Abstract
Systemic lupus erythematosus (SLE) is characterized by the existence of a heterogeneous group of autoantibodies such as anti-DNA, chromatin, histone, and ribonucleoprotein antibodies (Abs). Although the B-cell antigenic determinants have been well characterized, very limited data about the T-cell epitopes of self-antigen (Ag) have been reported. In the present study, we analysed auto-T-cell epitopes using bone marrow-derived dendritic cells (BM-DCs) pulsed with murine U1A (mU1A) protein capable of activating autoreactive T cells from unprimed MRL/lpr mice in vitro. The data suggested that there are at least four T-cell epitopes on the U1A protein, U1A31−50, U1A61−80, U1A201−220 and U1A271−287, and U1A31−50 had the most significant T-cell proliferative response. In addition, the main responsive T cells are the CD4− CD8− double-negative subgroup of T cells. Furthermore, we also demonstrated that the activation of double-negative T cells is major histocompatibility complex class II restricted. The study here provides information on T-cell epitope analysis of the U1A antigen using BM-DCs as the effective antigen-presenting cells.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with unknown aetiology. The presence of multiple autoantibodies against nuclear components such as nucleic acid and proteins in the sera is the characteristic finding in SLE. One of the possible mechanisms for this coexistence is that a certain molecule among the composite antigens stimulates autoreactive T cells and breaks down self-tolerance.1 Thus, these activated T cells provide help to B cells, resulting in the production of autoantibodies not only the T-cell-stimulating antigen but also each member of the composite antigens.
In a recent report, Arbuckle reported that the interval between the first positive test for antinuclear ribonucleoprotein antibodies and the initial clinical manifestation of disease is significantly shorter than that for anti-Ro, anti-La, antiphospholipid and antinuclear antibodies (ANA) in human SLE.2 U1snRNP is one of the U-type small nuclear ribonucleoproteins (UsnRNPs) that constitute a spliceosome. Human SLE and MRL/lpr mice, one strain of spontaneous lupus mice, often produce immunoglobulin G (IgG) antibodies against components of the U1snRNP particle.3 U1A is the immunodominant antigen of U1snRNP, which has been demonstrated in previous studies and the same group reported that these epitopes spread into other constituents of self-UsnRNPs through intermolecule/intramolecular help.4,5 Therefore, U1A may play an important role in the initial step of SLE development. In MRL/lpr mice, massive lymphoproliferation caused by accumulation of double-negative T cells (DNT cells) bearing the CD4– CD8– TCR+ B220+ phenotype is another characteristic finding, but the role of the DNT cells remains unclear.6
In this study, we used bone marrow-derived dendritic cells (BMDCs) as powerful antigen-presenting cells, pulsed with U1A protein or peptides following by coculture with T cells to elucidate the existence of T-cell epitopes on U1A protein. We also clarified the involvement of DNT cells in epitope recognition.
Materials and methods
Mice and their serum samples
Female MRL/lpr (H-2k), C3H (H-2k) and BALB/c (H-2d) mice were obtained from and maintained by a pathogen-free facility at the Animal Center, College of Medicine, National Taiwan University. MRL/lpr mice were purchased from Jackson Laboratories (Bar Harbor, ME). BALB/c and C3H mice were of the non-autoimmune strain, and C3H mice bore major histocompatibility complex (MHC) class II molecules identical to those of MRL/lpr (H-2k) mice. Female MRL/lpr and C3H mice, 6–8 weeks of age, were used as the source of BMDCs. Mice, 3–4 months of age, were used as the source of T cells. Blood samples of about 100 μl were collected from each mouse by retro-orbital puncture at the age of 8 weeks, and thereafter regularly at 4-week intervals until the age of 16 weeks. Sera were separated from whole blood by centrifugation at 1500 g for 10 min in a microcentrifuge and ∼40 μl from each mouse at each time-point was kept frozen at −20° until use.
Immunization of the mice
MRL/lpr and C3H mice at 6–8 weeks of age were immunized intraperitoneally with Freund's complete adjuvant (FCA) (Difco Laboratories, Inc., Detroit, MI) alone or with an emulsion of 10 μg of ovalbumin (OVA) in FCA. Antigens in Freund's incomplete adjuvant (FIA) (Difco) were used for subsequent booster immunization 2–4 weeks later. After 5 days, T cells were purified for the proliferation assays.
