Abstract
The inhibitory effect on antibody production by immune complexes has been shown to depend on co-ligation of the B-cell antigen receptor (BCR) with the low-affinity receptor for immunoglobulin G (IgG) (FcγRIIb, CD32). Here we report that immunoglobulin E (IgE) synthesis, induced in a BCR-independent manner by interleukin-4 (IL-4) and anti-CD40 antibody, was inhibited by CD32 ligation. The observed effect was specific for CD32 as, first, antibodies directed against other B-cell surface structures had no inhibitory effect, and, second, treatment with anti-CD32 of cells that had been in culture for 2 days was ineffective owing to the down-regulation of CD32 expression. IgE inhibition was also observed in cells stimulated by IL-4/CD40 F(ab′)2 or IL-4 plus soluble CD40 ligand, demonstrating that co-cross-linking of CD32 and CD40 was not necessary to induce inhibition. Mechanistic studies into the IgE class switch process demonstrated that IL-4/anti-CD40-induced IgE germline gene transcription and B-cell proliferation were not affected by CD32 ligation. The data demonstrate that the negative regulatory role of the CD32 molecule is not restricted to BCR-induced B-cell activation, but is also functional on other B-cell activation pathways mediated by CD40 and IL-4.
Keywords: allergy, B lymphocytes, CD32, IgE, isotype switching
Introduction
The family of immunoglobulin Fc receptors (FcR) plays an important role in a number of biological processes, such as phagocytosis, clearance of immune complexes, antigen presentation, antibody-mediated cellular cytotoxicity, production of inflammatory mediators and regulation of immunoglobulin synthesis. The presence of FcRs for both immunoglobulin E (IgE) (CD23) and immunoglobulin G (IgG) (CD32) on virgin human B cells is intriguing and has been implicated in the regulation of allergic diseases.1–3 Molecular cloning has revealed six CD32 isoforms, which show a high degree of homology in their extracellular and transmembrane regions but are significantly divergent in their cytoplasmic domains.4,5 The members of this class are encoded by three different genes: CD32a, b and c.6–9 Of those, CD32a and b have been shown to be present on human B cells.10,11 B-cell proliferation and immunoglobulin synthesis induced by engagement of the B-cell receptor (BCR) can be suppressed by CD32b.11–13
This effect has been reported to depend on CD32 co-cross-linking with the BCR, consistent with the fact that CD32 has a high avidity to immune complexes consisting of antigen and immunoglobulin, but only a low affinity to monovalent IgG. The inhibitory potential is brought about by a short sequence in the CD32 intracytoplasmic region, the immunoreceptor tyrosine-based inhibitory motif (ITIM).14,15
Inhibitory activity by CD32b can also be displayed by aggregation of the receptor independently of BCR signalling. In this case an inhibitory signal is generated through Bruton's tyrosine kinase (Btk) and Jun kinase (Jnk), independently of the ITIM.16 This signal can be blocked by molecules recruited upon cell activation.17
Multimerization of CD40 molecules on B cells by binding CD40 ligand on activated T cells culminates in cell proliferation, regulation of programmed cell death, immunoglobulin isotype switching and transcriptional regulation.18 Co-induction of CD40 and interleukin-4 (IL-4) not only synergizes to amplify B-cell proliferation,19 but also induces immunogloblulin class switching towards IgE synthesis.20–22
The present study provides evidence that IgE class switching induced by stimulation with CD40/IL-4 is subject to negative regulation upon treatment with CD32 antibodies without affecting B-cell proliferation. The data suggest that not only BCR-triggered B-cell activation pathways are subject to Fc-receptor modulation but also a subset of CD40 and/or IL-4-mediated signals.
Materials and methods
Culture medium and cells
Purified tonsillar B cells were cultured in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Rockville, MD), supplemented with 10% (v/v) fetal calf serum (FCS), 100 U/ml penicillin and 100 µg/ml streptomycin, 10 µg/ml transferrin and 1·25% soyabean lipids.
Human B lymphocytes were purified from tonsils by T-cell depletion, by rosetting with sheep red blood cells (Immuno AG, Vienna, Austria).23 The resulting B-cell population was ≈ 90% positive for CD19 expression. The human B-lymphoblastoid cell lines, CESS and CFB4.2,24 were maintained in IMDM containing 10% (v/v) FCS (Hyclone, Logan, UT), 10 U/ml penicillin (Life Technologies) and 100 µg/ml streptomycin (Life Technologies).
Cytokines and monoclonal antibodies
Purified human recombinant IL-4, with a specific activity of 0·5 U/ng, was obtained from Novartis AG (Basle, Switzerland). The hybridoma cell line producing mouse monoclonal anti-human CD40 was obtained from Dr Shu man Fu (University of Virginia, Charlotteville, VA). For flow cytometry, a mouse anti-human CD19 fluorescein isothiocyanate (FITC)-conjugated antibody (Immunotech, Marseille, France) was used. Negative control stainings were performed with a FITC/phycoerythrin-conjugated mouse γ1/γ2a antibody from Becton Dickinson (BD, Immunocytometry Systems, San Jose, CA). Antibodies to CD32 were obtained from Medarex, Inc. (Annandale, NJ) (IV:3), Serotec Ltd (Oxford, UK) (AT10) and Immunotech (2E1). Anti-human CD22 (Coulter clone B3), anti-human CD19 (Coulter clone B4) and anti-human SLAM (gift of Dr G. Aversa) were used as specificity controls. Mouse IgG was purchased from Pierce (Rockford, IL).
