Abstract
The nuclear factor (NF)-κB transcriptional system is a major effector pathway involved in inflammation and innate immune responses. The flavonoid luteolin is found in various herbal extracts and has shown anti-inflammatory properties. However, the mechanism of action and impact of luteolin on innate immunity is still unknown. We report that luteolin significantly blocks lipopolysaccharide (LPS)-induced IκB phosphorylation/degradation, NF-κB transcriptional activity and intercellular adhesion molecule-1 (ICAM-1) gene expression in rat IEC-18 cells. Using chromatin immunoprecipitation, we demonstrate that LPS-induced RelA recruitment to the ICAM-1 gene promoter is significantly reduced in luteolin-treated cells. Moreover, in vitro kinase assays show that luteolin directly inhibits LPS-induced IκB kinase (IKK) activity in IEC-18 cells. Using bone-marrow derived dendritic cells (BMDCs) isolated from interleukin (IL)-10−/− mice or from recently engineered transgenic mice expressing the enhanced green fluorescent protein (EGFP) under the transcriptional control of NF-κB cis-elements (cis-NF-κBEGFP), we found that luteolin blocks LPS-induced IκB phosphorylation and IKK activity, and decreases EGFP, IL-12 and tumour necrosis factor-α gene expression. Moreover, intraperitoneal administration of luteolin significantly inhibited LPS-induced EGFP expression in both peripheral blood mononuclear cells and splenocytes isolated from cis-NF-κBEGFP mice. These results indicate that luteolin blocks LPS-induced NF-κB signalling and proinflammatory gene expression in intestinal epithelial cells and dendritic cells. Modulation of innate immunity by natural plant products may represent an attractive strategy to prevent intestinal inflammation associated with dysregulated innate immune responses.
Keywords: luteolin, NF-κB, IκB kinase, intestinal epithelial cell, bone marrow-derived dendritic cell
Introduction
The intestinal mucosa is constantly exposed to a myriad of antigens, including bacteria and bacterial products (lipopolysaccharide (LPS), peptidoglycans), viruses, parasites and dietary antigens. The host has evolved sophisticated mechanisms to maintain homeostasis in the face of such a hostile environment.1–4 First and foremost, the intestinal luminal contents are isolated from the host by a single layer of cells, the intestinal epithelial cells (IECs). These cells form a tight barrier that prevents potential toxic luminal products from breaching the mucosal layer and activating the underlying resident immune cells and/or gaining access to the systemic blood circulation.2 In addition, an array of regulatory mechanisms such as the production of anti-inflammatory molecules (transforming growth factor (TGF)-β, interleukin (IL)-10, etc.), immunoglobulin A (IgA) synthesized by immune cells, and various mucins produced by IECs participate in the maintenance of host homeostasis.5–7
Among the immune cells present in the intestinal mucosa, dendritic cells (DCs) play a pivotal role in sampling enteric antigens and presenting various microbial antigens to T lymphocytes, which then mature into either effector and/or regulatory cells.8 The outcomes of these interactions determine whether the intestine initiates tolerance toward its luminal contents or conversely, proceeds to trigger an inflammatory process. Dysregulated and/or constant activation of innate immunity leading to improper activation of effector T cells is associated with the development of intestinal inflammatory disorders such as inflammatory bowel diseases (IBD).9 Thus, DCs are at the interface of innate and adaptive immunity and are active participants in intestinal homeostasis.
IECs and DCs have the potential to trigger a proinflammatory gene transcriptional programme when challenged with the bacterial product LPS.10–12 This genetic programme is activated through Toll-like receptor (TLR)-4-induced signal transduction, which then targets various down-stream effector pathways such as the transcription factor nuclear factor (NF)-κB.13,14 This transcription factor regulates the expression of numerous pro-inflammatory and immune-related genes and is a central constituent of many effector pathways induced by the innate immune system. TLR-4-mediated signal transduction operates through MyD88-dependent mechanisms involving IL-1 receptor-associated kinase (IRAK) phosphorylation and recruitment of tumour necrosis factor (TNF) receptor-associated factor-6 (TRAF6) and TGF-β activated kinase-1 (TAK1).13,14 The signals then converge upon the IκB kinase (IKK) complex, which phosphorylates the NF-κB inhibitor IκB on serine residues 32 and 36 causing its ubiquitination and degradation. Elimination of IκB liberates NF-κB from its inhibitory effect and permits the nuclear translocation of the transcription factor, binding to κB-promoter elements and induction of gene transcription.15,16 Because NF-κB promotes intestinal inflammation, targeting this signalling pathway may represent a therapeutic avenue for the treatment of intestinal inflammatory disorders.17
Complementary and alternative medicine (CAM) regroups diagnostic and therapeutic approaches not included within allopathic medicine. Among the various CAM, ‘herbal medicine’ is the most popular and fastest growing approach used to treat various ailments worldwide, specifically in the United States.18 Herbal medicine generally refers to extracts or active components obtained from plants, barks, roots, leaves, flowers, and fruit used to alleviate medical conditions. Although these products have been used for centuries as remedies to treat numerous medical conditions in various countries, lack of empirical data showing efficacy and mechanisms of action precludes their incorporation into mainstream medicine. Interestingly, many pharmaceutical products currently available originated from plant extracts (e.g. salicylate from willow bark, quinone from cinchona, digitalis from foxglove leaves and taxol from Taxus brevifolia), suggesting that some herbal extracts may be effective in treating some medical conditions.19 Whether plant extracts modulate innate immune responses and intestinal inflammatory disorders is not clear.
Flavonoids are naturally occurring polyphenolic compounds, present in many plants and plant-based foods that possess potent antioxidant, anticarcinogenic, immunomodulating and antibacterial activities.20–23 For example, the flavonoids curcumin and luteolin have anti-inflammatory properties both in vivo and in vitro.24–28 With respect to innate signalling, luteolin (3′,4′,5,7-tetrahydroxyflavone), a flavonoid found in many herbal extracts including celery, green pepper, perilla leaf and seeds and chamomile, inhibits LPS-induced TNF-α secretion by macrophages in vitro and has anti-inflammatory activity in mice.28 In addition, luteolin inhibits LPS-induced NF-κB activity in rat-1 fibroblasts.27
However, the mechanism through which luteolin mediates its anti-inflammatory effects is not completely understood, much less its impact on innate signalling. Using IECs and bone-marrow derived dendritic cells (BMDCs), we investigated the effect of luteolin on LPS-induced NF-κB signalling and proinflammatory gene expression. We report that luteolin blocks the activation of NF-κB signalling by targeting IKK in both cell types in vitro, and in immune cells in vivo using novel transgenic mice expressing the enhanced green fluorescent protein (EGFP) under the transcriptional control of NF-κB cis-elements (cis-NF-κBEGFP). These findings indicate that luteolin may represent a new class of flavonoid capable of inhibiting NF-κB activity both in vivo and in vitro.
Materials and methods
Cell culture and treatment of IECs
The rat non-transformed small intestinal cell line IEC-18 (American Type Culture Collection CRL 1589, Manassas, VA) was used between passages 5 and 15. Cells were grown as described previously.29 Luteolin (Sigma, St Louis, MO) was dissolved in dimethyl sulphoxide (DMSO; Sigma) to a final concentration of 10 mm. Cells were pretreated for 1 hr with various concentration of luteolin (0–100 µm) or with DMSO vehicle (0·5%), after which they were stimulated with LPS (10 µg/ml; from Escherichia coli serotype O111:B4, Sigma), IL-1β (10 ng/ml) or TNF-α (5 ng/ml) (both from R & D Systems, Minneapolis, MN).
