Abstract
Full scale B-cell activation requires not only B-cell receptor (BCR) engagement with antigen, but also costimulatory signals provided by T helper cells through the CD40–CD40 ligand (CD40L) interaction. It is hypothesized that a fusion protein of an antigen and soluble CD40L (CD40LT) would selectively target the costimulation to antigen-specific B cells, leading to synergy in the antibody response. This hypothesis was investigated by fusing green fluorescence protein (GFP), a generic antigen, with mouse CD40LT. Studies revealed that immunization in mice with the plasmid encoding GFP–CD40LT fusion protein led to synergistic induction of GFP-specific antibodies, while control plasmid(s) for GFP, CD40LT, or GFP plus CD40LT did not. Immunization with a single dose of the fusion protein also provoked a vigorous GFP-specific immunoglobulin G1 antibody response, but not other antibody isotypes. These results suggest that GFP–CD40LT fusion protein induces a GFP-specific B-cell activation and antibody response in an antigen-guided fashion. The potential application of this novel strategy in vaccine development is discussed.
Keywords: CD40, CD40L, antibody, B lymphocyte, fusion protein
Introduction
Emerging evidence supports the notion of a two-phase B-cell activation: the initial encounter with antigen via B-cell receptor (BCR) and subsequent activation upon T-cell contact.1 B cells specifically recognizing a particular antigen receive transmembrane signalling through the BCR, which consists of membrane immunoglobulin (mIg) and other transmembrane polypeptides.2,3 Signalling through the BCR induces early events in B-cell activation, including increased expression of major histocompatibility complex (MHC) class II molecules. In the absence of antigen-reactive helper T cells, activated B cells will proliferate and differentiate at a low level to produce some antigen-specific immunoglobulin M (IgM). mIg not only defines the antigen specificity of B cells, but also facilitates the endocytosis of bound antigen. B cells, along with other professional antigen-presenting cells (APCs), digest the internalized antigen in the endosomes and present the resulting antigenic peptides to CD4+ T cells in the context of newly synthesized MHC class II molecules. This leads to CD4 T-cell activation and up-regulation of several important cell surface molecules, such as CD40 ligand (CD40L), resulting in further activation of the antigen-specific B cells through the CD40L–CD40 interaction.4,5 This is one of the most important costimulatory signals necessary for B-cell activation, clonal expansion, germinal centre formation, isotype switching and the generation of B cell memory.6
CD40, a 50 000 MW type I transmembrane protein, was originally thought to be restricted to B cells, dendritic cells and basal epithelial cells, but later was demonstrated to be functionally expressed on other cells, including macrophages, Langerhans cells, endothelial cells and thymic epithelial cells.4,5 In contrast, CD40L (CD154, TRAP, gp39), a type II transmembrane protein, is predominantly expressed on activated CD4+ T cells.5,6 Like other members of tumour necrosis factor receptor (TNFR)/tumour necrosis factor (TNF) family, CD40L forms a homotrimer, which binds to trimerized CD40, an essential step for CD40 pathway activation.7 The extracellular domain of CD40L or soluble CD40L (CD40LT) is fully capable of stimulating B cells and is believed to self-associate through a trimerization domain in its extracellular region.8–10 Defects in the CD40L–CD40 pathway result not only in abnormal B-cell activation, but also in flawed in vivo priming and clonal expansion of antigen-specific CD4+ T cells, as seen both in clinical cases11 and genetically engineered animal models.12–15 Thus, full B-cell activation requires a primary signal from antigen binding to the BCR, as well as a costimulatory signal from CD40L–CD40 interaction, provided by the antigen-specific T helper cells.
Here evidence is presented to demonstrate that a fusion protein of antigen–CD40LT can activate B cells, presumably by providing simultaneous stimulation of BCR and CD40. Green fluorescent protein (GFP) was chosen as a generic antigen in this study. While a mixture of GFP and CD40LT failed to induce anti-GFP antibodies, the GFP-CD40LT fusion protein provoked synergistic GFP-specific IgG responses. This strategy may be valuable for vaccine development.
Materials and methods
Materials
Anti-polyhistidine monoclonal antibody (mAb), anti-FLAG mAb agarose were from Sigma (St. Louis, MO). Restriction enzymes were purchased from New England Biolab (Beverley, MA). All other reagents were from Sigma.