Protein antigens and peptides
A full-length cDNA encoding murine U1A was subcloned into the vector pET28a(+) (Novagen, Madison, WI). Histidine-tagged U1A was expressed by isopropylthio-β-d-galactoside (IPTG) induction and purified on nickel-agarose (His-Bind resin), according to methods described by the manufacturer. The U1A protein was analysed for the purity on a Coomassie Brilliant Blue-stained polyacrylamide gel. The molecular weight of histidine-tagged U1A is ∼35 000. Purified U1A proteins were passed through an endotoxin column (Pierce Chemical Co., Rockford IL) to remove potentially contaminating bacterial endotoxins acquired during preparation. No proliferative response was observed when endotoxin-free U1A was cocultured with splenocytes from naïve or antigen-immunized BALB/c mice (data not shown).
The overlapping peptides of U1A were designed to be 20-amino-acid residues in length, overlapping by 10-amino-acid residues. These series of peptides, OVA323−339 and the histidine TAG control peptides (32 amino acids) were synthesized and purified by high-performance liquid chromatography (HPLC) by the Genemed Synthesis Company (South San Francisco, CA). OVA (Sigma Chemical Co., St Louis, MO) was used as a foreign antigen and was dissolved in phosphate-buffered saline and stored at −20°.
Enzyme-linked immunosorbent assay (ELISA) for determination of the level of autoantibody
Antibodies specific for U1A were measured in serum samples by using a standard ELISA, as previously described.7 Briefly, U1A protein was diluted with phosphate-buffered saline to a concentration of 5 μg/ml. After overnight incubation at 4°, the plates were washed and blocked with gelatin post-coating solution. Diluted serum was then added to the appropriate wells, which were incubated at room temperature for 2 hr. Horseradish peroxidase-conjugated goat anti-mouse γ-chain-specific antibodies (Sigma) were added. After 2 hr of incubation, 2,2′-azino-bisC3-ethylbenzthiazoline-6-sulphonic acid (ABTS) solution (Sigma) was used as substrate and the absorbance was measured at 405 nm. The level of anti-U1A IgG was calculated as ELISA units/ml (EU/ml) by comparison with the antihistidine TAG monoclonal antibody (mAb; AD 1·1·1) (Serotec Ltd, Kidlington, Oxford, UK). The absorbance value generated by 250 ng/ml of antihistidine TAG mAb was defined as 1 EU/ml of anti-U1A IgG.
Generation of dendritic cells from bone marrow cultures
BMDCs were prepared as described previously.8 Bone marrow cells from femurs and tibias were depleted of red cells by using an ACK lysis buffer. Approximately 1 × 106 of cells were placed in 24-well plates in 1 ml of medium supplemented with recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF) (750 U/ml) and interleukin-4 (IL-4; 1000 U/ml) (Pepro Tech Inc., Rocky Hill, NJ). The culture medium was RPMI-1640 supplemented with 5% heat-inactivated fetal calf serum, 4 mm l-glutamine, 25 mm HEPES (pH 7·2), 50 μm 2-mercaptoethanol and 100 U/ml of amphotericin. On alternate days, medium was aspirated off to remove lymphocytes and then fresh medium containing GM-CSF and IL-4 was added. The purity of BMDCs was > 65% reported by Suen et al.9 On day 8 of culture, non-adherent cells (BMDCs) were collected and analysed by flow cytometry for the expression of MHC class II, B7-1, B7-2, CD11c and 33D1. BMDCs were also tested for their ability to activate naïve allogeneic T cells in vitro using a mixed lymphocyte reaction assay.
BMDCs were incubated with different doses of the protein antigens (15 or 30 μg/ml) on day 6 and then harvested on day 8. For peptide pulsing, BMDCs were incubated with peptides (100 μg/ml) for 3 hr at 37° on day 8. Cells were washed extensively to remove free antigens and irradiated with 2500 rads, then resuspended in AIM-5 medium (Gibco/BRL, Gaithersburg, MD) containing 1 × TCM (mouse serum replacement; Celox, St Paul, MN) to avoid non-specific stimulation. In addition, no peptide inhibited the mitogen responses when purified T cells were cocultured with peptide-pulsed BMDCs (100 μg/ml) or with irradiated splenocytes in the presence of U1A peptides (25 μg/ml).
The enrichment of DNT cells
The splenic CD4+ T cells were acquired from splenic T cells enriched by the nylon wool method and followed by positive selection through magnetic beads coated with anti-CD4 mAbs from the Becton-Dickinson Company (Worldwide Inc., Taiwan Branch, Taiwan). The purity of CD4+ T cells was over 96% confirmed by flow cytometry (data not shown). Using a similar method with CD4+ T cells, the isolation of DNT cells was performed by negative selection with magnetic beads coated with anti-CD4 and anti-CD8 mAbs with LD column (Miltenyi Biotec, Auburn, CA) from splenic T cells enriched by the nylon wool method. These cells were stained and analysed by flow cytometry. We gated on CD3+ B220+ cells then determined the percentage of CD4– CD8– cells. The percentage of CD4+ T cells in the DNT-cell population was lower than 3% (data not shown).