Animals
Cis-NF-κBEGFP mice (BALB/c background) and IL-10−/− mice (C57BL/6 × 129/Ola mixed background) were used between 8 and 10 weeks of age. Cis-NF-κBEGFP mice are recently engineered transgenic mice which express the EGFP under the transcriptional control of NF-κB cis-elements.30 EGFP gene expression is specifically induced by NF-κB inducers which correlates with RelA binding to the transgene promoter, and responds in vivo to stimulation with LPS and anti-CD3.30 Animal experiments were performed in accordance with the guidelines of the institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Isolation and culture of BMDCs
BMDCs were generated from the femur and tibia of cis-NF-κBEGFP and IL-10−/− mice. Bone marrow cells were flushed and depleted of red blood cells (RBC) using RBC lysing buffer (Sigma), and then cultured in ultra low-adherence 24-well plates (Costar, Corning, NY) in complete media (RPMI-1640 supplemented with 10% fetal calf serum, 2 mm l-glutamine, 50 µm 2-mercaptoethanol, 10 ng/ml murine recombinant granulocyte—macrophage colony-stimulating factor (GM-CSF) and 10 ng/ml murine recombinant IL-4 (both from PeproTech, Rocky Hill, NJ). Half of complete media was refreshed on the 3rd and 5th day. On the 7th day of culturing, non-adherent cells were collected as BMDCs. The resulting population was greater than 93% CD11c+ and CD11b+ as determined by flow cytometry. Cells were stimulated with LPS (1 µg/ml) for various times to determine the expression of IL-12 p40, TNF-α and EGFP.
Real-time reverse transcription—polymerase chain reaction (RT—PCR) analysis and enzyme-linked immunosorbent assay (ELISA)
RNA was isolated using Trizol (Invitrogen, Carlsbad, CA), and 1 µg of total RNA was reverse-transcribed as described previously.24 Real-time RT—PCR was performed using an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA) with specific primers for rat ICAM-1, mouse IL-12 p40 and mouse TNF-α. As an endogenous control, 18S ribosomal RNA primers were used. All primers were designed by Primer Express v2.0 (Applied Biosystems). The primer sequences used in the present study are shown in Table 1. PCR was conducted using SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's instruction. Thermal cycler conditions were as follows: one cycle of 10 min, 95 °C, and 40 cycles of denaturation (15 s, 95 °C) and combined annealing/extension (1 min, 60 °C). The specificity of the amplicon was tested via melting curve analysis. A serial dilution of an external standard (positive samples; LPS treated) was used to generate a calibration curve to determine signal intensity of each sample according to their threshold cycle. The amplifications were performed in triplicates and the data was normalized to the 18S ribosomal subunit.
Table 1.
Specific primer sequences used in the real-time RT—PCR and chromatin immunoprecipitation
| mRNA species | Oligonucleotides (5′→3′) |
|---|---|
| Rat ICAM-1 | |
| (S) | CGGGATGGTGAAGTCTGTCAA |
| (AS) | TGCACGTCCCTGGTGATACTC |
| Mouse IL-12 p40 | |
| (S) | GGAAGCACGGCAGCAGAATA |
| (AS) | AACTTGAGGGAGAAGTAGGAATGG |
| Mouse TNF-α | |
| (S) | CATCTTCTCAAAAATCGAGTGACAA |
| (AS) | TGGGAGTAGACAAGGTACAACCC |
| 18S-rRNA | |
| (S) | CGCCGCTAGAGGTGAAATTCT |
| (AS) | CATTCTTGGCAAATGCTTTCG |
| Rat ICAM-1 promoter | |
| (S) | CTTCTCTCCCGGACTCTCCT |
| (AS) | ATGAGGGCTTCGGTATTTCC |
An ELISA for mouse IL-12 p40 and TNF-α was performed using culture supernatants from luteolin-treated BMDCs according to the manufacturer's instructions (R & D Systems).
Adenoviral infection and NF-κB-luciferase reporter assay
IEC-18 cells were infected for 16 hr with Ad5κB-LUC as described previously.11 Where indicated IEC-18 cells were coinfected for an additional 12 hr with Ad5IκBαAA, Ad5dnIKKβ, or Ad5wtNIK at a multiplicity of infection (m.o.i) of 50. The Ad5dnIKKβ and Ad5wtNIK constructs were described previously.31 The Ad5GFP containing GFP was used as a viral negative control. The Ad5CMV-LUC containing constitutively active cytomegalovirus (CMV) promoter-driven luciferase was used as a luciferase reporter control. The adenoviruses were washed off, and fresh medium containing serum was added. Cells were stimulated with LPS (10 µg/ml) for 12 hr in the presence or absence of various concentration of luteolin. Cell extracts were prepared using luciferase cell lysis buffer (PharMingen, San Diego, CA). Luciferase assays were performed using an Lmax luminometer microplate reader (Molecular Devices, Sunnyvale, CA), and results were normalized for extract protein concentrations measured with the Bio-Rad protein assay kit (Bio-Rad).
Western blot analysis
IEC-18 cells and BMDCs were stimulated with LPS (1–10 µg/ml) for various times (0–1 hr). The cells were lysed in 1× Laemmli buffer, and 20 µg of protein was subjected to electrophoresis on 10% sodium dodecyl sulphate (SDS)—polyacrylamide gels as described previously.11 Where indicated IEC-18 cells and BMDCs were pretreated for 1 hr with 50 µm luteolin. Anti-phosphoserine IκBα (Cell Signalling, Beverly, MA), anti-IκBα (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phosphoserine RelA (S536, Cell Signalling), anti-IRAK-1 (a generous gift from D. K. Miller, Merck), anti-p38 (Cell Signalling), anti-phosphoserine p38 (Cell Signalling), anti-phosphoserine Janus kinase (JNK; PharMingen), anti-NIK (Santa Cruz Biotechnology), anti-IKK-β (Santa Cruz Biotechnology) and anti-β-actin (ICN, Costa Mesa, CA) were used to detect immunoreactive phospho-IκBα, IκBα, phospho-RelA, IRAK-1, p38, phospho-p38, phospho-JNK, NIK, IKK-β and β-actin, respectively, using an enhanced chemiluminescence detection kit (Amersham Biosciences, Arlington Heights, IL).
Immunofluorescence
Luteolin (50 µm)-pretreated IEC-18 cells were stimulated with LPS (10 µg/ml) for 30 min, after which they were fixed with 100% ice-cold methanol. RelA immunofluorescence was performed as described previously.24 Briefly, cells were blocked with 10% non-immune goat serum (NGS) for 30 min, then probed with rabbit anti-RelA antibody (Rockland, Gilberville, PA; diluted 1 : 200) in 10% NGS for 45 min, followed by rhodamine isothiocyanate-conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA; diluted 1 : 100) in 10% NGS for 30 min. RelA was visualized with a fluorescent light microscope.
Chromatin immunoprecipitation (ChIP) assay
After IEC-18 cells were stimulated with LPS (10 µg/ml) for 30 min in the presence or absence of luteolin (50 µm), cells were washed in cold phosphate-buffered saline (PBS) and fixed by adding formaldehyde to a final concentration of 1%. Nuclear extraction and chromatin immunoprecipitation were performed as described previously.32 Briefly, cells were lysed after formaldehyde fixation in L1 lysis buffer (50 mm Tris (pH 8·0), 2 mm ethylenediaminetetra-acetic acid (EDTA), 0·1% Nonidet P-40, and 10% glycerol) supplemented with protease inhibitors. Nuclei were pelleted and resuspended in 300 µl of L2 lysis buffer (50 mm Tris (pH 8·0), 0·1% SDS, and 5 mm EDTA). Chromatin was sheared by sonication (three times for 10 s at one-fifth of maximum power), centrifuged, and diluted in dilution buffer (50 mm Tris (pH 8·0), 5 mm EDTA, 0·2 m NaCl, and 0·5% Nonidet P-40). Extracts were precleared for 3 hr with salmon sperm-saturated protein A/G-agarose (ssProtein A/G). Immunoprecipitation was carried out overnight at 4° using 5 µl of anti-phosphoserine RelA (Cell Signalling). Immune complexes were collected with ssProtein A/G for 30 min and washed three times in washing buffer (20 mm Tris (pH 8·0), 0·1% SDS, 0·5 m NaCl, 2 mm EDTA, and 1% Nonidet P-40) and once in 0·5 m LiCl, followed by three washes with TE buffer. Immune complexes were extracted three times with 100 µl of extraction buffer (TE buffer containing 2% SDS). DNA cross-links were reverted by heating for 8 hr at 65°. After proteinase K (100 µg for 2 hr) digestion, DNA was extracted with phenol/chloroform and precipitated in ethanol. DNA isolated from an aliquot of the total nuclear extract was used as a loading control for the PCR (input control). PCR was performed with total DNA (1 µl, input control) and immunoprecipitated DNA (2 µl) using the following rat ICAM-1 promoter-specific primers, as shown in Table 1.