Preparation of heat-aggregated human IgG and F(ab′)2 fragments
Human IgG (Sigma, St. Louis, MO), at 10 mg/ml, was heated to 63° for 25 min and then centrifuged (20 min, 30 000 g, 4°). Aliquots of the supernatant (soluble aggregates) were stored at −70°. Anti-CD40 F(ab′)2 fragments were prepared by pepsin digestion, and the resulting protein fragments were purified by ion-exchange column chromatography. The purity of the preparation was verified by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE).
Immunoglobulin induction, ELISA and cell-proliferation assays
Tonsillar B cells were cultured at a density of 5 × 105 cells/ml in IMDM, as described above, at 37° in a humidified atmosphere containing 5% CO2. Nine replicates per sample were stimulated with 50 ng/ml IL-4 and 500 µg/ml anti-CD40 monoclonal antibodies (mAbs) to induce polyclonal IgE synthesis. After a 9-day incubation period, supernatants from nine replicates were collected and pooled into three aliquots. IgE, IgG1, immunoglobulin A (IgA) and immunoglobulin M (IgM) levels were quantified by conventional sandwich enzyme-linked immunosorbent assay (ELISA).1
CFB4.2 and CESS cells were thoroughly washed with phosphate-buffered saline (PBS) and incubated for 48 hr before measuring total IgG.
B-cell proliferation was quantified by the incorporation of [3H]thymidine ([3H]TdR) into DNA. During the last 12 hr of a 4-day culture period, cells were labelled with 1 µCi of [3H]TdR (Amersham Life Sciences, Bucks., UK), harvested and counted in a liquid scintillation counter.
The IgE germline promoter reporter gene assay was carried out as described previously.25 Briefly, BL-2 cell transfectants carrying the human IgE germline promoter linked to a luciferase gene were cultured with 10 ng/ml IL-4, 1 µg/ml anti-CD40 mAb and 1 µg/ml sheep anti-mouse IgG for 48 hr in the presence or absence of increasing concentrations of anti-CD32 (IV.3). Luciferase expression was measured in cell lysates according to the manufacturer's instructions (Promega, Madison, WI).
Results
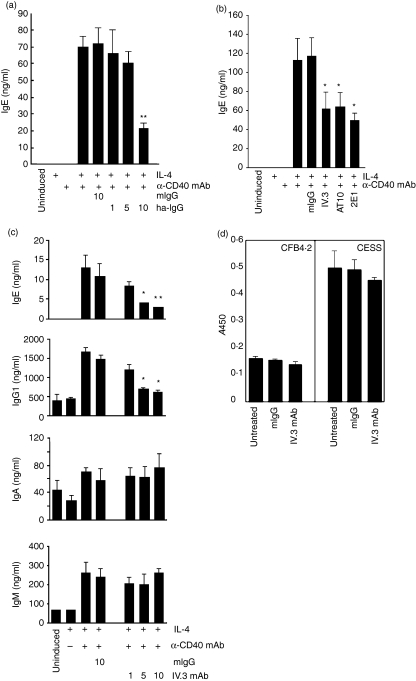
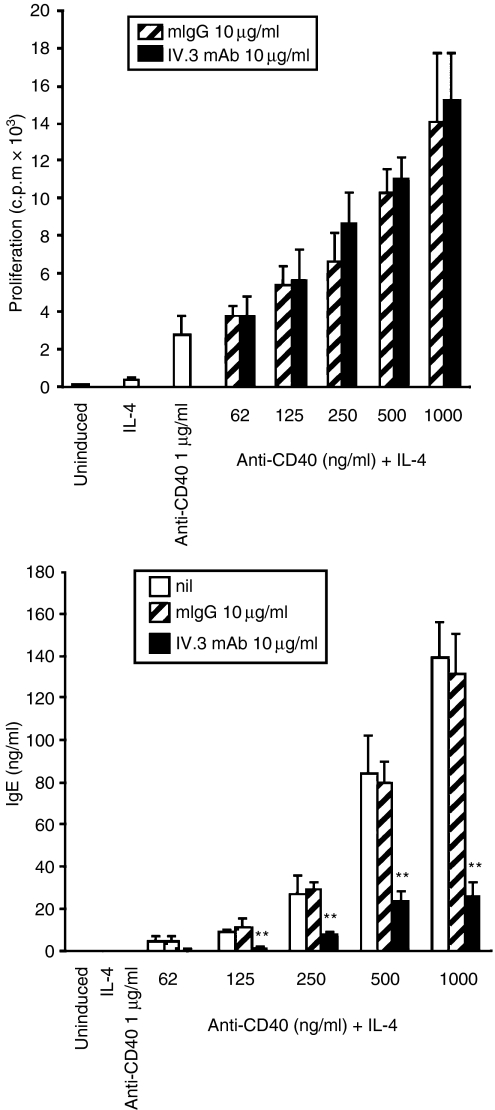
The interaction of CD40 ligand (CD40L) with its receptor, CD40, can be mimicked by agonistic anti-CD40 immunoglobulin or by recombinant soluble CD40L preparations. To induce IgE class switching and/or production, human B lymphocytes purified from tonsils were incubated with monoconal anti-CD40 and IL-4. To assess the potential influence of FcγRII cross-linking on immunoglobulin synthesis in this system, human heat-aggregated IgG was added at increasing concentrations at the start of the culture. Isotype-specific ELISA analysis of the supernatants revealed that IgE production was inhibited in a dose-dependent manner (Fig. 1a). Total mouse IgG had no effect. A similar degree of inhibition was observed when a panel of anti-CD32 mAbs was used (Fig. 1b). These data showed that signals mediated through the CD32 molecule can influence IgE class switching in this activation system. Analysis of a larger number of different donor batches showed that the degree of IgE inhibition varied between 66 and 84%(Table 1).