Plasmid construction
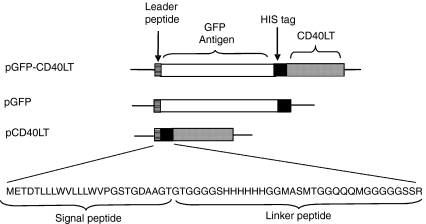
Murine Igκ chain leader peptide was generated by annealing primers of 5′-GAAGCTTCGCCACCATGGAGACAGACACACTCCTGCTATGGGTACTGCTGCTCTG-3′ and 5′-TGGTACCGGCCGCGTCACCAGTGGAACCTGGAACCCAGAGCAGCAGTACCCATAG-3′. The annealed primers were extended by Taq, digested with KpnI and HindIII (Underlines in the primers indicates the cutting sites), and inserted into pEGFP-N1 (BD Bioscience, Inc., San Jose, CA) at HindIII site to yield plasmid Igκ-LP (pIgκ-LP). Murine CD40LT was generated by reverse transcription–polymerase chain reaction (RT–PCR) from C57BL/6 mouse spleen using primers 5′-AGGTACCTCATCTAGAGGTGATGAGGATCCTCAAATTGC-3′ and 5′-AGCGGCCGCTTCAACTAGTTCAGAGTTTGAGTAAGCCAAAAGATG-3′, digested with NotI and XbaI, and inserted into pIgκ-LP to yield pIgκ-CD40LT. A flexible linker sequence, including polyhistidine tag and T7 tag, was generated by annealing primers of 5′-GGGTACCGGAGGTGGAGGCTCACATCACCATCACCATCACGGAGGTATGGCTAGCATGAC-3′ and 5′-CTCTAGATGAGCCACCACCTCCACCCATTTGCTGTCCACCAGTCATGCTAGCCATACCTC-3′. The annealed primers were extended by Taq, digested with KpnI and XbaI, and inserted into pIgκ-CD40LT at KpnI and XbaI sites to yield pCD40LT (Fig. 1). GFP was amplified by PCR from pEGFP-N1 (BD Bioscience) using primers of 5′-CGGTACCATGGTGAGCAAGGGCGAGGAG-3′ and 5′-CGGTACCCTTGTACAGCTCGTCCATGC-3′, digested with KpnI, and inserted into pCD40LT to yield pGFP–CD40LT. To generate control plasmid, pGFP-CD40LT was digested with XbaI and SpeI, and self-ligated to yield pGFP (Fig. 1).
Figure 1.
Constructs of plasmids expressing the recombinant fusion protein and control proteins. Leader peptide, murine Igκ chain leader peptide to increase efficiency for extracellular targeting; GFP, enhanced green fluorescent protein as a generic antigen; His tag, a linker region with six polyhistidine residues.
To generate additional controls, the extracellular domain of mouse BAFF (from Gln69 to Leu309, GenBank accession # AF119383), soluble BAFF (sBAFF), was generated by RT–PCR from C57BL/6 mouse spleen using primer 5′-CTCTAGAGCTTTCCAGGGACCAGAGGAAAC-3′ and 5′-AGCGGCCGCTTCAACTAGTTTACAGCAGTTTTAGGGCACCAAAG-3′, digested with NotI and XbaI, and inserted into pGFP-sCF40L at NotI and XbaI site to replace CD40LT and yield control pGFP–sBAFF. pGFP–ΔsBAFF with a truncated BAFF extracellular domain (from Ala127 to Leu309) was generated in a similar fashion. All coding sequences were designed in frame and were verified by DNA sequencing. All plasmids for in vivo study were purified with Qiagen endotoxin-free Maxi-prep kits.
GFP purification
Recombinant GFP necessary for enzyme-linked immunosorbent assay (ELISA) was expressed and purified as follows. GFP-FLAG fusion protein was generated by PCR and inserted into pAdTrack-CMV shuttle vector.16 Recombinant adenovirus expressing GFP-FLAG was generated as previously described.16 HEK.293 cell line was infected with Ad.GFP-FLAG for 2 days, harvested and lysed by freeze–thaw cycles. GFP-FLAG in the supernatant was purified with an anti-FLAG mAb affinity column, as previously described.16 The adsorption and elution of GFP-FLAG were directly monitored under a handheld UV light. The purified protein was dialysed against PBS and verified on a sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) for its purity (>95%). Because GFP-FLAG used in ELISA did not have any sequence homologous to the linker region in pGFP-CD40LT fusion protein, the antibody detected in ELISA should recognize only GFP, but not FLAG tag.
