Abstract
To define the role of memory T cells in a non-persistent viral infection, we have delineated the phenotype of memory CD8+ T cells specific for influenza A virus (FluA; matrix protein M158−66) based on the expression of several memory/effector lineage markers and relevant chemokine receptors. We found a majority of FluA-specific CD8+ T cells expressed CD27 and CD28, and variably expressed CD45RA, CD62L, CD94 and granzyme A. A majority of FluA-specific CD8+ T cells expressed high levels of CXCR3, and moderate levels of CCR5 and CXCR4, whereas a limited proportion expressed CCR7, CCR6 and CXCR5. A phenotypic profile based on these observations showed that there are both immature and mature memory CD8+ T cells specific for FluA.
Keywords: EBV, flow cytometry, influenza, phenotyping, tetramer, CD8+, T cells
Introduction
Generation of virus-specific memory CD8+ T cells appears to be a multistage developmental process, characterized by phenotypic alterations associated with changes in T-cell functional and migratory capacity.1 Most of our knowledge of memory CD8+ T-cell differentiation is based on phenotypic analyses of CD8+ T cells specific for persistent viruses, including herpesviruses and lentiviruses. The predominant phenotype of CD8+ T cells specific for non-persisting viruses, such as influenza A virus (FluA), has not been well characterized.
FluA infection causes severe acute airway infection associated with substantial mortality.2 Although CD8+ T cells specific for the immunodominant FluA matrix epitope (FluM158−66) persist after acute infection, their frequency in the circulation remains barely detectable by major histocompatibility complex (MHC) class I tetrameric analysis.3,4 This low frequency makes it difficult to perform accurate multiparametric flow cytometric analysis with FluA-specific MHC class I tetramers. Hence, there little is known about the range of the phenotypic diversity of circulating FluA-specific CD8+ T cells.
We have therefore determined the phenotype of FluA-specific CD8+ T cells by using four-colour flow cytometric analysis employing HLA-A*0201 FluM1 tetramer, together with an extensive set of lineage/maturation markers including several chemokine receptors, on CD8+ T cells from asymptomatic individuals. We found that FluA-specific CD8+ T cells largely consisted of a phenotype of immature memory cells based on expression of CD27 and CD28. However, a significant proportion of FluA-specific CD8+ T cells also expressed CXCR3, CCR5, CD94 and granzyme A, suggesting a heterogeneous population of more mature, effector memory cells. Thus, FluA-specific CD8+ T cells display a degree of phenotypic profile that is indicative of a highly heterogeneous population of phenotypically mature memory and primed effector CD8+ T cells.
Materials and methods
Study subjects and isolation of peripheral blood mononuclear cells
Nine HLA-A*0201 positive healthy volunteers (median age 52 years, range 26–54 years, all male Caucasians) were divided into two groups based on their Flu M1 tetramer or Epstein–Barr virus (EBV) BMLF1 tetramer reactivity: i.e. a FluM1 tetramer-positive group (donors 01 to 06), and an EBV BMLF1 tetramer-positive group (donors 01, and 07 to 09). Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples by centrifugation on Ficoll–Hypaque (Sigma, St Louis, MO) density gradients. Except for CD62L staining, the PBMC were immediately frozen in 10% dimethylsulphoxide and 90% fetal calf serum by a controlled rate cryogenic freezer (Gordinier Electronics, Roseville, MI) and stored at −135° for later use.
Antibodies and HLA-A*0201 tetramers
The following antibodies were used: fluorescein isothiocynate (FITC)-conjugated mouse anti-human CD27, CD28, CD62L, CD94 HLA-DR, CCR5, granzyme A, and perforin monoclonal antibodies (mAbs), unlabelled mouse anti-human CCR6, CXCR4, CXCR5, CXCR6 mAbs (Becton Dickinson, San Jose, CA), FITC-conjugated goat anti-mouse polyclonal F(ab′)2 antibodies (DAKO, Carpinteria, CA), Spectral red™ (SPRD)-conjugated goat F(ab′)2 anti-mouse mAb (Southern Biotechnology Birmingham, AL), FITC-conjugated mouse anti-rat polyclonal antibody (Jacksonimmuno, West Grove, PA), and FITC-conjugated mouse anti-human CD45RA, phycoerythrin (PE)-Cy5 conjugated mouse anti-human T-cell receptor-αβ (TCR-αβ), ECD-conjugated mouse CD8 mAbs (Coulter-Immunotech, Miami, FL). Rat anti-human CCR7 mAb (3D12) was kindly provided by Dr Reinhold Forster (Max-Delbruck-Center for Molecular Medicine, Berlin, Germany). Appropriate isotype-matched mAbs (BD and Coulter-Immunotech) in the fluorescent minus one (FMO) control5 were used throughout the course of the study.
