Abstract
Age-related macular maculopathy (ARM) and age-related macular degeneration (AMD) are the leading causes of blindness in the Western world. Despite the magnitude of this clinical problem, very little is known about the pathogenesis of the disease. In this study, we analysed the sera (using indirect immunohistochemistry and Western blot analysis) from a very large cohort of such patients and normal age-matched controls to detect circulating anti-retinal antibodies. Patients with bilateral drusen (n = 64) and with chorioretinal neovascularization (CNV) (n = 51) were recruited in addition to age-matched control subjects (n = 39). The sera were analysed for anti-retinal immunoglobulins on retinal sections. The data were then correlated with the clinical features graded according to the International Classification and Grading System of ARM and AMD. The sera of patients with drusen (93·75%) and CNV (82·27%) were found to have a significantly (P = 0·02) higher titre of autoantibodies to the retina in comparison with controls (8·69%), indicating significant evidence of involvement of the immune process in early stages of AMD. Subsequent statistical analysis of the drusen group showed significant progressive staining (P = 0·0009) in the nuclei layers from early to late stages of ARM. Western blotting confirmed the presence of anti-retinal immunoglobulins to retinal antigens. As anti-retinal immunoglobulins are present in patients with bilateral drusen and exudative AMD, these antibodies could play a significant role in the pathogenesis of AMD. Whilst we do not have evidence that these antibodies precede disease onset, the possibility that their presence might contribute to disease progression needs to be investigated. Finally, the eventual identification of the target antigens detected by these antibodies may permit the future development of new diagnostic methods for ARM and AMD.
Keywords: age-related macular degeneration, aging, auto-antibodies, drusen, retina, retinal pigment epithelium
Introduction
Age-related macular degeneration (AMD) is a common macular disease affecting elderly people in the Western world and is a leading cause of blindness. In the ageing population of the developed world, several studies have shown that the prevalence of visual loss caused by AMD increases exponentially from 70 to 85 years of age, with 3·5% of the population > 75 years of age having visual impairment. As the disease primarily impacts on central vision, it is the leading cause of blindness in people over 60 years of age and accounts for ≈ 50% of blind registration.1,2 The early phase of the disease is age-related maculopathy (ARM), characterized by drusen and pigmentary abnormalities, with choroidal neovascularization (CNV) and geographical atrophy (GA) representing the terminal stages of AMD.
The combination of risk factors in AMD, such as age, hypertension, smoking and genetic and environmental factors, may disrupt the maintenance of self-tolerance within the retina. Several studies have implicated the immune system as playing an important role in the progression of AMD. Dendritic cells, potent antigen-presenting cells and macrophages are intimately associated with drusen development.3 The precise role of retinal autoimmunity in this eye disease is uncertain. It could simply represent an epiphenomenon that develops after retinal damage caused by physical or immunological insult. Autoantibodies have been detected in the sera of AMD patients, using both human and rat retina, but it is not clear whether these autoantibodies play a direct role in the aetiology of AMD or represent a non-specific response to retinal damage.4–6 The identification of autoantibodies during the course of other diseases, such as type 1 diabetes and thyroiditis, has been shown to be helpful in making a diagnosis and understanding the mechanisms of pathogenesis, identifying therapeutic strategies and monitoring treatment.7,8 The detection of autoantibodies may allow subtyping of the disease, according to the antibody profiles, which may help to define subgroups in terms of pathogenesis and therapy. Our intention was to identify (using indirect immunohistochemistry and Western blotting) autoantibodies in the sera of patients with ARM or AMD and in those without ARM or AMD, and to correlate patterns with disease progression.
Materials and methods
Patients
Patients from the AMD clinic were invited to participate in this study that followed the protocol as defined by the Declaration of Helsinki and for which local ethical approval was granted. Subjects of ≥ 50 years of age, with ARM or AMD in one or both eyes, were recruited to the study. Exclusion criteria included visual loss from other retinal disorders, in particular uveitis. Control patients (i.e. without ARM or AMD) were selected from cataract clinics or were the unaffected spouses of AMD patients. The patients had a detailed discussion with attending consultants following which informed consent was obtained. Stereo colour photographs of both fundi were taken, and blood samples were obtained, through venepuncture, into Vacuette™ vials containing serum clot activator. The blood samples were centrifuged at 4200 g for 10 min after which the supernatant serum was separated and tested for immunoglobulin subclass levels, followed by storage at −20°.
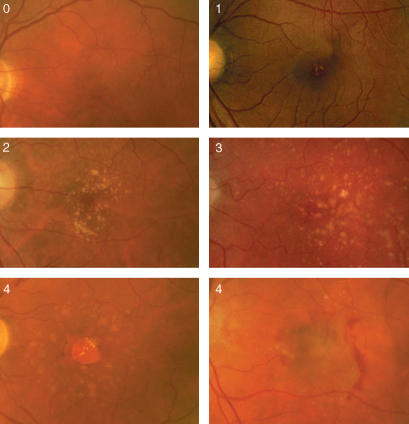
The stereo colour photographs were taken centred on the fovea (Topcon fundus camera; Topcon Corporation, Tokyo, Japan). Then, the photographs were graded and classified blind by a retinal specialist using the International Classification System for the clinical features of ARM and AMD, described previously, as illustrated in Fig. 1 and described in Table 1.9
Figure 1.
