Abstract
We previously demonstrated that lipoteichoic acid (LTA) might activate phosphatidylcholine-phospholipase C (PC-PLC) and phosphatidylinositol-phospholipase C (PI-PLC) to induce protein kinase C activation, which in turn initiates nuclear factor-κB (NF-κB) activation and finally induces inducible nitric oxide synthase (iNOS) expression and nitric oxide (NO) release in RAW 264.7 macrophages. In this study, we further investigated the roles of tyrosine kinase, phosphatidylinositiol 3-kinase (PI3K)/Akt, and p38 mitogen-activated protein kinase (MAPK) in LTA-induced iNOS expression and NO release in RAW 264.7 macrophages. Tyrosine kinase inhibitors (genistein and tyrphostin AG126), PI3K inhibitors (wortmannin and LY 294002), and a p38 MAPK inhibitor (SB 203580) attenuated LTA-induced iNOS expression and NO release in concentration-dependent manners. Treatment of RAW 264.7 macrophages with LTA caused time-dependent activations of Akt and p38 MAPK. The LTA-induced Akt activation was inhibited by wortmannin, LY 294002, genistein, and tyrphostin AG126. The LTA-induced p38 MAPK activation was inhibited by genistein, tyrphostin AG126, wortmannin, LY 294002, and SB 203580. The LTA-induced formation of an NF-κB-specific DNA–protein complex in the nucleus was inhibited by wortmannin, LY 294002, genistein, tyrphostin AG126, and SB 203580. Treatment of macrophages with LTA caused an increase in κB-luciferase activity, and this effect was inhibited by tyrphostin AG126, wortmannin, LY 294002, the Akt dominant negative mutant (AktDN), and SB 203580. Based on those findings, we suggest that LTA might activate the PI3K/Akt pathway through tyrosine kinase to induce p38 MAPK activation, which in turn initiates NF-κB activation, and ultimately induces iNOS expression and NO release in RAW 264.7 macrophages.
Keywords: lipoteichoic acid, inducible nitric oxide synthase, nitric oxide, PI3K, p38 MAPK, NF-κB, RAW 264.7 macrophages
Introduction
Nitric oxide (NO) is a highly reactive nitrogen radical implicated in multiple biological processes, including regulation of vascular tone, platelet and leukocyte adhesion, and neurotransmission, and mediation of excessive vasodilatation and cytotoxic actions of macrophages against microbes and tumour cells.1,2 Its formation is regulated by a family of enzymes, known as nitric oxide synthase (NOS), which oxidize the guanidine moiety of l-arginine, resulting in the equimolar production of NO and l-citrulline. Two major classes of NOS have been described based on their expression and regulation. The constitutive form present in neurons (nNOS) or endothelial cells (eNOS) is a calcium-dependent enzyme. The inducible form (iNOS), on the other hand, present in macrophages and other cells, is regulated at the transcriptional level in response to lipopolysaccharide (LPS) or certain proinflammatory cytokines and does not require calcium for its activity.3,4 The production of large amounts of NO by iNOS has been implicated in the genesis of septic and cytokine-induced circulatory shock.5
Septic shock can be defined as sepsis with hypotension resulting in impaired tissue perfusion despite adequate fluid resuscitation. Traditionally recognized as a consequence of Gram-negative bacteraemia, septic shock is also caused by Gram-positive organisms, fungi, and probably viruses and parasites. Although relatively rare in the 1970s, the incidence of Gram-positive septic shock has increased markedly over the past 15 years, and today between one-third and one-half of all cases of sepsis are caused by Gram-positive organisms.6 Endotoxin, a component of the outer membrane of Gram-negative bacteria, has been identified as the prime initiator of Gram-negative bacterial septic shock. In contrast to endotoxic shock, we know relatively little about the mechanisms of Gram-positive bacteria-induced septic shock. However, lipoteichoic acid (LTA) from Staphylococcus aureus causes the induction of iNOS in murine macrophages7,8 and vascular smooth muscle cells.9 LTA also produces circulatory failure (hypotension and vascular hyporeactivity to vasoconstrictor agents) in rats by induction of the iNOS protein and an increase in iNOS activity.10
Recently, we showed that LTA activates phosphatidylcholine-phospholipase C (PC-PLC) and phosphatidylinositol-phospholipase C (PI-PLC) to induce protein kinase C (PKC) activation, which in turn initiates nuclear factor-κB (NF-κB) activation, and finally induces iNOS expression and NO release in RAW 264.7 macrophages.8 Akt, a serine/threonine kinase, is a direct downstream effector of phosphatidylinositiol 3-kinase (PI3K).11 Akt can be modulated by multiple intracellular signalling pathways and acts as a transducer for many pathways initiated by growth factor receptor-activated PI3K. In addition, Akt can stimulate signalling pathways that up-regulate the activity of NF-κB in T cells or embryonic kidney 293 cells.12,13 A role for PI3K/Akt in iNOS induction has been implied.14,15 However, the role of the PI3K/Akt pathway in LTA-induced iNOS expression and NO release has not yet been determined. In this study, we therefore studied the signalling pathway of LTA-induced phosphatidylinositol 3-kinase (PI3K)/Akt activation and their roles in LTA-mediated NF-κB activation, iNOS expression, and NO release in RAW264.7 macrophages. Our results show that LTA might activate the PI3K/Akt pathways through tyrosine kinase activation to induce p38 mitogen-activated protein kinase (MAPK) activation, which in turn initiates NF-κB activation, and ultimately induces iNOS expression and NO release in RAW 264.7 macrophages.