In vitro IKK and IRAK-1 kinase assay
IEC-18 cells were pretreated for 1 hr with luteolin (50 µm) and then stimulated with LPS (10 µg/ml) for 20 min or infected with Ad5wtNIK (m.o.i 50) for 16 hr. IKK activity on serine IκBα phosphorylation was determined by immunocomplex kinase assay as described previously.24,31 IEC-18 cells were lysed in Triton lysis buffer containing protease and phosphatase inhibitors and then cleared by cenrifugation at 18 000 g for 10 min. Eight hundred micrograms of whole cell extract was immunoprecipitated with anti-IKKγ (Santa Cruz Biotechnology) or IRAK-1 (Santa Cruz Biotechnology)/protein-A beads. The kinase reactions were performed by incubating 25 µl of kinase buffer containing 20 mm Hepes (pH 7·7), 10 mm MgCl2, 5 mm dithiothreitol, 50 µm ATP, and 5 µCi of [γ-32P]ATP (ICN) with GST-IκBα substrate (amino acid 1–54; IKK activity) or buffer alone (IRAK-1 auto-phosphorylation) for 30 min at 30°. Substrate protein was resolved by gel electrophoresis, and phosphate incorporation was assessed by autoradiography and PhosphorImager analysis (Amersham Biosciences).
Alternatively, the effect of luteolin on IKK activity and IRAK-1 auto-phosphorylation was directly measured. Immunoprecipitated IKK complexes or IRAK-1 from LPS (1–10 µg/ml)-stimulated IEC-18 cells and BMDCs were incubated with various concentrations of luteolin or control DMSO vehicle, and the kinase reactions were performed as described above.
Spectrofluorometry and flow cytometric analysis
BMDCs isolated from cis-NF-κBEGFP mice were lysed by lysis buffer (50 mm Tris (pH 7·5), 5 mm EDTA, 0·2 m NaCl, and 0·1% Nonidet P-40), and cellular debris was removed by centrifugation at 13 000 g for 10 min. The fluorescence of EGFP in cell lysates was measured using a SpectraMax Gemini XS spectrofluorometer (Molecular Devices), and results were normalized for extract protein concentrations measured with the Bio-Rad protein assay kit. The excitation and emission settings were 488 nm and 509 nm, respectively.
For the detection of EGFP in peripheral blood mononuclear cells (PBMCs) and splenocytes isolated from cis-NF-κBEGFP mice, flow cytometry determination was performed as previously described.30 Briefly, 5 × 105 RBC-depleted PBMCs or splenocytes were washed with fluorescence-activated cell sorting (FACS) buffer and EGFP expression was measured on a FACscan (Becton-Dickinson, Mountain View, CA) using the FL1 channel to detect EGFP fluorescence. All flow cytometric analyses were conducted on living cells within 30 min of isolation.
Cytotoxicity assay
BMDCs were incubated with various concentrations of luteolin in the presence or absence of LPS. Cytotoxicity was measured using the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR) according to the manufacturer's specifications. Positive control dead cells were generated by treating IEC-18 cells or BMDCs with methanol for 30 min. Fluorescence in cell samples was measured with appropriate excitation and emission filters using a SpectraMax Gemini XS spectrofluorometer (Molecular Devices) according to the manufacturer's instructions.
In vivo LPS endotoxaemia study
Cis-NF-κBEGFP mice were pretreated intraperitoneally with DMSO vehicle or luteolin (0·2 mg/kg, 100 µl) for 2 hr before the intraperitoneal challenge of LPS (40 mg/kg, 100 µl) and killed 18 hr poststimulation. Peripheral blood was obtained by cardiac puncture and placed into a sodium heparin-coated vial. RBCs were lysed using RBC lysing buffer and PBMCs were pelleted by density-gradient centrifugation over Histopaque-1083 (Sigma) for 20 min at 800 g. Cells were resuspended in FACS analysis buffer (0·1% BSA/0·01% sodium azide in PBS) just prior to FACS analysis. Splenocytes were isolated by flushing the spleen through a 70 µm cell strainer (Becton-Dickinson, Franklin Lakes, NJ) and RBC lysed as described above. Cells were pelleted and resuspended in FACS analysis buffer just prior to FACS analysis.
Statistical analysis
All data were expressed as the means for a series of experiments ± SEM. Data were analysed by non-parametric t-tests or Wilcoxon rank sum tests. A 2-tailed P-value of <0·05 was considered statistically significant.
Results
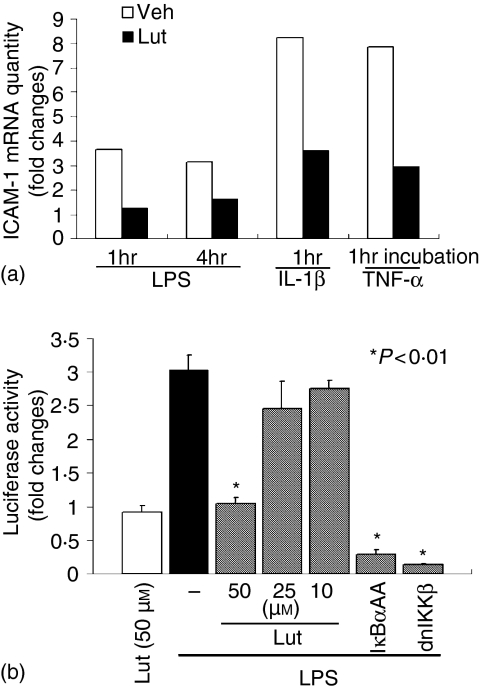
Luteolin inhibits LPS-induced ICAM-1 mRNA accumulation and NF-κB transcriptional activity in IEC-18 cells
The flavonoid luteolin has been shown to possess anti-inflammatory properties both in vitro and in vivo, although the inhibitory mechanism is not completely understood. We first analysed the effects of luteolin on signal-induced ICAM-1 gene expression in the rat non-transformed intestinal cell line IEC-18. Cells were preincubated with luteolin (50 µm) for 1 hr, stimulated with LPS (10 µg/ml) IL-1β and TNF-α (10 and 5 ng/ml, respectively) for 1 hr and 4 hr and ICAM-1 gene expression was measured by semiquantitative RT—PCR using an ABI Prism 7700 sequence detection system. As seen in Fig. 1(a), LPS, IL-1β and TNF-α induced ICAM-1 mRNA expression is inhibited by luteolin treatment (66, 56, and 63% inhibition at 1 hr poststimulation, respectively). Moreover, LPS-induced ICAM-1 mRNA accumulation is still inhibited 4 hr stimulation (48% inhibition). The transcription factor NF-κB is a common down-stream signal effector pathway utilized by LPS, IL-1β and TNF-α, and plays an essential role in signal-induced ICAM-1 gene expression in IEC.33 Therefore, we next sought to determine whether luteolin prevents LPS-induced NF-κB activity. IEC-18 cells were infected for 16 hr with an adenoviral vector encoding an NF-κB-luciferase reporter gene (Ad5-κB-LUC), pretreated with various doses of luteolin for 1 hr and then stimulated with LPS (10 µg/ml) for 12 hr. In addition, cells were infected with Ad5IκBαAA and Ad5dnIKKβ to selectively block NF-κB activation. As shown in Fig. 1(b), luteolin dose-dependently inhibited LPS-induced NF-κB transcriptional activity in IEC-18 cells. However, CMV promoter-driven luciferase activity is not blocked by luteolin, suggesting that this flavonoid is not inhibiting luciferase activity per se (data not shown). As expected, Ad5IκBαAA and Ad5dnIKKβ also blocked LPS-induced NF-κB activity in IEC-18 cells.
Figure 1.