HLA-A*0201 EBV BMLF1280−288 (GLCTLVAML) and FluAM158−66 (GILGFVFTL) tetrameric agents were obtained from the NIH Tetramer Synthesis Facility. These tetramers were labelled with PE, and used at a 1/50 dilution for staining 2 × 106−5 × 106 cells.
Preparation of stained PBMC
HLA-A*0201 restricted EBV BMLF1280−288 and Flu AM158−66 specific CD8+ T cells were stained as described in a protocol obtained from the NIH Tetramer Synthesis Facility with a few modifications. Briefly, for staining CD27, CD28, CD45RA, CD62L, HLA-DR, CD94 and CCR5, 2 × 106−5 × 106 fresh (for staining CD62L) or thawed frozen PBMC were resuspended in 50 μl of phosphate-buffered saline with 4% heat-inactivated fetal calf serum and 0·1% sodium azide, and were first stained with 1 μl of the appropriate tetramer at 4° for 30 min. Then, the cells were washed and incubated with PE-Cy5 mouse anti-human TCR αβ and ECD anti-human CD8 mAbs for 30 min at 4°. Cells were fixed with 1% paraformaldehyde (PFA) and analysed with an Elite XL flow cytometer (Beckman-Coulter) immediately after staining.
For detection of CCR6, CXCR3, CXCR4 and CXCR5, PBMC were first stained with the primary mAbs, washed, and incubated with goat anti-mouse antibody for 30 min at 4°. Cells were then washed and blocked with 10% mouse serum (Sigma) for 15 min at room temperature. The tetramer, PE-Cy5 anti-human TCR αβ, and ECD anti-human CD8 mAbs were added, and subsequently the cells were fixed with 1% PFA. For CCR7 staining, PBMC were first treated with 5% mouse serum for 15 min at room temperature, and then stained with rat anti-CCR7 mAb for 30 min at 4°. The cells were then stained with FITC-conjugated mouse anti-rat polyclonal antibody, and the rest of the procedure was carried out as described.
For intracellular staining of granzyme A and perforin, 2 × 106−5 × 106 fresh or frozen-thawed PBMC were first stained with the tetramer panel as described. After the initial wash, cells were resuspended in 300 μl of OrthopermFix (OrthoDiagnostics, Raritan, NJ) or PermiFlow (Invirion, Frankfort, MI) for 60 min at room temperature. Subsequently, the cells were washed and stained with anti-human granzyme A or perforin mAbs for 40 min at 4°, fixed with 1% PFA and immediately read on the flow cytometer.
Flow cytometric and statistical analysis
The flow cytometer was calibrated daily for colour compensation and laser fluctuation. A four-colour compensation matrix was created for acquisition of tetramer-stained samples and subsequent software compensation by singly and FMO-staining PBMC from donors. We followed a flow cytometic analysis for rare eventing described by Hoffman et al.4 and spectral compensation by Roderer.5 Approximately 2 × 105− 1 × 106 total events were collected for the fully stained sample and FMO isotype control (the same number of events was collected for both samples). This resulted in more than 200 tetramer, CD8high and TCR-αβ+ events based on a compounded gating scheme of Hoffman et al.4 The frequencies of FluM1- and BMLF1-specific CD8 T cells are shown in Table 1. Data analysis and graphic representations were performed using flowjo (TreeStar, Cupertino, CA).
Table 1. Frequency of BMLF1 and FluM1 tetramer positive cells detected ex vivo in peripheral blood.
| Antigen | Donors | Tetramer frequency1 (%TCR-αβ+ CD8+) |
|---|---|---|
| FluM1 | 01 | 0.16 |
| 02 | 0.13 | |
| 03 | 0.23 | |
| 04 | 0.56 | |
| 05 | 0.11 | |
| 06 | 0.14 | |
| BMLF1 | 01 | 4.91 |
| 07 | 0.40 | |
| 08 | 0.32 | |
| 09 | 1.08 |
Average frequency of FMO isotype control; 01 BMLF1 (n = 7, SD = 0·36), 01 FluM1 (n = 7, SD = 0·03), 02 BMLF1 (n = 7, SD = 0·09), 03 BMLF1 (n = 6, SD = 0·05), 04 BMLF1 (n = 7, SD = 0·06), 05 FluM1 (n = 8, SD = 0·02), 06 FluM1 (n = 10, SD = 0·02), 07 FluM1 (n = 7, SD = 0·05), 08 FluM1 (n = 7 SD = 0·01), and 09 FluM1 (n = 5, SD = 0·05).
