Abstract
Secretion of immunomodulatory molecules is a key strategy employed by pathogens to enable their survival in host organisms. For example, arthropod-transmitted filarial nematodes, which achieve longevity within the infected host by suppressing and modulating the host immune response, produce excretory–secretory (ES) products that have been demonstrated to possess immunomodulatory properties. In this review we discuss the immunomodulatory effects of the phosphorylcholine-containing filarial nematode-secreted glycoprotein ES-62 and describe the intracellular signal transduction pathways it targets to achieve these effects.
Keywords: ES-62, filarial nematodes, immunomodulation, signal transduction
Introduction
Filarial nematodes are arthropod-transmitted parasites of vertebrates including humans. Of the eight species known to infect humans, three –Wuchereria bancrofti, Brugia malayi and Onchocerca volvulus– are a major cause of morbidity in the Tropics. It is currently estimated that about 150 million people are infected with one or more of these worms and a significant proportion of these suffer debilitating health problems including severe skin lesions, elephantiasis and eye damage that may lead to blindness.1 Infection with filarial nematodes is long-term, with individual worms surviving for ∼ 10 years.2 Parasite longevity reflects suppression or modulation of the host immune system (reviewed in refs 3,4) and there is increasing evidence that such immunomodulation can be mediated by bioactive molecules secreted by the worms (reviewed in refs 5,6). In this review we will discuss the results of studies in our laboratories over the last 10 years, which have examined immunomodulation by ES-62, a secreted product of the rodent filarial nematode Acanthocheilonema viteae.
The immune response to filarial infection
Filarial parasites are transmitted to mammalian hosts by an arthropod vector. They develop inside the vector from microfilariae to infective larvae, before migrating to the arthropod mouth parts for transmission to the mammalian host when the arthropod feeds. The larvae then develop into adult worms and the life-cycle is completed with the generation of microfilariae, which are ingested again in the arthropod blood-meal. A spectrum of disease states exists in areas where filarial infection is endemic (reviewed in ref. 7). Aggressive immune responses to filarial nematodes occur in some individuals, resulting in chronic pathology, such as elephantiasis. However, the majority of individuals, although having detectable microfilariae in their bloodstreams, are otherwise apparently asymptomatic and have been described as being immunologically tolerant to the parasite. As alluded to earlier, this is thought to be the result of immunomodulation to achieve a situation conducive to both parasite survival and host health. Such people often have dramatically increased levels of immunoglobulin G4 (IgG4) and interleukin-10 (IL-10), with reduced interferon-γ (IFN-γ) production.8–11 Levels of IgE, which may be protective against infection with filarial nematodes and other helminths, are elevated in patients with chronic pathology.8 In contrast, individuals with detectable microfilariae tend to have a higher IgG4 : IgE ratio. IgG4 has been shown to compete with IgE for epitope recognition and hence may limit the induction of pathology.12
ES-62, a phosphorylcholine-containing glycoprotein
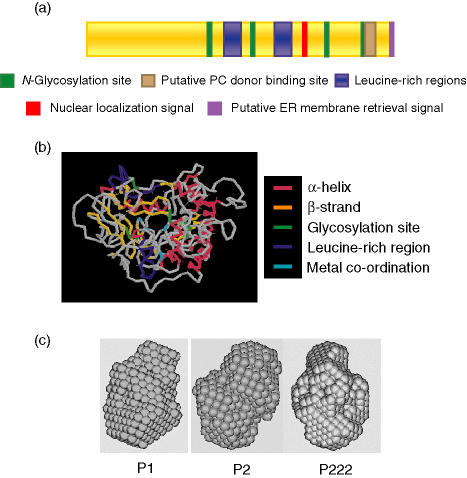
ES-62 is produced by the post-infective life-cycle stages (L4 larvae and adult worms) of A. viteae and can be detected in the serum of infected jirds.13,14 However, the ES-62 gene is transcribed throughout the A. viteae life-cycle, although mRNA levels are considerably higher in adult worms than in L3 larvae (∼ 5% adult levels) and microfilariae (< 0·2% adult levels).15 ES-62 mRNA is translated into a glycoprotein with a molecular weight of 62 000 (including post-translational modifications) that has phosphorylcholine (PC) moieties attached via N-type glycans (reviewed in ref. 16; see Fig. 1). The number of PC-containing glycans present on each molecule is currently unknown but the number of PC groups per glycan has been shown to be variable.
Figure 1.
ES-62 structural studies. (a) The location of key residues within the ES-62 sequence, including N-glycosylation sites, a possible site for interaction with PC donors,23 leucine-rich regions (likely to be involved in protein–protein interaction), and regions containing subcellular targeting motifs. (b) Prediction of a tertiary structure for the ES-62 monomer, obtained using dragon66 indicating α-helices and β-strands, as well as glycosylation sites, leucine-rich regions and residues involved in metal ion co-ordination (ES-62 shows homology with aminopeptidases that contain a divalent cation in their active site17). (c) A low-resolution dummy atom model of the ES-62 tetramer, which is likely to be slightly elongated, obtained using dammin67 under three symmetry conditions (P1, no symmetry; P2, two-point symmetry; P222, 222-point symmetry).