Fundus photographs illustrating the various progressive stages of age-related maculopathy (ARM) and age-related macular degeneration (AMD), and the terminal stages of AMD as geographical atrophy (GA) and chorioretinal neovascularization (CNV).
Table 1.
International Classification and Grading System of age-related maculopathy (ARM) and age-related macular degeneration (AMD)
| Stage | Definition |
|---|---|
| 0a | No signs of ARM |
| 0b | Hard drusen (< 63 µm) only |
| 1a | Soft indistinct drusen (≥ 63 µm) |
| 1b | Pigmentary abnormalities only |
| 2a | Soft indistinct drusen (≥ 125 µm) or reticular drusen only |
| 2b | Soft indistinct drusen (≥ 63 µm) with pigmentary abnormalities |
| 3 | Soft indistinct drusen (≥ 125 µm) or reticular drusen with pigmentary abnormalities |
| 4 | Geographical or neovascular AMD |
Indirect immunohistochemistry and confocal microscopy
All experimental procedures conformed to both the Association for Research in Vision and Ophthalmology (ARVO) statement for use of animals in ophthalmic and vision research and our own Institution's guidelines. BALB/c mice were killed and enucleated, the eyes were frozen in OCT compound in dry ice and stored at −80° before use. Cryosections of 5–7 microns were cut from this tissue, mounted on slides, then air-dried at room temperature for 30 min. The tissues were fixed in 100% acetone at 4° for 10 min followed by washing in phosphate-buffered saline (PBS) (Gibco, Life Technology Ltd, Paisley, UK). The slides were blocked with 5% normal rabbit serum (Sigma-Aldrich, Gillingham, Dorset, UK) in PBS for 30 min. Human sera from patients and controls were diluted (1 : 10 and 1 : 100) in PBS containing 1% bovine serum albumin (BSA) (Sigma) and 0·01% azide. These concentrations of antibody were determined after the careful titration (1 : 10 to 1 : 1000 dilutions) of multiple serum samples to establish dilutions that would provide specific immunoreactivity only in a subset of serum samples. A total of 115 serum samples from patients with ARM or AMD were analysed by immunohistochemistry (IHC), as were 39 samples from age-matched controls. The 1 : 100 dilution used in the immunohistochemical experiments resulted in only 8·27% staining among the age-matched controls, indicating that there was minimal non-specific staining at this dilution. The sections were incubated with the diluted sera for 1 hr. A secondary antibody, Cy5-conjugated™* AffiniPure F(ab′)2 fragment goat anti-human immunoglobulin G (IgG) (H+L) (Code no. 309-176-006; Jackson Immunoresearch Laboratory Inc., Soham, Cambridgeshire, UK), was diluted (1 : 1000) in PBS containing 1% BSA and the sections were incubated for 1 hr followed by washing with PBS and distilled water. In the control slides, PBS only was used followed by incubation with secondary antibody. Confocal microscopy (Carl Ziess, Welwyn Garden City, Herts) was performed on all slides by optimizing the red channel (633 nm). The digital images were read in a blinded manner by an investigator, and the level of staining in all layers of the retina, including the choroid, Bruchs membrane, retinal pigment epithelium (RPE), outer plexiform layer (OPL), outer nuclear layer (ONL), inner nuclear layer (INL), inner plexiform layer (IPL) and retinal ganglion cell layer (RGL), was recorded. Positive staining at a particular retinal layer was recorded on a database as 1, whilst negative staining was recorded as 0.
Western blotting
Retinal tissue from BALB/c mice was isolated followed by snap-freezing in liquid nitrogen. Total protein extract was obtained by adding 100 mg of tissue to 100 µl of 10 mm PBS containing 5%β-mercaptoethanol, 1 mm phenylmethylsulphonyl fluoride (PMSF) and benzamidine, and centrifugation (150 000 g, 4°, 20 min) followed by boiling at 100° for 10 min. The sample was then diluted with sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer (125 mm Tris–HCl, pH 6·8, containing 2% SDS, 10% glycerol and 0·005% bromophenol blue) and placed on ice prior to use. The protein concentration in this homogenate was 0·9 mg/ml, as determined by using the BCA Protein Assay Kit (Pierce Biotech., Rockford, IL).
Cell culture
ARPE19 cells, Y79 cells, HeLa cells and Jurkat cells were maintained as monolayer cultures in medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin, 100 µg/ml of streptomycin and 1% GlutaMAX™ (Invitrogen, Paisley, UK). ARPE19 and Y79 cells were cultured in F-12 Nutrient Mixture medium. HeLa cells were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM). Jurkat cells were cultured in RPMI-1640 containing 25 mm HEPES. Tissue culture media and cell culture supplies were from Life Technologies Ltd. (Paisley, UK). Cells were lysed in a buffer containing 150 mm NaCl, 1% Nonidet P-40 (NP-40), 0·25% sodium deoxycholic acid, 50 mm Tris–HCl (pH 7·4), 1 mm NaF, 1 mm Na3VO4, and one complete Mini™ (Roche) protease inhibitor cocktail tablet.