The phenotypic profile was constructed as follows. The per cent marker expression of the tetramer-positive and -negative CD8+ T cells was derived by the number of events in the upper right (tetramer-marker double-positives) or bottom right [tetramer negative (bulk CD8+ T cells)-marker positives] quadrant of plots divided by number of events in the bottom right quadrant or the bottom left quadrant of the same plots, respectively. The Student's t-test for paired samples was used to show significant difference (P < 0·05) in per cent marker expression between tetramer-positive and tetramer-negative CD8+ T cells. Statistical analysis and graphical representation used jmp v5·0 software (JMP Sales, Cary, NC).
Results
Surface and intracellular expression of conventional functional and CD antigen markers on FluM1-specific CD8+ T cells
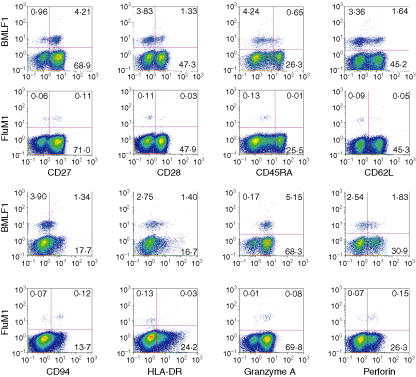
To phenotypically characterize the memory-effector stage of FluM1-specific CD8+ T cells, we first examined expression of CD27, CD28, CD45RA, CD62L, CD94, HLA-DR and granzyme A (Fig. 1). Immature and mature phenotypes were verified by the presence or absence of costimulatory molecules CD27 and CD28,6,7 naïve8 and central memory marker9 CD62L, and effector cell markers granzyme A10 and perforin.11 We found that a majority of FluM1-specific CD8+ T cells expressed CD27 (median 98%, range 58–100%) and CD28 (median 90%, range 58–100%) (Table 2), though a moderate proportion of FluM1-specific CD8+ T cells expressed the naïve cell/central memory marker, CD62L (median 40%, range 28–69%) (Table 2). Granzyme A expression was found in a relatively larger proportion of FluM1-specific CD8+ T cells (median 63%, range 20–98%), although limited expression was seen in most of a lower FluM1-specific CD8+ T cells (median 16%, range 4–68%) (Table 2).
Figure 1.
Surface and intracellular expression of memory/effector markers on BMLF1- or FluM1-specific TCR-αβ+ CD8+ cells. Fresh or frozen PBMC from these volunteers were stained with PC5-anti-TCR-αβ, ECD-anti-CD8, PE-BMLF1- or FluM1-specific tetramers, and a panel of FITC-labelled mAbs against these surface markers. The figure shows all cells with gates set on CD8high TCR-αβ+ and a lymph-gate on FS and SS-Log. The y and x axes of each graph represent the intensity of tetramer staining for each marker shown. Numbers in the quadrant indicate percentage positives in the each quadrant. This (donor 01) is representative of two groups (BMFL1 and FluM1) of three healthy volunteers and two experiments from the same PBMC samples.
Table 2. Per cent TCR-αβ+ CD8+ tetramer and per cent bulk TCR-αβ+ CD8+ (tetramer minus) in parentheses expressing each marker among six volunteers from the FluM1 group12.