Materials and methods
Materials
Lipoteichoic acid (LTA derived from Staphylococcus aureus), pyrrolidine dithiocarbamate (PDTC), wortmannin, genistein, sulphanilamide, N-(1-naphthyl)-ethylenediamine, Trizma base, dithiothreitol (DTT), glycerol, phenylmethylsulphonyl fluoride (PMSF), pepstatin A, leupeptin, and sodium dodecyl sulphate (SDS) were purchased from Sigma Chem. (St. Louis, MO). LY 294002, SB 203580, and tyrphostin AG126 were purchased from Calbiochem-Novabiochem (San Diego, CA). Penicillin/streptomycin, fetal calf serum (FCS), T4 polynucleotide kinase, and Dulbecco's modified Eagle's minimal essential medium (DMEM)/Ham's F-12 were purchased from Life Technologies (Gaithersburg, MD). Rabbit polyclonal antibodies specific for iNOS, Akt, p38 MAPK, and anti-rabbit immunoglobulin G (IgG)-conjugated alkaline phosphatase were purchased from Santa Cruz Biochemicals (Santa Cruz, CA). Antibodies specific for phospho-p38 MAPK and phospho-Akt were purchased from New England Biolabs (Beverly, MA). Anti-mouse IgG-conjugated alkaline phosphatase was purchased from Jackson Immuno Research Laboratories (West Grove, PA). The NF-κB probe was purchased from Promega (Madison, WI). 4-Nitro blue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) were purchased from Boehringer Mannheim (Mannheim, Germany). pGL2-ELAM-Luc (which is under the control of one NF-κB binding site) and pBK-CMV-Lac Z were kindly provided by Dr W.-W. Lin (National Taiwan University, Taipei, Taiwan). An Akt dominant negative mutant (AktDN) was provided by Dr C.-M. Teng (National Taiwan University, Taipei, Taiwan). [α-32P]ATP (6000 Ci/mmol) was purchased from Amersham Pharmacia Biotech (Amersham, UK). GenePORTERTM 2 was purchased from Gene Therapy System (San Diego, CA). All materials for SDS-PAGE were purchased from Bio-Rad (Hercules, CA). Protein assay reagents were purchased from Bio-Rad.
Cell culture
The mouse macrophage cell line, RAW 264.7, was obtained from American Type Culture Collection (Livingstone, MT), and cells were maintained in DMEM/Ham's F-12 nutrient mixture containing 10% FCS, 100 U/ml of penicillin G, and 100 µg/ml streptomycin in a humidified 37° incubator. After reaching confluence, cells were seeded onto either 6-cm dishes for immunoblotting assays, 96-well plates for nitrite assays, or 12-well plates for transfection and κB luciferase assays.
Measurement of NO concentration
NO production was assayed by measuring nitrite (a stable degradation product of NO) in supernatants of cultured RAW 264.7 cells using the Griess reagent. Briefly, RAW 264.7 macrophages were cultured in 24-well plates. After reaching confluence, the culture medium was changed to phenol red-free DMEM. Cells were pretreated with specific inhibitors as indicated followed by 10 µg/ml LTA and then were incubated in a humidified incubator at 37° for 24 hr. The supernatant was collected, mixed with an equal volume of Griess reagent (1% sulphanilamide, 0·1%N-(1-naphthyl)-ethylenediamine, 2% phosphoric acid), and incubated at room temperature for 10 min. The absorbance measured at 550 nm in a microplate reader was used as an indication of the nitrite concentration. Sodium nitrite (NaNO2) was used to produce a standard curve of nitrite concentrations.
Protein preparation and Western blot analysis
The expressions of iNOS, α-tubulin, Akt phosphorylated at Ser473, Akt, p38 MAPK phosphorylated at Thr180/Tyr182, and p38 MAPK in RAW 264.7 cells were evaluated, and the preparation of total proteins and Western blotting were performed as described previously.16 Briefly, RAW 264.7 cells were cultured in 10-cm Petri dishes. After reaching confluence, cells were pretreated with specific inhibitors as indicated followed by LTA (10 µg/ml), and then incubated in a humidified incubator at 37°. After incubation, cells were washed twice in ice-cold phosphate-buffered saline (PBS, pH 7·4) and solubilized in extraction buffer containing 10 mm Tris (pH 7·0), 140 mm NaCl, 2 mm PMSF, 5 mm DTT, 0·5% NP-40, 0·05 mm pepstatin A, and 0·2 mm leupeptin. Samples of equal amounts of protein (60 µg) were subjected to SDS–polyacrylamide gel electrophoresis, then transferred onto a polyvinylidene difluoride membrane, which was then incubated in TBST buffer (150 mm NaCl, 20 mm Tris-HCl, and 0·02% Tween 20; pH 7·4) containing 5% non-fat milk. Proteins were visualized by specific primary antibodies and then incubated with horseradish peroxidase- or alkaline phosphatase-conjugated second antibodies. Immunoreactivity was detected using enhanced chemiluminescence (ECL) or NBT/BCIP following the manufacturer's instructions. Quantitative data were obtained using a computing densitometer with scientific imaging systems (Kodak, Rochester, NY).
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
RAW 264.7 macrophages were cultured in 10-cm culture Petri dishes. After reaching confluence, cells were pretreated with 100 nm wortmannin, 30 µm LY 294002, 30 µm tyrphostin AG126, 30 µm genistein, or 10 µm SB 203580 for 30 min followed by LTA (10 µg/ml), and then incubated in a humidified incubator at 37° for 30 min. The cytosolic and nuclear protein fractions were then separated as described previously.17 Briefly, cells were washed with ice-cold PBS, and then centrifuged. The cell pellet was resuspended in hypotonic buffer (10 mm HEPES (pH 7·9), 10 mm KCl, 0·5 mm DTT, 10 mm aprotinin, 10 mm leupeptin, and 20 mm PMSF) for 15 min on ice, and vortexed for 10 s. The nuclei were pelleted by centrifugation at 15 000 g for 1 min. Supernatants containing the cytosolic proteins were collected. A pellet containing nuclei was resuspended in hypertonic buffer (20 mm HEPES (pH 7·6), 25% glycerol, 1·5 mm MgCl2, 4 mm ethylenediaminetetra-acetic acid (EDTA), 0·05 mm DTT, 20 mm PMSF, 10 mm aprotinin, and 10 mm leupeptin) for 30 min on ice. The supernatants containing the nuclear proteins were collected by centrifugation at 15 000 g for 2 min and stored at −70°.