Protein extracts (10–15 µl per lane) were fractionated by SDS–PAGE (10% gel) and electroblotted onto poly(vinylidene difluoride) (PVDF) membranes (Immobilon™; Millipore, Bedford, MA). The membranes were blocked with Tris-buffered saline containing Tween (TBS-T), 5% skimmed milk and 5% BSA for 1 hr at room temperature and then incubated for 1 hr with a 1 : 500 dilution of human sera (from ARM, AMD and control patients) in TBS-T containing 5% skimmed milk and 5% BSA. The membranes were washed and incubated with a 1 : 1000 dilution of peroxidase-conjugated rabbit anti-human horseradish IgG, specific for the gamma chains of human IgG origin (DakoCytochem, Hamburg, Germany), for 45 min at room temperature followed by washing with TBS-T. These dilutions of the sera and secondary antibody were used following extensive titration and after repeated experiments to confirm positive findings. Bound antibodies were visualized using the ECL Western blot detection kit (Amersham Pharmacia Biotech, Piscataway, NJ).
Results
In total, 154 patients, including age-matched controls, were entered into the study. The ARM group consisted of 64 patients (41·56%), the CNV group comprised 51 patients (33·12%) and the control group comprised 39 patients (25·32%). See Table 2 for further demographic details.
Table 2.
Demographic distribution of patient and control groups
| Control (n = 39) | ARM (n = 64) | CNV (n = 51) | |
|---|---|---|---|
| Age range (years) | 60–90 | 50–88 | 65–95 |
| Mean age (years) | 75·39 | 74·56 | 82·2 |
The above data show the demographic distribution of age and gender in all groups. The age range is younger in the age-related maculopathy (ARM) group in comparison to the choroidal neovascularization (CNV) group; this reflects the end stage of the disease process in older patients.
Using a two-sample Wilcoxon rank-sum (Mann–Whitney) test that adjusted for variance in age and gender (z = 0·012), there was no evidence of a significant difference in age between the sexes in all groups. The mean serum immunoglobulin levels were within the normal range in all age-groups, and no statistical difference was observed between the drusen stages (Table 3). Analysis of immunocytochemistry results showed the presence of autoantibodies to retinal tissue in the majority of the ARM and AMD sera compared with the sera of controls that stained specifically to the choroid, Bruchs membrane, RPE, photoreceptor ONL, and INL and RGL. Following analysis using statistical software, the staining in these regions of the retina were significant in comparison to the staining of other layers, such as the photoreceptor and plexiform layers, which were not statistically significant on further analysis using Fisher's exact test. There was mild staining of other layers, such as the photoreceptor and plexiform layers; however, these were not statistically significant on further analysis. The outer nuclear membrane stains strongly, as shown in Fig. 2(a), whereas the entire inner nuclei stain completely. The sera of some patients contained specific anti-retinal immunoglobulins to antigens within the nucleoli. This was confirmed by the similar staining patterns observed for both 1 : 10 and 1 : 100 dilutions. In Fig. 2(b), there is distinct staining of the nucleoli in the inner nuclear layer with diffuse staining at the INL, in contrast to Fig. 2(c), which shows specific staining of nucleoli in the ONL. Figure 2(d) shows a retinal section incubated with control patient sera showing an absence of specific staining in comparison to ARM and AMD sera. Some of the affected patient sera stained the retinal pigment epithelium as well the inner segment of the photoreceptor layer, as shown in Figs 3 and 4. The control group showed background fluorescence with the exception of a few patients who showed mild, non-specific staining of the retina (data not shown).
Table 3.
Mean serum immunoglobulin levels in affected patients and controls
| IgG (7–18·6 g/l) | IgA (0·78–4·80 g/l) | IgM (0·49–2·00 g/l) | |
|---|---|---|---|
| Control | 12·182 | 2·376 | 1·064 |
| Drusen | 11·506 | 2·166 | 1·095 |
| CNV | 11·088 | 2·677 | 1·383 |
The mean levels of serum immunoglobulin isoclasses in all groups were within the normal range, indicating that the systemic immune system appears to be stable during the progression of this disease. There was no significant difference in any group following statistical analysis.CNV; choroidal neovascularization; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
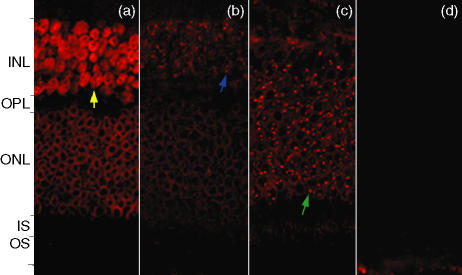
Figure 2.
Confocal microscopy, using the red channel (633 nm) to view anti-human immunogobulin G (IgG) conjugated to Cy5, on retinal sections incubated in the sera of patients with age-related maculopathy (ARM) and age-related macular degeneration (AMD). (a) Patient with chorioretinal neovascularization (CNV), stage 4. The outer nuclear membrane stains strongly, whereas the entire inner nuclei stain completely (yellow arrow). (b) Patient with ARM, stage 3. There is distinct staining of the nucleoli (blue arrow) in the inner nuclear layer with diffuse staining at the inner nuclear layer compared to (c) (ARM patient, stage 2a), which shows specific staining of nucleoli in the outer nuclear layer (green arrow). (d) Retinal sections incubated with control sera showing an absence of specific staining in comparison to the sera from patients with ARM and AMD. INL, inner nuclear layer; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segment.