| Subject ID | ||||||
|---|---|---|---|---|---|---|
| Marker | 01 | 02 | 03 | 04 | 05 | 06 |
| CD27 | 58 (71) | 91 (92) | 97 (85) | 99 (81) | 99 (57) | 100 (72) |
| CD28 | 24 (48) | 74 (82) | 93 (73) | 86 (76) | 96 (58) | 98 (67) |
| CD45RA | 6 (25) | 7 (54) | 16 (38) | 1 (28) | 7 (32) | 3 (41) |
| CD62L | 35 (45) | 38 (47) | 40 (46) | 41 (63) | 28 (48) | 70 (60) |
| CD94 | 63 (14) | 60 (2) | 32 (9) | 82 (15) | 45 (7) | 11 (17) |
| HLA-DR | 20 (24) | 19 (12) | 12 (26) | 15 (28) | 6 (34) | 1 (14) |
| Granzyme A | 79 (70) | 73 (33) | 52 (55) | 98 (56) | 20 (65) | 53 (38) |
| Perforin | 68 (27) | 27 (5) | 5 (14) | 27 (20) | 5 (29) | 4 (33) |
| CXCR3 | 32 (24) | 62 (36) | 83 (53) | 99 (85) | 85 (74) | 90 (57) |
| CXCR4 | 19 (18) | 39 (64) | 23 (32) | 52 (41) | 97 (88) | 59 (52) |
| CXCR5 | 6 (4) | 6 (2) | 9 (4) | 1 (2) | 12 (1) | 7 (1) |
| CCR5 | 57 (34) | 20 (13) | 42 (30) | 91 (41) | 29 (37) | 44 (37) |
| CCR6 | 18 (7) | 6 (13) | 4 (6) | 2 (10) | 15 (10) | 3 (6) |
| CCR7 | 12 (22) | 27 (64) | 16 (35) | 2 (40) | 21 (17) | 33 (38) |
Per cent marker expression is derived from the number of marker-positive tetramer + events (upper right quadrant of plots in Figs 1 and 2) or tetramer– (bulk CD8+ T cells) events (bottom right quadrant) divided by number of total tetramer+ or tetramer– (bulk CD8+ T cells) events (Upper or bottom right and left quadrants).
Markers shown in bold or underlined across the donor indicate that the mean per cent marker expression of the tetramer + CD8+ T cell is significantly lower (bold) or higher (underline) than that of the tetramer– CD8+ T cell (bulk CD8+ T cells) with P < 0·05, determined by the paired Student's t-test. See the graphical representation of these values in Fig. 3.
CD45RA expression has been found on both naïve and effector, bulk CD8+ T cells10 and CD27– CD45RA+ has been shown to be an effector phenotype.12 We observed that a small proportion of FluM1-specific CD8+ T cells (except subject 02) expressed CD45RA (median 7%, range 1–14%) (Table 2). Thus, a majority of FluM1-specific CD8+ T cells appear to be CD27+ CD45RA–, indicating that FluM1-specific CD8+ T cells do not display an effector phenotype.
In addition, we assessed CD94 expression, because of its known association with terminal differentiation13,14 and HLA-DR, a late activation marker.15 A relatively higher proportion of FluM1-specific CD8+ T cells expressed CD94 (median 53%, range 11–82%) whereas a consistently lower proportion of FluM1-specific CD8+ T cells expressed HLA-DR (median 14%, range 1–20%) (Table 2).
In summary, the phenotype of the majority of FluM1-specific CD8+ T cells circulating in asymptomatic donors appears to be CD27+ CD28+ CD45RA– HLA-DR–, and a moderate proportion of FluM1-specific CD8+ T cells express CD62L, CD94, and granzyme A.
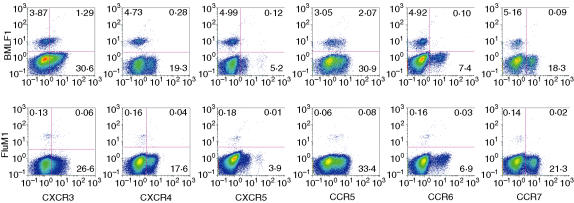
Surface expression of chemokine receptors on FluM1-specific CD8+ T cells
To further delineate memory and effector phenotypes among FluM1-specific CD8+ T cells, we determined surface expression of six chemokine receptors: CXCR3, CXCR4, CXCR5, CCR5, CCR6 and CCR7 (Fig. 2). CXCR4 appears to be expressed preferentially on naïve CD8+ T cells;16,17 in contrast, expression of CCR5 or CXCR3 is known to be associated with activated memory cells.16 CCR5 also appears to be expressed primarily on the late memory to effector stage of viral-specific CD8+ T cells.18 Thus, these latter two chemokine receptors serve as potential mature memory/primed effector markers. We found that a moderate proportion of FluM1-specific CD8+ T cells expressed CCR5 (median 43%, range 20–92%), and a higher proportion expressed CXCR3 (median 84%, range 32–99%), whereas there was a variable proportion of CXCR4 expression (median 45%, range 19–97%) (Table 2).