Figure 1.
CD32 triggering inhibits interleukin-4 (IL-4)/anti-CD40 monoclonal antibody (mAb)-induced immunoglobulin synthesis. (a) Inhibition of immunoglobulin E (IgE) production by human heat-aggregated immunoglobulin G (ha-IgG). IgE synthesis was induced by culturing tonsillar B cells with IL-4/anti-CD40 mAb. Mouse IgG (mIgG; control) or ha-IgG were added at the start of the culture at increasing concentrations (µg/ml) and IgE was quantified in the supernatants by enzyme-linked immunosorbent assay (ELISA). (b) Cells were activated, as described above, in the presence of 10 µg/ml of three different CD32 mAbs. (c) CD32 ligation inhibits the inducible portion of IgE and IgG1 synthesis, but not that of immunoglobulin A (IgA) or immunoglobulin M (IgM). An example is shown in which all four isotypes were induced to various degrees. The data were analyzed for statistical significance by using the Student's t-test (*P < 0·05, **P < 0·01). All data shown represent the mean immunoglobulin concentration of pooled triplicate measurements and standard deviations. (d) No influence of CD32 antibodies on the constitutive IgG synthesis in two human B-lymphoblastoid cell lines. Cells were incubated for 48 hr in the absence or presence of 10 µg/ml mIgG or IV.3 anti-CD32. Total IgG was determined in the supernatants by ELISA and is presented as the absorbance at 450 nm.
Table 1.
Immunoglobulin concentrations in cell supernatants from uninduced or interleukin-4 (IL-4)/CD40 monoclonal antibody (mAb)-stimulated cells, in ng/ml, for three different B-cell donors
| IgE | IgGI | IgA | IgM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Uninduced | Induced | 10 µg/ml IV.3 | Uninduced | Induced | 10 µg/ml IV.3 | Uninduced | Induced | 10 µg/ml IV.3 | Uninduced | Induced | 10 µg/ml IV.3 |
| 1 | <0·08 | 118 | 40 (66) | 209 | 161 | 134 (NS) | 27 | 28 | 25 (NS) | 56 | 124 | 130 (NS) |
| 2 | <0·08 | 561 | 133 (76) | 56 | 697 | 319 (54) | 6 | 52 | 37 (NS) | 28 | 451 | 332 (NS) |
| 3 | <0·08 | 140 | 22 (84) | 390 | 331 | 283 (NS) | 209 | 163 | 161 (NS) | 220 | 300 | 188 (NS) |
Numbers in parenthesis denote the percentage of inhibition in the presence of 10 µg/ml IV.3 antibody. NS, non-significant.
Treatment with IL-4 and CD40 mAb also induced IgG1, IgM and IgA synthesis to variable extents, depending on the donor. IgM and IgA synthesis were not affected, indicating a certain degree of isotype specificity of the negative CD32 signal. In contrast, anti-CD32 treatment led to a significant block of inducible IgG1 production in donors responding to the stimuli, while it had no effect on the constitutive IgG1 synthesis in non-responding cells (Fig. 1c and Table 1). The constitutive IgG1 production of two IgG1-producing B-cell lines (CESS, CFB4.2) was also not inhibited by CD32 antibody treatment (Fig. 1d), thus confirming our data in primary B cells.
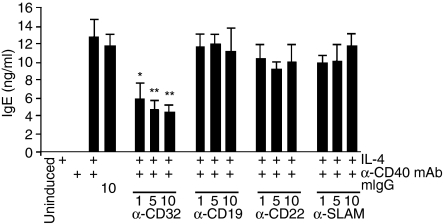
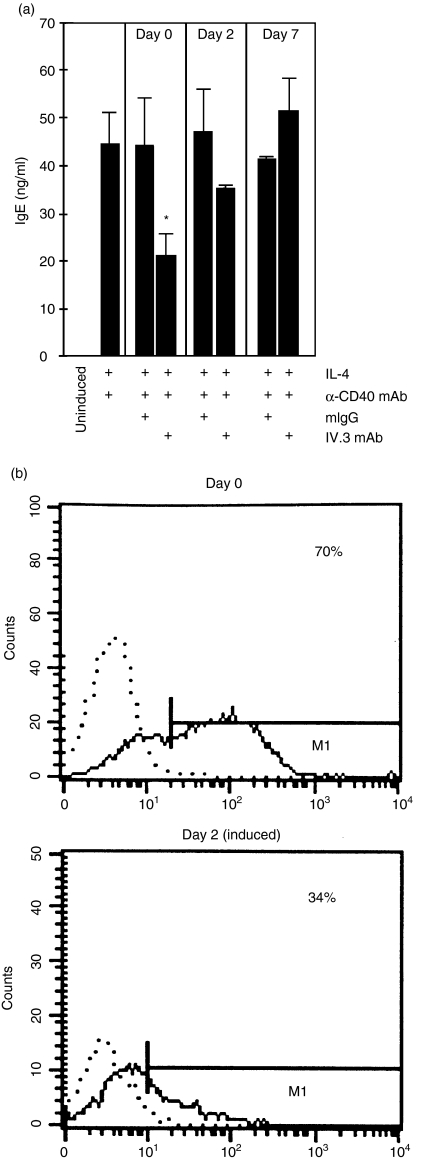
To assess the specificity of the inhibitory effect, antibodies directed against the B-cell surface markers CD19,26 CD2227 and SLAM,28 were used in parallel to the IV.3 anti-CD32 antibody. While the IV.3 antibody inhibited IgE secretion by 65% none of the three other reagents had a negative influence (Fig. 2). Further support for CD32 being critically involved in mediating the inhibitory effect came from time-course experiments. Tonsillar B cells were induced on day 0, as described previously. At different time-points afterwards, anti-CD32 antibody was added and IgE production was quantified in the cell supernatants on day 9. In parallel, CD32 expression was measured by fluorescence-activated cell sorting (FACS) (Fig. 3). IgE synthesis was blocked when the IV.3 antibody was given simultaneously with the IL-4/anti-CD40 mAb induction cocktail. Addition of anti-CD32 on day 2 resulted in significant loss of the inhibitory effect and was completely ineffective when added on day 7 (Fig. 3a). FACS measurements at the start of the culture period using the IV.3 antibody showed that between 55 and 70% of the cells expressed CD32 on the cell surface. On day 2, a strong reduction of IV.3 reactivity, both at the level of positive cells as well as receptor density, was observed (Fig. 3b). Taken together, these results suggested that the loss of the anti-CD32 mAb effect was caused by the progressive loss of CD32 surface expression, thus further confirming the specificity of CD32 to mediate the inhibitory effect.