Western blot
COS-7 cells were transfected with various plasmids or PBS with LipofectAmine Plus reagents (Invitrogen, Inc., Carlsbad, CA) according to the manufacturer's protocol. Two days after transfection, the cells were harvested, washed with phosphate-buffered saline (PBS) twice and lysed with a lysis buffer (50 mm Tris-HCl, pH 7·4, 2 mm ethylenediaminetetraacetic acid, 1 mm phenylmethylsulfonyl fluoride (PMSF), 80 µg/ml benzamide, 0·5% Triton-X-100). After centrifugation at 13 000 g for 10 min, the supernatant was analysed by Western blot using antipolyhistidine mAb.
Flow cytometry
Supernatant from COS-7 cells transfected with pGFP-CD40LT or PBS was collected and concentrated with Centricon YM-3 filter units (Millipore, Inc., Billerica, MA). B cells were purified from C57BL/6 mouse spleen by magnetic-activated cell sorting with anti-B220 mAb (Miltenyi Biotec., Auburn, CA) and incubated with the concentrated supernatants. GFP-CD40LT binding to B cells was analysed by flow cytometry.
Animal immunization
C57BL/6 or BALB/c mice (male, 5–7 weeks old, the Jackson Laboratories, Bar Harbor, ME) were injected s.c. in their hind footpads with 50 µg plasmid DNA plus 50 µg carrier DNA (the plasmid with the same backbone, but no coding sequence) in 50 µl of sterile PBS. Additionally, in some groups, pGFP (50 µg) was combined with pCD40LT (50 µg) and injected. Some mice were boosted 14 days later with their initial regimen, while others received no booster. Blood was collected at day 21 through the tail vein, starting before immunization.
ELISA
ELISA was performed, as previously described, with the following modifications: 96-well plates were coated with purified GFP-FLAG (10 µg/ml) in PBS. After blocking, serum from immunized mice in serial dilutions was incubated in the coated plates for 1 hr. After washing, biotin-conjugated anti-mouse immunoglobulin antibodies (Caltag Laboratories, Burlingame, CA) were added to the plates and incubated for 1 hr. The bound biotin-labelled antibodies were revealed by streptavidin-conjugated horseradish peroxidase (HRP), followed by colorometric assay using o-phenylenediamine as a substrate. The signals were detected at 490 nm with Synergy HT Multi-Detection Microplate Reader with KC4 software (Bio-Tek Instructment, Inc., Winooski, VT).
Results
Characterization of fusion proteins
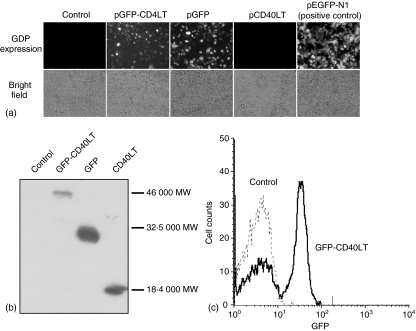
Plasmids encoding the GFP-CD40LT fusion protein (pGFP-CD40LT) and control proteins, GFP (pGFP) and CD40LT (pCD40LT), were constructed (Fig. 1). The fusion protein contains a signal peptide derived from the murine Igκ chain to increase the efficiency for extracellular targeting. Additionally, a flexible peptide linker of 45 aa was inserted between GFP and CD40LT to increase the flexibility of the two protein domains. A polyhistidine tag was also included in the linker to provide a convenience for protein detection. The two control proteins contained the same signal peptide and peptide linker. To verify the protein expression, plasmids were transfected into COS-7 cells. The GFP expression by pGFP-CD40LT and pGFP was verified under a fluorescent microscope (Fig. 2a). Furthermore, recombinant protein expression was verified by Western blot analysis, using anti-polyhistidine mAb. The respective sizes for GFP, CD40LT and GFP-CD40LT (with the linker) were ∼18 000 MW, ∼30 000 MW and ∼46 000 MW, as predicted by their aa sequences (Fig. 2b). GFP-CD40LT binding to B cells was demonstrated by flow cytometry (Fig. 2c).
Figure 2.