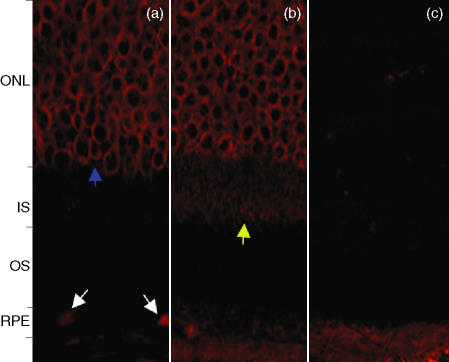
Figure 3.
(a) Patient with chorioretinal neovascularization (CNV), stage 4. There is specific staining of the retinal pigment epithelium (white arrows) with strong staining of the outer nuclear membrane layer (blue arrow). (b) Patient with CNV, stage 4. Specific staining of the outer nuclear layer and the inner segment (IS), of the photoreceptor layer (yellow arrow), accompanied by mild staining of the retinal pigment epithelium (RPE). (c) Retinal sections incubated with control sera showing an absence of specific staining in comparison to sera from patients with age-related maculopathy (ARM) and age-related macular degeneration (AMD). ONL, outer nuclear layer; OS, outer segment.
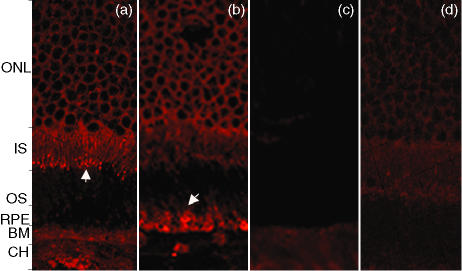
Figure 4.
(a) Patient with chorioretinal neovascularization (CNV), stage 4. The inner segment of the photoreceptor layer stains strongly in addition to the outer nuclear layer (ONL). (b) Patient with CNV, stage 4. Localized labelling of retinal pigment epithelium (RPE) cells accompanied by staining of the outer nuclear and the inner segment of photoreceptor layers. (c) Retinal sections incubated with control sera showing an absence of specific staining in comparison to sera from patients with age-related maculopathy (ARM) and age-related macular degeneration (AMD). (d) The negative control, consisting of secondary antibody only, shows mild background fluorescence. BM, basement membrane; CH, choroid; IS, inner segment; OS, outer segment.
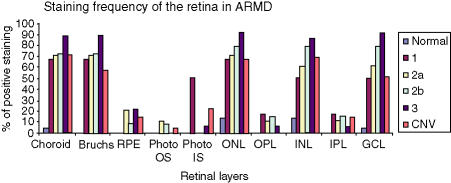
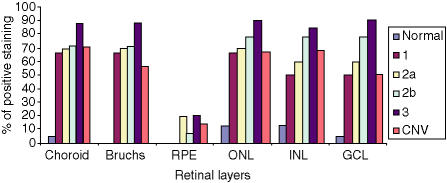
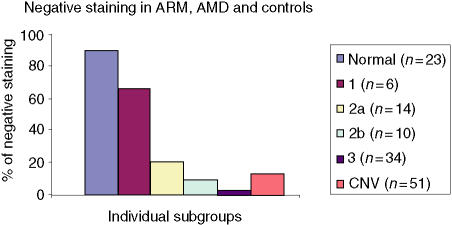
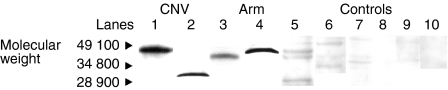
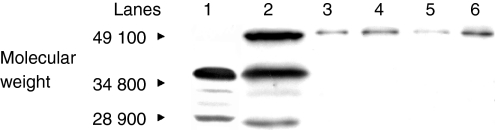
The frequency of staining in the CNV group was significantly greater in comparison to controls, with staining in the choroid, Bruchs membrane, ONL, INL and RGL. The drusen group stained in a similar pattern predominantly in the choroid, Bruchs membrane and nuclei in some of the retinal layers (Fig. 5). Subsequent statistical analysis of this data identified a significant difference between the drusen and control group using Fisher's test (P = 0·02). Similarly, a significant difference was found between the CNV group and the control group (P = 0·01). However, there was no statistical difference between the drusen group and the CNV group. There was significant staining at the choroid, Bruchs membrane, RPE and ganglion cell layer (GCL) in ARM and AMD patients in comparison to controls. Further analysis using the χ2-test showed a significantly (P < 0·05) increased proportion of staining from the early (stage 2) to late (stage 3) stages of ARM in the INL, ONL and GCL. There was staining in the earlier stages (stages 1a and 1b) of ARM but, owing to low numbers (n = 6) in these groups, the results cannot be interpreted accurately (Fig. 6). In order to evaluate the significance of staining, we developed a unique method to observe staining patterns. The study patients were subdivided into three different groups as follows: those with no staining; those with staining in the outer retina only, as defined by choroids/Bruchs membrane/retinal pigment epithelium and photoreceptor layers (Group 1); and those staining in the inner and outer retina (as defined by the outer and inner nuclear and including the retinal ganglion cell layer) (Group 2). The data were then analysed using statistical software and a significant difference was found between the progressive stages of ARM from stage 2 to stage 3 using this system (P < 0·05). There is a significant, progressive increase in immune reactivity to the retina from stage 2 to stage 3. Although the data shows a high frequency of staining in stage 1, as a result of the low numbers (n = 6), a high percentage in this group may not indicate any relevance (Fig. 7). Analysis of the negative-staining group shows a strongly positive correlation between control patients and the absence of staining (91·73% specificity). As drusen becomes more established owing to the progression of ARM, negative staining decreases, but shows a slight increase in the CNV group (Fig. 8). Western blotting using sera from patients with ARM and AMD confirmed the presence of anti-retinal autoantibodies to perhaps more than one target antigen whose molecular weight (MW) ranges between 28 400 and 49 100 (Fig. 9).