Proliferation assays
Responder T cells were purified by either nylon wool alone or followed by magnetic-activated cell sorter (MACS) methods. The enriched non-B cells, isolated by passing splenocytes over nylon wool columns, were incubated at 37° for 1 hr to remove macrophages. The purity of these T cells was analysed by flow cytometry: there were < 5% B cells and > 80%T cells. Purified T cells (1 × 105−2 × 105 cells/well) were cocultured with BMDCs (2500 cells/well) in the presence or absence of anti-IAd (ANS-32·1; PharMingen, San Diego, CA) or anti-IAk (11-5·2; PharMingen) for 4 or 5 days. The T-cell proliferation assays were conducted 4–7 days after coculture of purified T cells and syngeneic BMDCs. When the optimal proliferation appeared at 4–6 hr of culture, 1 μCi of [3H]thymidine was added to each well. The cells were collected onto glass-fibre filters using an automated multisample harvester. [3H]Thymidine incorporation was then measured in a dry scintillation counter (Packard Instrument Co., Meridan, CT). The stimulation index (SI) was calculated by dividing the mean counts per minute (c.p.m.) incorporated in cultures of T cells plus antigen-pulsed BMDCs (in the presence or absence of blocking mAb) by the mean c.p.m. in control cocultures of T cells plus non-antigen-pulsed BMDCs. A positive response was defined as an SI of > 2·0.
Statistical analysis
We used the Wilcoxon test to identify significant differences in the level of anti-U1A IgG in MRL/lpr mice of different ages. The Mann–Whitney U-test was used to identify statistically significant differences in the proliferation assays between different groups. A P-value of < 0·01 was considered to be statistically significant.
Results
Development of specific antibody response to U1A protein and dsDNA in MRL/lpr mice
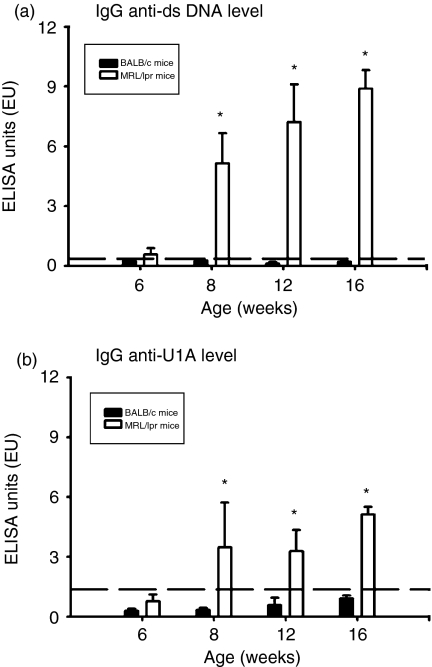
Because the objective of this study was to investigate the role of U1A protein in the pathogenesis of murine lupus, we first checked for the existence of antibodies to U1A at different time-points. Because anti-U1A IgG is non-existent in MRL/lpr mice at 6 weeks of age but had appeared by 8 weeks of age in our experiments, mice were bled monthly, starting at 6 weeks of age and the anti-dsDNA IgG levels in their sera were determined by ELISA. As shown in Fig. 1(a), anti-dsDNA IgG was detected at significant levels in MRL/lpr mice from 8 weeks of age up to 16 weeks of age, although this was not found in age-matched BALB/c mice (P < 0·01). In addition, the levels of anti-U1A IgG at different time-points had a similar pattern to anti-dsDNA IgG as shown in Fig. 1(b). This antibody was also detected at significant levels in MRL/lpr mice from 8 weeks of age to 16 weeks compared to age-matched BALB/c mice (P < 0·01). Therefore, the concentrations of anti-dsDNA and anti-U1A IgG were significantly elevated from 8 weeks to 16 weeks of age. According to a previous description of the reciprocal T-B-determinant spreading in SLE,10–14 T cells that specifically recognize U1A protein can be activated when the disease initiates and spreads to systemic organ systems in lupus-prone MRL/lpr mice.
Figure 1.
The level of autoantibodies in MRL/lpr mice over time with age. Sera obtained from five BALB/c and five MRL/lpr mice at different time-points were tested for anti-dsDNA IgG (a) and anti- U1A IgG (b) by ELISA. Sera were diluted 1 ≕ 100 for detecting these two kinds of autoantibodies. Values that were greater than the mean + 3SD (horizontal dash line) from 4-month-old BALB/c mice (n = 5) were regarded as positive. *Indicates P < 0·01 when compared to age-matched BALB/c mice.
