Abstract
LacZ (Gal)-reactive immune cells were transferred into athymic nu/nu mice inoculated with Gal-expressing syngeneic tumour cells (ESbL-Gal) in order to study tumour-protective T-cell memory. This transfer prevented tumour outgrowth in recipients and resulted in the persistence of a high frequency of Gal-specific CD8+ T cells in the bone marrow and spleen. In contrast, such Ag-specific memory CD8+ T cells were not detectable by peptide–major histocompatibility complex (MHC) multimer staining in animals that had not previously received an antigenic challenge. Even though CD44hi memory T cells from the bone marrow showed a significantly higher turnover rate, as judged by bromodeoxyuridine (BrdU) incorporation, than respective cells from spleen or lymph nodes, as well as in comparison to CD44lo naïve T cells, these findings suggest that tumour-associated antigen (TAA) from residual dormant tumour cells are implicated in maintaining high frequencies of long-term surviving Gal-specific memory CD8+ T cells. Memory T cells could be recruited to the peritoneal cavity by tumour vaccination of immunoprotected nu/nu mice and exhibited ex vivo antitumour reactivity. Long-term immune memory and tumour protection could be maintained over four successive transfers between tumour-inoculated recipients, which involved periodic antigenic restimulation in vivo prior to reisolating the cells for adoptive transfer. Using a cell line (ESbL-Gal-BM) that was established from dormant tumour cells isolated from the bone marrow of immunoprotected animals, it could be demonstrated that the tumour cells had up-regulated the expression of MHC class I molecules and down-regulated the expression of several adhesion molecules during the in vivo passage. Our results suggest that the bone marrow microenvironment has special features that are of importance for the maintenance of tumour dormancy and immunological T-cell memory, and that a low level of persisting antigen favours the maintenance of Ag-specific memory T cells over irrelevant memory T cells.
Keywords: adoptive immunotherapy, bone marrow, CD8+ T-cell memory, tumour dormancy
Introduction
CD8+ T lymphocytes play a central role in the generation of a protective immune response and long-term memory. This has been described in detail for microbial infections,1,2 but much less is known about protective immune responses and long-term memory in tumour systems.1 Only a few model systems have been used for studying CD8+ T-cell memory work with cell-bound antigen (Ag), for example tumour-associated antigen (TAA). As viral Ag induces several-fold stronger responses than TAA, it is important to elucidate whether the same rules apply to long-term persistence and maintenance of CD8+ T-cell memory specific for viral or cell-bound Ag. Understanding the mechanisms by which long-term tumour-specific T-cell memory can be generated should facilitate the development of effective tumour vaccines3,4 and adoptive immune cell transfer therapies.5,6
Generally speaking, live viruses or attenuated vaccines generate larger numbers of cytotoxic T lymphocytes (CTL) than do non-replicating vectors, DNA vaccines or protein subunit vaccines. In the murine lymphocytic choriomeningitis virus (LCMV) model, 5–10% of the CD8+ T cells present during the peak immune response go on to become memory cells7–9 and the frequency of antigen-specific CTL appears to be a function of both the initial antigenic load and persistence of the viral antigen.7,9,10 Furthermore, there appear to exist qualitative differences in the types of CTL responses induced by replicating versus non-replicating vaccines.11,12 In the present study, the immunization protocol involved live replication competent tumour cells for priming13 and a replication-incompetent γ-irradiated tumour cell vaccine for boosting and attraction of TAA-specific immune cells to the peritoneal cavity.14–16 This protocol has been demonstrated to result in tumour protection and long-term survival of the animals, and to induce a primary CD8+ T-cell response3,17 as well as a tumour dormancy state in the bone marrow microenvironment.18,19 As the intraperitoneal (i.p.) boosting vaccination consists of irradiation-inactivated tumour cells, and the immune peritoneal exudate cells (iPEC) contain highly Gal-reactive CD8+ T cells, the possibility that live tumour cells are transferred with the iPEC during adoptive immunotherapy (ADI) is negligible.