(a) Expression of fusion proteins. COS-7 cells were transfected with indicated plasmids for 48 hr with LipofectAmine plus reagent. The expression of GFP was revealed under a fluorescent microscope.(b)Western blot. Transfected COS-7 cells were analysed by Western blot using anti-polyhistidine mAb.(c)GFP-CD40LT binding to B cells. Purified mouse B cells were incubated with the supernatant of COS-7 cells transfected with pGFP-CD40LT or mock transfection.
Antigen-guided B-cell activation
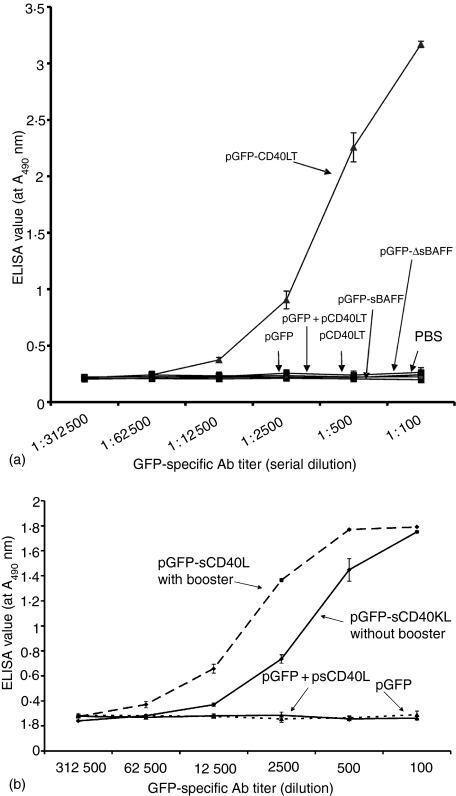
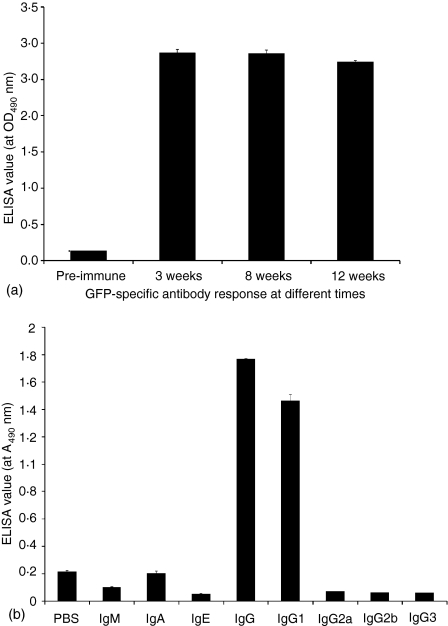
To overcome many complicated issues related to recombinant protein expression and purification, such as protein purity, stability and degradation, mice were directly immunized with the plasmid(s), as seminally reported by Wolff et al.17 and widely used for vaccination studies in animal models.18–20In vivo studies indicated that mice immunized with pGFP, pCD40LT or pGFP plus pCD40LT did not have detectable anti-GFP antibodies, even with 1 : 100 diluted sera, suggesting that GFP has a relatively low antigenicity in the absence of adjuvants. However, pGFP-CD40LT induced significant anti-GFP antibodies (Fig. 3), suggesting that the fusion protein GFP-CD40LT induced GFP-specific B-cell activation and antibody responses, as proposed in Fig. 6. Similar GFP-specific antibody responses were induced by pGFP-CD40LT in C57BL/6 and BALB/c mice. The enhancement of the antibody responses by the fusion protein compared to GFP plus CD40LT was ∼10 000-fold. Immunization with a single dose of pGFP-CD40LT induced significant anti-GFP antibodies, though an additional booster injection may further enhance the antibody titer (Fig. 4a). Minimal decrease in antibody titer was demonstrated up to 12 weeks post immunization (Fig. 4b).
Figure 3.
(a) Synergistic induction of GFP-specific antibodies by pGFP-CD40LT. Mice (5–7 weeks old, five mice/group) were immunized s.c. with indicated plasmid(s) (50 µg/plasmid/mouse) at day 0 and day 14. Blood was collected at day 21. Anti-GFP antibody titre was analysed by ELISA using biotin-labelled anti-mouse total IgG secondary antibody, as described in Methods. (b) pGFP-CD40LT induced high level anti-GFP antibodies without booster. Mice were immunized with indicated plasmid with or without booster, as indicated. Blood was collected at day 21 and analysed for anti-GFP antibodies. These data represent three separate experiments.