T cells show the proliferative response to U1A protein presented by BMDCs in vitro in MRL/lpr mice but not in C3H mice
The potential role of BMDCs as the antigen-presenting cells has been shown in the report by Suen et al. in 2001.9 They demonstrated that antigen-specific T cells isolated from DBA-2 × NZW F1 mice responded to antigen-pulsed syngeneic BMDCs in vitro.
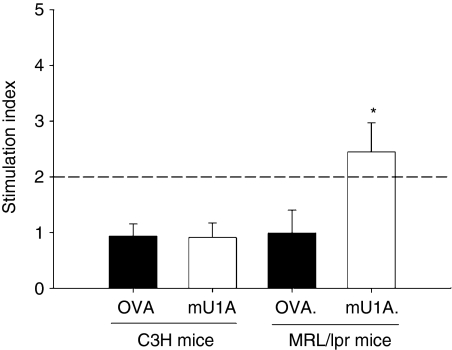
To determine the existence of U1A-specific T cells in MRL/lpr mice, the different concentrations of U1A were presented by syngeneic BMDCs and then cocultured with freshly isolated splenic T cells, then the existence of a proliferative response was detected by [3H]thymidine incorporation. When BMDCs pulsed with 15 μg/ml U1A protein, no T-cell proliferative responses were detected in C3H mice but were detected in three of five MRL/lpr mice (Fig. 2). When we increased the concentration of U1A protein to 30 μg/ml, there was still no T-cell proliferative response in C3H mice. However, four of five MRL/lpr mice developed a significant T-cell proliferative response to U1A protein (data not shown).
Figure 2.
The T-cell proliferation against U1A protein by syngeneic bone marrow-derived dendritic cells (BMDCs). T cells from C3H (a) or MRL/lpr mice (b) at 3–4 months of age were cocultured with OVA (10 μg/ml) or U1A (10 μg/ml) -pulsed syngeneic BMDCs for 5 days. The data are presented as mean ± SD, n = 3 in C3H mice, n = 5 in MRL/lpr mice. These results were obtained from two independent experiments. When SI (stimulation index) was > 2 (horizontal line), we referred to it as positive. *Indicates P < 0·01 when compared to mU1A on C3H mice group.
Auto-T-cell epitopes in U1A protein are found in MRL/lpr mice, but not in C3H mice
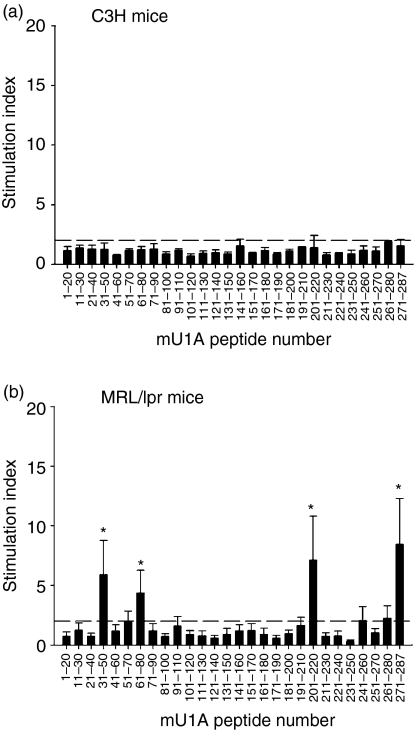
Next, we identified the T-cell-epitope(s) of U1A protein. A panel of 20-mer peptides was synthesized, each overlapping its neighbour by 10 amino acids and spanning the entire sequence of the U1A protein. BMDCs pulsed with peptides were then cocultured with splenic T cells enriched by the nylon wool method from MRL/lpr and C3H mice. As shown in Fig 3, T cells from some MRL/lpr mice proliferated significantly after BMDCs were pulsed with peptides 4, 7, 21 and 28, U1A31−50, U1A61−80, U1A201−220 and U1A271−287, respectively (Fig. 3b). There was no T-cell proliferative response to U1A peptides in all of the C3H mice (Fig. 3a). The control peptide OVA323−339, a known IAd-binding peptide, also did not elicit any proliferative response in MRL/lpr and C3H mice. Therefore, auto-T-cell epitopes in U1A protein can be found in MRL/lpr mice but not in C3H mice.
Figure 3.
Identification of auto-T-cell epitopes in the U1A protein by using bone marrow-derived dendritic cells (BMDCs) as antigen-presenting cells. T cells from C3H (a) or MRL/lpr mice (b) at 3–4 months of age were cocultured with overlapping peptides of U1A-pulsed syngeneic BMDCs (100 μg/ml). Results are expressed as mean ± SD, n = 3 in C3H mice, n = 4 in MRL/lpr mice. These results were obtained from two independent experiments. Dotted horizontal line indicates an SI of 2·0; *indicates P < 0·01 when compared to each peptide on C3H mice group.