Figure 2.
Antibodies against other B-cell surface structures do not inhibit immunoglobulin E (IgE) synthesis. The concentration of antibodies added together with interleukin-4 (IL-4)/anti-CD40 monoclonal antibodies (mAbs) are shown in µg/ml. IgE was quantified in supernatants harvested at day 9. The data were analysed for statistical significance by using the Student's t-test (*P < 0·05, **P < 0·01). mIgG, mouse immunoglobulin G.
Figure 3.
Loss of the inhibitory effect of CD32 at later time-points is caused by the down-regulation of CD32 expression. (a) Tonsillar B cells were cultured with interleukin-4 (IL-4)/anti-CD40 monoclonal antibody (mAb) for 9 days, starting on day 0. A total of 10 µg/ml mouse immunoglobulin G (IgG) (mIgG) or IV.3 antibody was added on days 0, 2 or 7. Immunoglobulin E (IgE) production was measured in the cell supernatants by enzyme-linked immunosorbent assay (ELISA). (b) Loss of CD32 surface expression upon cell culture, as measured by fluorescence-activated cell sorter (FACS) analysis. The reactivity profile against an isotype-matched control antibody (dotted line) and for the IV.3 mAb (normal line) is shown. The number denotes the percentage of IV.3-reactive cells. The data were analysed for statistical significance by using the Student's t-test (*P < 0·05).
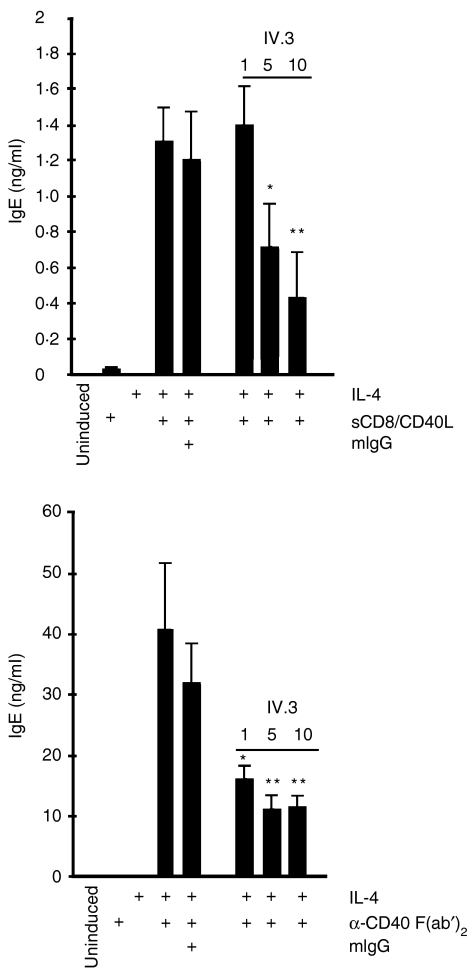
Down-regulation of BCR-mediated B-cell activation has been documented to require co-cross-linking of the BCR and CD32.4 As B-cell activation in our system is driven by CD40-mediated signalling, the question was raised if the inhibitory effect was dependent on co-cross-linking of CD40 with CD32. Cells were induced with IL-4 and CD40 F(ab′)2 fragments or with a fusion protein consisting of murine CD8α and soluble CD40 ligand (sCD8/CD40L).29 Fig. 4 demonstrates that the addition of anti-CD32 IV.3 antibody led to the inhibition of IgE synthesis in both types of activation to an extent comparable to that observed when the cells were incubated with intact anti-CD40 antibody. These results demonstrate that CD40/CD32 co-cross-linking is probably not required to mediate the inhibitory effect on IgE synthesis.
Figure 4.
Co-cross-linking of CD32 and CD40 is not involved in the inhibitory effect on immunoglobulin E (IgE) synthesis. Tonsillar B cells were induced with interleukin-4 (IL-4) and soluble CD40 ligand (sCD8/CD40L) (upper panel) or with anti-CD40 F(ab′)2 fragments (lower panel) in the absence or presence of increasing concentrations (µg/ml) of intact CD32 antibody. IgE levels were quantified after 9 days by enzyme-linked immunosorbent assay (ELISA). The data were analysed for statistical significance by using the Student's t-test (*P < 0·05, **P < 0·01).