A double-stranded oligonucleotide probe containing NF-κB sequences (5′-AGTTGAGGGGACTTTCCCAGGC-3′; Promega) was purchased and end-labelled with [α-32P]ATP using T4 polynucleotide kinase. The nuclear extract (2·5–5 µg) was incubated with 1 ng of 32P-labelled NF-κB probe (50 000–75 000 c.p.m.) in 10 µl of binding buffer containing 1 µg poly(dI-dc), 15 mm HEPES (pH 7·6), 80 mm NaCl, 1 mm EDTA, 1 mm DTT, and 10% glycerol at 30° for 25 min. DNA/nuclear protein complexes were separated from the DNA probe by electrophoresis on 6% polyacrylamide gels; then the gels were vacuum-dried and subjected to autoradiography with an intensifying screen at −80°. Quantitative data were obtained using a computing densitometer with Image-Pro plus software (Media Cybernetics, MD).
Transfection and κB-luciferase assays
RAW 264.7 cells at 2 × 105 were cultured in 12-well plates, and then transfected the following day using GenePORTERTM 2 with 0·5 µg of pGL2-ELAM-Luc and 0·5 µg of pBK-CMV-Lac Z. After 24 hr, the medium was aspirated and replaced with fresh DMEM/Ham's F12 containing 10% FBS. Cells were treated with vehicle and LTA (10 µg/ml) or pretreated with specific inhibitors as indicated for 30 min before incubation of LTA for 24 hr. To assay the effect of the Akt dominant negative mutant (AktDN), cells were cotransfected with AktDN, pGL2-ELAM-Luc, and pBK-CMV-Lac Z for 24 h and then treated with LTA for another 24 hr. Luciferase activity was determined with a luciferase assay system (Promega), and was normalized on the basis of Lac Z expression. The level of induction of luciferase activity was compared as a ratio of cells with and without stimulation.
Statistical analysis
Results are expressed as the mean ± the standard error of the mean (SEM) from three to four independent experiments. One-way analysis of variance (anova) followed by, when appropriate, Bonferroni's multiple range test was used to determine the statistical significance of the difference between means. A P-value of less than 0·05 was taken as statistical significance.
Results
The role of tyrosine kinase in LTA-induced iNOS expression and NO release
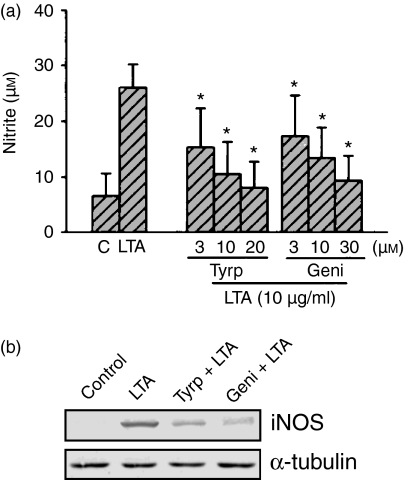
We previously demonstrated that LTA might activate PC-PLC and PI-PLC to induce PKC activation, which in turn initiates NF-κB activation, and finally induces iNOS expression and NO release in RAW 264.7 macrophages.8 To determine whether tyrosine kinase activation is involved in the signal transduction pathway leading to iNOS expression and NO production caused by LTA, the tyrosine kinase inhibitors, genistein and tyrphostin AG126, were used. Pretreatment of cells for 30 min with tyrphostin AG126 (3–20 µm) and genistein (3–30 µm) attenuated LTA-induced nitrite production in a concentration-dependent manner (Fig. 1a). Treatment of RAW 264.7 cells with tyrphostin AG126 (20 µm) and genistein (30 µm) alone had no effect on basal nitrite production (data not shown), but they almost completely abolished LTA-induced nitrite release (Fig. 1a). LTA-induced iNOS expression was also inhibited by 20 µm tyrphostin AG126 and 30 µm genistein (Fig. 1b).
Figure 1.
Effects of tyrphostin and genistein AG126 on LTA-mediated nitrite release and iNOS expression in RAW 264.7 macrophages. In (a), cells were pretreated with various concentrations of tyrphostin AG126 or genistein for 30 min followed by LTA (10 µg/ml) treatment for 24 hr. The supernatant was collected for nitrite measurement. Results are expressed as the mean ± SEM of three independent experiments performed in triplicate. *P < 0·05 as compared with the LTA-treated group. In (b), cells were pretreated with tyrphostin AG126 (20 µm) or genistein (30 µm) for 30 min followed by LTA (10 µg/ml) treatment for 24 hr, and then immunodetected using specific antibodies against iNOS or α-tubulin as described in ‘Materials and methods’. Typical traces represent three experiments with similar results. Equal loading in each lane is demonstrated by similar intensities of α-tubulin. Tyrp, tyrphostin AG126; Geni, genistein.