Most studies make use of T-cell receptor (TCR) transgenic mice, focusing on responses to a single, well-defined antigenic epitope. We recently described a novel model system for the study of anti-tumour T-cell memory.15,16 This model simulates a physiological situation in that several T-cell clones with different specificities and ligand affinities compete for survival factors and space as a result of the natural mechanisms of T-cell homeostasis. The model consists of the adoptive transfer of tumour iPEC, including Ag-specific T cells, and antigen-presenting cells (APCs)3 to preirradiated, tumour-cell-inoculated, syngeneic T-cell-deficient athymic BALB/c nu/nu mice, resulting in the long-term survival of the animals and in the prevention of tumour growth. Such athymic nude mice provide an optimal environment for studying the fate of adoptively transferred antigen-specific T cells, as, by nature, they are T-cell deficient. Thus, T cells specific for a given antigenic epitope occur at higher frequencies than when using normal mice as recipients, because the T-cell pool of nude recipients consists exclusively of the Ag-experienced T cells transferred during ADI.
We now set out to exploit this model system in order to analyse various aspects of CD8+ T-cell memory, including the possible involvement of persistent TAA for the long-term maintenance of Ag-specific memory. The results indicate that T-cell memory is largely radio-resistant and that CD8+ memory T cells survive in the absence of the specific Ag, although TAA persistence is necessary to maintain high frequencies of TAA-specific CD8+ T cells.
Materials and methods
Animals
Female DBA/2 (H-2d) mice (4–6 weeks of age) were obtained from Iffa Credo (Lyon, France), and BALB/c nu/nu (H-2d) mice were obtained from Iffa Credo (l'Arbresle, France). All animals were housed under specific pathogen-free conditions.
Cells
P815-Gal, commonly known as P13.1, is a lacZ-transduced variant of the DBA/2 mouse-derived mastocytoma, P815.20 ESbL-Gal (clone L-CI.5s) is a lacZ-transduced variant of the highly metastatic DBA/2 mouse-derived T-lymphoma, ESb.21 ESbL-Gal-BM is a tumour-cell line derived from dormant tumour cells isolated from the bone marrow of ESbL-Gal-immunized DBA/2 mice. To create the cell line ESbL-Gal-BM, bone marrow was isolated from femur and tibia. Single-cell suspensions were washed twice in prewarmed, pure RPMI-1640 (Gibco, Karlsruhe, Germany), then resuspended in minimal nutrient medium supplemented with 10 µg/ml gentamycin. The cells were transferred to a tissue culture flask, which was placed in an upright position at 37° in 5% CO, in order to prevent outgrowth of adherent cells. After 2 days of culture, 500 µg/ml of G418 was added to select for Gal-expressing cells. After a further 2 days, the culture medium was changed to complete culture medium and the cells were allowed to expand for 2–3 weeks undisturbed. Dendritic cells (DC) were grown from the bone marrow of naïve DBA/2 mice, as described previously.22 The cell line, Ag8653,23 was used to obtain granulocyte–macrophage colony-stimulating factor (GM-CSF)-containing culture supernatant for the maturation of DC.
Media and reagents
Minimal nutrient medium consisted of RPMI-1640, 0·05 mmβ-mercaptoethanol, 10 mm HEPES, 50 IU/ml penicillin and 50 µg/ml streptomycin (all from Gibco), and was used for the primary culture of ex vivo tumour cells to generate tumour cell lines. Complete culture medium was further supplemented with 5% (v/v) fetal calf serum (FCS) (Biochrom KG, Berlin, Germany) and 2 mm l-glutamine (Gibco) and was used for the culture of all established tumour cell lines, as well as for co-cultures of immune cells with target cells for cytolytic assays. For ex vivo organ preparation and immunofluorescent labelling procedures the following buffer was prepared (subsequently referred to as staining buffer): phosphate-buffered saline (PBS) (Biochrom), 1% (v/v) FCS, and 0·01% (w/v) NaN3 (Merck, Darmstadt, Germany). Ag8653 and DC were cultured in X-vivo 20 serum-free medium (Bio-Whittaker, Verviers, Belgium). For the maturation of DC the medium was supplemented with 10% (v/v) GM-CSF-containing culture supernatant.
Peptides
The immunodominant β-galactosidase peptide, TPHPARIGL (amino acids 876–884),24 and the murine chorio-meningitis virus p89 peptide, YPHFMPTNL (amino acids 168–176),25 were obtained from the Peptide Facility of the Deutsches Krebsforschungszentrum (DKFZ; Heidelberg, Germany).