Luteolin inhibits LPS-induced ICAM-1 mRNA accumulation and NF-κB transcriptional activity in IEC-18 cells. (a) IEC-18 cells were pretreated with luteolin (50 µm) for 1 hr, and then stimulated with LPS (10 µg/ml), IL-1β (10 ng/ml) or TNF-α (5 ng/ml) for 1 hr. Total RNA (1 µg) was extracted, reverse-transcribed, and amplified with an ABI Prism 7700 sequence detection system using specific rat ICAM-1 primers. Relative quantification was performed by comparison of threshold cycles values of samples with 18S ribosomal RNA. mRNA levels are expressed as fold changes over control determined as the mean of one experiment performed in triplicate. Results are representative of three independent experiments. (b) IEC-18 cells were infected for 16 hr with Ad5κB-LUC, and cells were coinfected for an additional 12 hr with Ad5IκBαAA, Ad5dnIKKβ, and control Ad5GFP (m.o.i. of 50). Cells were stimulated with LPS (10 µg/ml) for 12 hr in the presence or absence of various concentrations of luteolin. Cell extracts were prepared, luciferase assays performed on an Lmax microplate reader, and results were normalized to extract protein concentrations. LPS-induced luciferase activity is expressed as fold changes over control determined as the mean and SEM of three independent experiments measured in triplicate. Veh, DMSO vehicle; Lut, Luteolin.
Luteolin blocks LPS-induced IκBα phosphorylation/degradation, RelA nuclear translocation, and NF-κB recruitment to the ICAM-1 promoter in IEC-18 cells
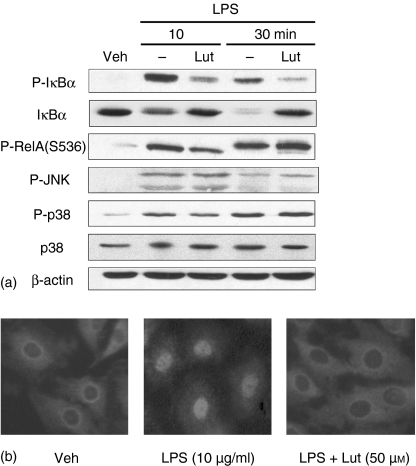
To dissect the effect of luteolin of LPS-induced signal transduction, we determined the phosphorylation levels of various down-stream signalling proteins involved in NF-κB activation using Western blot analysis. Figure 2(a) shows that LPS strongly induced IκBα phosphorylation (1st panel) and triggered IκBα degradation in IEC-18 cells (2nd panel), which is blocked in luteolin-treated cells. Recent studies have shown that RelA phosphorylation, JNK and the p38 pathway increase NF-κB transcriptional activity in various cell systems.34–37 We next tested the effect of luteolin on LPS-induced RelA (S536), JNK and p38 phosphorylation. As shown in Fig. 2(a) (3rd, 4th and 5th panels), LPS-induced RelA (S536), JNK and p38 phosphorylation were not inhibited by luteolin treatment. These suggest that luteolin exerts some level of specificity on LPS signal transduction in IEC-18 cells.
Figure 2.
Luteolin blocks LPS-induced IκBα phosphorylation/degradation and RelA nuclear translocation. (a) IEC-18 cells were stimulated with LPS (10 µg/ml) for various times (0–30 min). Where indicated, IEC-18 cells were pretreated for 1 hr with luteolin (50 µm). Total protein was extracted, and 20 µg of protein was subjected to SDS—PAGE followed by phospho-IκBα, IκBα, phospho-RelA (S536), phospho-JNK, phospho-p38, p38, and β-actin immunoblotting using the ECL technique. Results are representative of three independent experiments. (b) IEC-18 cells were pretreated with luteolin (50 µm) for 1 hr, and then stimulated with LPS (10 µg/ml) for 30 min. RelA localization was visualized using an anti-RelA primary antibody followed by a rhodamine-conjugated detection antibody. This immunofluorescence is representative of two independent experiments. Veh, DMSO vehicle; Lut, Luteolin.
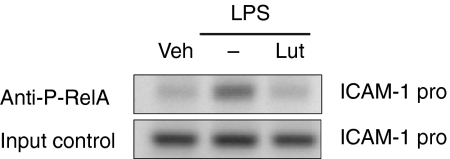
To measure the impact of luteolin-mediated inhibition of IκBα phosphorylation and degradation on NF-κB activation, we evaluated RelA nuclear translocation by immunofluorescence. As seen in Fig. 2(b), luteolin significantly inhibited LPS-induced RelA nuclear translocation in IEC-18 cells, suggesting that this critical NF-κB subunit is not present in the nucleus to induce gene transcription. To address this possibility, we carried out ChIP analysis of the ICAM-1 gene promoter using a phosphoserine (536) RelA antibody. IEC-18 cells were stimulated with LPS (10 µg/ml) in the presence or absence of luteolin (50 µm), and RelA recruitment to the ICAM-1 promoter region determined by PCR. As seen in Fig. 3, LPS-induced RelA recruitment to the ICAM-1 promoter was blocked by luteolin pretreatment. Similar levels of ICAM-1 promoter were amplified from the total pool of genomic DNA (input control), demonstrating that equal amounts were used for the ChIP assay (Fig. 3, lower panel). Non-specific IgG or protein A/Gbead alone showed no signal (data not shown). These findings indicate that luteolin blocks NF-κB signalling through inhibition of IκBα degradation, RelA nuclear translocation and binding to the ICAM-1 gene promoter.
Figure 3.
Luteolin blocks LPS-induced NF-κB recruitment to the ICAM-1 promoter in IEC-18 Cells. IEC-18 cells were stimulated with LPS for 30 min in the presence or absence of luteolin (50 µm), and ChIP assay was performed using an antiphospho-RelA antibody as described under Materials and Methods. PCR was performed using specific primers for the rat ICAM-1 promoter. Results are representative of two independent experiments. Veh, DMSO vehicle; Lut, Luteolin.
Luteolin directly inhibits LPS-induced IκB kinase activity in IEC-18 cells
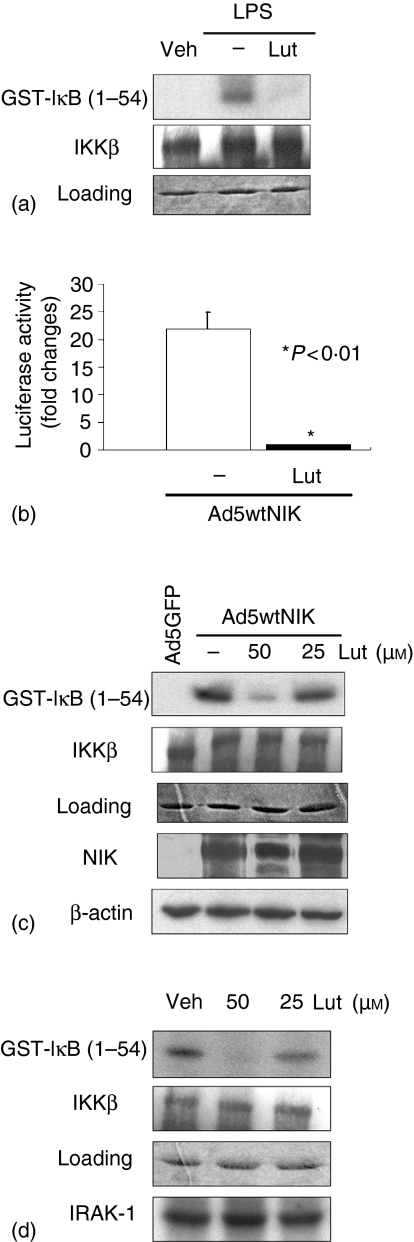
The above data indicates that luteolin inhibits a signalling event leading to IκB phosphorylation. LPS-induced serine IκBα phosphorylation is mediated by activation of the IKK complex in numerous cell systems. To determine the impact of luteolin on IKK activity, we performed an in vitro kinase assay. IEC-18 cells were pretreated with luteolin (50 µm), stimulated with LPS (10 µg/ml) for 20 min and IKKγ was immunoprecipitated. IKKα/β kinase activity was measured using GST-IκB (1–54) as a substrate. As shown in Fig. 4(a), luteolin significantly inhibited LPS-induced IKK activity in IEC-18 cells. Use of non-specific IgG or no antibody-bead alone controls showed no signal (data not shown).