ES-62 has highest sequence homology with a recently found family of aminopeptidases and carboxypeptidases (e.g. 38% and 37% identity with mouse and human aminopeptidases) and has been shown to possess some, albeit weak, aminopeptidase activity in vitro against synthetic substrates.17 Interestingly, the biologically active forms of many aminopeptidases are dimeric or tetrameric18,19 and consistent with this, gel filtration studies and sedimentation equilibrium data demonstrated that ES-62 is a tightly bound tetramer formed from dimers.16,20,21 Furthermore, divalent cations are known to be critical to the function of aminopeptidases and ES-62 has a putative metal co-ordination motif in its sequence; indeed, a strong magnesium (Mg2+) signal was detected in its atomic emission spectrum.21 Although a function for the aminopeptidase component of ES-62 has not yet been convincingly demonstrated, the molecule has been shown to display a variety of immunomodulatory properties, many of which have been attributed to the presence of PC. PC is a molecular pattern associated with pathogen products from a diverse range of organisms, including bacteria, fungi and protozoa, as well as filarial and gastrointestinal nematodes (reviewed in ref. 22). It enables the detection of pathogens by the host (for example via antibodies or C-reactive protein), but can also function to promote pathogen survival via modulation of the host immune response.23
ES-62 exerts its immunomodulatory effects on a variety of cells of the murine immune system including B and T lymphocytes as well as antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages.23–30 Broadly, rather than acting in an immunosuppressive manner, the molecule induces a T helper type 2 (Th2)/anti-inflammatory phenotype, characterized by the production of IL-10, with reduced levels of IL-12, IFN-γ and pro-inflammatory cytokines, and IgG1 rather than IgG2a antibodies. These effects (summarized in Fig. 2) and the signalling pathways targeted by ES-62 to achieve this immunomodulation are described below.
Figure 2.
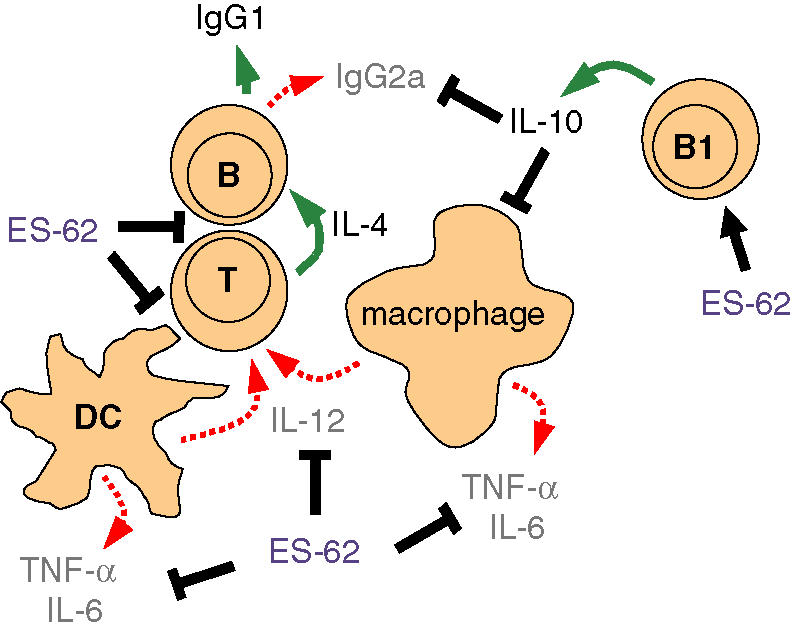
Immunomodulation by ES-62. ES-62 targets multiple cells of the immune system [black arrows (+) and T bars (–)] to achieve immunomodulation, broadly biasing the immune response to a Th2/anti-inflammatory response characterized by the production of low levels of IL-12, IFN-γ, TNF-α and IL-6 (red arrows), secretion of IL-4 (green arrow) by Th2 cells and production of the Th2-associated antibody isotypes IgG1 (mouse) and IgG4 (human). IL-10 production by B1 cells (green arrow) also contributes to this response. ES-62 alters costimulatory molecule expression on DCs and targets the signalling pathways triggered following cross-linking of the B- and T-cell antigen receptors (BCR and TCR), hence disrupting the responses of these cells to specific antigen.
Immunomodulation by ES-62
B-cell activation and antibody production
At high concentrations (25–50 μg/ml), ES-62 can act as a weak mitogen when incubated alone with murine splenic B cells.24 However, at concentrations comparable to those found in the bloodstream of infected humans (0·2–2 μg/ml),31in vitro stimulation with ES-62 substantially inhibits the proliferation of splenic B cells activated via the B-cell receptor (BCR).24 Furthermore, the same effect is observed when such B cells exposed to ES-62 in vivo by release from subcutaneously implanted osmotic pumps are activated ex vivo.26,27 This inhibition appears to be direct and can be mimicked by PC alone or by PC conjugated to bovine serum albumin (BSA), indicating that the PC moiety may be responsible for this immunomodulatory effect.24 Indeed, B cells from mice exposed to PC in vivo are hyporesponsive to BCR cross-linking compared to B cells from control animals.23
Rather paradoxically, and despite its apparent ability to desensitize B lymphocytes, ES-62 induces an antibody response during natural infection in jirds.32 Indeed, BALB/c mice injected with ES-62 also mount an antibody response; subcutaneous injection with ES-62 induces the production of the Th2-associated isotype IgG1, but not IgG2a (Th1).23 Moreover, this paradox is not simply found experimentally but is reflected by the literature in that many human studies reveal an indirect association between the presence of circulating filarial nematode products and levels of parasite-specific IgG1, IgG2 and IgG3 whilst the IgG4 subclass is usually found to be elevated and often to a considerable degree.8 One possible explanation relates to the generation of IL-4, a cytokine that would promote IgG4 production, and which is a frequently recorded feature of filariasis. We have previously shown during in vitro studies that IL-4 actually synergises with ES-62 to cause B-lymphocyte proliferation rather than hyporesponsiveness.23 Thus it is possible that although ES-62 renders conventional B lymphocytes hyporesponsive in vivo such that their ability to produce antibody is impaired, in an environment that contains IL-4, the cells may in fact be induced to produce antibodies of the IgG4 subclasses. Such a scenario could also help explain why total, in addition to specific, levels of this subclass are greatly increased in filariasis patients. Indeed, we find experimentally that ES-62 and IL-4 are comitogenic for B cells and that such IL-4-mediated rescue appears to arise as a consequence of exposure to IL-4 preventing the degradation of protein kinase C-α (PKC-α), an important enzyme in mitogenic activation of B lymphocytes, that is normally driven by ES-62.33 In addition, the observed Th2 bias of the anti-ES-62 response is dependent on IL-4 because IL-4 knockout mice fail to produce IgG1; an IL-10-dependent role for PC in blocking the IgG2a response has also been implicated.29 Thus the main effect of PC-containing molecules such as ES-62 on antibody responses during filarial nematode infection may not be so much to inhibit them as to polarize them. Consistent with this, we have recently shown that the murine antibody response to ES-62 is converted from solely IgG1 (a Th2 antibody) to mixed IgG1/IgG2a (the latter, a Th1 antibody) when the PC moiety is removed.29
B1 lymphocytes, which reside in the pleural and peritoneal cavities, respond to PC-containing filarial nematode molecules by producing IL-10 and specific IgM antibody.34 In contrast to the splenic B cells (B2 phenotype) described above, exposure of B1 cells to ES-62 released from osmotic pumps results in their proliferation and IL-10 production, even in the absence of further stimuli.27 However, ES-62-exposed B1 cells show further proliferation and IL-10 production following subsequent in vitro ligation of the antigen receptor or lipopolysaccharide (LPS) stimulation. Since up to 10% of B1 cells would be expected to bind PC via their antigen receptor35 the spontaneous B1-cell proliferation may simply reflect this. Interestingly, these data raise the possibility that the anti-PC IgM response frequently observed in filaria-infected humans and in animal models of filariasis (reviewed in ref. 36) may be the result of B1-cell activation rather than B2-cell activation.