Figure 2.
Surface expression of chemokine receptors on BMLF1- or FluM1-specific TCR-αβ+ CD8+ cells. Refer to Fig. 1 legend for a description of the groups.
A previous report showed that expression of CCR6 was restricted to the memory subset (CD45RO+) of CD8+ T cells.19 In contrast, we found that CCR6 was minimally expressed on FluM1-specific CD8+ T cells (median, 5%, range 2–18%) (Table 2).
The expression of CCR7 differentiates CCR7+ central memory T cells and CCR7– effector/memory T cells.20 We found a lower proportion of CCR7 expression on FluM1-specific T cells (median 20%, range 2–33%) (Table 2). A similar, low level of CCR7 expression has been observed in HIV-specific and CMV-specific CD8+ T cells.21–23
Thus, our data show that a large proportion of FluM1-specific, memory CD8+ T cells express moderate levels of chemokine receptors CCR5, CXCR3 and CXCR4 while lacking major expression of CXCR5, CCR6 and CCR7 receptors. These T cells fit the conventional definition of memory/effector cells based on chemokine receptor expression.
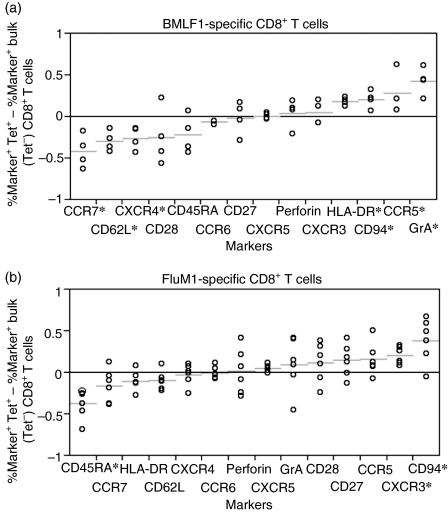
Comparison of the phenotypic profile between FluM1- and BMLF1-specific CD8+ T-cell maturation
We next determined the extent of FluM1-specific CD8+ T-cell maturation in relation to bulk CD8+ T cells. For this, we constructed the phenotypic profile by comparing differences in the per cent marker expression on tetramer-positive CD8+ T cells and corresponding tetramer-negative CD8+ T cells (bulk CD8+ T cells). Then, we compared the phenotypic profiles of FluM1-specific CD8+ T cells to BMLF1-specific CD8+ T cells (Table 3) as an example of more mature CD8+ T cells. As expected, expression of early-stage memory markers (CD27, CD28, CD62L, CCR7 and CXCR4) on FluM1-specific CD8+ T cells was favoured in the bulk CD8+ T cells more than in BMLF1-specific CD8+ T cells, whereas expression of some more mature memory markers (HLA-DR, granzyme A, and CCR5) was slightly more extensive in BMLF1-specific CD8+ T cells (Fig. 3). Furthermore, FluM1-specific CD8+ T cells displayed a higher degree of a skewed CD45RA– phenotype (from the bulk CD8+ T cells) than BMLF1-specific CD8+ T cells (Fig. 3). In contrast, except for CD45RA, CD94 and CXCR3, there were no statistically significant differences in per cent marker expression between FluM1-specific CD8+ T cells and bulk CD8+ T cells. These data suggest that BMLF1-specific CD8+ T cells are skewed toward a more mature phenotype than FluM1-specific CD8+ T cells.
Table 3. Per cent TCR-αβ+ CD8+ tetramer+ and per cent bulk TCR-αβ+ CD8+ (tetramer–) in parentheses expressing each marker among five volunteers from the BMLF1 group1, 2.