Figure 4.
Luteolin directly inhibits LPS-induced IκB kinase activity in IEC-18 cells. (a) and (c), IEC-18 cells were pretreated with luteolin (50 µm) for 1 hr, and then stimulated with LPS (10 µg/ml) for 20 min (a) or infected with Ad5wtNIK (m.o.i 50) for 16 hr (c). Whole cell extract was immunoprecipitated with anti-IKKγ/protein-A beads and the kinase reaction was performed using GST-IκB (1–54) as a substrate as described under ‘Materials and methods’. Substrate protein was resolved by gel electrophoresis, and phosphate incorporation was assessed by autoradiography and PhosphorImager analysis. Coomassie Blue staining and Western blot for IKKβ show equal loading (2nd and 3rd blot, respectively). (b) IEC-18 cells were coinfected with Ad5κB-LUC and Ad5wtNIK in the presence or absence of luteolin (50 µm). Luciferase assay was measured using an Lmax microplate reader, and results were normalized for extract protein concentrations. (d) to evaluate whether luteolin inhibits IKK or IRAK-1 directly, immunoprecipitated IKK or IRAK-1 from LPS-stimulated IEC-18 cells were aliquoted into kinase reaction buffer in presence of various concentrations of luteolin or DMSO vehicle. IKK or IRAK-1 assay was performed as described in the Materials and Methods. These results are representative of at least two independent experiments. Veh, DMSO vehicle; Lut, Luteolin.
To dissect in more detail the mechanism of luteolin-mediated inhibition, we investigated the effect of this flavonoid on NIK-induced NF-κB activity. NIK has been positioned as an up-stream kinase involved in activation of the IKK complex. We recently showed that adenoviral gene delivery of NIK (Ad5wtNIK) strongly induced IKK activity and NF-κB transcriptional activity in IEC.31 IEC-18 cells were coinfected with Ad5κB-LUC and Ad5wtNIK in the presence or absence of luteolin (50 µm) and luciferase activity measured. Ad5wtNIK induced a 21-fold increase in NF-κB activity that was significantly suppressed in luteolin-treated cells (Fig. 4b). To measure the impact of luteolin on NIK-induced IKK activity, IEC-18 cells were infected with Ad5wtNIK for 12 hr in the presence or absence of luteolin. Ad5wtNIK significantly induced IKK activity in IEC-18 cells, which was dose-dependently blocked in luteolin-pretreated cells (Fig. 4c). Luteolin did not decrease NIK expression in Ad5wtNIK-infected cells. This suggests that luteolin acts at the level of, or downstream of NIK and might directly inhibit IKK activity. To test this hypothesis, we directly added various concentrations of luteolin to LPS-stimulated IKKγ or IRAK-1 immunoprecipitated extracts and then determined IKK or IRAK-1 activity in a cell-free system. As shown in Fig. 4(d), LPS-induced IKK activity, but not IRAK-1 autophosphorylation is blocked by luteolin in a dose-dependent manner in a cell-free system. This suggests that luteolin prevents LPS signalling to NF-κB through the direct blockade of IKK activity in IEC-18 cells.
Luteolin inhibits LPS-dependent NF-κB transcriptional activity in BMDCs isolated from cis-NF-κBEGFP
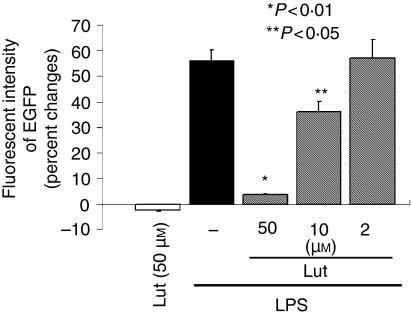
To expand this observation to another cell type, we studied the effects of luteolin on BMDCs. These cells play a pivotal role in the induction and in the control of host innate immune responses. To directly measure NF-κB activity in vivo, we recently generated a transgenic mouse expressing the EGFP under the transcriptional control of NF-κB cis-elements (cis-NF-κBEGFP).31 Cells and tissues derived from that mouse allow for the measurement of NF-κB dependent transcription through the evaluation of EGFP expression levels. BMDCs were isolated from the cis-NF-κBEGFP mice, pretreated with various concentrations of luteolin for 1 hr and then stimulated with LPS (1 µg/ml) for an additional 18 hr followed by EGFP quantification using a Gemini XS fluorescent microplate reader. As shown in Fig. 5, luteolin significantly decreased LPS-induced EGFP expression in BMDCs in a dose-dependent manner. This suggests that luteolin-mediated NF-κB inhibition is not cell-type specific and operates in mouse primary cells.
Figure 5.
Luteolin inhibits LPS-dependent NF-κB transcriptional activity in BMDCs isolated from cis-NF-κBEGFP. Bone marrow-derived dendritic cells (BMDCs) isolated from cis-NF-κBEGFP mice were pretreated with various concentrations of luteolin for 1 hr, and then stimulated with LPS (1 µg/ml) for 18 hr. Cells were lysed using Nonidet P-40 lysis buffer and EGFP levels measured using a Gemini XS microplate reader. The results were normalized to protein extract concentrations. The excitation and emission settings were 488 nm and 509 nm, respectively. Data are expressed as percent changes over control determined as the means of three independent experiments. Veh, DMSO vehicle; Lut, Luteolin.
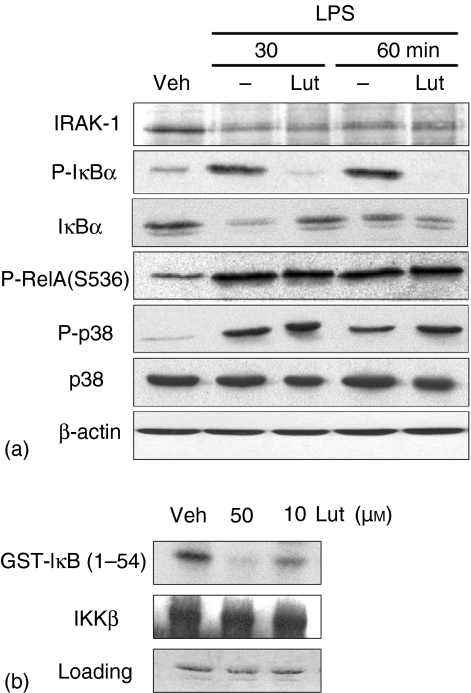
We next investigated the effect of luteolin on LPS-induced NF-κB signalling in BMDCs. IRAK-1 is a signalling molecule involved in the IL-1R/TLR pathway that is degraded following LPS and IL-1 stimulation.38 To investigate the effect of luteolin on LPS-induced IRAK-1 degradation, BMDCs were pretreated (1 hr) with luteolin (50 µm), stimulated with LPS (1 µg/ml) for various time points and IRAK-1 protein levels were determined by Western blot analysis. As seen in Fig. 6(a), IRAK-1 protein levels decreased in LPS-stimulated BMDCs and this process was not affected by luteolin (1st panel). However, luteolin significantly blocked LPS-induced IκBα phosphorylation/degradation in BMDCs (Fig. 6a, 2nd and 3rd panels), without impairing the phosphorylation of RelA, p38 (Fig. 6a, 4th and 5th panels) and JNK (data not shown). Thus, similarly to IEC-18, luteolin blocks LPS-induced NF-κB signalling through decreased IκB phosphorylation and degradation. Consistent with the findings presented in Fig. 2(a), luteolin did not significantly impair LPS-induced p38 and RelA phosphorylation in BMDCs. Because luteolin directly blocked LPS-induced IKK activity in IEC-18, we tested whether this flavonoid exerts a similar effect in BMDCs. Interestingly, luteolin treatment of IKK immunoprecipitates dose-dependently inhibited kinase activity in BMDCs (Fig. 6b). This indicates that luteolin blocks NF-κB activity by interfering with IKK activity in both IEC-18 cells and BMDCs.
Figure 6.