DNT cells as the major responders to U1A peptide
When autoimmune disease developed in MRL/lpr mice, lymphoproliferation with DNT-cell accumulation was obviously noted. Therefore, we tried to examine whether the CD4+ T cells or DNT cells could recognize and respond to U1A peptide(s).
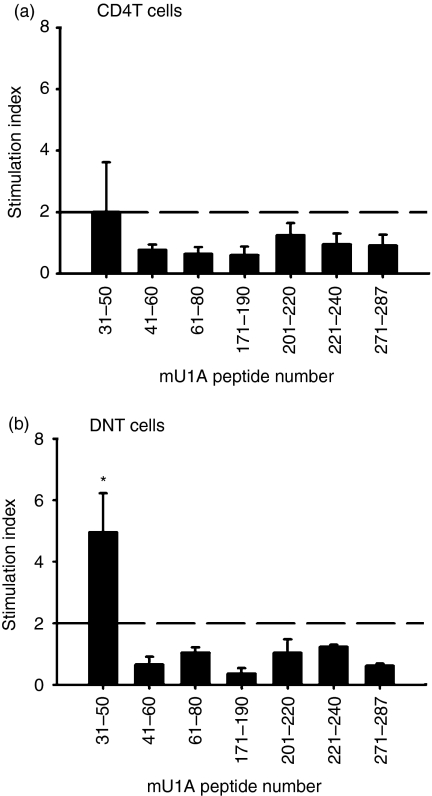
When BMDCs were pulsed with U1A31−50 cells, the proliferative response was induced in both CD4+ T cells and DNT cells in MRL/lpr mice, as shown in Fig. 4; they did not respond to other peptides. Thus we suggested that U1A31−50 was the immunodominant T-cell epitope of U1A in MRL/lpr mice. CD4+ T cells recognized and proliferated to U1A31−50 were only found in two of five mice (Fig. 4a). However, the same response was noted in all four mice, as shown in Fig. 4(b). Among the T-cell repertories, the major responders to U1A31−50 may be the subgroup of DNT cells.
Figure 4.
Identification of auto-T cell epitopes in the U1A protein recognized by CD4T cells or DNT cells by using bone marrow-derived dendritic cells (BMDCs) as antigen-presenting cells. CD4T cells (a) or DNT cells (b) were acquired from MRL/lpr mice at 3–4 months of age and cocultured with some peptides of U1A-pulsed syngeneic BMDCs. The data are shown as mean ± SD, n = 5 in CD4T cells group, n = 4 in DNT cells group. These results were obtained from two independent experiments. The dotted horizontal line indicates an SI of 2·0; *represents P < 0·01 when compared to each peptide on CD4+T-cell group.
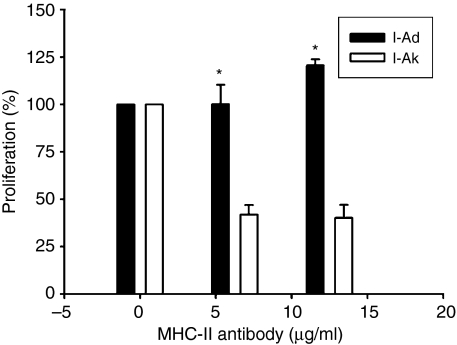
The response of DNT cells to U1A31−50 was MHC class II restricted
Next, we assessed whether the response of DNT cells to U1A31−50 was MHC class II restricted or not. After preparation of BMDCs and DNT cells, anti-MHC class II mAbs were added with BMDCs at 37° for 30 min before coculturing with DNT cells. The c.p.m. when BMDCs were pulsed with U1A31−50 alone without MHC blocker was classed as 100% and the other data were presented as per cent proliferation in Fig. 5. Adding the anti-IAk antibodies inhibited the proliferative response of DNT cells to U1A31−50 greatly, but not completely, c. 60%. Adding anti-IAd antibodies at a lower dose did not cause any inhibition. In contrast, adding anti-IAd antibodies in a higher dose slightly enhanced the proliferative response. According to our results, we considered that the response of the DNT cells to U1A31−50 was MHC class II restricted.
Figure 5.
The per cent of DNT cell proliferation in response to coculture with U1A31−50-pulsed bone marrow-derived dendritic cells (BMDCs) together with MHC class II blocker, I-Ad (act as negative control) and I-Ak. The concentrations of MHC class II blocker were 6·25 and 12·5 μg/ml. The data were shown as the c.p.m. of T-cell proliferation in groups with MHC class II blocker added or not. (The value of per cent of proliferation was defined as 100% in DNT cells cocultured with U1A31−50-pulsed BMDCs alone). Results from three mice were presented as mean ± SD. *Indicates P < 0·01 when compared to that of I-Ad control.