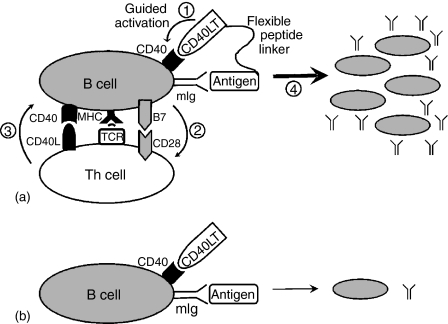
Figure 6.
(a) Antigen-guided B-cell activation. Antigen-CD40LT fusion protein induces an antigen-specific antibody response in synergy with the following proposed sequential events. (1) The fusion protein binds to antigen-specific mIg and guides CD40LT to selectively exert its stimulation on antigen-specific B cells; (2) Activated B cells up-regulate the expression of class II and B7 molecules, uptake the bound antigen, digest the protein, present antigenic peptide to T cells; (3) Activated T cells further increase CD40L expression on its surface and interact with B cells. (4) Large-scale B cell activation through T-cell–B-cell interaction results in antigen-specific B cell proliferation and vigorous antibody response. Thus, the fusion protein only tips the balance at the initial stage and generates critical momentum for the immune response. Both T and B cells are required for the synergistic antibody response. (b) Unguided B-cell activation. The mixture of CD40LT and antigen lacks selectivity on antigen-specific B cells, leading to minimal B-cell activation and antibody production in an unguided fashion.
Figure 4.
(a) Long-term antibody response induced by GFP-CD40LT. GFP-specific antibody response was analysed by ELISA with antiserum dilution at 1 : 500.(b)Anti-GFP antibody isotype profile. Mice were immunized with pGFP-CD40LT, as described in Fig. 3. Blood was collected and analysed for anti-GFP antibodies using isotype-specific biotin-labelled secondary antibodies, as indicated. Anti-GFP sera were diluted at 1 : 100.
It is possible that the increase in protein size enhances its antigenicity and antibody response. This possibility was investigated by fusing GFP to the extracellular domain of BAFF, a B-cell activating factor that has been demonstrated to promote B-cell maturation and survival.21,22 Similar to CD40L, BAFF is a type II membrane protein of the TNF family. Two different BAFF fusion constructs were generated: The first one, GFP-sBAFF of ∼62 000 MW, contains the full-length extracellular domain (Gln69 to Leu309) of mouse BAFF, including two metalloproteinase cleavage sites. The second construct, GFP-ΔsBAFF of ∼48 000 MW (Ala127 to Leu309), lacks the cleavage sites. Both constructs had the same leader sequence and linker region as GFP-CD40LT. Fusion protein expression was verified, as described for GFP-CD40LT (not shown). In vivo studies revealed that both fusion proteins of sBAFF failed to induce GFP-guided antibody production (Fig. 3), suggesting that the synergistic induction of GFP-specific antibody response by antigen-guided B-cell activation only works through the CD40 pathway and is not dependent on the size of the immunogen.
In general, T helper 1 cells (Th1) induce immunoglobulin isotype switching to IgG2a, while Th2 skew the response toward IgG1. Although the strategy proposed in this study is quite different from the traditional T cell-dependent B-cell activation, it is interesting to define the isotype profile of GFP-specific antibodies. Our results indicate that pGFP-CD40LT mainly induces an IgG1 response, while other Ig isotypes were barely detectable both in C57BL/6 (Fig. 5) and BALB/c mice (not shown).
Figure 5.
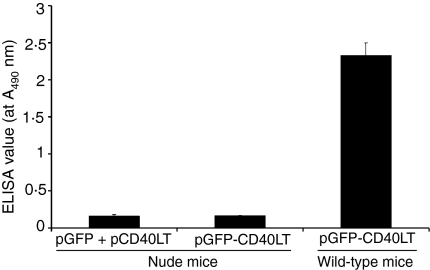
GFP-CD40LT could not induce GPF-specific antibody response in nude mice. Nude mice (5 weeks old, five mice/group) were immunized with pGFP-CD40LT, pGFP plus pCD40LT, as described in Fig. 3. GFP-specific antibody response was analysed at day 21 with serum dilution of 1 : 100.