Luteolin blocks LPS-induced IκBα phosphorylation/degradation and IκB kinase activity directly. (a) BMDCs isolated from cis-NF-κBEGFP mice were pretreated with luteolin (50 µm) for 1 hr, and then stimulated with LPS (1 µg/ml) for various times (0–60 min). Total protein was extracted, and 20 µg of protein was subjected to SDS—PAGE followed by IRAK-1, phospho-IκBα, IκBα, phospho-RelA, phospho-p38, p38, and β-actin immunoblotting using the ECL technique. Results are representative of three independent experiments. (b) BMDCs isolated from cis-NF-κBEGFP mice were stimulated with LPS (1 µg/ml) for 30 min. Whole cell extracts were immunoprecipitated with an anti-IKKγ/protein-A beads, and kinase reactions performed as described in the legend to Fig. 4. These results are representative of two independent experiments. Veh, DMSO vehicle; Lut, Luteolin.
Luteolin blocks LPS-induced IL-12 p40 and TNF-α in BMDCs isolated from cis-NF-κBEGFP and IL-10−/− mice
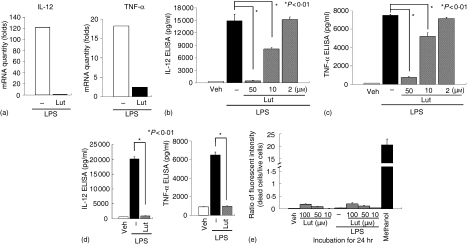
We next verified the inhibitory effect of luteolin on LPS-induced proinflammatory gene expression in BMDCs isolated from cis-NF-κBEGFP mice. BMDCs were pretreated with luteolin (50 µm) for 1 hr, stimulated with LPS (1 µg/ml) and then IL-12 p40 and TNF-α gene expression measured by real-time semiquantitative RT—PCR and ELISA. As expected, luteolin strongly inhibited LPS-induced IL-12 p40 and TNF-α mRNA expression in BMDCs (Fig. 7a) Accordingly, LPS-induced IL-12 p40 and TNF-α secretion was dose-dependently inhibited in luteolin-treated BMDCs (Fig. 7b, c).
Figure 7.
Luteolin blocks LPS-induced IL-12 p40 and TNF-α in BMDCs isolated from cis-NF-κBEGFP and IL-10−/− mice. (a) BMDCs isolated from cis-NF-κBEGFP were pretreated with luteolin (50 µm) for 1 hr, and then stimulated with LPS (1 µg/ml) for 5 hr. Total RNA was extracted, reverse-transcribed, and amplified using an ABI Prism sequence detection system and specific IL-12 p40 and TNF-α primers as described in Materials and methods. (b—d) BMDCs isolated from cis-NF-κBEGFP (b, c) or from IL-10−/− (d) mice were pretreated with various concentrations of luteolin for 1 hr, and then stimulated with LPS (1 µg/ml) for 24 hr. ELISAs for mouse IL-12 p40 and TNF-α were performed using culture supernatants from luteolin-treated BMDCs. (e) BMDCs isolated from cis-NF-κBEGFP were pretreated with various concentrations of luteolin for 1 hr, and then stimulated with LPS (1 µg/ml) for 24 hr. Simultaneous two-colour fluorescence determination of live and dead cells was carried out with two probes that measured two recognized parameters of cell viability: calcein AM for intracellular esterase activity and ethidium homodimers for plasma membrane integrity. Cells were incubated with methanol (100%) for 30 min to generate a positive control for cell death. Fluorescence in cell samples was measured with appropriate excitation and emission filters using a spectrofluorometer. The data shown are mean and SEM of at least three independent experiments.
Interleukin-10 gene deficient (IL-10−/−) mice develop a spontaneous T helper 1-mediated colitis when housed under specific pathogen-free (SPF) conditions,39 which is in large part caused by the dysregulated production of IL-12 by antigen-presenting cells.40 Moreover, LPS-induced IL-12 p40 secretion is stronger in BMDCs isolated from IL-10−/− mice than wild type mice.41 Thus, we next tested the effect of luteolin in cells displaying dysregulated innate responses to LPS. Interestingly, luteolin (50 µm) blocked LPS-induced IL-12 p40 and TNF-α protein secretion in BMDCs isolated from IL-10−/− mice (Fig. 7d). To investigate whether inhibition of LPS-induced proinflammatory gene expression by luteolin is caused by cytotoxic effects, we measured luteolin-induced cytotoxicity in LPS-stimulated BMDCs. Luteolin-treated BMDCs (Fig. 7e) and IEC-18 cells exhibited minimal cytotoxicity (data not shown) as compared to methanol-treated control, suggesting that the flavonoid is not significantly altering cell viability.
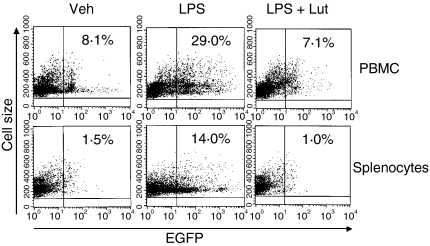
Luteolin blocked LPS-induced NF-κB activation in peripheral blood mononuclear cells and splenocytes in vivo
To test the physiological relevance of luteolin-mediated inhibition of NF-κB activity in vivo, we used cis-NF-κBEGFP transgenic mice. Mice were injected intraperitoneally (i.p) with DMSO vehicle or luteolin (0·2 mg/kg), and 2 hr later, injected i.p. with LPS (40 mg/kg) for 18 hr. PBMCs and splenocytes were isolated and EGFP expression quantified by FACS analysis. EGFP expression levels were strongly induced in both PBMC (29% versus 8·1%) and splenocytes (14% versus 1·5%) in LPS-injected cis-NF-κBEGFP transgenic mice (Fig. 8). Interestingly, luteolin totally blocked LPS-induced EGFP expression in both PBMC and splenocytes. These findings indicate that luteolin blocked LPS-induced NF-κB signalling both in vitro and in vivo. This effect appears to be mediated through direct inhibition of IKK activity.
Figure 8.
Luteolin blocked LPS-induced NF-κB activation in peripheral blood mononuclear cells (PBMCs) and splenocytes in vivo. Cis-NF-κBEGFP mice were pretreated intraperitoneally (i.p) with DMSO vehicle or luteolin (0·2 mg/kg, 100 µl) for 2 hr prior to i.p. challenge with LPS (40 mg/kg, 100 µl). Mice were killed 18 hr after LPS administration. Isolation of PBMCs and splenocytes was performed as described under Materials and methods. Cells were pelleted, resuspended in FACS buffer, and EGFP expression was measured on a FACscan using the FL1 channel to detect EGFP fluorescence. The numbers represent the percentage of EGFP positive cells. These results are representative of two independent experiments (n = 4).
Discussion
In this study, we investigated the impact of the flavonoid luteolin on LPS signalling and elucidated the mechanism of action using two different cell systems, IECs and BMDCs. Both cell types are important players in the regulation of intestinal homeostasis by virtue of their abilities to produce proinflammatory mediators, activate lamina propria mononuclear cells recruit peripheral blood immune cells and present antigens.8,42 In this study, luteolin strongly inhibited LPS-mediated NF-κB activation in both IEC-18 and BMDCs. We found that luteolin blocked LPS-induced IκBα phosphorylation/degradation and RelA nuclear translocation. Interestingly, LPS-induced RelA, JNK and p38 phosphorylation were not inhibited in luteolin-treated cells. Also IRAK-1, a signalling molecule involved in TLR-4 pathway, is degraded in LPS-stimulated BMDCs and that this process was not affected by luteolin, which was confirmed by in vitro kinase assay. These findings suggest then that luteolin is not a general inhibitor of protein phosphorylation and also indicate that this flavonoid acts down-stream of IRAK-1 but upstream of IκB phosphorylation. Cytokine and bacterial product signalling converge on the IKK complex to trigger IκB phosphorylation and ultimately NF-κB activity in numerous cell systems. We recently found that NIK strongly induced IKK activity and NF-κB transcriptional activity in IECs.31 Using adenoviral gene delivery, we found that luteolin blocked NIK-induced NF-κB transcriptional activity in IEC-18 cells, suggesting that this flavonoid mediates its suppressive effects downstream of NIK. Because luteolin strongly blocked LPS-induced IKK activity in vivo and in vitro in both IEC-18 and BMDCs, this suggests that this flavonoid mainly mediates its effects through this kinase complex. This is in line with the inhibitory effect of luteolin on IL-1β, TNF-α and LPS-induced ICAM-1 gene expression, which all utilize IKK as a common signal transductor to induce NF-κB activity.