Macrophage and DC activation and polarization of immune responses
In contrast to its ability to profoundly modulate B-cell responses, ES-62 exhibits only marginal direct effects on T-cell function, at least in terms of antigen/mitogen-driven proliferation and cytokine secretion. However, ES-62 polarizes T-cell responses indirectly via modulation of the maturation and function of DCs28 and macrophages.30,37 These specialized APCs are required for the priming and activation of CD4+ T lymphocytes and are capable of directing the subsequent differentiation and function of T cells via both interaction with costimulatory molecules expressed on the APC surface and the secretion of cytokines. With respect to the former, ES-62 and indeed PC modulate the surface expression of costimulatory molecules to generate DCs with an immature phenotype, which are capable of driving the development of Th2 cells.28,38 Therefore, immature DCs exposed to ES-62 in the tissues during a natural filarial nematode infection could contribute to the Th2/anti-inflammatory phenotype observed in these infections. However, we have recently shown that exposure to ES-62 in vivo can act even earlier; specifically, it can subvert the development of DC and macrophage progenitors in the bone marrow. In vivo exposure of bone marrow progenitors to ES-62 released from osmotic pumps biases their subsequent ex vivo differentiation to generate DCs and macrophages that are hyporesponsive to subsequent activation by LPS.38 Thus the immunomodulatory effects of ES-62 are retained throughout the ex vivo development of these cells from their precursors, suggesting that the effects of ES-62 can be long-lived. Once again, these effects can be mimicked by PC-BSA/PC-ovalbumin. Production of the Th1-inducing cytokine IL-12 and the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and IL-6 in response to pathogen molecules other than LPS, e.g. bacterial lipopeptide (BLP), as well as CpG oligonucleotides, is also significantly reduced following the exposure, either in vitro or in vivo, of macrophages and DCs to ES-62.30,39 Again, inhibition is also seen following in vitro or in vivo exposure to PC, either unconjugated or conjugated to ovalbumin or BSA.38
Intriguingly, in spite of these anti-Th1/anti-inflammatory effects, treatment of macrophages and DCs with ES-62 alone induces low levels of IL-6, IL-12 and TNF-α production.30 The precise reason for this is not known but we postulate that it may be the consequence of abortive signalling, which renders cells hyporesponsive to subsequent (or even simultaneous) stimulation with other ligands30,37 and interestingly is compatible with the finding that pro-inflammatory cytokines dominate the early immune response to filarial parasites.40
Modulation of intracellular signal transduction
B-cell signalling
The best understood mechanism of ES-62 action is the disruption of B-cell activation, which results in the suppression of proliferation following BCR cross-linking. The BCR comprises a clonatypic antigen-binding component (surface immunoglobulin, sIg) and its accessory immunoreceptor tyrosine-based activatory motif (ITAM)-containing signal transducing molecules Igα and Igβ. Ligation of the BCR triggers protein tyrosine kinase (PTK) activity, resulting in tyrosine phosphorylation of the ITAMs (reviewed in refs 41,42) and the recruitment of a number of key signal-transducing pathways implicated in cellular activation and proliferation (Fig. 3). These include the phospholipase C (PLC)-γ, phosphoinositide-3-kinase (PI-3-K) and the Ras-Erk (extracellular-regulated kinase) mitogen-activated protein kinase (MAP kinase) signalling cascades.
Figure 3.