It has been reported that the complete process of heavy chain class switching requires a few rounds of cell divisions to be completed.30 As CD32 signalling led to the abrogation of cell proliferation induced via the BCR,12,13 it was investigated whether the inhibitory effect on anti-CD40/IL-4-induced IgE production was a consequence of impaired cell proliferation. Purified B cells were induced with increasing amounts of anti-CD40 antibody and/or IL-4, as described above. One aliquot of cells was used for measuring cell proliferation at day 4, while the other half was cultured further for the determination of IgE in the supernatants. The results shown in Fig. 5 demonstrate that anti-CD40 triggering alone induced B-cell proliferation. IL-4 alone had little effect, but synergized with anti-CD40 mAb, as described previously.19 The addition of anti-CD32 antibody had no adverse effect. Similarly to cell proliferation, IgE synthesis was induced with increasing anti-CD40 concentrations, but only in the presence of the cytokine. In contrast to proliferation, anti-CD32 antibody significantly inhibited IgE synthesis, independently of the CD40 antibody concentration. These data demonstrated that the negative effect on IgE production was not a consequence of a CD32-mediated block in cell proliferation.
Figure 5.
CD32 triggering does not affect interleukin-4/anti-CD40 monoclonal antibody (mAb)-induced B-cell proliferation. Tonsillar B cells were cultured with IL-4 or anti-CD40 mAb separately (open bars) or with IL-4 and increasing concentrations of anti-CD40 mAb, as shown on the x-axis. A total of 10 µg/ml mouse immunoglobulin G (IgG) (mIgG) or IV.3 mAb was added at the start of the culture. DNA synthesis was measured on day 4 by [3H]thymidine incorporation (upper panel). Other cell aliquots were cultured further until day 9 after which immunoglobulin E (IgE) concentrations were quantified in the cell supernatants (lower panel). The data were analysed for statistical significance by using the Student's t-test (*P < 0·05, **P < 0·01).
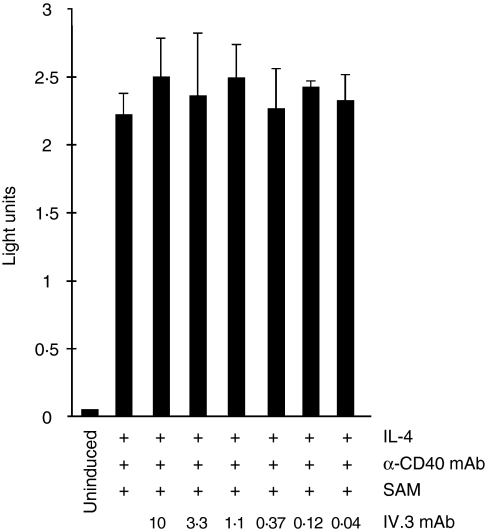
The process of IgE isotype class switching can, in general, be divided into two consecutive steps.31 Within a few hours, IL-4 induces the activation of IgE germline gene transcription. Co-engagement of the CD40 receptor synergizes with IL-4, but has no activation potential on its own. IL-4/CD40 stimulation also leads to activation of the recombination machinery, in particular the gene encoding activation-induced cytidine deaminase (AID). In a second step, DNA recombination brings the IgE Cε gene in close proximity to the preassembled VDJH gene segments, thus allowing for transcription and translation of a fully functional IgE molecule. To determine whether CD32 engagement was acting at the level of IgE germline gene induction to inhibit IgE switching, CD32 antibodies were added to IL-4/anti-CD40 mAb-induced BL-2 cell transfectants, which carry the human IgE germline promoter linked to a luciferase reporter gene. The results demonstrated that activation of IgE germline transcripts was not impaired by the presence of CD32 antibodies (Fig. 6). These data were substantiated by measuring endogenous IgE germline transcripts in tonsillar B cells stimulated with IL-4/anti-CD40mAb (data not shown). Therefore, we concluded that the blocking effect of CD32 ligation on IgE class switching was not caused by an early block of IgE germline gene activation.
Figure 6.
CD32 ligation does not inhibit interleukin-4 (IL-4)/anti-CD40 monoclonal antibody (mAb)-induced immunoglobulin E (IgE) germline gene transcription. BL-2 cells, transfected with an IgE germline promoter luciferase construct, were stimulated with IL-4 and anti-CD40 mAb for 48 hr in the absence or presence of CD32 antibody (µg/ml) before luciferase was measured.
Discussion
The negative regulatory role of the CD32b molecule on various aspects of B-lymphocyte function upon BCR signalling has been convincingly demonstrated to depend on the co-cross-linking of these two surface molecules.4,5,9,15,32 This report demonstrates that BCR-independent B-cell activation, leading to IgE class switching, can also be counter-regulated through CD32. While it cannot be ruled out that CD32a is mediating this effect, the following overwhelming arguments favour the concept that CD32b is the isoform responsible for inhibiting IgE: first, there is no literature for a negative role of CD32a on B cells; and, second, CD32b is the main isoform expressed on tonsillar B cells, while CD32a was found only in very small quantities.33 In addition, the reactivity of the IV-3 anti-CD32 antibody is not restricted to the CD32a isoform, as previously reported, but also recognizes the CD32b molecule as efficiently as the AT10 antibody. This was analysed in Chinese hamster ovary (CHO) cells stably transfected with human CD32b (a kind gift of J. Ravetch, Rockefeller University, New York, NY) (data not shown).