Involvement of PI3K/Akt in LTA-induced iNOS expression and NO release
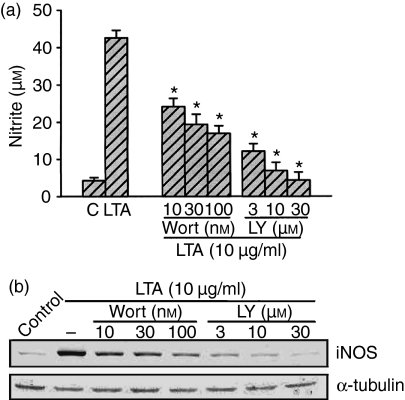
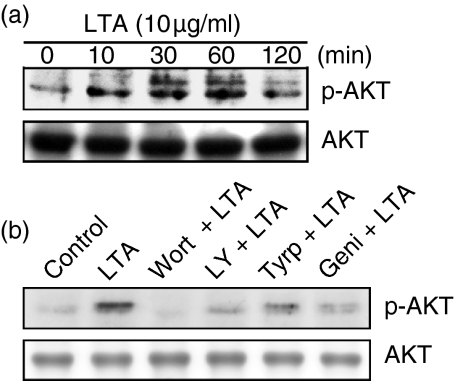
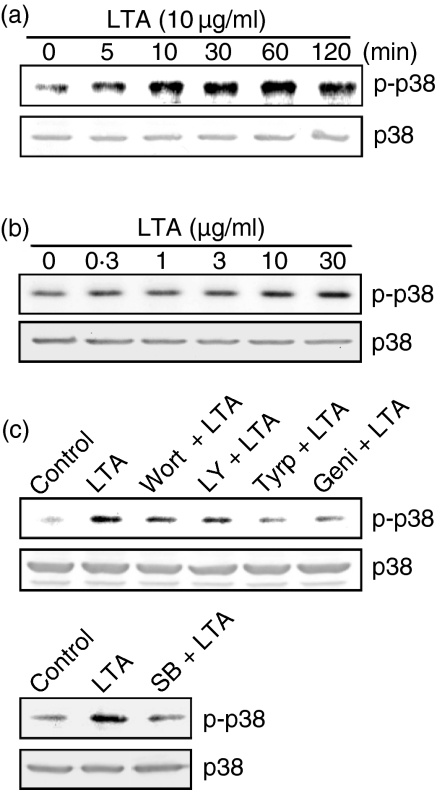
To explore whether PI3K might mediate LTA-induced iNOS expression and NO release, the PI3K inhibitors, wortmannin and LY 294002, were used. Figure 2(a) shows that LTA-induced nitrite release was inhibited by wortmannin (10–100 nm) and LY 294002 (3–30 µm) in a concentration-dependent manner. When cells were treated with 100 nm wortmannin or 30 µm LY 294002, LTA-induced nitrite release was inhibited by 61 ± 8% and 93 ± 6%, respectively (n = 3). However, 100 nm wortmannin or 30 µm LY 294002 had no effect on the basal nitrite release (data not shown). As shown in Fig. 2(b), pretreatment of RAW 264.7 macrophages with wortmannin (10–100 nm) and LY 294002 (3–30 µm) concentration-dependently inhibited LTA-induced iNOS expression. Next, we directly measured Akt activation in response to LTA. Because serine/threonine phosphorylation of residue 473 in Akt causes enzymatic activation18 the antibody specific against the phosphorylated Akt (Ser473) was used to examine Akt phosphorylation, an index of kinase activation. Treatment of RAW 264.7 macrophages with LTA (10 µg/ml) caused marked activation of Akt with a maximal response after 30–60 min of treatment. After 120 min of treatment, the LTA-induced response had declined (Fig. 3a, upper panel). The protein level of Akt was not affected by LTA treatment (Fig. 3a, bottom panel). LTA-induced Akt activation was markedly inhibited by pretreatment of cells for 30 min with 100 nm wortmannin, 30 µm LY 294002, 20 µm tyrphostin AG126, and 30 µm genistein (Fig. 3b, upper panel). None of these treatments had any effect on Akt expression (Fig. 3b, bottom panel).
Figure 2.
Effects of wortmannin and LY 294002 on LTA-mediated nitrite release and iNOS expression in RAW 264.7 macrophages. In (a), cells were pretreated with various concentrations of wortmannin or LY 294002 for 30 min followed by 10 µg/ml LTA treatment for 24 hr, and then the media were collected for nitrite measurement. Results are expressed as the mean ± SEM of three independent experiments performed in triplicate. *P < 0·05 as compared with the LTA-treated group. In (b), cells were pretreated with various concentrations of wortmannin or LY 294002 for 30 min followed by 10 µg/ml LTA treatment for 24 hr, and then immunodetected using specific antibodies against iNOS or α-tubulin as described in ‘Materials and methods’. Typical traces represent three experiments with similar results. Equal loading in each lane is demonstrated by similar intensities of α-tubulin. Wort, wortmannin; LY, LY 294002.
Figure 3.
Tyrosine kinase and PI3K's involvement in LTA-mediated Akt activation in RAW 264.7 macrophages. In (a), cells were incubated with 10 µg/ml LTA for various time intervals. Whole-cell lysates were prepared and subjected to Western blot analysis using antibodies specific for phosphorylated Akt (p-Akt) or Akt as described in ‘Materials and methods’. In (b), cells were pretreated with 100 nm wortmannin, 30 µm LY 294002, 20 µm tyrphostin AG126, or 30 µm genistein for 30 min before incubation with LTA (10 µg/ml) for 30 min. Whole-cell lysates were prepared and subjected to Western blot analysis using antibodies specific for phosphorylated Akt (p-Akt) or Akt as described in ‘Materials and methods’. Typical traces represent three experiments with similar results. Wort, wortmannin; LY, LY 294002; Tyrp, tyrphostin AG126; Geni, genistein.
Involvement of p38 MAPK in LTA-induced iNOS expression and NO release
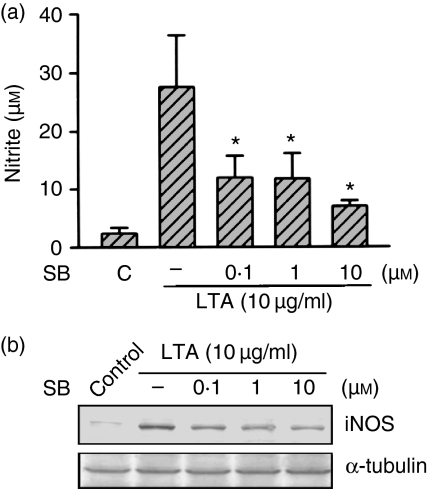
In order to examine whether p38 MAPK is also involved in the LTA-induced signal transduction pathway leading to iNOS expression and NO release, cells were pretreated with a p38 MAPK inhibitor (SB 203580). Pretreatment of RAW 264.7 macrophages for 30 min with SB 203580 (0·1–10 µm) inhibited the LTA (10 µg/ml)-induced nitrite release in a concentration-dependent manner. When cells were treated with 10 µm SB 203580, LTA-induced nitrite release was inhibited by 81 ± 4% (n = 3) (Fig. 4a). However, 10 µm SB 203580 had no effect on the basal nitrite release (data not shown). The LTA-induced increase in the iNOS protein level was also concentration-dependently inhibited by pretreatment of cells with SB 203580 (0·1–10 µm) (Fig. 4b). Because activation of p38 MAPK requires phosphorylation at the threonine and tyrosine residues, immunoblot analysis was performed to examine p38 MAPK phosphorylation using an antibody specific for phospho-p38 MAPK. Stimulation of cells with LTA (10 µg/ml) for various time intervals caused activation of p38 MAPK which peaked at 60 min (Fig. 5a). However, after 120 min of treatment with LTA, activation of p38 MAPK had decreased. Treatment with LTA (0·3–30 µg/ml) for 30 min induced p38 MAPK activation in a concentration-dependent manner, with a maximum effect at 10–30 µg/ml treatment (Fig. 5b). The protein level of p38 MAPK was not affected by LTA treatment (Fig. 5a, b). The LTA-induced p38 MAPK activation was inhibited by pretreatment of cells for 30 min with wortmannin (100 nm), LY 294002 (30 µm), tyrphostin AG126 (20 µm), genistein (30 µm), or SB 203580 (10 µm) (Fig. 5c). None of these treatments had any effect on p38 MAPK expression (Fig. 5c).