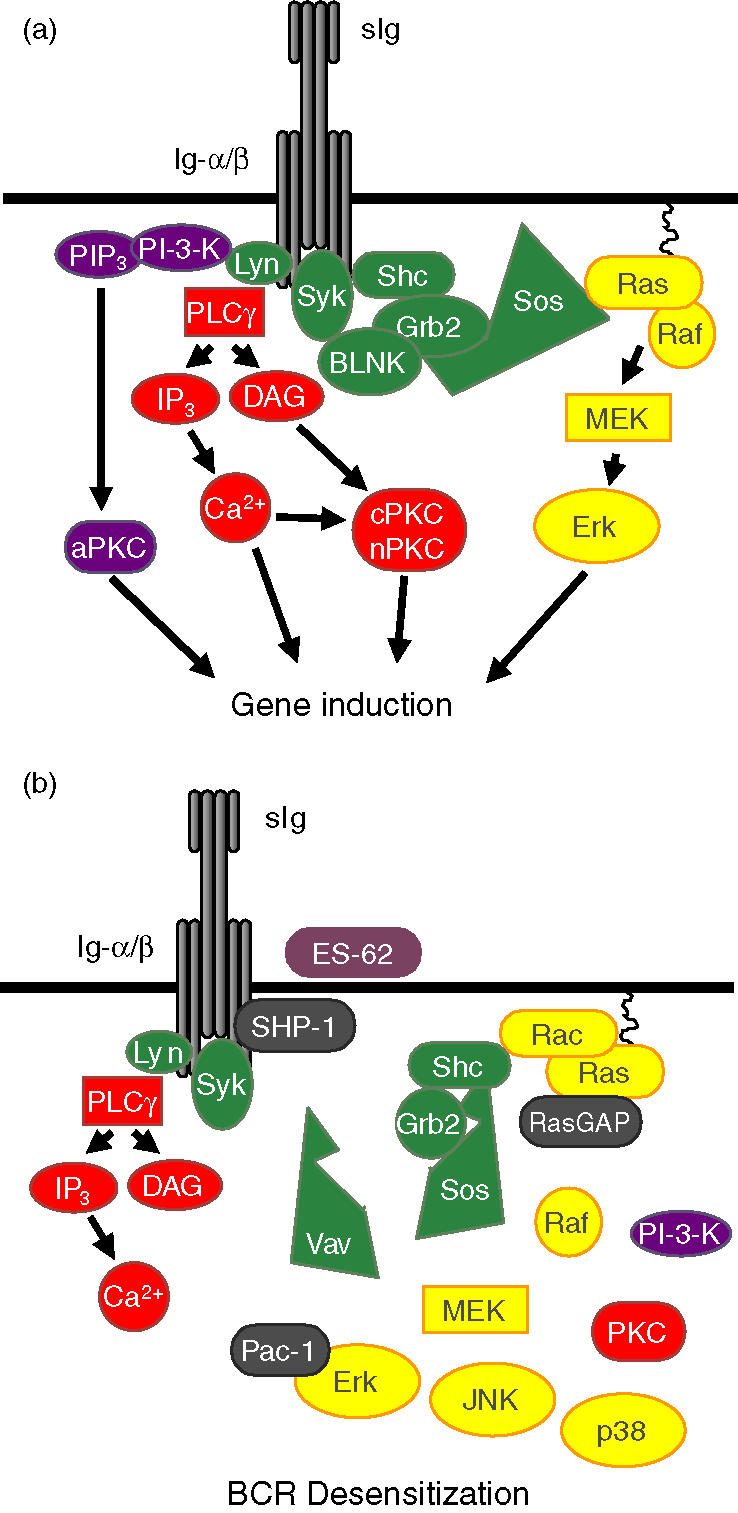
BCR signalling in untreated (a) and ES-62-exposed (b) B cells. Following ligation of the B-cell antigen receptor (BCR) of untreated B cells (a) the kinase, Lyn, tyrosine phosphorylates the immunoreceptor tyrosine activation motifs (ITAMs) on the accessory transducing molecules Ig-α and Ig-β resulting in the recruitment and activation of the PI-3-K and PLC-γ-signalling pathways. Whilst PI-3-K activation results in the activation of atypical PKC isoforms (aPKC), PLC-γ activation induces inositol trisphosphate (IP3) and diacylglycerol (DAG) generation, ultimately resulting in activation of classical (cPKC) and novel (nPKC) PKC isoforms. Binding of the adaptor proteins Shc and BLNK to the phosphorylated ITAMs leads to the recruitment of the Grb2Sos complexes (Grb2 is an adaptor protein which binds Sos, a guanine nucleotide exchange factor) required for activation of the GTPase, Ras. Active Ras initiates the Erk MAP kinase cascade by binding and activating the ser/thr kinase, Raf leading to stimulation of the thr/tyr kinase MEK and consequent activation and nuclear translocation of the ser/thr kinase Erk. ES-62/PC signalling (b) disrupts BCR coupling to the PI-3-K cascade as well as targeting major negative regulatory sites in the control of the Erk, p38 and JNK MAP kinase cascades. First, ES-62 signalling promotes the BCR-activation of SHP-1 tyrosine phosphatase to prevent initiation of BCR signalling by maintaining the ITAMs in a resting, dephosphorylated state and hence prevents recruitment of the ShcGrb2Sos complexes required to activate the Ras- and Rac-MAP kinase cascades. Second, ES-62 signalling promotes the BCR-mediated recruitment of RasGAP to terminate ongoing Ras signals. In addition, ES-62 is also likely to target MAP kinase activation by down-regulating PKC isoform expression. Finally, ES-62-signalling promotes the BCR-driven association of the nuclear MAP kinase dual (thr/tyr) phosphatase, Pac-1 with Erk to terminate any ongoing Erk signals. This multipronged mechanism results in a rapid and profound desensitization of BCR coupling to the MAP kinase cascades.
Our studies have shown that ES-62 selectively targets these key signalling events following BCR ligation to disrupt the activation and proliferation of B cells. For example, it does not target the early BCR-coupled PLC-γ-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate, which generates the second messengers, inositol trisphosphate and diacylglycerol that mobilize intracellular stores of calcium and activate protein kinase C isoforms.24 Rather, ES-62 appears to selectively modulate the expression and activity of certain PKC isoforms in resting and BCR-stimulated B cells.24,33 Thus, whilst ES-62 selectively down-regulates the expression of the α, β, ζ, δ and ι/λ isoforms, predominantly by stimulating proteolytic degradation, it up-regulates the expression of PKC-γ and PKC-ε in murine splenic B cells.33 In addition, ES-62 acts to modulate PKC signalling resulting from antigen-receptor ligation of B cells by disrupting the normal activation and nuclear translocation patterns of the PKC-α and PKC-ι/λ isoforms.33 These data are consistent with proposals that PKC-α, -β and -ι/λ transduce key activation signals involved in the regulation of antigen-driven DNA synthesis and proliferation in B cells43 such as phosphorylation of the nuclear protein lamin B and the induction and activation of NF-κB, Fos, Egr-1 and Myc.44–48
In addition to its effects on PKC signalling, pre-exposure to ES-62 selectively inhibits BCR-mediated recruitment of key proliferative pathways, such as the PI-3-K and Ras/Erk MAP kinase cascades.49 An intriguing feature of these results was that stimulation of B cells with ES-62 alone did not induce activation of Ras or PI-3-K despite the fact that ES-62 can induce activation of the PTKs Lyn and Syk (upstream regulators of Ras and PI-3-K) and Erk MAP kinase (downstream effector of Ras).49 This apparent discrepancy was resolved by our finding49,50 that ES-62 does not mediate uncoupling of the BCR from the PI-3-K or Ras/Erk MAP kinase cascades by targeting activation of Syk or Lyn. Instead, it primes for the induction of the tyrosine phosphatase SHP-1, which negatively regulates activation via the BCR complex by dephosphorylating the Igα/β-ITAMs, thereby preventing recruitment of the Ras/Erk MAP kinase cascade. Moreover, ES-62 recruits additional negative regulatory elements of this pathway, namely RasGAP and the dual (thr/tyr) phosphatase Pac-1, to terminate any residual coupling of the BCR to Ras and Erk activity, respectively. This multipronged mechanism provides for a rapid and profound desensitization of BCR-stimulated Erk MAP kinase signalling (Fig. 3).