| Subject ID | ||||
|---|---|---|---|---|
| Marker | 01 | 07 | 08 | 09 |
| CD27 | 81 (73) | 83 (88) | 64 (93) | 95 (78) |
| CD28 | 26 (49) | 24 (81) | 35 (77) | 92 (70) |
| CD45RA | 13 (28) | 20 (63) | 90 (84) | 2 (38) |
| CD62L | 33 (47) | 16 (59) | 7 (44) | 41 (67) |
| CD94 | 26 (19) | 26 (6) | 25 (3) | 44 (11) |
| HLA-DR | 34 (18) | 27 (7) | 32 (9) | 19 (8) |
| Granzyme A | 93 (74) | 95 (51) | 91 (31) | 91 (48) |
| Perforin | 42 (33) | 26 (8) | 16 (8) | 24 (23) |
| CXCR3 | 25 (32) | 71 (59) | 55 (63) | 81 (61) |
| CXCR4 | 6 (20) | 13 (56) | 35 (67) | 2 (16) |
| CXCR5 | 2 (13) | 3 (0) | 8 (3) | 1 (3) |
| CCR5 | 40 (33) | 43 (22) | 29 (7) | 89 (27) |
| CCR6 | 2 (8) | 6 (15) | 1 (6) | 4 (5) |
| CCR7 | 2 (18) | 5 (68) | 7 (59) | 18 (53) |
Per cent marker expression is derived from the number of marker-positive tetramer + events (upper right quadrant of plots in Figs 1 and 2) or tetramer– (bulk CD8+ T cells) events (bottom right quadrant) divided by number of total tetramer+ or tetramer– (bulk CD8+ T cells) events (upper or bottom right and left quadrants).
Markers shown in bold or underlined across the donor indicate that the mean per cent marker expression of the tetramer + CD8+ T cell is significantly lower (bold) or higher (underline) than that of the tetramer– CD8+ T cell (bulk CD8+ T cells) with P < 0·05, determined by the paired Student's t-test. See the graphical representation of these values in Fig. 3
Figure 3.
Comparison of the proportion of marker expression between tetramer+ and tetramer– (bulk) CD8+ T cells. A distribution plot shows the differences between percentages of BMLF1 (4.A) or FluM1 (4.B) tetramers, and corresponding BMLF1– or FluM1– bulk CD8+ T cells expressing surface and intracellular antigens from Tables 2 and 3. The FluM1-responsive donors (01 through 06), and the BMLF1-responsive donors (01, 07 through 09) (4.A) are represented by circles. Short horizontal lines indicate the mean difference of each marker examined. Markers on the horizontal axes are arranged by amount of mean difference, from the lowest (left) to the highest (right). *Indicates that markers with the difference between the mean per cent marker expression of the tetramer+ CD8+ T cells and the mean per cent marker expression of corresponding bulk (tetramer–) CD8+ T cell is statistically significant (P < 0·05).
Discussion
In the present study, we characterized phenotypes of FluM1-specific CD8+ T cells for a large number of phenotypic markers and chemokine receptors. Our data show that FluM1-specific CD8+ T cells appear to be CD27+CD28+ CD45RA–. This phenotypic enrichment is suggestive of a less mature or early stage of memory-effector cells. Consistent with our findings, the only other phenotypic study on the FluM1-specific CD8+ T cell has recently shown that circulating FluM1-specific CD8+ T cells (CD28+ CCR7– CD45RA–/low) are less mature than the CD8+ T cells specific for persistent virus infection.13
Our analysis shows only slight differences in marker expression between FluM1-specific and BMLF1-specific CD8+ T cells despite well known, distinct differences in underlying viral infection and persistent antigen burden. This is not surprising because EBV-specific CD8+ T cells have been shown to be phenotypically enriched in the early stage of CD8+ T-cell maturation.7 However, persistent EBV infection causes repeated stimulation of BMLF1-specific CD8+ T cells, and this undoubtedly drives BMLF1-specific CD8 T cells to skew towards a more mature phenotype than FluM1-specific CD8+ T cells. This may reflect our observation that although BMLF1-specific CD8+ T cells show a greater degree of down-modulation of early-stage memory markers (CCR7, CD28, CD62L and CXCR4), FluM1-specific CD8+ T cells appear to show a similar degree of differences in the per cent of some effector markers (perforin, CCR5 and CXCR3) from the bulk CD8+ T cells. Thus, it is possible that down-modulation of some phenotypic markers such as CD27 and CD28 may not be strongly correlated with the development of the effector cell phenotype.
Expression of effector and activation markers could also be modulated by the nature of viral infection rather than memory CD8+ T-cell maturation. For instance, preferential CD94 expression on FluM1-specific CD8+ T cells may have little to do with terminal differentiation of such cells. Possibly, expression of the CD94-NKG2A heterodimer averts over-activation of FluM1-specific CD8+ T cells to prevent a fatal pulmonary oedema as a result of acute infection. Such regulation is presumably not necessary for persistent EBV infection. Similarly, persistent EBV infection appears to cause constitutive activation of a small number of antigen-specific CD8+ T cells, indicated by a higher proportion of HLA-DR+ BMLF1-specific CD8+ T cells.