Figure 4.
Effect of SB 203580 on LTA-mediated nitrite release and iNOS expression in RAW 264.7 macrophages. In (a), cells were pretreated with various concentrations of SB 203580 for 30 min followed by 10 µg/ml LTA treatment for 24 hr, and then the media were collected for nitrite measurement. Results are expressed as the mean ± SEM of three independent experiments performed in triplicate. *P < 0·05 as compared with the LTA-treated group. In (b), cells were pretreated with various concentrations of SB 203580 for 30 min followed by 10 µg/ml LTA treatment for 24 hr, and then immunodetected using specific antibodies against iNOS or α-tubulin as described in ‘Materials and methods’. Typical traces represent three experiments with similar results. Equal loading in each lane is demonstrated by similar intensities of α-tubulin. SB, SB 203580.
Figure 5.
Tyrosine kinase and PI3K's involvement in LTA-mediated p38 MAPK activation in RAW 264.7 macrophages. In (a) and (b), cells were incubated with LTA (10 µg/ml) for various time intervals (a) or various concentrations of LTA for 30 min (b). Whole-cell lysates were prepared and subjected to Western blot analysis using antibodies specific for phosphorylated p38 MAPK (p-p38) or p38 MAPK (p38) as described in ‘Materials and methods’. In (c), cells were pretreated with 100 nm wortmannin, 30 µm LY 294002, 20 µm tyrphostin AG126, 30 µm genistein, or 10 µm SB 203580 for 30 min before incubation with LTA (10 µg/ml) for 30 min. Whole-cell lysates were prepared and subjected to Western blot analysis using antibodies specific for phosphorylated p38 MAPK (p-p38) or p38 MAPK (p38) as described in ‘Materials and methods’. Typical traces represents three experiments with similar results. Wort, wortmannin; LY, LY 294002; Tyrp, tyrphostin AG126; Geni, genistein; SB, SB 203580.
Tyrosine kinase, PI3K/Akt, and p38 MAPK mediated LTA-induced NF-κB activation
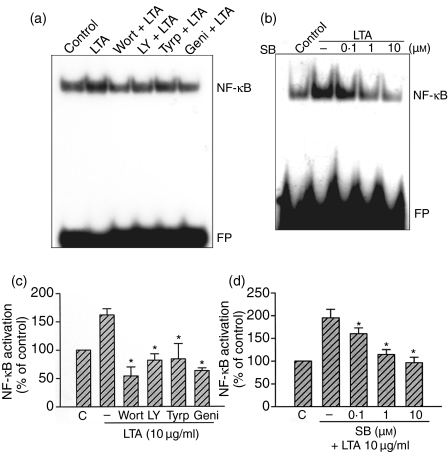
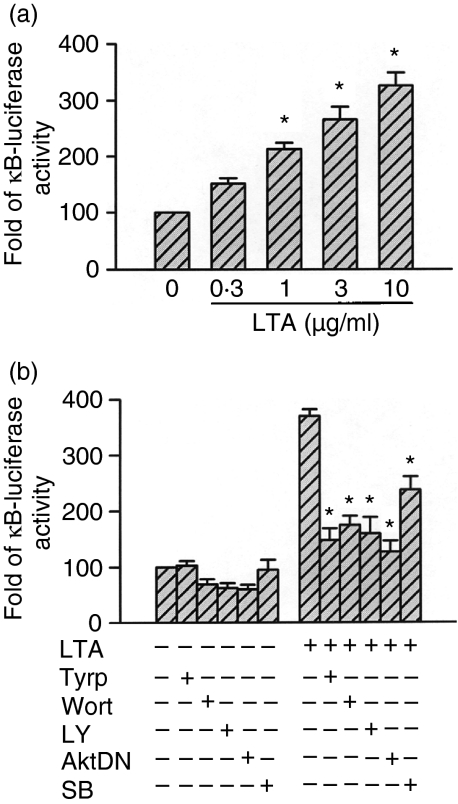
Previously, we demonstrated that NF-κB activation is critical for the induction of iNOS protein caused by LTA in RAW 264.7 macrophages.8 We further examined whether NF-κB activation occurred through the tyrosine kinase, PI3K/Akt, and p38 MAPK signalling pathways. In nuclear extracts of unstimulated cells, light staining of NF-κB-specific DNA–protein complex formation was observed. In our previous study, we demonstrated that stimulation of cells with 10 µg/ml LTA for 30 min resulted in marked activation of NF-κB-specific DNA–protein complex formation. Furthermore, we also demonstrated that the formation of the NF-κB complex was completely inhibited by the addition of the 50× cold NF-κB consensus DNA sequence, indicating that the DNA–protein interactions are sequence specific.8 In the present study, pretreatment of cells for 30 min with wortmannin (100 nm), LY 294002 (30 µm), tyrphostin AG126 (20 µm), or genistein (30 µm) markedly inhibited the LTA-induced activation of NF-κB-specific DNA–protein complex formation. Moreover, pretreatment of cells with SB 203580 (0·1–10 µm) also inhibited the LTA-induced activation of NF-κB-specific DNA–protein complex formation in a concentration-dependent manner (Fig. 6a–d). To directly determine the level of NF-κB activation after LTA treatment, RAW 264.7 macrophages were transiently transfected with pGL2-ELAM-κB-luciferase as an indicator of NF-κB activation. As shown in Fig. 7(a), macrophage treatment with LTA (0·3–10 µg/ml) for 24 hr caused a concentration-dependent increase in κB-luciferase activity. The LTA-induced increase in κB-luciferase activity was also inhibited by pretreatment of cells for 30 min with tyrphostin AG126 (20 µm), wortmannin (100 nm), LY 294002 (30 µm), or SB 203580 (10 µm) by 69 ± 7%, 60 ± 6%, 63 ± 10%, and 47 ± 8%, respectively (n = 3), (Fig. 7b). To further confirm the role of Akt in LTA-mediated NF-κB activation, an Akt dominant negative mutant (AktDN) was tested. Transfection of RAW 264.7 cells with 1 µg AktDN inhibited the LTA-induced increase in κB-luciferase activity by 75 ± 7% inhibition (n = 3), (Fig. 7b).