The data showed that the IL-4/anti-CD40 mAb-induced IgE synthesis in primary human B lymphocytes was dose-dependently inhibited by heat-aggregated human IgG. The inhibitory effect of heat-aggregated IgG could be mimicked by using agonistic anti-CD32. It was further demonstrated that the blockade of IgE was not a consequence of diminished B-cell proliferation. This is in agreement with previous work demonstrating that the occupancy of CD32 by immune complexes inhibited antibody synthesis, but not B-cell proliferation induced by F(ab′)2 anti-µ and cytokines.34,35 Inhibition of IgE synthesis has also been reported by Sigman et al., using intravenous antibody (IVIG). However, in their study, treatment with IVIG resulted in decreased B-cell proliferation as the underlying cause of decreased IgE production.36 Owing to the very high affinity of specific antibodies to CD32, maximal inhibition was observed at concentrations of 5–10 µg/ml, whereas IVIG had to be used at 1000× higher concentrations (5–10 mg/ml). The high doses of antibody may account for the antiproliferative effect observed by Sigman et al. The effects of anti-CD32 antibody in our study clearly implicate CD32 as a regulator of IgE production. This is in contrast with Zhuang et al.,33 who argue for a CD32-independent mechanism. This is based on data showing that treatment with KB61, a CD32b-specific antibody, could not reverse the inhibitory effect of IVIG on B-cell proliferation and IgE synthesis. Although the authors argue that the suppressive effect of IVIG is not mediated by CD32b, because preincubation with the KB61 antibody did not reverse the inhibitory effects of IVIG, the observed effect may rather be explained by the inhibitory function of the antibody itself and therefore would be in line with our observations.
The down-regulation of CD40-mediated IgG production has also been documented by Ravanel et al,37 who reported that cross-linking of CD32 by the nucleocapsid protein of measles virus inhibited IL-2/IL-10/CD40-ligand-induced IgG, IgA and IgM synthesis to an extent very similar to that reported in the present study using anti-CD32. The differences in results relating to the inhibition of IgA and IgM may be explained by the different cytokines used for directing class switching.
The degree of inhibition was variable and not complete. This may be explained by the different activation status of the individual B-cell preparations from various donors. In addition, it is possible that not all cells which switch to IgE production are susceptible to the CD32-mediated regulatory effect, simply because they do not express the receptor or lose it during the culture period. This assumption is supported by the FACS analysis showing that CD32 expression is rapidly diminished upon culture. Modulation of Fcγ receptors by IL-4 has been described previously. Similarly to our data, IL-4 treatment led to the loss of CD32b2 and CD32a expression on B cells and monocytes.10,38–40 CD32 down-regulation may also help to explain the results of Sigman et al.36 In agreement with our observations, IVIG had to be added during the first 48 hr to achieve inhibition of IgE.
While IgE synthesis was inhibited in all donors examined, IgG1 was blocked only in those donors in whom the stimuli caused an increase in IgG1 production over the constitutive levels found in all donors. In addition, IgA and IgM levels were not inhibited. These findings may be explained by the fact that the increase of these two isotypes, and partly also IgG1, is caused by the IL-4/anti-CD40 mAb-induced cell proliferation of postswitched B cells present in human tonsils. As IL-4/anti-CD40mAb-driven B-cell proliferation is not influenced by treatment with anti-CD32 mAb, no effect on IgM, IgA and partly IgG1 can be expected. Only virgin B cells can respond to IL-4 and anti-CD40 mAb with a class switch to IgE or IgG1 and represent targets for the CD32 mAb effect. Therefore, the signalling pathway(s) leading to IgE and IgG class switching may be modulated by the inhibitory CD32 signal.
One of the first steps in the IgE class switch process is the activation of transcription through the Cε gene locus from the IgE germline gene promoter, thereby opening this region for the subsequent DNA recombination step and guiding the recombinase machinery towards this locus.31 The data indicate that the activation of IgE germline gene transcription by IL-4/anti-CD40 mAb was not affected by treatment with CD32 antibody, demonstrating that CD32 signalling must affect other aspects of IgE class switching. As also cell proliferation, another prerequisite for successful class switching, was not inhibited by CD32 antibodies, we currently favour the concept that events directly related to DNA recombination may be targeted by the negative CD32 signal.
The inhibitory effect was still observed when the cells were induced with anti-CD40 F(ab′)2 fragments or soluble CD40L. This demonstrated that, in contrast to BCR-mediated activation, co-cross-linking of CD40 and CD32 was not a requirement to block IgE synthesis. The signal-transducing molecules of the BCR contain an immunoreceptor tyrosine-based activation motif (ITAM) that is involved in signal transduction.41 This sequence appears to be the molecular target of the negative signal through the CD32 receptor. Interestingly, no such ITAM was identified in the cytoplasmic domains of the CD40 and IL-4 receptor molecules. One may speculate that the inhibition of signalling through these structures follows an ITAM-independent mechanism – possibly clustering of CD32b. It has been reported that cross-linking of CD32b alone is sufficient to induce negative signalling in B cells17 without involvement of an ITAM motif.
Antigen-specific immune complexes have been successfully used in the treatment of chronic bronchial asthma.42,43 Interestingly, antigen-specific IgE and IgG were significantly inhibited. It is probable that the inhibitory effect was caused by co-cross-linking of the BCR and CD32 on allergen-specific B cells. In addition, it is possible that the immune complexes also cross-linked CD32 on non-specific IgE producers, leading to the inhibition of total IgE and IgG. Such a scenario remains to be experimentally addressed.