In conclusion, the present study shows that circulating FluM1-specific CD8+ T cells are composed of multiple subsets, each displaying a distinct set of naïve, memory and effector markers. Conceivably, such multiple subsets could be generated as a result of the antigen-specific CD8+ T-cell maturation24 or they could simply signify subsets with distinct migratory and functional capacities. These results suggest that phenotypic maturation may not always accurately ascribe functional maturation.
Acknowledgments
We are grateful to the blood donors for their cooperation, to Dr Reinhold Foerster for providing the rat anti-human CCR7 monoclonal antibody (3D12), to Dr Laila Gamadia for technical help and discussion of the manuscript, to Dr Albert Donnenberg for assistance with flow cytometry and data analysis, to Dr Andrea Gambotto for helpful discussions and to Bonnie Colleton and Dr Pawel Kalinski for critical review of the manuscript. This work is supported by NIH grants R01-AI41870 and U01-AI37984. This work was in partial fulfilment of the requirements for a PhD from the University of Pittsburgh for AH.
Abbreviations
- EBV
Epstein–Barr virus
- TCR-αβ,T-cell
receptor-αβ chain
References
- 1.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 2.Palache AM. Influenza vaccines. A reappraisal of their use. Drugs. 1997;54:841–56. doi: 10.2165/00003495-199754060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Dunbar PR, Smith CL, Chao D, et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. J Immunol. 2000;165:6644–52. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann TK, Donnenberg VS, Friebe-Hoffmann U, et al. Competition of peptide-MHC class I tetrameric complexes with anti-CD3 provides evidence for specificity of peptide binding to the TCR complex. Cytometry. 2000;41:321–8. [PubMed] [Google Scholar]
- 5.Roederer M. Spectral compensation for flow cytometry. visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.De Rosa SC, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry. Identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–8. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 7.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 8.Picker LJ, Treer JR, Ferguson-Darnell B, et al. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor 1-selection on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–21. [PubMed] [Google Scholar]
- 9.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 10.Baars PA, Ribeiro Do Couto LM, Leusen JH, et al. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+ CD45RA+ CD27– human T cells. J Immunol. 2000;165:1910–17. doi: 10.4049/jimmunol.165.4.1910. [DOI] [PubMed] [Google Scholar]
- 11.Veiga-Fernandes H, Walter U, Bourgeois C, et al. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 12.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speiser DE, Lienard D, Pittet MJ, et al. In vivo activation of melanoma-specific CD8(+) T cells by endogenous tumor antigen and peptide vaccines. A comparison to virus-specific T cells. Eur J Immunol. 2002;32:731–41. doi: 10.1002/1521-4141(200203)32:3<731::AID-IMMU731>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Botet M, Bellon T, Llano M, et al. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol. 2000;61:7–17. doi: 10.1016/s0198-8859(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 15.Ho HN, Hultin LE, Mitsuyasu RT, et al. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–9. [PubMed] [Google Scholar]
- 16.Rabin RL, Park MK, Liao F, et al. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162:3840–50. [PubMed] [Google Scholar]
- 17.Faint JM, Annels NE, Curnow SJ, et al. Memory T cells constitute a subset of the human CD8+ CD45RA+ pool with distinct phenotypic and migratory characteristics. J Immunol. 2001;167:212–20. doi: 10.4049/jimmunol.167.1.212. [DOI] [PubMed] [Google Scholar]
- 18.Fukada K, Sobao Y, Tomiyama H, et al. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J Immunol. 2002;168:2225–32. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- 19.Liao F, Rabin RL, Smith CS, et al. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–94. [PubMed] [Google Scholar]
- 20.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Shankar P, Lange C, et al. CD8 T cells specific for human immunodeficiency virus, Epstein–Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–64. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 22.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 23.Gamadia LE, Rentenaar RJ, Baars PA, et al. the differentiation of cytomegalovirus specific CD8 positive T cells in healthy and immunosuppressed virus carriers. Blood. 2001;96:754–61. doi: 10.1182/blood.v98.3.754. [DOI] [PubMed] [Google Scholar]
- 24.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–7. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]