T-cell dependent B-cell activation
If the fusion protein is fully capable of activating B cells directly through antigen–BCR and CD40LT–CD40 interactions, one may expect a T-cell independent antibody response induced by the fusion protein. To elucidate the role of T cells in the B-cell activation mediated by the fusion protein, nude mice were immunized with the plasmid encoding the GFP-CD40LT fusion protein. Surprisingly, our results indicate that this construct failed to induce a GFP-specific IgG response in these athymic mice (Fig. 6), suggesting that CD4+ T helper cells are still required for B-cell activation induced by the fusion protein.
Discussion
The induction of a GFP-specific antibody response by GFP-CD40LT, but not by GFP or GFP plus CD40LT, suggests that B cells can be directly activated by the antigen–CD40LT fusion protein (Fig. 6). Such antigen-guided B-cell activation constitutes a novel approach for eliciting an IgG antibody response in vivo. Similar to antibody-guided, toxin-conjugated cancer immune therapy23, our strategy uses a guided system to selectively activate B-cell clones specifically recognizing the antigen, resulting in substantially higher levels of antigen-specific antibodies. Because B cells are the only cells that coexpress antigen-specific mIg and CD402–5, it is our expectation that antigen-CD40LT fusion protein will selectively activate antigen-specific B cells (Fig. 6a), while B cells with other antigen specificities will receive minimal activation (Fig. 6b). CD40 expression was originally thought to be restricted to B cells, dendritic cells and basal epithelial cells, but later was demonstrated on other cells as well.4,5 The broad distribution of CD40 increases the range of tissues that CD40L can influence, and may be responsible for the mortality in transgenic mice with widespread ectopic expression of CD40L.15 Therefore, the therapeutic application of CD40L has to be carefully orchestrated to increase its selectivity and minimize any unintended effect on other cells types, as the one proposed in this study.
The results from T-cell deficient nude mice suggest that T cells are required for the antibody response induced by GFP-CD40LT. One of the possible explanations for these results is that the B cells initially activated by the fusion protein through an antigen-guided fashion may further stimulate GFP-specific T cells through antigen presentation in the context of MHC class II. This, in turn, would mobilize B cells through additional CD40 interaction with CD40L on the activated T cells, prolonging the signal provided initially by the fusion protein and resulting in a vigorous GFP-specific IgG response (Fig. 6a). Because this reaction loop is disrupted in nude mice, the fusion protein fails to induce a GFP-specific antibody response. Thus, it is concluded that T cells play a critical role in the antibody response induced by GFP-CD40LT. This conclusion was further supported by previous studies of targeting antigen to dendritic cells by antigen–CD40L.24–26 On the other hand, a previous study demonstrated that coadministration of anti-CD40 antibody and antigen resulted in T-cell independent antibody response27, suggesting that more vigorous stimulation of CD40 may bypass T-cell pathway for antibody response.
A previous study with a carcinoembryonic antigen (CEA) fused to the C-terminus of CD40L demonstrated that oral immunization with S. typhimurium harbouring the DNA vector expressing this fusion protein had only a marginal effect of preventing tumour growth.28 However, the CEA-specific antibody titre was not reported in that study. Furthermore, the above construct may not stimulate CD40 efficiently, because CD40L is a type II membrane protein with the receptor-binding domain located on the extracellular C-terminal region.4,5,29 In contrast, GFP was fused to the N-terminus of CD40L in this study, so that its receptor-binding domain remained free and available for the interaction with CD40. Other recent studies with antigen–CD40L or antigen–anti-CD40 antibody fusion protein corroborated the findings of our study.30–33
Another published study has demonstrated that coimmunization with DNA plasmids expressing β-galactosidase (pβ-gal) and CD40LT led to substantial enhancement of β-gal-specific antibody production.20 However, our results indicated that coimmunization with pGFP and pCD40LT did not induce GFP-specific antibodies. The discrepancy between the outcomes of the two studies is unknown. Relatively low antigenicity of GFP may be responsible for the discrepancy. It is possible that β-gal has relatively high antigenicity, because mice may have been primed by the β-gal released from intestinal Escherichia coli. Thus, the subsequent coimmunization with β-gal and CD40LT led to enhancement of anti-β-gal antibody induction. An alternative explanation is that pCD40LT in the previous study has a GCN4 trimerization domain at the N-terminus of CD40LT, which significantly enhances its biological activity.34 Thus, GCN4-CD40LT is capable of enhancing the β-gal-specific antibody response through coimmunization, while our wild-type CD40LT fails to facilitate an antibody response induced by GFP.