ES-62 also modulates the activation of the two other major MAP kinase subfamilies, p38 and c-Jun N-terminal kinase (JNK).51 The precise targets of ES-62 in these pathways are unclear but our results, like those of others,52 suggest that the BCR modulates p38 and JNK signalling in a Vav- and Rac-dependent manner.51 The finding that ES-62 alone can selectively stimulate Syk, Lyn and MAP kinase activation, whilst it does not appear to modulate Ras, Rac, or PI-3-K activity33,49 also suggests that MAP kinases can be activated in B cells via alternative PTK-dependent pathways. This proposal is consistent with the increasing evidence for Ras-independent pathways of Erk MAP kinase activation involving lipid second messengers and PKC.53 We have not, however, found any evidence to support the proposal that ES-62 or BCR stimulates Erk MAP kinase activity via lipid second messengers derived from phosphatidylcholine-specific phospholipase C (PtdCho-PLC), PtdCho-phospholipase D (PtdCho-PLD) or sphingomyelinase-dependent pathways.49 However, PKC-α has been reported to mediate activation of Erk MAP kinase via Raf and MAP kinase kinase (MEK) pathways54,55 and this is consistent not only with reports that PKC activity plays a role in coupling the BCR to ErkMAP kinases56 but also provides a rationale for our finding that, whilst prolonged pretreatment with ES-62 acts to reduce this PKC activity, ES-62 initially up-regulates PKC-α expression.24,33
T-cell signalling
Although ES-62 exerts few, if any, direct effects on antigen-/mitogen-driven proliferation or cytokine secretion, in a manner analogous to that observed for the suppression of antigen receptor stimulation of B cells, the protein suppresses anti-CD3-induced proliferation of Jurkat T cells and also promotes concanavalin A-induced growth arrest of these cells25 and this can be mimicked by PC.23 The precise sequence of signalling events underlying these responses has not been fully elucidated, but it is clear that ES-62-mediated desensitization of TCR signalling is associated with disruption of TCR coupling to PLD, PKC, PI-3-K and Ras-Erk MAP kinase signalling but, as with B cells, not the PLC-mediated generation of inositol phosphates.25 Again, PC appears to be the active moiety, because culture with PC or PC-BSA has comparable effects to ES-62 on the coupling of the TCR to PTK activation (ZAP-70, Lck and Fyn recruitment) and the Ras-Erk MAP kinase signalling cascades.22,23,25 These findings are consistent with an earlier report showing that PC-containing molecules of the human filarial nematode, B. malayi, inhibit the response of human T cells to mitogens.57
Macrophage and DC signalling
Induction of cytokine production by macrophages and DCs in response to many pathogen products occurs following ligation of a family of recently identified pattern recognition receptors, known as Toll-like receptors (TLRs; reviewed in ref. 58). TLRs are thought to recognize specific molecular motifs of host as well as pathogen origin, including pathogen-associated molecular patterns (PAMPs). For example, TLR4 is required for the detection of and response to bacterial LPS whereas BLP and CpG DNA motifs are recognized by TLR2 and TLR9, respectively. TLR signals are transduced via adaptor molecules including MyD88 (reviewed in refs 59,60) resulting in the activation of various signalling pathways including the MAP kinase cascades and NF-κB.
Treatment of macrophages with ES-62 induces tyrosine phosphorylation of a number of proteins51 and modulates the activation of members of all three major MAP kinase subfamilies (Erk, p38 and JNK), which are involved in the regulation of cytokine production.30,37 For example, suppression of IL-12 by ES-62 is likely to be partly the result of Erk MAP kinase-mediated suppression of the transcription of the p40 subunit of this heterodimeric cytokine, because ES-62 inhibition can be rescued by pretreatment with the Erk MAP kinase kinase (MEK-1) inhibitor PD98059.37 ES-62 treatment also suppresses the activation of the p3837 and JNK MAP kinases51 which are required for the production of IL-12 as well as IL-6 and TNF-α, suggesting another mechanism whereby suppression of these cytokines is likely to be achieved. Furthermore, preliminary analysis indicated that ES-62 regulates gene induction by modulating the activation and gene promoter binding of the transcription factors NF-κB and IFN regulatory factor-1 (IRF-1).51
We have recently shown that ES-62 achieves its modulation of macrophage and DC activation in a TLR4-dependent manner.39 Low-level cytokine induction by ES-62 alone was abolished in macrophages/DCs from TLR4 knockout mice and also MyD88 knockout mice. Similarly ES-62-mediated suppression of cytokine induction by TLR ligands (BLP and CpG) was dependent on the presence of TLR4. In contrast, ES-62 effects were TLR2 and TLR-6 independent. Modulation of surface expression of major histocompatibility complex class II and costimulatory molecules (CD40, CD80, CD86) was also suppressed in DCs from TLR4 knockout mice, although these latter effects were only partially dependent on the presence of MyD88. Interestingly, macrophages and DCs from C3H/HeJ mice, which are unresponsive to LPS because of their production of a defective form of TLR4 resulting from a point mutation in the intracellular TIR domain, remain responsive to ES-62 as evidenced by the modulation of cytokine production and costimulatory molecule expression. Thus it appears that TLR4 must be present but not necessarily fully functional for ES-62 responsiveness. This may be because of ‘non-classical’ coupling to downstream signal transduction pathways from TLR4 or the recruitment of a signalling coreceptor.