Adoptive transfer of antitumour protection
Tumour iPEC14 were obtained and adoptive transfer experiments were performed as previously described.16 Briefly, naïve DBA/2 mice were primed with 5 × 104 live ESbL-Gal at a non-tumorigenic site, the ear pinna (i.e.)26 and restimulated 7 days later with 1 × 107 100 Gy-irradiated ESbL-Gal administered i.p. Three days later, d3 iPEC were isolated by peritoneal lavage. BALB/c nu/nu mice received sublethal whole-body irradiation, at a dose of 4·5 Gy from a Cobalt 60 source (Gammatron F80S; Siemens, Braunschweig, Germany), 1 day prior to receiving an intravenous (i.v.) tumour inoculum of 1 × 105 live ESbL-Gal. One day later, 1 × 107 d3 iPEC were adoptively transferred i.v. For repeated tumour-protection transfers, immunoprotected BALB/c nu/nu mice were challenged with 1 × 107 100 Gy-irradiated ESbL-Gal i.p. 3 days before isolating memory peritoneal exudate cells (mPEC) by peritoneal lavage and transferring them, at the indicated cell number per mouse, to preconditioned BALB/c nu/nu mice (sublethally whole-body irradiated and tumour inoculated).
Phenotypization by flow cytometry
For the detection of Gal-specific CD8+ T cells, total T cells were enriched from single-cell suspensions of ex vivo-isolated immune organs using mouse Thy-1.2 MACS Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). These were then incubated for 15 min with anti-CD8αFITC (53-6.7) monoclonal antibodies (mAbs) (BD Biosciences, Heidelberg, Germany) at 4°. After washing the samples with staining buffer, cells were incubated for 30 min with phycoerythrin (PE)-labelled H2-Ld/Gal876−884 (TPHPARIGL) multimers (NIAID Tetramer Facility and NIH AIDS Research and Reference Reagent Program, Bethesda, MD) at 4°. Shortly before flow cytometric analysis, propidium iodide (PI) (Sigma, Deisenhofen, Germany) was added to each probe. The following mAbs were used for the phenotypic characterization of parental (ESbL-Gal) and dormancy-derived (ESbL-Gal-BM) tumour cell lines: anti-CD3εFITC (145-2C11); anti-L-selectinFITC (Mel 14); anti-CD45R/B220PE (RA3-6B2); anti-H2-Dd PE (34-2-12); anti-I-Ad PE (AMS-32.1); antiβ7-integrin rat IgG (Fib27) (all from BD Biosciences); donkey anti-rat IgGPE (Dianova, Hamburg, Germany); anti-H2-Ld PE (19.191) (G Hämmerling, DKFZ); anti-ICAM-1 rat IgG (YN.1/1.7); anti-LFA-1 rat IgG (Tib 213); and anti-PSGL-1 rat IgG (2PH-1) (P. Altevogt, DKFZ). In order to verify Gal expression, tumour cells were taken up in staining buffer and heated at 37° for 10 min before adding an equal volume of a preheated 2 µm fluorescein di-β-d-galactopyranoside (FDG) (Molecular Probes, Leiden, the Netherlands) solution diluted in distilled H2O. Following incubation for 1 min at 37°, the samples were diluted with ice-cold staining solution, equilibrated for 10 min on ice, washed twice and then analysed by flow cytometry using a fluorescence-activated cell sorter (FACS Scan) and cellquest software (BD Biosciences).
Cytotoxicity assay
A standard 4-hr 51Cr-release assay was performed using ex vivo-isolated d3 iPEC as effector cells and 51Cr-labelled ESbL-Gal and P815-Gal as target cells. Effectors (E) and targets (T) were co-incubated in serial dilutions prepared in triplicate, with a maximum E:T ratio of 50 : 1. Cell-free supernatants were evaluated by using an Automatic Gamma Counter (Wallac, Turku, Finland), and percentage specific lysis was calculated using the following formula:
 |
From this, the relative specific lysis was calculated, where 1·0 equals the maximum lytic activity measured.