Cytokine-induced NF-κB activity has been shown to require the production of radical oxygen species in some cell systems.43,44 Flavonoids possess antioxidant properties which may in part explain their anti-inflammatory action. However, the involvement of radical oxygen species in signal-induced NF-κB activation is controversial and the subject of intense debate.45–48 For example, a recent report found that endogenously produced reactive oxygen species failed to activate NF-κB and that the antioxidant N-acetyl-l-cysteine and pyrrolidine dithiocarbamate inhibit NF-κB activation independently of their antioxidative properties.49 Thus, it appears unlikely that luteolin-mediated NF-κB inhibition proceeds through a REDOX-sensitive mechanism.
Our data clearly show that luteolin directly interferes with activation of IKK activity. This is consistent with a recent finding showing inhibition of TNF-α-induced IKK activity by luteolin in respiratory epithelial cells.50 The IKK complex is controlled by the structural regulatory protein IKKγ, also known as NF-κB essential modifier that directs the activation of the catalytic IKKα and IKKβ subunits, which then phosphorylates IκBα at serine residue 32 and 36. The IKKα and IKKβ subunits each contain a protein kinase domain, leucine zipper motifs and helix-loop-helix motifs whereas IKKγ has three α-helical regions capable of forming a coiled-coil which may help in the recruitment of upstream IKK activators.51 The kinase activity of IKKβ, the predominant subunit involved in signal-induced IκB phosphorylation, has been shown to be dependent on phosphorylation of serine residues 177 and 181 present in the activation loop of the kinase domain.52 In addition, interaction between the regulatory IKKγ and the catalytic IKKβ subunit appears to be mediated by a short length of amino acids (44–86).53 Although luteolin directly blocks IKK activity, it is not clear whether this effect is mediated through decreased IKK phosphorylation and/or interference with the recruitment of up-stream coactivators. Alternatively, luteolin may affect critical residues present within the activation loop of the kinase or may interfere with the ATP binding sites. Further studies would be necessary to precisely identify the molecular mechanism of luteolin-mediated inhibition.
Recent evidence indicates that phosphorylation of serines 276, 529 and 536 on RelA increase NF-κB transcriptional activity.34,35,54–56 Interestingly, luteolin failed to block LPS-induced RelA S536 phosphorylation in IEC and BMDCs. This suggests that luteolin mainly modulates NF-κB activity through RelA shuttling and likely not by interfering with the transactivating ability of the subunit. In addition, the lack of inhibitory effect on IRAK-1 auto-phosphorylation and on p38 and JNK phosphorylation indicates that luteolin is not a pan-kinase inhibitor but rather exerts some level of specificity. IKK has been shown to induce RelA phosphorylation at serine residue 536.55,57 Thus, the blockade of LPS-induced IKK activity and IκBα phosphorylation mediated by luteolin, in the absence of impaired RelA S536 phosphorylation is a surprising finding. However, the IKK/RelA S536 phosphorylation axis is mostly observed in TNF-α treated cells.55,57 Although a recent paper showed a role for IKKβ in LPS-induced RelA S536 phosphorylation in mouse embryonic fibroblasts,58 it is possible that LPS-induced IKK activity and RelA phosphorylation are two uncoupled events and that luteolin selectively interferes with the former in IECs and BMDCs. Further investigation will be necessary to identify the kinase responsible for RelA S536 phosphorylation in these cells.
Dysregulated innate responses to the endogenous microflora are a hallmark of intestinal inflammation such as that observed in IBD and blockade of innate signal transduction may help restore host homeostasis and alleviate inflammation.9,59 Using BMDCs isolated from IL-10−/− mice, which develop a spontaneous IBD-like colitis, we show that luteolin strongly inhibits LPS-induced IL-12 and TNF-α protein secretion, two key mediators of intestinal inflammation in IBD. This finding clearly indicates the potent down-modulating effects of this flavonoid on proinflammatory gene expression in disease relevant cells. Because luteolin evokes minimal toxicity (< 11%) it is unlikely that blockade of LPS-induced gene expression is caused by global decrease in protein synthesis and/or increase in cell death.
To investigate the physiological impact of luteolin on NF-κB activity in vivo, we used cis-NF-κBEGFP transgenic mice recently engineered in our laboratory. This approach allows the dynamic assessment of NF-κB activity through measurement of EGFP expression levels. Using this approach, we demonstrate for the first time that luteolin blocks LPS-induced NF-κB activation in PBMCs and splenocytes in vivo. Considering that LPS-mediated multiple organ dysfunction syndrome and septic shock have a high mortality of up to 60% despite antibiotic therapy and intensive care support,60 luteolin or other derivatives may be possible candidate for the development of an antisepsis agent. Further investigations are needed to clarify the long-term effect of luteolin on immune cells and vital end organs of endotoxaemic mice. We are currently investigating the effect of luteolin on experimental models of intestinal inflammation. In addition, this flavonoid may have a beneficial impact on the prevention of cancers and or as an adjuvant to chemotherapy in the treatment of cancers. Future studies will examine the ability of luteolin to sensitive transformed cell lines and tumours to chemotherapeutic agents. We are currently testing the effect of various plant extracts and pure compounds on an animal model of colon cancer.
In conclusion, we demonstrate that luteolin blocks LPS-induced NF-κB signalling and proinflammatory gene expression in intestinal epithelial cells and dendritic cells through the inhibition of IKK activity. Modulation of innate immunity by compounds isolated from natural plant extracts may represent an attractive strategy towards the prevention and treatment of intestinal and systemic inflammation associated with dysregulated innate immune responses.
Acknowledgments
The authors wish to thank Dr Acharan Narola for suggesting the study on luteolin, Brigitte Allard for technical expertise, and Charlotte Walters of the ImmunoTechnology Core of the Center for Gastrointestinal Biology and Disease, University of North Carolina at Chapel Hill, for cytokine measurements. We also thank Dr Humberto Jijon for critical reading of the manuscript. This work was supported by NIH ROI grants DK 47700, the Broad Medical Research Program of The Eli and Edythe L. Broad Foundation to C. Jobin and by NIH P30 DK34987 for the CGIBD.