It is not currently clear whether TLR4 is required for the direct recognition of ES-62. Preliminary evidence suggests that the PC moiety of ES-62 is at least in part required for its recognition (unpublished data) and hence it is tempting to speculate that TLR4 may recognize this molecular pattern, especially because other PC-containing molecules have been reported to act via TLR4.61–63
Conclusions and future prospects
In summary, our studies over the past decade have demonstrated extensively how a single parasite glycoprotein can cause profound modulation of the host immune response to enable coexistence of host and parasite, via co-ordinated targeting of multiple cells of the immune system. Importantly, ES-62 is not unique to A. viteae; homologues of ES-62 are found in other filarial nematodes, including the human parasites B. malayi and O. volvulus.14,15,64 For example, A. viteae ES-62 shares 77% homology with an ES-62 cDNA of B. malayi (see Fig. 4). In addition, PC-containing molecules are produced by a diverse range of pathogens (reviewed in ref. 22); hence PC represents an important molecular pattern in the detection of and response to pathogens.
Figure 4.
Alignment of Acanthocheilonema viteae ES-62 with homologues from Brugia malayi and Brugia pahangi. Alignment of A. viteae and B. malayi cDNA sequences and an incomplete sequence derived from two B. pahangi PCR products demonstrates significant homology between these filarial nematodes. Lower case letters represent the signal peptide cleavage sites. Putative N-glycosylation sites are coloured red and underlined. Per cent homology of the sequences was calculated for the regions indicated between the asterisks.
Of additional interest, dissection of the mechanisms of immune modulation by ES-62 ultimately provides information that can be utilized in the development of novel strategies not only for the treatment of filariasis and other infections, but also for the treatment of pathological conditions caused by excessive or inappropriate immune responses resulting from immune dysfunction. Indeed, we recently demonstrated that exposure to ES-62 prevented the initiation of arthritis in a murine collagen-induced arthritis model of rheumatoid arthritis and also suppressed the progression of established disease.65 These effects correlated with the inhibition of collagen-specific pro-inflammatory/Th1 cytokine production (TNF-α, IL-6 and IFN-γ). In human studies, ES-62 was also able to suppress the in vitro release of pro-inflammatory cytokines by synovial cells derived from patients with rheumatoid arthritis. Thus, ES-62 constitutes a pathogen-derived immunomodulator with significant therapeutic potential.
Acknowledgments
Funding was kindly provided by the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, the Leverhulme Trust and the Medical Research Council.
References
- 1.WHO. Filariasis. Geneva: World Health Organisation; 2000. [Google Scholar]
- 2.Subramanian S, Stolk WA, Ramaiah KD, et al. The dynamics of Wuchereria bancrofti infection: a model-based analysis of longitudinal data from Pondicherry. India Parasitol. 2004;128:467–82. doi: 10.1017/s0031182004004822. 10.1017/S0031182004004822. [DOI] [PubMed] [Google Scholar]
- 3.King CL. Transmission intensity and human immune responses to lymphatic filariasis. Parasite Immunol. 2001;23:363–71. doi: 10.1046/j.1365-3024.2001.00395.x. 10.1046/j.1365-3024.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- 4.Brattig NW. Pathogenesis and host responses in human onchocerciasis. Impact of Onchocerca filariae and Wolbachia endobacteria. Microbes Infect. 2004;6:113–28. doi: 10.1016/j.micinf.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Maizels RM, Blaxter ML, Scott AL. Immunological genomics of Brugia malayi: filarial genes implicated in immune evasion and protective immunity. Parasite Immunol. 2001;23:327–44. doi: 10.1046/j.1365-3024.2001.00397.x. 10.1046/j.1365-3024.2001.00397.x. [DOI] [PubMed] [Google Scholar]
- 6.Harnett W, Parkhouse RME. Nature and function of parasitic nematode surface and excretory-secretory antigens. In: Sood ML, Kapur J, editors. Perspectives in Nematode Physiology and Biochemistry. New Delhi: Narendra Publication House; 1995. pp. 207–42. [Google Scholar]
- 7.Lawrence RA. Immunity to filarial nematodes. Vet Parasitol. 2001;100:33–44. doi: 10.1016/s0304-4017(01)00481-2. 10.1016/S0304-4017(01)00481-2. [DOI] [PubMed] [Google Scholar]
- 8.Kurniawan A, Yazdanbakhsh M, van Ree R, Aalberse R, Selkirk ME, Partono F, Maizels RM. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941–50. [PubMed] [Google Scholar]
- 9.Mahanty S, Nutman TB. Immunoregulation in human lymphatic filariasis. The role of interleukin 10. Parasite Immunol. 1995;17:385–92. doi: 10.1111/j.1365-3024.1995.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 10.Mahanty S, Mollis SN, Ravichandran M, Abrams JS, Kumaraswami V, Jayaraman K, Ottesen EA, Nutman TB. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–73. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 11.Ravichandran M, Mahanty S, Kumaraswami V, Nutman TB, Jayaraman K. Elevated IL-10 mRNA expression and downregulation of Th1-type cytokines in microfilaraemic individuals with Wuchereria bancrofti infection. Parasite Immunol. 1997;19:69–77. doi: 10.1046/j.1365-3024.1997.d01-185.x. 10.1046/j.1365-3024.1997.d01-185.x. [DOI] [PubMed] [Google Scholar]
- 12.Hussain R, Hamilton RG, Kumaraswami V, Adkinson NF, Jr, Ottesen EA. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981;127:1623–9. [PubMed] [Google Scholar]
- 13.Harnett W, Worms MJ, Kapil A, Grainger M, Parkhouse RM. Origin, kinetics of circulation and fate in vivo of the major excretory-secretory product of Acanthocheilonema viteae. Parasitology. 1989;99:229–39. doi: 10.1017/s0031182000058686. [DOI] [PubMed] [Google Scholar]