In summary, our data provide and extend evidence that negative signals mediated by CD32 inhibit IgE production induced by CD40 triggering in the presence of IL-4.33,36 The inhibitory effect does not require co-cross-linking of CD32 with CD40, and may affect IgE class switching at the level of DNA recombination.
Acknowledgments
The authors wish to thank Fritz Effenberger for preparation of the anti-CD40 F(ab′)2 fragments and Maximilian Horejs for help with illustration and figures.
Abbreviations
- BCR
B-cell receptor
- CD40L
CD40 ligand
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- FcR
Fc receptor
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- ha-IgG
heat aggregated immunoglobulin G
- IgE
immunoglobulin E
- IgG
immunoglobulin G
- IL-4
interleukin-4
- IMDM
Iscove's modified Dulbecco's medium
- ITAM
immunoreceptor tyrosine-based activating motif
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- IVIG
intravenous immunoglobulin
- mAb
monoclonal antibody
References
- 1.Bheekha Escura R, Wasserbauer E, Hammerschmid F, Pearce A, Kidd P, Mudde GC. Regulation and targeting of T-cell immune responses by IgE and IgG antibodies. Immunology. 1995;86:343–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Mudde GC, Bheekha R, Bruijnzeel-Koomen CA. Consequences of IgE/CD23-mediated antigen presentation in allergy. Immunol Today. 1995;16:380–3. doi: 10.1016/0167-5699(95)80005-0. 10.1016/0167-5699(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 3.Mudde GC, Reischul IG, Corvaia N, Hren A, Poellabauer EM. Antigen presentation in allergic sensitization. Immunol Cell Biol. 1996;74:167–73. doi: 10.1038/icb.1996.23. [DOI] [PubMed] [Google Scholar]
- 4.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 5.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–92. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 6.Qiu WQ, de Bruin D, Brownstein BH, Pearse R, Ravetch JV. Organization of the human and mouse low-affinity Fc gamma R genes: duplication and recombination. Science. 1990;248:732–5. doi: 10.1126/science.2139735. [DOI] [PubMed] [Google Scholar]
- 7.Warmerdam PA, van den Herik-Oudijk IE, Parren PW, Westerdaal NA, van de Winkel JG, Capel PJ. Interaction of a human Fc gamma RIIb1 (CD32) isoform with murine and human IgG subclasses. Int Immunol. 1993;5:239–47. doi: 10.1093/intimm/5.3.239. [DOI] [PubMed] [Google Scholar]
- 8.Hibbs ML, Bonadonna L, Scott BM, McKenzie IF, Hogarth PM. Molecular cloning of a human immunoglobulin G Fc receptor. Proc Natl Acad Sci USA. 1988;85:2240–4. doi: 10.1073/pnas.85.7.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks DG, Qiu WQ, Luster AD, Ravetch JV. Structure and expression of human IgG FcRII (CD32). Functional heterogeneity is encoded by the alternatively spliced products of multiple genes. J Exp Med. 1989;170:1369–85. doi: 10.1084/jem.170.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarmay G, Rozsnyay Z, Koncz G, Danilkovich A, Gergely J. The alternative splicing of human Fc gamma RII mRNA is regulated by activation of B cells with mIgM cross-linking, interleukin-4, or phorbolester. Eur J Immunol. 1995;25:262–8. doi: 10.1002/eji.1830250143. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Herik-Oudijk IE, Westerdaal NA, Henriquez NV, Capel PJ, Van De Winkel JG. Functional analysis of human Fc gamma RII (CD32) isoforms expressed in B lymphocytes. J Immunol. 1994;152:574–85. [PubMed] [Google Scholar]
- 12.Uhr JW, Moller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- 13.Chan PL, Sinclair NR. Regulation of the immune response. V. An analysis of the function of the Fc portion of antibody in suppression of an immune response with respect to interaction with components of the lymphoid system. Immunology. 1971;21:967–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–91. doi: 10.1016/s0167-5699(97)80025-4. 10.1016/S0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 15.Cambier JC. Inhibitory receptors abound? Proc Natl Acad Sci USA. 1997;94:5993–5. doi: 10.1073/pnas.94.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 17.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity. 1999;10:753–60. doi: 10.1016/s1074-7613(00)80074-6. 10.1016/S1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- 18.Van Kooten C, Banchereau J. CD40–CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 19.Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–2. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 20.Gauchat JF, Aversa G, Gascan H, de Vries JE. Modulation of IL-4 induced germline epsilon RNA synthesis in human B cells by tumor necrosis factor-alpha, anti-CD40 monoclonal antibodies or transforming growth factor-beta correlates with levels of IgE production. Int Immunol. 1992;4:397–406. doi: 10.1093/intimm/4.3.397. [DOI] [PubMed] [Google Scholar]
- 21.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–70. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 22.Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8:199–205. doi: 10.1016/s0952-7915(96)80058-6. 10.1016/S0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 23.Thompson PA, Jelinek DF, Lipsky PE. Regulation of human B cell proliferation by prostaglandin E2. J Immunol. 1984;133:2446–53. [PubMed] [Google Scholar]