It is noteworthy that no anti-GFP antibody was detected in the group immunized with the GFP-expressing plasmid. However, it is important to point out that the immunization with the DNA did not have any adjuvant, which is necessary for high titre antibody production for some antigens. Furthermore, DNA vaccine usually expresses lower levels of encoded protein because of poor transfection efficiency by direct DNA injection, rather than by liposome- or virus-mediated gene transfer. Moreover, protein antigenicity may also be an important factor to determine the level of antibody response.
Although our strategy has yet to be demonstrated with other antigens for its general applicability, it is conceivable that it has broad applications for vaccine development for infectious diseases and tumour, and for antibody production in research. Despite the enormous success in vaccine development, many infectious diseases still lack effective vaccines. Most vaccine development is time-consuming and requires enormous efforts on a case-by-case basis. Such efforts are often outpaced by the rapid emergence of new infectious pathogens and drug-resistant strains. A variety of strategies have been successfully used in vaccine development, including inactivated/attenuated vaccine, recombinant protein or DNA vaccines. In general, pathogen-based vaccines elicit more effective immune protection, but have significant safety concerns, while protein-based vaccines are relatively safer, but less effective, and thus may require additional adjuvants that may also trigger some detrimental effects by themselves. Our strategy provides an alternative approach for protein-based vaccines with efficacy and minimal safety concerns related to pathogen-based vaccines or adjuvants.
In summary, our studies demonstrated that immunization with GFP-CD40LT fusion protein leads to synergistic induction of GFP-specific Abs in an antigen-guided fashion. The application of this novel strategy in vaccine development is yet to be explored.
Acknowledgments
The author wish to thank Dr Brigitte Huber for scientific discussion and manuscript reviewing, Drs Elizabeth Fini, Richard Riley, Bonnie Blomberg, Natalie Sutkowski, Abbie Meyer and Laising Yen for discussion, and Dr Yuejin Li for technical assistance. This work is partially supported with the NIH grant EY13698, P30-EY014801, Grant-in-Aid from Fight for Sight and unrestricted grant from Research to Prevent Blindness. Dr W. Li is a recipient of the Career Development Award from RPB.
Abbreviations
- CD40LT
soluble CD40 ligand
- GFP
green fluorescent protein
- pGFP
plasmid encoding GFP
- pGFP-CD40LT
plasmid encoding GFP-CD40LT fusion protein
- pGFP plus pCD40LT
a mixture of two plasmids encoding GFP and CD40LT, respectively
References
- 1.Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000;176:171–80. doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 2.Kurosaki T. Functional dissection of BCR signaling pathways. Curr Opin Immunol. 2000;12:276–81. doi: 10.1016/s0952-7915(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 3.Matsuuchi L, Gold MR. New views of BCR structure and organization. Curr Opin Immunol. 2001;13:270–7. doi: 10.1016/s0952-7915(00)00215-6. [DOI] [PubMed] [Google Scholar]
- 4.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 6.Gray D, Siepmann K, Wohlleben G. CD40 ligation in B cell activation, isotype switching and memory development. Semin Immunol. 1994;6:303–10. doi: 10.1006/smim.1994.1039. [DOI] [PubMed] [Google Scholar]
- 7.Peitsch MC, Jongeneel CV. A 3-D model for the CD40 ligand predicts that it is a compact trimer similar to the tumor necrosis factors. Int Immunol. 1993;5:233–8. doi: 10.1093/intimm/5.2.233. [DOI] [PubMed] [Google Scholar]
- 8.Pietravalle F, Lecoanet-Henchoz S, Blasey H, Aubry JP, Elson G, Edgerton MD, Bonnefoy JY, Gauchat JF. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J Biol Chem. 1996;271:5965–7. doi: 10.1074/jbc.271.11.5965. [DOI] [PubMed] [Google Scholar]
- 9.Mazzei GJ, Edgerton MD, Losberger C, et al. Recombinant soluble trimeric CD40 ligand is biologically active. J Biol Chem. 1995;270:7025–8. doi: 10.1074/jbc.270.13.7025. [DOI] [PubMed] [Google Scholar]
- 10.Park SM, Kim HS, Choe J, Lee TH. Differential induction of cytokine genes and activation of mitogen-activated protein kinase family by soluble CD40 ligand and TNF in a human follicular dendritic cell line. J Immunol. 1999;163:631–8. [PubMed] [Google Scholar]
