PubMed] [Google Scholar]
- 17.Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 18.Park AY, Hondowicz BD, Scott P. IL-12 is required to maintain a Th1 response during Leishmania major infection. J Immunol. 2000;165:896–902. doi: 10.4049/jimmunol.165.2.896. [DOI] [PubMed] [Google Scholar]
- 19.Gerosa F, Nisii C, Righetti S, Micciolo R, Marchesini M, Cazzadori A, Trinchieri G. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–34. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Song CH, Kim CH, et al. Profiles of IFN-gamma and its regulatory cytokines (IL-12, IL-18 and IL-10) in peripheral blood mononuclear cells from patients with multidrug-resistant tuberculosis. Clin Exp Immunol. 2002;128:516–24. doi: 10.1046/j.1365-2249.2002.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popi AF, Lopes JD, Mariano M. Interleukin-10 secreted by B-1 cells modulates the phagocytic activity of murine macrophages in vitro. Immunology. 2004;113:348–54. doi: 10.1111/j.1365-2567.2004.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S, Mohanty M, Mangla A, George A, Bal V, Rath S, Ravindran B. Macrophage effector functions controlled by Bruton's tyrosine kinase are more crucial than the cytokine balance of T cell responses for microfilarial clearance. J Immunol. 2002;168:2914–21. doi: 10.4049/jimmunol.168.6.2914. [DOI] [PubMed] [Google Scholar]
- 23.Riggs J, Howell K, Matechin B, Matlack R, Pennello A, Chiasson R. X-chromosome-linked immune-deficient mice have B-1b cells. Immunology. 2003;108:440–51. doi: 10.1046/j.1365-2567.2003.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Juarrero M, Turner OC, Turner J, Marietta P, Brooks JV, Orme IM. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect Immun. 2001;69:1722–8. doi: 10.1128/IAI.69.3.1722-1728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–9. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]