- 24.van Reijsen FC, Bruijnzeel-Koomen CA, Kalthoff FS, Maggi E, Romagnani S, Westland JK, Mudde GC. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol. 1992;90:184–93. doi: 10.1016/0091-6749(92)90070-i. 10.1016/0091-6749(92)90070-I. [DOI] [PubMed] [Google Scholar]
- 25.Berger M, Albrecht B, Berces A, Ettmayer P, Neruda W, Woisetschläger M. S(+)-4-(1-Phenylethylamino) quinazolines as inhibitors of human immunoglobuline E synthesis: potency is dictated by stereochemistry and atomic point charges at N-1. J Med Chem. 2001;44:3031. doi: 10.1021/jm010888h. [DOI] [PubMed] [Google Scholar]
- 26.Hippen KL, Buhl AM, D'Ambrosio D, Nakamura K, Persin C, Cambier JC. Fc gammaRIIB1 inhibition of BCR-mediated phosphoinositide hydrolysis and Ca2+ mobilization is integrated by CD19 dephosphorylation. Immunity. 1997;7:49–58. doi: 10.1016/s1074-7613(00)80509-9. 10.1016/S1074-7613(00)80509-9. [DOI] [PubMed] [Google Scholar]
- 27.Doody GM, Dempsey PW, Fearon DT. Activation of B lymphocytes. integrating signals from CD19, CD22 and Fc gamma RIIb1. Curr Opin Immunol. 1996;8:378–82. doi: 10.1016/s0952-7915(96)80128-2. 10.1016/S0952-7915(96)80128-2. [DOI] [PubMed] [Google Scholar]
- 28.Punnonen J, Cocks BG, Carballido JM, Bennett B, Peterson D, Aversa G, de Vries JE. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J Exp Med. 1997;185:993–1004. doi: 10.1084/jem.185.6.993. 10.1084/jem.185.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell F. Soluble CD40 ligand can replace the normal T cell-derived CD40 ligand signal to B cells in T cell-dependent activation. J Exp Med. 1993;177:1209–13. doi: 10.1084/jem.177.4.1209. 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenter AL, Watson JV. Cell cycle kinetics model of LPS-stimulated spleen cells correlates switch region rearrangements with S phase. J Immunol Methods. 1987;97:111–7. doi: 10.1016/0022-1759(87)90112-8. 10.1016/0022-1759(87)90112-8. [DOI] [PubMed] [Google Scholar]
- 31.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev. 2003;3:721. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 32.O'Rourke L, Tooze R, Fearon DT. Co-receptors of B lymphocytes. Curr Opin Immunol. 1997;9:324–9. doi: 10.1016/s0952-7915(97)80077-5. 10.1016/S0952-7915(97)80077-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang Q, Bisotto S, Fixman ED, Mazer B. Suppression of IL-4- and CD40-induced B-lymphocyte activation by intravenous immunoglobulin is not mediated through the inhibitory IgG receptor FcgammaRIIb. J Allergy Clin Immunol. 2002;110:480–3. doi: 10.1067/mai.2002.127284. 10.1067/mai.2002.127284. [DOI] [PubMed] [Google Scholar]
- 34.Uher F, Lamers MC, Dickler HB. Antigen–antibody complexes bound to B-lymphocyte Fc gamma receptors regulate B-lymphocyte differentiation. Cell Immunol. 1985;95:368–79. doi: 10.1016/0008-8749(85)90324-7. 10.1016/0008-8749(85)90324-7. [DOI] [PubMed] [Google Scholar]
- 35.Uher F, Dickler HB. Cooperativity between B lymphocyte membrane molecules. independent ligand occupancy and cross-linking of antigen receptors and Fc gamma receptors down-regulates B lymphocyte function. J Immunol. 1986;137:3124–9. [PubMed] [Google Scholar]
- 36.Sigman K, Ghibu F, Sommerville W, Toledano BJ, Bastein Y, Cameron L, Hamid QA, Mazer B. Intravenous immunoglobulin inhibits IgE production in human B lymphocytes. J Allergy Clin Immunol. 1998;102:421–7. doi: 10.1016/s0091-6749(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 37.Ravanel K, Castelle C, Defrance T, Wild TF, Charron D, Lotteau V, Rabourdin-Combe C. Measles virus nucleocapsid protein binds to FcgammaRII and inhibits human B cell antibody production. J Exp Med. 1997;186:269–78. doi: 10.1084/jem.186.2.269. 10.1084/jem.186.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.te Velde AA, Huijbens RJ, Heije K, de Vries JE, Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990;76:1392–7. [PubMed] [Google Scholar]
- 39.Snapper CM, Hooley JJ, Atasoy U, Finkelman FD, Paul WE. Differential regulation of murine B cell Fc gamma RII expression by CD4+ T helper subsets. J Immunol. 1989;143:2133–41. [PubMed] [Google Scholar]
- 40.Laszlo G, Dickler HB. IL-4 induces loss of B lymphocyte Fc gamma R II ligand binding capacity. J Immunol. 1988;141:3416–21. [PubMed] [Google Scholar]
- 41.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–4. 10.1038/338383b0. [PubMed] [Google Scholar]
- 42.Machiels JJ, Somville MA, Lebrun PM, Lebecque SJ, Jacquemin MG, Saint-Remy JM. Allergic bronchial asthma due to Dermatophagoides pteronyssinus hypersensitivity can be efficiently treated by inoculation of allergen–antibody complexes. J Clin Invest. 1990;85:1024–35. doi: 10.1172/JCI114532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machiels JJ, Lebrun PM, Jacquemin MG, Saint-Remy JM. Significant reduction of nonspecific bronchial reactivity in patients with Dermatophagoides pteronyssinus-sensitive allergic asthma under therapy with allergen–antibody complexes. Am Rev Respir Dis. 1993;147:1407–12. doi: 10.1164/ajrccm/147.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]