Figure 5.
Staining frequency of the retina in age-related maculopathy (ARM) and end-stage-related macular degeneration (AMD) in comparison to controls. The bar chart shows clear progressive staining of the choroid, Bruchs membrane, inner nuclear layer (INL), outer nuclear layer (ONL) from stage 1 through stage 2 and up to stage 3 (stages are as indicated in the Figure key) using the International Classification System of drusen (ARM). The retinal pigment epithelium (RPE), outer segment (OS) and inner segment (IS) photoreceptor layers, as well as the plexiform layers, show minimal staining with no specific pattern. GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer.
Figure 6.
Immunohistochemistry, using sera from patients with drusen, on murine BALB/c eye sections shows that the drusen group demonstrate a greater frequency of staining in the choroid, Bruchs outer and inner nuclear layers (ONL, INL) in comparison to those of the chorioretinal neovascularization (CNV) and control groups, indicating significant evidence of involvement of the immune process in the early stage of AMD. There is a gradual, statistically significant increase in retinal autoantibody titre from stages 2–3 ONL, INL and RGL (the stages are as indicated in the figure key). GCL, ganglion cell layer; RPE, retinal pigment epithelium.
Figure 7.
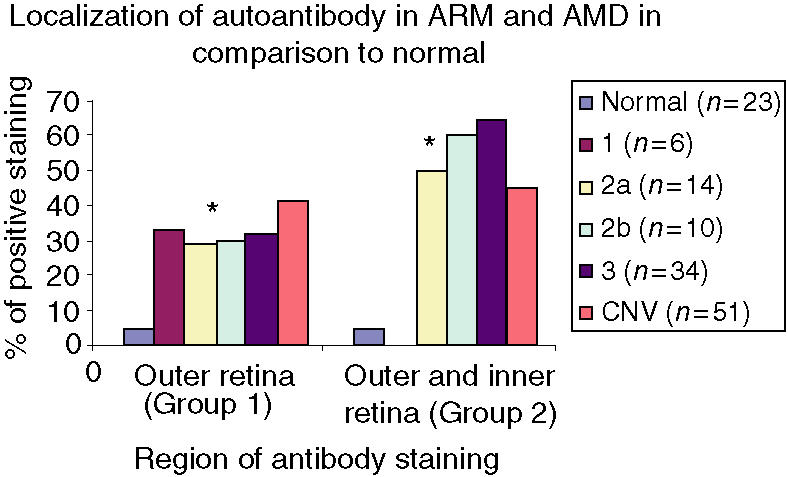
The bar chart shows the two different groups of specific staining. Group 1 consists of the outer retina comprising choroid, Bruchs, retinal pigment epithelium and photoreceptor layers. Group 2 consists of those that stained both in the outer retina as well as in the inner retina with staining in the outer and inner nuclear layers, including the retinal ganglion cell layer. The stages of age-related maculopathy (ARM) are as indicated in the Figure key. CNV, chorioretinal neovascularization. *P < 0·05.
Figure 8.
Bar chart showing the distribution of negative staining in patients with age-related maculopathy (ARM), age-related macular degeneration (AMD) and in controls. Using indirect immunohistochemistry, the data show good correlation between controls and negative staining. The stages of ARM are as indicated in the Figure key. CNV, chorioretinal neovascularization.
Figure 9.
Western blot analysis of BALB/c mouse retinal lysate incubated with human sera of patients with chorioretinal neovascularization (CNV; stage 4) (lanes 1 and 2) and age-related maculopathy (ARM; stages 3, 2 and 1; corresponding lanes 3, 4 and 5) show the presence of several retinal antigens of molecular weight 28 400–49 100. The majority of controls (lanes 6–10) showed an absence of autoantibody to retinal antigens, with the exception of two patients (lanes 7–10).