Discussion
The major targets in human and murine SLE are located in the nucleus, for example SnRNP and the anti-SnRNP antibody appear early, before autoimmune disease develops. As we know, U1A protein has been reported as the immunodominant portion of SnRNP. In our results, the anti-U1A levels were significantly elevated in MRL/lpr mice from 8 weeks of age. At that time, proteinuria, splenomegaly and lymphoproliferation were still not developed. Thus, U1A protein might be one of the initial targets in the development of SLE.
Since these pathogenic autoantibodies are mostly of the IgG isotype, it is likely that T cells are essentially providing help to the autoantibody-producing B cells. Evidence for T-cell involvement in disease pathogenesis is illustrated by the association of SLE with certain MHC class II alleles and affinity maturation of IgG autoantibody production.15 Therefore, tolerance to self-antigens was broken down and autoreactive T cells were activated, resulting in sequential immune responses that damage the systemic tissues. Thus, to clarify the T-cell epitopes on important targets, antigens became critical for the further understanding of the pathogenic mechanisms of lupus. In 1993, Fatenejad et al. reported the discovery of epitopes within amino acids 161–220 and 221–287 of U1A protein in MRL/lpr mice.4 They strongly suggested that intact U1 particles might be targets of the immune response in this disease. Our data from MRL/lpr mice revealed four T-cell epitopes on U1A protein. They were U1A31−50, U1A61−80, U1A201−220 and U1A271−287. According to the results of T-cell proliferation studies, U1A31−50 had the most significant T-cell proliferative response. The result here is actually similar to the data published by another group.16 They demonstrated a subset of lupus patients with anti-nRNP A reactivity producing autoantibodies primarily against two major epitopes of nRNP A (also known as U1A in this study). These sequences span amino acids 44–56 (A3 epitope) and amino acids 103–115 (A6 epitope) by analysis with maximally overlapping octapeptides using solid-phase ELISA. In a later study, they reported that immunization of animals with the A3 peptide of nRNP A, but not the A6 peptide, induced an extensive immune response against multiple snRNP antigens similar to that seen in human disease. In addition, animals immunized with A3 peptide develop significantly impaired renal function and leucopenia, but those immunized with A6 peptide did not.17 The suggested explanation for this phenomenon arises from the three-dimensional structure of nRNP A. The A3 epitope is postulated on the surface of the molecules in vivo and is probably available to be bound by specific antibodies, but the A6 epitope is masked in the crystallized structure. They therefore concluded that structural availability influences the capacity of autoantigenic epitopes to induce a widespread lupus-like autoimmune response. We still do not have the complete crystallized structure of murine U1A, the above evidence may help to explain the possible importance of U1A31−50. In human lupus, T-cell epitopes on U1A protein have also been reported in amino acids 1–11, 35–58 and 257–282. The different sequence of T-cell epitopes on U1A protein might be species-specific and MHC-dependent.
In addition to lupus nephritis, lymphoproliferation with accumulation of DNT cells was the characteristic finding in MRL/lpr mice. Yamagima et al. demonstrated that the number and proportion of CD4– CD8– B220+ αβT cells in the appendix was extremely high even before the onset of autoimmune disease in lpr mice, After the onset of disease, such DN B220+ αβT cells became prominent in all immune organs of lpr mice.18 However, conflicting results have been obtained about whether or not there is an increase in DNT cells in patients with SLE.19–21 Besides, the role of DNT cells in the development of disease remained unclear. Liu et al. showed no relation to the establishment of disease22 and Chesnutt et al. suggested that autoantibody production in MRL/lpr mice is dependent on the expression of CD4 and not on the accumulation of DNT cells.23 Another study proposed that they possess a regulatory role in this disease.24 One study demonstrated that although there was no significant increase in absolute circulating DNT cells, there was a greater proportion of the DNT cell population in SLE. Besides, these cells were more activated than control DNT cells as determined by a number of activation markers.25 Their data also indicated that there was a higher frequency of αβ DNT cell in about one-third of patients with SLE that constitutively expressed IL-4 than in control individuals. This increased IL-4 was more accentuated in αβ DNT cells than in CD4+ and CD8+ T-cell populations, suggesting that the DNT cell compartment could be functionally more important than the conventional αβ T cells in these patients with SLE.26 We identified DNT cells, among the T-cell repertoires, as the responders to the most important T-cell epitope, U1A31−50. This is the first study to report that DNT cells played a role as autoreactive T cells in response to the U1A antigen. CD4+ T cells may also respond to U1A31−50, but the intensity is lower than that of DNT cells. We propose that CD4+ T cells may be a major help to DNT cells but are not the major responder to U1A31−50.