Ag loading of DC, and the IFN-γ ELISPOT assay
Approximately 1 × 106 DCs per ml of X-vivo 20 medium were seeded out in a six-well plate and cultured overnight in the presence of 0·2 µg/ml of peptide. Cells were washed twice in fresh medium and used as Ag-presenting cells in an interferon-γ (IFN-γ) enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). The murine IFN-γ ELISPOT Kit (Diaclone, Besançon Cedex, France) was used according to the manufacturer's instructions. Briefly, 5 × 104 T cells were co-incubated with peptide-pulsed or mock-pulsed DCs, at a ratio of 5 : 1, in a 96-well plate bottomed with 0·45-µm cellulose ester membranes (Millipore, Eschborn, Germany) that had been precoated with IFN-γ capture antibody. Twenty-four hour co-cultures were performed at 37°, in an atmosphere of 5% CO2, in 200 µl per well of X-vivo 20 medium. After washing off the cells, secreted IFN-γ was revealed with a biotinylated detection antibody, alkaline phosphatase-conjugated streptavidin and an alkaline phosphatase conjugate substrate kit (Biorad, Hercules, CA). The results were evaluated using an Axioplan 2 imaging microscope (Carl Zeiss, Göttingen, Germany) in combination with a 3 CCD video camera (Sony Deutschland GmbH, Köln-Ossendorf, Germany) and the KS ELISPOT software (Carl Zeiss).
BrdU-uptake assay
Twenty-four hours after the addition of bromodeoxyuridine (BrdU) (0·8 mg/ml) to the drinking water, female DBA/2 mice (4–6 months old) were killed and their lymph nodes, spleen and bone marrow isolated. Single-cell suspensions of these tissues were prepared for BrdU detection. Cells were first stained against CD3ε (clone 145-2C11; BD Biosciences) and CD44 (clone IM7; BD Biosciences). The detection of BrdU (BrdU Flow Kit; BD Biosciences) was performed according to the manufacturer's protocol and cellular staining was detected using a FACS Scan with cellquest software. Data were analysed using flowjo software (Tree Star, San Carlos, CA).
Results
Long-term maintenance of Gal-specific reactivity and repetitive adoptive transfer of protective anti-tumour immunity
In order to determine whether donor T cells retain their in vivo antitumour reactivity even after a long period within the animals, BALB/c nu/nu mice, successfully treated with ADI (hereafter referred to as memory nude mice), were restimulated i.p. 1 month after ADI-transfer. The terms iPEC and mPEC were chosen to describe PEC isolated from mice whose first antigen exposure through prime/boost vaccination was only 1 week or was longer than 1 month, respectively, prior to i.p. challenge and PEC isolation. iPEC and mPEC thus differ in the resting period in which the Ag-specific T cells underwent following the first round of stimulation in DBA/2 mice before being restimulated in the peritoneal cavity for enrichment and isolation for ADI and/or analysis. mPEC were isolated 3 days after i.p. challenge and transferred to secondary ESbL-Gal tumour-inoculated nude mice (see Fig. 1 for a schematic representation of the memory transfer protocol). As summarized in Table 1, Gal-specific memory T cells retained their reactivity and protective capacities over a long period of time (test duration 8 months), transferring antitumour immune protection to four subsequent groups of hosts, while being repeatedly stimulated to proliferate and expand. Even half the dose of mPEC (5 × 106) that was normally administered for ADI, efficiently conferred antitumour protection. Indeed, ADI with mPEC isolated from the tertiary host, and iPEC from the original immune cell donor, DBA/2, resulted in a comparable survival of tumour-bearing BALB/c nu/nu mice (P = 0·64; data not shown).
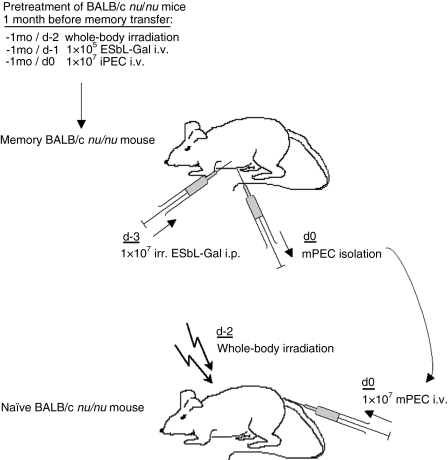
Figure 1.