Figure 6.
Tyrosine kinase, PI3K, and p38 MAPK's involvement in LTA-mediated NF-κB-specific DNA-protein complex formation in nuclear extracts of RAW 264.7 macrophages. RAW 264.7 macrophages were pretreated with 100 nm wortmannin, 30 µm LY 294002, 20 µm tyrphostin AG126, 30 µm genistein (a), or 0·1–10 µm SB 203580 (b) for 30 min before incubation with 10 µg/ml LTA for 30 min. Nuclear extracts were prepared for determination of NF-κB-specific DNA-protein binding activity by EMSA as described in ‘Materials and methods’. In (c) and (d), the extent of NF-κB activation was quantitated using a densitometer with Image-Pro plus software. Results are expressed as the mean ± SEM (n = 3). *P < 0·05 as compared with the LTA-treated group. Wort, wortmannin; LY, LY 294002; Tyrp, tyrphostin AG126; Geni, genistein; SB, SB 203580; FP, free probe.
Figure 7.
Tyrosine kinase, PI3K/Akt, and p38 MAPK's involvement in LTA-mediated increase in κB-luciferase activity in RAW 264.7 macrophages. In (a), RAW 264.7 macrophages were transiently transfected with 0·5 µg of pGL2-ELAM-Luc and 1 µg of pBK-CMV-Lac Z for 24 hr, and then cells were incubated with 0·3–10 µg/ml LTA for another 24 hr. Luciferase activities were determined as described in ‘Materials and methods’. The level of induction of luciferase activity was compared to that of cells without LTA treatment. Data represent the mean ± SEM of three experiments performed in duplicate. *P < 0·05 as compared to the control without LTA treatment. In (b), RAW 264.7 macrophages were transiently transfected with 0·5 µg of pGL2-ELAM-Luc and 1 µg of pBK-CMV-Lac Z for 24 hr. Cells were cotransfected with 1 µg of the Akt dominant negative mutant or pretreated with 20 µm tyrphostin AG126, 100 nm wortmannin, 30 µm LY 294002, or 10 µm SB 203580 for 30 min, and then stimulated with 10 µg/ml LTA for another 24 hr. Cells were harvested for the luciferase assay as described in ‘Materials and methods’. Data represent the mean ± SEM of three experiments performed in duplicate. *P < 0·05 as compared to the LTA-treatment group. AktDN, Akt dominant negative mutant; Tyrp, tyrphostin AG126; Wort, wortmannin; LY, LY 294002; SB, SB 203580.
Discussion
Our previous study indicated that PC-PLC, PI-PLC, PKC, and transcription factor NF-κB might be involved in LTA-mediated signalling pathways leading to the expression of iNOS protein and NO release in RAW264.7 macrophages.8 In those published data, we proved that neither LTA-induced iNOS expression nor NO release is inhibited by polymyxin B, which binds and neutralizes LPS. These results indicate that the inductions of iNOS protein and NO release caused by LTA are not caused by LPS contamination.
In the present study, we demonstrated that LTA-mediated iNOS expression and NO release were prevented by tyrosine kinase inhibitors (tyrphostin AG 126 and genistein), indicating that activation of tyrosine kinase is involved in LTA-mediated iNOS expression and NO release in RAW 264.7 macrophages. Similar results were also seen in murine J744.2 macrophages.7 PI3K activation leads to phosphorylation of phosphatidylinositides, which then activate the downstream main target, Akt, which seems to have important roles in regulating cellular growth, differentiation, adhesion, and the inflammatory reaction.19,20 Activation of PI3K/Akt plays an important role in iNOS expression in vascular smooth muscle cells and peritoneal macrophages.14,15 In this study, we demonstrated that the LTA-induced iNOS expression and NO release were inhibited by PI3K inhibitors (wortmannin and LY 294002). A previous report indicated that the PI3K inhibitor, LY 294002 (50 µm), inhibits the serine/threonine-specific kinase, CK2, with similar potency. In contrast, wortmannin (1 µm), another inhibitor of PI3K, does not inhibit CK2.21 Therefore, we used two structurally unrelated PI3K inhibitors, wortmannin (100 nm) and LY 294002 (30 µm), to minimize the chance for a non-specific effect of a drug in the present study. This result suggests that PI3K activation is involved in the signal transduction leading to iNOS expression and NO release induced by LTA. Furthermore, we found that LTA-induced Akt activation was inhibited by PI3K inhibitors (wortmannin and LY 294002) and tyrosine kinase inhibitors (tyrphostin AG 126 and genistein), indicating that activations of tyrosine kinase and PI3K occur upstream of LTA-induced Akt activation.
Activation of p38 MAPK has been demonstrated to be involved in LTA-induced cyclo-oxygenase-2 expression and prostaglandin E2 release in human pulmonary epithelial cells.22 A previous report also showed that p38 MAPK activation is involved LPS-induced iNOS expression and NO release in RAW 264.7 macrophages.17 We found herein that treatment of RAW 264.7 macrophages with LTA resulted in marked activation of p38 MAPK. Furthermore, a previous report showed that the pyridinyl imidazole, SB 203580, is a remarkably specific inhibitor of p38 MAPK, and at a concentration of up to 100 µm still does not affect the activities of a number of other protein kinases, including closely related MAPK family members.23 In the present study, SB 203580 (10 µm) was used to inhibit LTA-induced iNOS expression and NO release, indicating that activation of p38 MAPK is indeed involved in LTA-mediated iNOS expression and NO release. Furthermore, LTA-mediated p38 MAPK activation was separately inhibited by wortmannin, LY 294002, genistein, tyrphostin AG 126, and SB 203580. These findings suggest that LTA-induced p38 MAPK activation occurs downstream of the signal of tyrosine kinase and PI3K.