Abbreviations
- LPS
lipopolysaccharide
- IEC
intestinal epithelial cell
- DC
dendritic cell
- BMDC
bone-marrow derived dendritic cell
- IBD
inflammatory bowel diseases
- TLR
Toll-like receptor
- NF-κB
nuclear transcription factor-κB
- IRAK
interleukin-1 receptor-associated kinase
- TRAF
TNF receptor-associated factor
- TAK
transforming growth factor-β-activated kinase
- IKK
IκB kinase
- EGFP
enhanced green fluorescent protein
- DMSO
dimethyl sulphoxide
- TNF
tumour necrosis factor
- IL-1β
interleukin-1β
- GM-CSF
granulocyte—macrophage colony-stimulating factor
- RT—PCR
reverse transcription—polymerase chain reaction
- ICAM-1
intercellular adhesion molecule-1
- Ad
adenoviral
- dn
dominant negative
- wt
wild type
- NIK
NF-κB-inducing kinase
- NGS
non-immune goat serum
- ChIP
chromatin immunoprecipitation
- PBMC
peripheral blood mononuclear cells
- RBC
red blood cell
- FACS
fluorescence-activated cell sorter
- GST
glutathione S-transferase
- SEM
standard error of mean
References
- 1.Jobin C, Sartor RB. The IκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol. 2000;278:C451–62. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 2.Haller D, Jobin C. Interaction between resident luminal bacteria and the host: Can a healthy relationship turn sour? J Pediatr Gastroenterol Nutr. 2004;38:123–36. doi: 10.1097/00005176-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kraehenbuhl JP, Corbett M. Immunology. Keeping the gut microflora at bay. Science. 2004;303:1624–5. doi: 10.1126/science.1096222. 10.1126/science.1096222. [DOI] [PubMed] [Google Scholar]
- 4.Neish AS. The gut microflora and intestinal epithelial cells: a continuing dialogue. Microbes Infect. 2002;4:309–17. doi: 10.1016/s1286-4579(02)01543-5. 10.1016/S1286-4579(02)01543-5. [DOI] [PubMed] [Google Scholar]
- 5.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–9. doi: 10.1007/s002689900401. 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 6.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 7.Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589–94. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut. 2003;52:1522–9. doi: 10.1136/gut.52.10.1522. 10.1136/gut.52.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 10.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–72. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 11.Haller D, Russo MP, Sartor RB, Jobin C. IKKβ and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-κB activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–78. doi: 10.1074/jbc.M205737200. 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B, Du Hoebe KX, Ulevitch RJ. How we detect microbes and respond to them. the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74:479–85. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 14.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–8. doi: 10.1074/jbc.R300028200. 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 15.Karin M. The beginning of the end. IκB kinase (IKK) and NF-κB activation. J Biol Chem. 1999;274:27339–42. doi: 10.1074/jbc.274.39.27339. 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 17.Jobin C, Sartor RB. NF-κB signaling proteins as therapeutic targets for inflammatory bowel diseases. Inflamm Bowel Dis. 2000;6:206–13. doi: 10.1097/00054725-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004;116:478–85. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Woolf AD. Herbal remedies and children: do they work? Are they harmful? Pediatrics. 2003;112:240–6. [PubMed] [Google Scholar]
- 20.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–56. doi: 10.1056/NEJMra020398. 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 21.Gohil K, Packer L. Bioflavonoid-rich botanical extracts show antioxidant and gene regulatory activity. Ann N Y Acad Sci. 2002;957:70–7. doi: 10.1111/j.1749-6632.2002.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 22.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–77. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 23.Ross JA, Kasum CM. Dietary flavonoids. bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 24.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor IκB kinase activity. J Immunol. 1999;163:3474–83. [PubMed] [Google Scholar]
- 25.Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–22. doi: 10.1053/gast.2002.37050. 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- 26.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181–7. [PubMed] [Google Scholar]
- 27.Kim SH, Shin KJ, Kim D, et al. Luteolin inhibits the nuclear factor-κB transcriptional activity in Rat-1 fibroblasts. Biochem Pharmacol. 2003;66:955–63. doi: 10.1016/s0006-2952(03)00465-9. 10.1016/S0006-2952(03)00465-9. [DOI] [PubMed] [Google Scholar]
- 28.Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fotsis T, Papapetropoulos A, Roussos C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am J Respir Crit Care Med. 2002;165:818–23. doi: 10.1164/ajrccm.165.6.2101049. [DOI] [PubMed] [Google Scholar]
- 29.Jobin C, Haskill S, Mayer L, Panja A, Sartor RB. Evidence for an altered regulation of IκBα degradation in human colonic epithelial cells. J Immunol. 1997;158:226–34. [PubMed] [Google Scholar]
- 30.Magness ST, Jijon H, Van Houten N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD-3-induced NF-κB activation using a novel gene targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–70. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 31.Russo MP, Schwabe RF, Sartor RB, Jobin C. NF-κB-inducing kinase restores defective IκB kinase activity and NF-κB signaling in intestinal epithelial cells. Cell Signal. 2004;16:741–50. doi: 10.1016/j.cellsig.2003.11.007. 10.1016/j.cellsig.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Haller D, Holt L, Kim SC, Schwabe RF, Sartor RB, Jobin C. Transforming growth factor-beta 1 inhibits non-pathogenic Gram negative bacteria-induced NF-κB recruitment to the interleukin-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J Biol Chem. 2003;278:23851–60. doi: 10.1074/jbc.M300075200. 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
- 33.Jobin C, Hellerbrand C, Licato LL, Brenner DA, Sartor RB. NF-κB mediates cytokine-induced expression of ICAM-1 in IEC-6 cells, a process blocked by proteasome inhibitors. Gut. 1998;42:779–87. doi: 10.1136/gut.42.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Baldwin AS., Jr Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-α is mediated through phosphorylation of relA/p65 on serine 529. J Biol Chem. 1998;273:29411–6. doi: 10.1074/jbc.273.45.29411. 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 35.Mayo MW, Madrid LV, Westerheide SD, Jones DR, Yuan XJ, Baldwin AS, Jr, Whang YE. PTEN blocks tumor necrosis factor-induced NF-κB-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J Biol Chem. 2002;277:11116–25. doi: 10.1074/jbc.M108670200. 10.1074/jbc.M108670200. [DOI] [PubMed] [Google Scholar]
- 36.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–90. doi: 10.1074/jbc.273.6.3285. 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 37.Rahman A, Anwar KN, Uddin S, Xu NYeRD, Platanias LC, Malik AB. Protein kinase C-δ regulates thrombin-induced ICAM-1 gene expression in endothelial cells via activation of p38 mitogen-activated protein kinase. Mol Cell Biol. 2001;21:5554–65. doi: 10.1128/MCB.21.16.5554-5565.2001. 10.1128/MCB.21.16.5554-5565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamin T-T, Miller DK. The interleukin-1 receptor-associated kinase is degraded by the proteasomes following its phosphorylation. J Biol Chem. 1997;272:21540–7. doi: 10.1074/jbc.272.34.21540. 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- 39.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology. 2002;122:94–105. doi: 10.1053/gast.2002.30308. [DOI] [PubMed] [Google Scholar]
- 41.Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-κB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105::689–96. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- 42.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kono H, Rusyn I, Yin M, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.True AL, Rahman A, Malik AB. Activation of NF-κB induced by H2O2 and TNF-α and its effects on ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L302–11. doi: 10.1152/ajplung.2000.279.2.L302. [DOI] [PubMed] [Google Scholar]
- 45.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li N, Karin M. Is NF-κB the sensor of oxidative stress? FASEB J. 1999;13:1137–43. [PubMed] [Google Scholar]
- 47.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg R, Haenen GR, van den Berg H, Bast A. Transcription factor NF-κB as a potential biomarker for oxidative stress. Br J Nutr. 2001;86(Suppl. 1):121–7. doi: 10.1079/bjn2001340. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that reactive oxygen species do not mediate NF-κB activation. EMBO J. 2003;22:3356–66. doi: 10.1093/emboj/cdg332. 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-α-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: structure—activity relationships. Mol Pharmacol. 2004;66:683–93. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 51.Burke JR. Targeting IκB kinase for the treatment of inflammatory and other disorders. Curr Opin Drug Discov Devel. 2003;6:720–8. [PubMed] [Google Scholar]
- 52.Carter RS, Geyer BC, Xie M, Acevedo-Suarez CA, Ballard DW. Persistent activation of NF-κB by the tax transforming protein involves chronic phosphorylation of IκB kinase subunits IKKβ and IKKγ. J Biol Chem. 2001;276:24445–8. doi: 10.1074/jbc.C000777200. [DOI] [PubMed] [Google Scholar]
- 53.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science. 2000;289:1550–4. doi: 10.1126/science.289.5484.1550. 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 54.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–71. doi: 10.1016/s1097-2765(00)80066-0. 10.1016/S1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 55.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–6. doi: 10.1074/jbc.274.43.30353. 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 56.Prajapati S, Gaynor RB. Regulation of IκB kinase (IKK) γ/NEMO function by IKKβ-mediated phosphorylation. J Biol Chem. 2002;277:24331–9. doi: 10.1074/jbc.M201393200. 10.1074/jbc.M201393200. [DOI] [PubMed] [Google Scholar]
- 57.Sakurai H, Suzuki S, Kawasaki N, et al. Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–23. doi: 10.1074/jbc.M301598200. 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 58.Yang F, Tang E, Guan K, Wang CY. IKKβ plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 59.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 60.Balk RA. Pathogenesis and management of multiple organ dysfunction or failure in severe sepsis and septic shock. Crit Care Clin. 2000;16:337–52. doi: 10.1016/s0749-0704(05)70113-5. [DOI] [PubMed] [Google Scholar]