In Fig. 9, lane 1 corresponds to a retinal antigen of ≈ 46 000 MW that binds to anti-nuclear immunoglobulin which localizes to the INL layer and also binds to the nuclear membrane of the ONL (Fig. 2a). Lane 2 represents a retinal antigen with a MW of 30 100 from an anti-nucleolar immunoglobulin that localizes to the INL (Fig. 2b). Lane 3 corresponds to a retinal antigen with a MW of 35 200, which binds to an anti-nucleolar immunoglobulin that localizes to the INL and ONL. In lane 4, the antibody that localizes to the inner segment of the photoreceptor layer binds to retinal antigen with a MW of 38 400. Lane 5 represents a retinal antigen of MW between 28 900 and 38 400, to which the serum antibody localizes to the RPE. In lane 6, only one of the controls showed some very weak staining to retinal antigens in comparison to those patients with AMD and ARM who stain very strongly. Lanes 7–10 show no staining, indicating an absence of retinal autoantibodies in the sera of the majority of controls.
In Fig. 10, the Western blot from lysate containing cadaver human retinal, BALB/c retina and from cell culture lysate of ARPE19 cells, human photoreceptor cells (Y79) cells, fibroblast cells (HeLa), and human T cells (Jurkat), shows that the antigens may be preserved through species, as reported in previous studies.
Figure 10.
Western blot from lysate containing cadaver human retina (lane 1), BALB/c mouse retina (lane 2) and from culture lysate of ARPE19 cells (lane 3), human photoreceptor cells (Y79) (lane 4), fibroblast cells (HeLa) (lane 5), and human T cells (Jurkat) (lane 6) incubated with the sera of a patient with chorioretinal neovascularization (CNV) shows that antigens are similar in mouse and human tissue. Cultured retinal and immune-competent cells demonstrated the presence of antigen with strongly staining bands; however, fibroblast cells showed weak staining.
Discussion
AMD is the most important single cause of blindness among Caucasian individuals in developed countries. Age, hypertension, smoking and genetic predisposition are known risk factors for AMD. Subclinical atherosclerosis, which may be a consequence of this, may increase the risk of ARM progression. Several studies have implicated the immune system in playing an important role in the progression of this AMD.10
Anti-retinal immunoglobulins are initially identified using immunocytochemistry followed by a confirmatory assay, such as Western blotting. Autoantibodies to the retina have been detected in the sera of patients with AMD by using tests carried out on fresh tissue from human, mouse and rat eyes. The pattern of binding on the murine and rat retinae were found to be similar to that observed on human retina, indicating that the antigens were not species specific. However, it had not been made clear whether these autoantibodies play a direct role in the aetiology of AMD or represent a specific response to retinal damage. Specific antibodies against astrocytes have been detected previously in AMD sera and are thought to influence the permeability of the retina by affecting the blood–retinal barrier.11,12 These studies used high concentrations of sera (1 : 2 and 1 : 10) in their analysis; however, in our experiments the dilutions of the sera and secondary antibody concentrations were titrated several times to optimize the results, which were consistent over several replicate experiments. In addition, by using 5% normal rabbit sera we were able to obtain specific labelling with reduced background fluorescence.
Using a secondary antibody conjugated to a fluorochrome, such as Cy5 (633 nm, red channel), which is observed at the extreme of normal wavelength (490–760 nm), we were able to establish a sensitive method of detection. A researcher who was blind to the clinical phenotype of the patient conducted the experiments. The clinical phenotype was graded by a retinal specialist who was blinded to the experimental findings, further ruling out the possibility of bias. These data have shown that, in contrast to the control group, anti-retinal immunoglobulins are present in the sera of patients with ARM and AMD.
There were a few patients with ARM and CNV who did not stain at all using this method. The non-staining CNV group consisted of patients with end-stage disciform scars and significant fibrosis. We would expect some level of immune reactivity to the retina as a result of exposure of the blood–retinal barrier, but this was not observed. The ARM group that did not stain had stage 1 drusen, which represents the first stage of the disease process. It is possible that at this stage, local inflammation is only just beginning, hence the absence or low titre of anti-retinal autoantibodies. In this study, there were too few samples of early drusen (stage 1); therefore, an accurate interpretation of this cannot be made and further studies are needed. In the ARM group there was a statistically significant increase in titres of retinal auto-antibody from early (stage 2) to late (stage 3) phases of ARM. The results also show that there was no statistical difference between CNV and drusen staining. This indicates that the anti-retinal immunoglobulin may play a significant role in the progression of ARM; however, it is possible that anti-retinal immunoglobulins could also be a reactive response as a result of breach of the blood–retinal barrier. We can assume that in the early ARM stage, specific immune-privileged retinal antigens, which may be otherwise protected, are exposed to the host immune system.
Johnson et al. and Anderson et al. have shown that amyloid Beta protein is found within drusen and could be pathogenic, resulting in a pro-inflammatory response through activation of the complement cascade at the sub-RPE space.12,13 Previous histological studies on cadaver eyes with early drusen showed uniformly distributed immunoreactivity against immunoglobulin isoclass G, whereas irregular drusen showed varying degrees of positive reactivity. There was immunoreactivity against specific immunoglobulin isoclass G within drusen and in RPE cells adjacent to and overlying drusen. This implies that some RPE cells closely associated with drusen may be compromised and could consequently become targets for immune complex formation and complement-mediated attack. These cells may undergo degenerative changes contributing to drusen expansion.14
In a recent animal model of AMD, CCL2- and CCR2-deficient mice were found to have complement component C5 and IgG complexes in the RPE and choroid of knockout mice at a distribution similar to that found in eyes of humans with AMD.15 Mullins et al. demonstrated the presence of acute-phase proteins within drusen, such as coagulation proteins and immunoglobulin, as well as human leucocyte antigen-DR (HLA-DR), which suggests a direct or an indirect role of the humoral immune process in the aetiology of AMD.16,17 Further evidence that the immune-complex pathogenesis is important in AMD comes from studies on sera from patients: the sera recognized RPE protein homogenates from cultured cells using immunoblotting. However, none of the immunogenic molecules have yet been characterized, although beta amyloid has been shown to be significant.