The transcription factor, NF-κB, is critical to the induction of iNOS by LTA in macrophages.7,8 In the present study, we found that LTA-induced formation of the NF-κB-specific DNA–protein complex was separately inhibited by wortmannin, LY 294002, tyrphostin AG126, genistein, and SB 203580. Using transient transfection with pGL2-ELAM-κB-luciferase as an indicator of NF-κB activity, we also found that LTA induced an increase in NF-κB activity, this effect was inhibited by the Akt dominant negative mutant (AktDN) and these inhibitors. In addition, we also previously demonstrated that the PKC inhibitors, Ro 31-8220 and Go 6976, inhibit LTA-mediated activation of NF-κB-specific DNA–protein complex formation.8 These results indicate that, in addition to PKC, activations of tyrosine kinase, PI3K/Akt, and p38 MAPK occur upstream of NF-κB in the LTA-induced signalling pathway in RAW 264.7 macrophages.
In conclusion, LTA might activate the PI3K/Akt pathway through tyrosine kinase to elicit p38 MAPK activation, which in turn initiates NF-κB activation, and finally causes iNOS expression and NO release in RAW 264.7 macrophages. The present study, together with our previous report8 delineates, in part, the signal transduction pathways of LTA-induced iNOS expression and NO release. A schematic representation of the signalling pathway of LTA-induced iNOS expression and NO release in RAW 264.7 macrophages is shown in Fig. 8. Based on the results of the present study and previous reports,17,24 we found that the signal transduction pathway of LTA-induced iNOS expression is similar to that of LPS-induced iNOS expression in RAW 264.7 cells. One possible explanation for this similarity is that both LTA and LPS bind to the same receptor, CD14,25,26 which is a glycosylphosphatidylinositol-linked membrane receptor lacking an intracellular signalling domain. Moreover, a transmembrane coreceptor (signal transducing receptor) for CD14 has been postulated.27 Recently, Toll-like receptor 2 (TLR2) and TLR4 have been identified as the signal transducing receptors for LTA and LPS, respectively.28,29 Several reports have indicated that by interacting with plasma proteins (CD14 or TLR4), LPS can activate several signalling pathways, including activation of PLC, and increases in the intracellular Ca2+ concentration30 and activities of PKC,31 tyrosine kinase,32 p42/44 MAPK,31 JNK,33 p38 MAPK,34 and PI3K.35 Moreover, it has been demonstrated that LPS-mediated signalling via CD14, TLR4, and NF-κB is responsible for iNOS gene induction in macrophages.36 In contrast to LPS, a previous report indicated that in human tracheal smooth muscle cells, LTA-stimulated p42/44 MAPK activation is mediated through a TLR2 receptor and involves tyrosine kinase, PLC, PKC, Ca2+, MEK, and PI3K.37 Furthermore, LTA has been also shown to induce NF-κB activation through the TLR2 receptor in HEK293T cells or RAW 264.7 cells.38 Taken together, it seems very likely that TLR2 and TLR4 possess a similar signal transduction pathway. The results suggest that iNOS induction and the subsequent enhanced release of NO may be involved in inflammatory responses and septic shock elicited by Gram-positive organisms. By understanding these signal transduction pathways, we may be able to design therapeutic strategies to reduce inflammatory responses and septic shock caused by Gram-positive organisms.
Figure 8.
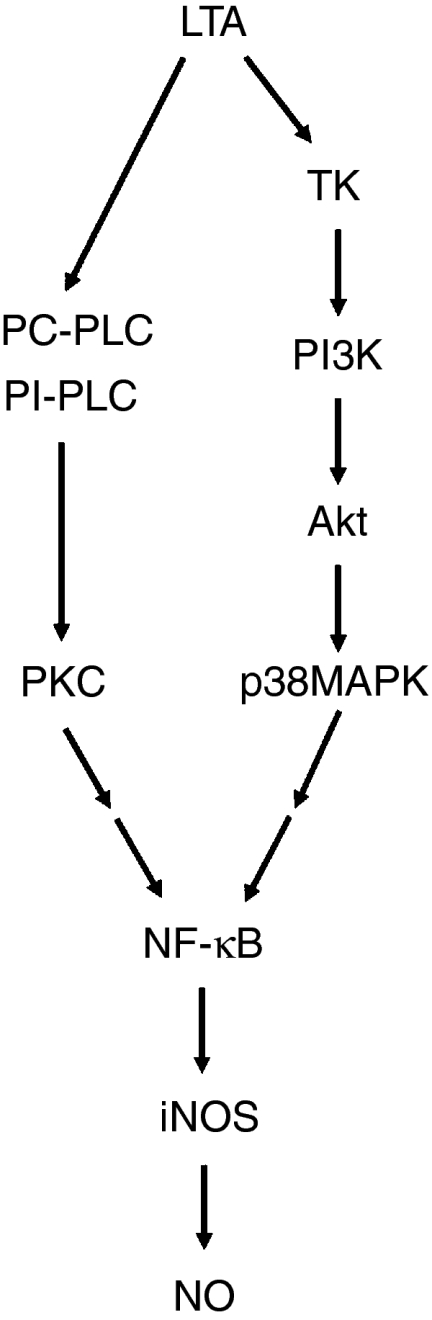
Schematic summary of signal transduction by LTA which induces iNOS expression and NO release in RAW 264.7 macrophages. Treatment of RAW 264.7 macrophages with LTA caused iNOS expression and NO release through two separate pathways: the phosphatidylcholine-phospholipase C (PC-PLC), phosphatidylinositol-phospholipase C (PI-PLC)/PKC/NF-κB cascade and the tyrosine kinase (TK)/phosphatidylinositiol 3-kinase (PI3K)/Akt/p38 MAPK/NF-κB cascade.
Acknowledgments
This study was supported by research grants from the Shin Kong Wu Ho-Su Memorial Hospital (SKH-TMU-08) and the National Science Council of the Republic of China (NSC91-2314-B-038–010).