- 14.Stepek G, Auchie M, Tate R, Watson K, Russell DG, Devaney E, Harnett W. Expression of the filarial nematode phosphorylcholine-containing glycoprotein, ES62, is stage specific. Parasitology. 2002;125:155–64. doi: 10.1017/s0031182002001920. 10.1017/S0031182002001920. [DOI] [PubMed] [Google Scholar]
- 15.Stepek G, Houston KM, Goodridge HS, Devaney E, Harnett W. Stage-specific and species-specific differences in the production of the mRNA and protein for the filarial nematode secreted product, ES-62. Parasitology. 2004;128:91–8. doi: 10.1017/s0031182003004220. 10.1017/S0031182003004220. [DOI] [PubMed] [Google Scholar]
- 16.Harnett W, Harnett MM, Byron O. Structural/functional aspects of ES-62 – a secreted immunomodulatory phosphorylcholine-containing filarial nematode glycoprotein. Curr Protein Pept Sci. 2003;4:59–71. doi: 10.2174/1389203033380368. 10.2174/1389203033380368. [DOI] [PubMed] [Google Scholar]
- 17.Harnett W, Houston KM, Tate R, et al. Molecular cloning and demonstration of an aminopeptidase activity in a filarial nematode glycoprotein. Mol Biochem Parasitol. 1999;104:11–23. doi: 10.1016/s0166-6851(99)00113-9. 10.1016/S0166-6851(99)00113-9. [DOI] [PubMed] [Google Scholar]
- 18.Taylor A. Aminopeptidases: towards a mechanism of action. Trends Biochem Sci. 1993;18:167–71. [PubMed] [Google Scholar]
- 19.Acosta D, Goni F, Carmona C. Characterization and partial purification of a leucine aminopeptidase from Fasciola hepatica. J Parasitol. 1998;84:1–7. [PubMed] [Google Scholar]
- 20.Harnett W, Houston KM, Amess R, Worms MJ. Acanthocheilonema viteae: phosphorylcholine is attached to the major excretory-secretory product via an N-linked glycan. Exp Parasitol. 1993;77:498–502. doi: 10.1006/expr.1993.1113. 10.1006/expr.1993.1113. [DOI] [PubMed] [Google Scholar]
- 21.Ackerman CJ, Harnett MM, Harnett W, Kelly SM, Svergun DI, Byron O. 19 A solution structure of the filarial nematode immunomodulatory protein, ES-62. Biophys J. 2003;84:489–500. doi: 10.1016/S0006-3495(03)74868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harnett W, Harnett MM. Phosphorylcholine: friend or foe of the immune system? Immunol Today. 1999;20:125–9. doi: 10.1016/s0167-5699(98)01419-4. 10.1016/S0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 23.Harnett W, Deehan MR, Houston KM, Harnett MM. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 1999;21:601–8. doi: 10.1046/j.1365-3024.1999.00267.x. 10.1046/j.1365-3024.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 24.Harnett W, Harnett MM. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J Immunol. 1993;151:4829–37. [PubMed] [Google Scholar]
- 25.Harnett MM, Deehan MR, Williams DM, Harnett W. Induction of signalling anergy via the T-cell receptor in cultured Jurkat T cells by pre-exposure to a filarial nematode secreted product. Parasite Immunol. 1998;20:551–63. doi: 10.1046/j.1365-3024.1998.00181.x. 10.1046/j.1365-3024.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilson EH, Deehan MR, Katz E, Brown KS, Houston KM, O'Grady J, Harnett MM, Harnett W. Hyporesponsiveness of murine B lymphocytes exposed to the filarial nematode secreted product ES-62 in vivo. Immunology. 2003;109:238–45. doi: 10.1046/j.1365-2567.2003.01661.x. 10.1046/j.1365-2567.2003.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson EH, Katz E, Goodridge HS, Harnett MM, Harnett W. In vivo activation of murine peritoneal B1 cells by the filarial nematode phosphorylcholine-containing glycoprotein ES-62. Parasite Immunol. 2003;25:463–6. doi: 10.1111/j.1365-3024.2003.00650.x. 10.1111/j.1365-3024.2003.00650.x. [DOI] [PubMed] [Google Scholar]
- 28.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 29.Houston KM, Wilson EH, Eyres L, Brombacher F, Harnett MM, Alexander J, Harnett W. Presence of phosphorylcholine on a filarial nematode protein influences immunoglobulin G subclass response to the molecule by an interleukin-10-dependent mechanism. Infect Immun. 2000;68:5466–8. doi: 10.1128/iai.68.9.5466-5468.2000. 10.1128/IAI.68.9.5466-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167:940–5. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 31.Lal RB, Paranjape RS, Briles DE, Nutman TB, Ottesen EA. Circulating parasite antigen (s) in lymphatic filariasis: use of monoclonal antibodies to phosphocholine for immunodiagnosis. J Immunol. 1987;138:3454–60. [PubMed] [Google Scholar]
- 32.Harnett W, Worms MJ, Grainger M, Pyke SD, Parkhouse RM. Association between circulating antigen and parasite load in a model filarial system, Acanthocheilonema viteae in jirds. Parasitology. 1990;101:435–44. doi: 10.1017/s0031182000060637. [DOI] [PubMed] [Google Scholar]
- 33.Deehan MR, Harnett MM, Harnett W. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J Immunol. 1997;159:6105–11. [PubMed] [Google Scholar]
- 34.Al-Qaoud KM, Fleischer B, Hoerauf A. The Xid defect imparts susceptibility to experimental murine filariosis – association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int Immunol. 1998;10:17–25. doi: 10.1093/intimm/10.1.17. 10.1093/intimm/10.1.17. [DOI] [PubMed] [Google Scholar]
- 35.Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988;168:687–98. doi: 10.1084/jem.168.2.687. 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harnett W. Molecular approaches to the diagnosis of Onchocerca volvulus in man and the insect vector. In: Kennedy MW, editor. Parasitic Nematodes – Antigens, Membranes and Genes. London: Taylor & Francis; 1991. p. 195. [Google Scholar]