In our study, the U1A31−50-induced proliferative response of DNT cells was largely inhibited after adding anti-MHC class II antibody. However, the coreceptor between DNT cells and the MHC molecule of antigen-presenting cells is yet to be defined. One previous study confirmed that the CD4 KO(CD4–/–) T cells lacking coreceptors can be activated after stimulation with MHC-peptide. These activated cells increased the expression of CD62L and the secretion of interferon-γ. However, the production of IL-2 was not detectable.27 Their results showed that if CD4 is not properly engaged, T cells could avoid activation-induced cell death. Variations in the interaction of the CD4 coreceptor with polymorphic MHC class II complex may affect the development of autoimmune diseases. They also found that CD4+/+ T cells completely disappeared during chronic stimulation whereas DNT cells survived. The DNT cells also up-regulated the level of B220 and persisted as antigen-specific CD4– CD8– B220+ T cells. Their data indicate a specific role for the CD4 coreceptor in mediating the apoptosis of activated T cells and a lack of co receptor allows prolonged survival of activated T cells. Thus, even DNT cells without CD4 coreceptor might act as effector cells in response to MHC–peptide. DNT cells might originate from CD4+ T cells with down-regulation of CD4 coreceptor.28 The results suggested that the effector function of DNT cells is MHC class II-restricted, which was similar to our previous finding in another lupus prone NZB × NZW F1 mice.29 Further effort is needed to assess the cytotoxic T-lymphocyte activity of DNT cells in the ability of tissue destruction. Such studies will provide more information on DNT cells involved in the pathogenesis of SLE.
In conclusion, we identified four T-cell epitopes, U1A31−50, U1A61−80, U1A201−220 and U1A271−287, on U1A protein with the help of BMDCs as the antigen-presenting cells. Among them, U1A31−50 had the most significant T-cell proliferative response, especially from DNT cells. Furthermore, the DNT-cell proliferative response to U1A antigen was inhibited by anti-MHC class II antibody. Although more studies are needed, the information here might provide help in further understanding the role of U1A self-antigen and its T-cell response in the pathogenesis of lupus.
References
- 1.Tan EM. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- 2.Melissa RA, Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin M, Van Venrooij WJ. Autoantibodies to the U RNP particles. relationship to clinical diagnosis and nephritis. Clin Exp Immunol. 1991;83:286–90. doi: 10.1111/j.1365-2249.1991.tb05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatenejad S, Brooks W, Schwartz A, Craft J. Pattern of anti-small nuclear ribonucleoprotein antibodies in MRL/MP-lpr/lpr mice suggests that the intact U1SnRNP particle is their autoimmunogenic target. J Immunol. 1994;152:5523–31. [PubMed] [Google Scholar]
- 5.Fatenejad S, Mamula MJ, Craft J. Role of intermolecular/intramolecular B- and T-cell determinants in the diversification of autoantibodies to ribonucleoprotein particles. Proc Natl Acad Sci USA. 1993;90:12010–14. doi: 10.1073/pnas.90.24.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen PL, Eisenberg RA. Lpr and gld. single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 7.Devi BS, Van Noordin S, Krausz T, Davies KA. Peripheral blood lymphocytes in SLE-hyperexpression of CD154 on T and B lymphocytes and increased number of double negative T cells. J Autoimmunol. 1998;11:471–5. doi: 10.1006/jaut.1998.0213. [DOI] [PubMed] [Google Scholar]
- 8.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suen JL, Wu CH, Chen YY, Wu WM, Chiang BL. Characterization of self-T-cell response and antigenic determinants of U1A protein with bone marrow-derived dendritic cells in NZB × NZW F1 mice. Immunology. 2001;103:301–9. doi: 10.1046/j.1365-2567.2001.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh RR, Hahn BH. Reciprocal T-B determinants spreading develops spontaneously in murine lupus: implications for pathogenesis. Immunol Rev. 1998;164:201–8. doi: 10.1111/j.1600-065x.1998.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 11.Moudgil KD. Diversification of response to hsp65 during the course of autoimmune arthritis is regulatory rather than pathogenic. Immunol Rev. 1998;164:175–84. doi: 10.1111/j.1600-065x.1998.