References
- 1.Moncada S, Palmer RM, Higgs EA. Nitric oxide. physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109. [PubMed] [Google Scholar]
- 2.Nathan C, Xie QW. Nitric oxide synthases. roles, tolls, and controls. Cell. 1994;78:915. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 3.Jaffrey SR, Snyder SH. Nitric oxide: a neural messenger. Annu Rev Cell Dev Biol. 1995;11:417. doi: 10.1146/annurev.cb.11.110195.002221. 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051. [PubMed] [Google Scholar]
- 5.Thiemermann C. The role of the 1-arginine: nitric oxide pathway in circulatory shock. Adv Pharmacol. 1994;28:45. doi: 10.1016/s1054-3589(08)60493-7. [DOI] [PubMed] [Google Scholar]
- 6.Bone RC. Gram-positive organisms and sepsis. Arch Intern Med. 1994;154:26. [PubMed] [Google Scholar]
- 7.Kengatharan M, De Kimpe SJ, Thiemermann C. Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br J Pharmacol. 1996;117:1163. doi: 10.1111/j.1476-5381.1996.tb16711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CT, Chiang LL, Lee CN, et al. Induction of nitric oxide synthase in RAW 264.7 macrophages by lipoteichoic acid from Staphylococcus aureus: involvement of protein kinase C- and nuclear factor-kB-dependent mechanisms. J Biomed Sci. 2003;10:136. doi: 10.1007/BF02256005. 10.1159/000068076. [DOI] [PubMed] [Google Scholar]
- 9.Auguet M, Lonchampt MO, Delaflotte S, Goulin-Schulz J, Chabrier PE, Braquet P. Induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in vascular smooth muscle cells. FEBS Lett. 1992;297:183. doi: 10.1016/0014-5793(92)80356-l. 10.1016/0014-5793(92)80356-L. [DOI] [PubMed] [Google Scholar]
- 10.De Kimpe SJ, Hunter ML, Bryant CE, Thiemermann C, Vane JR. Delayed circulatory failure due to the induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in anaesthetized rats. Br J Pharmacol. 1995;114:1317. doi: 10.1111/j.1476-5381.1995.tb13349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665. doi: 10.1126/science.275.5300.665. 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 12.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601. doi: 10.1016/s0960-9822(99)80265-6. 10.1016/S0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 13.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82. doi: 10.1038/43466. 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 14.Hattori Y, Hattori S, Kasai K. Lipopolysaccharide activates Akt in vascular smooth muscle cells resulting in induction of inducible nitric oxide synthase through nuclear factor-kappa B activation. Eur J Pharmacol. 2003;481:153. doi: 10.1016/j.ejphar.2003.09.034. 10.1016/j.ejphar.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Sodhi A, Biswas SK. fMLP-induced in vitro nitric oxide production and its regulation in murine peritoneal macrophages. J Leukoc Biol. 2002;71:262. [PubMed] [Google Scholar]
- 16.Lin CH, Kuan IH, Lee HM, Lee WS, Sheu JR, Ho YS, Wang CH, Kuo HP. Induction of cyclooxygenase-2 protein by lipoteichoic acid from Staphylococcus aureus in human pulmonary epithelial cells: involvement of a nuclear factor-kappa B-dependent pathway. Br J Pharmacol. 2001;134:543. doi: 10.1038/sj.bjp.0704290. 10.1038/sj.bjp.0704290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481. [PubMed] [Google Scholar]
- 18.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541. [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter CL, Cantley LC. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049. doi: 10.1126/science.287.5455.1049. 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 21.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95. doi: 10.1042/0264-6021:3510095. 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CH, Kuan IH, Wang CH, et al. Lipoteichoic acid-induced cyclooxygenase-2 expression requires activations of p44/42 and p38 mitogen-activated protein kinase signal pathways. Eur J Pharmacol. 2002;450:1. doi: 10.1016/s0014-2999(02)02002-2. 10.1016/S0014-2999(02)02002-2. [DOI] [PubMed] [Google Scholar]
- 23.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 24.Chen CC, Wang JK, Lin SB. Antisense oligonucleotides targeting protein kinase C-alpha-beta I, or -delta but not -eta inhibit lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages: involvement of a nuclear factor kappa B-dependent mechanism. J Immunol. 1998;161:6206. [PubMed] [Google Scholar]
- 25.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem Biophys Res Commun. 1997;233:375. doi: 10.1006/bbrc.1997.6462. 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 26.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 27.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437. doi: 10.1146/annurev.iy.13.040195.002253. 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 28.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406. doi: 10.1074/jbc.274.25.17406. 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443. doi: 10.1016/s1074-7613(00)80119-3. 10.1016/S1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 30.Waga I, Nakamura M, Honda Z, Ferby I, Toyoshima S, Ishiguro S, Shimizu T. Two distinct signal transduction pathways for the activation of guinea-pig macrophages and neutrophils by endotoxin. Biochem Biophys Res Commun. 1993;197:465. doi: 10.1006/bbrc.1993.2502. 10.1006/bbrc.1993.2502. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein SL, Sanghera JS, Lemke K, DeFranco AL, Pelech SL. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267:14955. [PubMed] [Google Scholar]
- 32.Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009. [PubMed] [Google Scholar]
- 33.Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774. doi: 10.1073/pnas.93.7.2774. 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739. doi: 10.1038/372739a0. 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 35.Herrera-Velit P, Knutson KL, Reiner NE. Phosphatidylinositol 3-kinase-dependent activation of protein kinase C-zeta in bacterial lipopolysaccharide-treated human monocytes. J Biol Chem. 1997;272:16445. doi: 10.1074/jbc.272.26.16445. 10.1074/jbc.272.26.16445. [DOI] [PubMed] [Google Scholar]
- 36.Dil N, Qureshi MA. Involvement of lipopolysaccharide related receptors and nuclear factor kappa B in differential expression of inducible nitric oxide synthase in chicken macrophages from different genetic backgrounds. Vet Immunol Immunopathol. 2002;88:149. doi: 10.1016/s0165-2427(02)00153-8. 10.1016/S0165-2427(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 37.Lee CW, Chien CS, Yang CM. Lipoteichoic acid-stimulated p42/p44 MAPK activation via Toll-like receptor 2 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L921. doi: 10.1152/ajplung.00124.2003. 10.1152/ajplung.00124.2003. [DOI] [PubMed] [Google Scholar]
- 38.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol. 2003;10:259. doi: 10.1128/CDLI.10.2.259-266.2003. 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]