- 37.Goodridge HS, Harnett W, Liew FY, Harnett MM. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and – independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology. 2003;109:415–25. doi: 10.1046/j.1365-2567.2003.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodridge HS, Marshall FA, Wilson EH, Houston KM, Liew FY, Harnett MM, Harnett W. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology. 2004;113:491–8. doi: 10.1111/j.1365-2567.2004.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodridge HS, Marshall FA, Else KJ, et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174:284–93. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 40.Babu S, Nutman TB. Proinflammatory cytokines dominate the early immune response to filarial parasites. J Immunol. 2003;171:6723–32. doi: 10.4049/jimmunol.171.12.6723. [DOI] [PubMed] [Google Scholar]
- 41.Pleiman CM, D'Ambrosio D, Cambier JC. The B-cell antigen receptor complex: structure and signal transduction. Immunol Today. 1994;15:393–9. doi: 10.1016/0167-5699(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 42.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–86. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 43.Brick-Ghannam C, Ericson ML, Schelle I, Charron D. Differential regulation of mRNAs encoding protein kinase C isoenzymes in activated human B cells. Hum Immunol. 1994;41:216–24. doi: 10.1016/0198-8859(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 44.Francis DA, Karras JG, Ke XY, Sen R, Rothstein TL. Induction of the transcription factors NF-kappa B, AP-1 and NF-AT during B cell stimulation through the CD40 receptor. Int Immunol. 1995;7:151–61. doi: 10.1093/intimm/7.2.151. [DOI] [PubMed] [Google Scholar]
- 45.Hornbeck P, Huang KP, Paul WE. Lamin B is rapidly phosphorylated in lymphocytes after activation of protein kinase C. Proc Natl Acad Sci USA. 1988;85:2279–83. doi: 10.1073/pnas.85.7.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klemsz MJ, Justement LB, Palmer E, Cambier JC. Induction of c-fos and c-myc expression during B cell activation by IL-4 and immunoglobulin binding ligands. J Immunol. 1989;143:1032–9. [PubMed] [Google Scholar]
- 47.Mittelstadt PR, DeFranco AL. Induction of early response genes by cross-linking membrane Ig on B lymphocytes. J Immunol. 1993;150:4822–32. [PubMed] [Google Scholar]
- 48.Seyfert VL, McMahon S, Glenn W, Cao XM, Sukhatme VP, Monroe JG. Egr-1 expression in surface Ig-mediated B cell activation. Kinetics and association with protein kinase C activation. J Immunol. 1990;145:3647–53. [PubMed] [Google Scholar]
- 49.Deehan MR, Frame MJ, Parkhouse RM, Seatter SD, Reid SD, Harnett MM, Harnett W. A phosphorylcholine-containing filarial nematode-secreted product disrupts B lymphocyte activation by targeting key proliferative signaling pathways. J Immunol. 1998;160:2692–9. [PubMed] [Google Scholar]
- 50.Deehan MR, Harnett W, Harnett MM. A filarial nematode-secreted phosphorylcholine-containing glycoprotein uncouples the B cell antigen receptor from extracellular signal-regulated kinase-mitogen-activated protein kinase by promoting the surface Ig-mediated recruitment of Src homology 2 domain-containing tyrosine phosphatase-1 and Pac-1 mitogen-activated kinase-phosphatase. J Immunol. 2001;166:7462–8. doi: 10.4049/jimmunol.166.12.7462. [DOI] [PubMed] [Google Scholar]
- 51.Goodridge HS, Deehan MR, Harnett W, Harnett MM. Subversion of immunological signalling by a filarial nematode phosphorylcholine-containing secreted product. Cell Signal. 2005;17:11–16. doi: 10.1016/j.cellsig.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–8. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 53.Buscher D, Hipskind RA, Krautwald S, Reimann T, Baccarini M. Ras-dependent and -independent pathways target the mitogen-activated protein kinase network in macrophages. Mol Cell Biol. 1995;15:466–75. doi: 10.1128/mcb.15.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berra E, Diaz-Meco MT, Lozano J, Frutos S, Municio MM, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. Embo J. 1995;14:6157–63. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelech SL. Kinase connections on the cellular intranet. Signalling pathways. Curr Biol. 1996;6:551–4. doi: 10.1016/s0960-9822(02)00540-7. [DOI] [PubMed] [Google Scholar]
- 56.Campbell KS. Signal transduction from the B cell antigen-receptor. Curr Opin Immunol. 1999;11:256–64. doi: 10.1016/s0952-7915(99)80042-9. [DOI] [PubMed] [Google Scholar]
- 57.Lal RB, Kumaraswami V, Steel C, Nutman TB. Phosphocholine-containing antigens of Brugia malayi nonspecifically suppress lymphocyte function. Am J Trop Med Hyg. 1990;42:56–64. doi: 10.4269/ajtmh.1990.42.56. [DOI] [PubMed] [Google Scholar]
- 58.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 59.O'Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–9. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 60.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 61.Walton KA, Hsieh X, Gharavi N, Wang S, Wang G, Yeh M, Cole AL, Berliner JA. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J Biol Chem. 2003;278:29661–6. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 62.Walton KA, Cole AL, Yeh M, et al. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol. 2003;23:1197–203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 63.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–8. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 64.Nor ZM, Houston KM, Devaney E, Harnett W. Variation in the nature of attachment of phosphorylcholine to excretory-secretory products of adult Brugia pahangi. Parasitology. 1997;114(3):257–62. doi: 10.1017/s0031182096008402. [DOI] [PubMed] [Google Scholar]
- 65.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–33. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 66.Aszodi A, Taylor WR. Secondary structure formation in model polypeptide chains. Protein Eng. 1994;7:633–44. doi: 10.1093/protein/7.5.633. [DOI] [PubMed] [Google Scholar]
- 67.Svergun DI. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys J. 1999;76:2879–86. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]