Dendritic cells, potent antigen-presenting cells and macrophages are intimately associated with drusen development and complement activation, both within drusen and along the RPE–choroid interface.3 Our data have shown that the sera of patients with ARM and AMD contain autoantibodies to retinal tissue which could be pathogenic in the progression of ARM. We note here that our data do not determine whether the antigens are retina-specific, and this is currently under investigation. Although there is an increasing proportion of positive staining from stage 0 to stage 3, it is not certain whether the presence of positive staining would signify a higher risk of developing CNV independent to the fundus image. If the latter is confirmed in the follow-up study, this would allow us to identify high-risk patients for future prophylaxis treatment before CNV formation.
Recently, sera from AMD patients demonstrated mean titres of anti-carboxyethylpyrrole (CEP) autoantibody that were higher than those of controls, confirming the role of the immune system in the pathogenesis of AMD and suggesting that the autoantibody titre may have diagnostic utility in predicting AMD susceptibility; this is comparable to the results of our study, which consisted of larger study numbers.18 From studies on visual paraneoplastic disorders, such as cancer-associated retinopathy (CAR), we know that antibodies are directed against the specific primary antigens recoverin (46 000 MW) and retinal enolase (23 000 MW), amongst others.19,20In vitro and in vivo studies indicate that these autoantibodies are cytotoxic to retinal cells and induce apoptotic death of retinal photoreceptor cells, which leads to the degeneration of the photoreceptor cell layer. Antibodies with other retinal specificities may induce their target retinal cell death by activating a caspase 3-dependent apoptotic pathway. Thus, autoantibody-induced photoreceptor cell death may be a common pathway that leads to blindness.21,22 Furthermore, retinal cells overlying both soft and hard drusen showed structural and molecular abnormalities indicative of photoreceptor degeneration.23 Specific nuclear autoantigens to photoreceptor cells have been detected in paraneoplastic retinopathy, with reactivity being observed to the outer and inner nuclear layers.24 These antigens could be similar to those detected in the present study. However, once again we emphasize that we have no evidence that the antigens detected in this study are retina-specific (although this is not a strict requirement for an antigen to play a significant role in immune pathology). In the ARM group we observed staining of the inner and outer nuclear layers in addition to the ganglion cell layer. This could be important as antibodies to these cells could lead to photoreceptor degeneration and increased RPE loading with lipofuscin deposition and drusen evolution.
Autoantibodies can be detected in the sera of high-risk patients prior to the onset of disease, as clearly demonstrated in Type 1 diabetes mellitus.25 In the BB rat model of Type 1 diabetes mellitus, autoantibodies were detected, in sera, to a 64 000 MW protein prior to the spontaneous onset of the disease.26 Approximately 70–80% of patients with newly diagnosed Type 1 diabetes have autoantibodies to islet-cell autoantibodies (ICA) and glutamic acid carboxylase (GAD65), and the presence of such autoantibodies years before the onset of clinical symptoms has made it possible to identify individuals at high risk of developing Type 1 diabetes and to initiate therapeutic intervention trials on relatively small numbers of subjects.27 We note here that autoantibodies detected in several diseases of clear autoimmune pathogenesis are often directed at antigens that are not tissue-specific. One clear example is systemic lupus erythematosis, where autoantibodies are directed at ubiquitous antigens such as histones and nucleic acid.28–31
Regardless of the tissue-specificity of the antigens detected by the patient sera, our data show that in patients with ARM, we can identify, using anti-retinal autoantibodies, those who are likely to progress to the late stages of AMD. Further research to identify the antigen, the location and its function could bring us closer to understanding the role of the immune system in AMD. Identification of the antigen by purification, sequencing and cloning, using molecular techniques, may permit the development of specific autoantibody assays.32
Analysis of antibody titre during treatment might aid in monitoring different modes of treatment for this visually disabling disease before vision is affected. Inactivation or removal of these antibodies, as a novel therapy, may have a therapeutic effect in reducing the progression of AMD.