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 12.Tian J, Olcott AP, Hanssen LR, Zekzer D, Middleton B, Kaufman DL. Infectious Th1 and Th2 autoimmunity in diabetes-prone mice. Immunol Rev. 1998;164:119–27. doi: 10.1111/j.1600-065x.1998.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 13.Vanderlugt CL, Begolka WS, Neville KL, Katz-Levy Y, Howard LM, Eagar TN, Bluestone JA, Miller SD. The functional significance of epitope spreading and its regulation by co-stimulatory molecules. Immunol Rev. 1998;164:63–72. doi: 10.1111/j.1600-065x.1998.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 14.Mamula MJ, Janeway CA., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993;14:151–2. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, et al. The. revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;1982(25):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 16.James JA, Harley JB. Human lupus anti-splicesome A protein autoantibodies bind contiguous surface structures and segregate into two sequential epitope binding patterns. J Immunol. 1996;156:4018–26. [PubMed] [Google Scholar]
- 17.McClain MT, Lutz CS, Kaufman KM, Faig OZ, Gross TF, James JA. Structural availability influences the capacity of autoantigenic epitopes to induce a widespread lupus-like autoimmune response. PNAS. 2004;101:3551–6. doi: 10.1073/pnas.0306267101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamagiwa S, Sugahara S, Shimizu T, et al. The primary site of CD4– CD8– B220+ αβT cells in lpr mice: the appendix in normal mice. J Immunol. 1998;160:2665–74. [PubMed] [Google Scholar]
- 19.Brooks EG, Balk SP, Aupeix K, Colonna M, Strominger JL, Groh-Spies V. Human T-cell receptor (TCR) alpha/beta+CD4–CD8–T cells express oligoclonal TCRs, share junctional motifs across TCR V beta-gene families, and phenotypically resemble memory T cells. Proc Natl Acad Sci USA. 1993;90:11787–91. doi: 10.1073/pnas.90.24.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon HU, Yousefi S, Dommann-Scherrer CC, et al. Expension of cytokine-producing CD4–CD8–T cells associated with abnormal Fas expression and hypereosinophilia. [see comments] J Exp Med. 1996;183:1071–82. doi: 10.1084/jem.183.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusonoki Y, Hirai Y, Kyoizumi S, Akiyama M. Evidence for in vivo clonal proliferation of unique population of blood CD4–CD8–T cells bearing T-cell receptor alpha and beta chains in two normal men. Blood. 1992;79:2965–72. [PubMed] [Google Scholar]
- 22.Liu MF, Li JS, Wen TH, Lei HY. Double-negative (CD4–CD8–) TCR alphabeta+ cells in patients with systemic lupus erythematosus. Scand J Rheumatol. 1998;27:130–4. doi: 10.1080/030097498441001. [DOI] [PubMed] [Google Scholar]
- 23.Chesnutt MS, Finck BK, Killeen N, Connolly MK, Goodman H, Wofsy D. Enhanced lymphoproliferation and diminished autoimmunity in CD4-deficient MRL/lpr mice. Clin Immunol Immunopathol. 1998;87:23–32. doi: 10.1006/clin.1997.4492. [DOI] [PubMed] [Google Scholar]
- 24.Ford MS, Young KJ, Zhang Z, Ohashi PS, Zhang L. The immune regulatory function of lymphoproliferive double negative T cells in vitro and in vivo. J Exp Med. 2002;196:261–7. doi: 10.1084/jem.20020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand A, Dean GS, Quereshi K, Isenberg DA, Lydyard PM. Characterization of CD3+CD4–CD8– (double negative) T cells in patients with systemic lupus erythematosus: activation markers. Lupus. 2002;11:493–500. doi: 10.1191/0961203302lu235oa. [DOI] [PubMed] [Google Scholar]
- 26.Dean GS, Anand A, Blofeld A, Isenberg DA, Lydyard PM. Characterization of CD3+CD4–CD8– (double negative) T cells in patients with systemic lupus erythematosus: production of IL-4. Lupus. 2002;11:501–7. doi: 10.1191/0961203302lu234oa. [DOI] [PubMed] [Google Scholar]
- 27.Hamad AR, Srikrishnan A, Mirmonsef P, Broeren C, June CH, Pardoll D, Schneck JP. Lack of coreceptor allows survival of chronically stimulated double-negativeα/β T cells: implications for autoimmunity. J Exp Med. 2001;193:1113–21. doi: 10.1084/jem.193.10.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laouar Y, Ezine S. In vivo CD4+ lymph node T cells from lpr mice generate CD4–CD8–B220+TCR-beta low cells. J Immunol. 1994;153:3948–55. [PubMed] [Google Scholar]
- 29.Chen Y-C, Ye Y-L, Chiang B-L. Establishment and characterization of cloned CD4– CD8–αβ-TCR bearing autoreactive T cells from autoimmune NZB/W F1 mice. Clin Exp Immunol. 1997;108:52–7. doi: 10.1046/j.1365-2249.1997.d01-971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]