- 11.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–64. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Foy TM, Laman JD, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–31. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 13.Renshaw BR, Fanslow WC3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 15.Sacco MG, Ungari M, Cato EM, et al. Lymphoid abnormalities in CD40 ligand transgenic mice suggest the need for tight regulation in gene therapy approaches to hyper immunoglobulin M (IgM) syndrome. Cancer Gene Ther. 2000;7:1299–306. doi: 10.1038/sj.cgt.7700232. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Huber B, Grand R, Li W. Recombinant Adenovirus Co-expressing covalent peptide/MHC class II complex and B7-1: in vitro and in vivo activation of myelin basic protein-specific T cells. J Immunol. 2001;167:1297–305. doi: 10.4049/jimmunol.167.3.1297. [DOI] [PubMed] [Google Scholar]
- 17.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 18.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application and optimization. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–48. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 20.Gurunathan S, Irvine KR, Wu CY, Cohen JI, Thomas E, Prussin C, Restifo NP, Seder RA. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161:4563–71. [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolink AG, Melchers F. BAFFled B cells survive and thrive: roles of BAFF in B-cell development. Curr Opin Immunol. 2002;14:266–75. doi: 10.1016/s0952-7915(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 23.Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Immunol. 1999;11:570–8. doi: 10.1016/s0952-7915(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 24.Chen HW, Huang HI, Lee YP, et al. Linkage of CD40L to a self-tumor antigen enhances the antitumor immune responses of dendritic cell-based treatment. Cancer Immunol Immunother. 2002;51:341–8. doi: 10.1007/s00262-002-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Tang Y, Akbulut H, Zelterman D, Linton PJ, Deisseroth AB. An adenoviral vector cancer vaccine that delivers a tumor-associated antigen/CD40-ligand fusion protein to dendritic cells. Proc Natl Acad Sci U S A. 2003;100:15101–6. doi: 10.1073/pnas.2135379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi T, Worgall S, Singh R, Moore MA, Crystal RG. Dendritic cells genetically modified to express CD40 ligand and pulsed with antigen can initiate antigen-specific humoral immunity independent of CD4+ T cells. Nat Med. 2000;6:1154–9. doi: 10.1038/80498. [DOI] [PubMed] [Google Scholar]
- 27.Dullforce P, Sutton DC, Heath AW. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nat Med. 1998;4:88–91. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- 28.Xiang R, Primus FJ, Ruehlmann JM, et al. A dual-function DNA vaccine encoding carcinoembryonic antigen and CD40 ligand trimer induces T cell-mediated protective immunity against colon cancer in carcinoembryonic antigen-transgenic mice. J Immunol. 2001;167:4560–5. doi: 10.4049/jimmunol.167.8.4560. [DOI] [PubMed] [Google Scholar]
- 29.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 30.Vega MI, Santos-Argumedo L, Huerta-Yepez S, Luria-Perez R, Ortiz-Navarrete V, Isibasi A, Gonzalez-Bonilla CR. A Salmonella typhi OmpC fusion protein expressing the CD154 Trp140-Ser149 amino acid strand binds CD40 and activates a lymphoma B-cell line. Immunology. 2003;110:206–16. doi: 10.1046/j.1365-2567.2003.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manoj S, Griebel PJ, Babiuk LA, van Drunen Littel-van den Hurk S. Modulation of immune responses to bovine herpesvirus-1 in cattle by immunization with a DNA vaccine encoding glycoprotein D as a fusion protein with bovine CD154. Immunology. 2004;112:328–38. doi: 10.1111/j.1365-2567.2004.01877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manoj S, Griebel PJ, Babiuk LA, van Drunen Littel-van den Hurk S. Targeting with bovine CD154 enhances humoral immune responses induced by a DNA vaccine in sheep. J Immunol. 2003;170:989–96. doi: 10.4049/jimmunol.170.2.989. [DOI] [PubMed] [Google Scholar]
- 33.Barr TA, McCormick AL, Carlring J, Heath AW. A potent adjuvant effect of CD40 antibody attached to antigen. Immunology. 2003;109:87–92. doi: 10.1046/j.1365-2567.2003.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris AE, Remmele RL, Jr, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154) J Biol Chem. 1999;274:418–23. doi: 10.1074/jbc.274.1.418. [DOI] [PubMed] [Google Scholar]