Abbreviations
- AMD
age-related macular degeneration
- ARM
age-related maculopathy
- BSA
bovine serum albumin
- CNV
choroidal neovascularization
- GA
geographical atrophy
- GCL
ganglion cell layer
- INL
inner nuclear layer
- IPL
inner plexiform layer
- MW
molecular weight
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- PBS
phosphate-buffered saline
- RGL
retinal ganglion cell layer
- RPE
retinal pigment epithelium
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
References
- 1.Owen CG, Fletcher AE, Donoghue M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol. 2003;87:312–7. doi: 10.1136/bjo.87.3.312. 10.1136/bjo.87.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.la Cour M, Kiilgaard JF, Nissen MH. Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging. 2002;19:101–33. doi: 10.2165/00002512-200219020-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE–Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. 10.1016/S1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Wu L, Pan S, Wu DZ. An immunologic study on age-related macular degeneration. Yan Ke Xue Bao. 1993;9:113–20. [PubMed] [Google Scholar]
- 5.Gurne DH, Tso MO, Edward DP, Ripps H. Antiretinal antibodies in serum of patients with age-related macular degeneration. Ophthalmology. 1991;98:602–7. doi: 10.1016/s0161-6420(91)32252-8. [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Wu DZ, Cao X, et al. [Study of pathogenesis of age-related macular degeneration] Yan Ke Xue Bao. 1996;12:58–63. [PubMed] [Google Scholar]
- 7.Zanone MM, Catalfamo E, Pietropaolo SL, et al. Glutamic acid decarboxylase and ICA512/IA-2 autoantibodies as disease markers and relationship to residual beta-cell function and glycemic control in young type 1 diabetic patients. Metabolism. 2003;52:25–9. doi: 10.1053/meta.2003.50003. 10.1053/meta.2003.50003. [DOI] [PubMed] [Google Scholar]
- 8.Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes. autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327–64. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 9.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The Int ARM Epidemiol Study Group Surv Ophthalmol. 1995;39:367–74. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 10.van Leeuwen R, Ikram MK, Vingerling JR, et al. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44:3771–7. doi: 10.1167/iovs.03-0121. 10.1167/iovs.03-0121. [DOI] [PubMed] [Google Scholar]
- 11.Penfold PL, Provis JM, Furby JH, et al. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1990;228:270–4. doi: 10.1007/BF00920033. 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- 12.Johnson LV, Leitner WP, Rivest AJ, et al. The Alzheimer's A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:11830–5. doi: 10.1073/pnas.192203399. 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson DH, Talaga KC, Rivest AJ, et al. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–56. doi: 10.1016/j.exer.2003.10.011. 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Johnson LV, Ozaki S, Staples MK, et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–9. doi: 10.1006/exer.1999.0798. 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 15.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–7. doi: 10.1038/nm950. 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–96. doi: 10.1006/exer.2001.1094. 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 17.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. Faseb J. 2000;14:835–46. [PubMed] [Google Scholar]
- 18.Gu X, Meer SG, Miyagi M, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–35. doi: 10.1074/jbc.M305460200. 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 19.Thirkill CE, Tait RC, Tyler NK, et al. The cancer-associated retinopathy antigen is a recoverin-like protein. Invest Ophthalmol Vis Sci. 1992;33:2768–72. [PubMed] [Google Scholar]
- 20.Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase-alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun. 1998;11:671–7. doi: 10.1006/jaut.1998.0239. 10.1006/jaut.1998.0239. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PT, Lewis GP, Talaga KC, et al. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003;44:4481–8. doi: 10.1167/iovs.03-0436. 10.1167/iovs.03-0436. [DOI] [PubMed] [Google Scholar]
- 22.Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2:63–8. doi: 10.1016/s1568-9972(02)00127-1. 10.1016/S1568-9972(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 23.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–42. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 24.Eichen JG, Dalmau J, Demopoulos A, et al. The photoreceptor cell-specific nuclear receptor is an autoantigen of paraneoplastic retinopathy. J Neuroophthalmol. 2001;21:168–72. doi: 10.1097/00041327-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Baekkeskov S, Nielsen JH, Marner B, et al. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982;298:167–9. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- 26.Baekkeskov S, Dyrberg T, Lernmark A. Autoantibodies to a 64-kilodalton islet cell protein precede the onset of spontaneous diabetes in the BB rat. Science. 1984;224:1348–50. doi: 10.1126/science.6374896. [DOI] [PubMed] [Google Scholar]
- 27.Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–52. doi: 10.1172/JCI14257. 10.1172/JCI200114257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes-Hernandez J, Ordi-Ros J, Labrador M, et al. Antihistone and anti-double-stranded deoxyribonucleic acid antibodies are associated with renal disease in systemic lupus erythematosus. Am J Med. 2004;116:165–73. doi: 10.1016/j.amjmed.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 29.Gioud M, Kaci MA, Monier JC. Histone antibodies in systemic lupus erythematosus. A possible diagnostic tool. Arthritis Rheum. 1982;25:407–13. doi: 10.1002/art.1780250408. [DOI] [PubMed] [Google Scholar]
- 30.Schett G, Smole J, Zimmermann C, et al. The autoimmune response to chromatin antigens in systemic lupus erythematosus: autoantibodies against histone H1 are a highly specific marker for SLE associated with increased disease activity. Lupus. 2002;11:704–15. doi: 10.1191/0961203302lu247oa. 10.1191/0961203302lu247oa. [DOI] [PubMed] [Google Scholar]
- 31.Shoenfeld Y, Segol O. Anti-histone antibodies in SLE and other autoimmune diseases. Clin Exp Rheumatol. 1989;7:265–71. [PubMed] [Google Scholar]
- 32.Lernmark A. Autoimmune diseases: are markers ready for prediction? J Clin Invest. 2001;108:1091–6. doi: 10.1172/JCI14234. 10.1172/JCI200114234. [DOI] [PMC free article] [PubMed] [Google Scholar]