Experimental scheme for recruitment, activation and transfer of memory T cells. To generate memory BALB/c nu/nu mice, naive DBA/2 mice were injected with a subtumorigenic dose of 5 × 104 ESbL-Gal at a non-tumorigenic site, the ear pinna (i.e.) and, 7 days later, were challenged intraperitoneally (i.p.) with 1 × 107 irradiation-inactivated (100 Gy) ESbL-Gal. Three days after inoculation with the second dose of tumour cells, immune peritoneal exudate cells (iPEC) were harvested. A total of 1 × 107 iPEC were transferred intravenously (i.v.) to BALB/c nu/nu mice that had been whole-body irradiated (4·5 Gy) 2 days earlier, and inoculated with 1 × 105 live ESbL-Gal tumour cells i.v. 1 day earlier. One to two months later, Gal-specific cells were recruited to the peritoneal cavity of memory BALB/c nu/nu mice by administering an i.p. challenge of 1 × 107 irradiation-inactivated (100 Gy) ESbL-Gal. After 3 days, memory peritoneal exudate cells (mPEC) were isolated and 1 × 107 mPEC were transferred to preirradiated (4·5 Gy), tumour-inoculated (memory transfer) or antigen-naive BALB/c nu/nu (‘parking experiment’). Illustrated is the transfer of mPEC from memory BALB/c nu/nu to antigen-naive BALB/c nu/nu mice.
Table 1.
Multiple transfers of memory cells
| Experimental group | ADI performed with | nu/nu host | Duration of PEC within host | Deaths: day and frequency* | Untreated control: death day and frequency* | ||
|---|---|---|---|---|---|---|---|
| I | 1 × 107 DBA/2 iPEC | 1° | 33 days | – | 0/21 | d9 | 5/5 |
| II | 1 × 107 group I mPEC | 2° | 43 days | – | 0/19 | d8 | 2/2 |
| III | 5 × 106 group II mPEC | 3° | 41 days | d40 | 1/10 | d9 | 2/2 |
| IV | 5 × 106 group III mPEC | 4° | 145 days | d39 | 1/3 | d10 | 2/2 |
| d78 | 1/3 | ||||||
| d145 | 1/3 | ||||||
All animals received 4·5 Gy whole-body irradiation before being inoculated intravenously with 1 × 105 ESbL-Gal. Group I animals were treated with 1 × 107 day 3 (d3) immune peritoneal exudate cells (iPEC) obtained from ESbL-Gal-immunized DBA/2 mice. Memory peritoneal exudate cells (mPEC) were obtained from adoptive immunotherapy (ADI)-treated BALB/c nu/nu mice by injecting 1 × 107 irradiated (100 Gy) ESbL-Gal cells intraperitoneally 3 days before isolation of mPEC. These were used to confer tumour protection to ESbL-Gal-inoculated BALB/c nu/nu mice (group II) following the standard ADI protocol. This procedure was repeated three times, leaving the immune cells within each host for a minimum of 1 month (time spans indicated) before reisolation and transfer to the next host.
Total duration of the experiment: 8 months.
Frequency indicates the number of dead animals in relation to the total number of animals per group.
Gal-specific IFN-γ production by mPEC in vitro
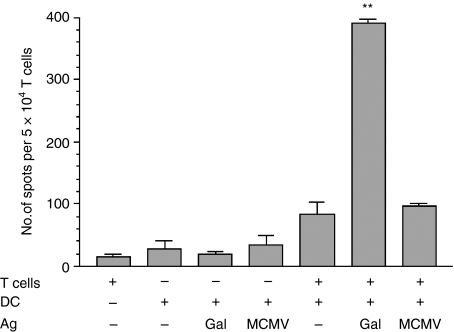
The capacity of mPEC to secrete IFN-γ upon in vitro stimulation with the immunodominant Gal-peptide was tested in an ELISPOT. To achieve this, T cells were enriched from DBA/2-derived mPEC (1·5 months after a prime/boost vaccination) and co-cultured with peptide-pulsed DCs that had been generated from the bone marrow.11 The original protocol for maturation of DCs was only modified in that the cells were cultured in serum-free X-vivo 20 medium in order to prevent eventual presentation of serum proteins, thus decreasing the background noise. Although the cells had been restimulated in vivo 3 days before isolation, this was not expected to cause a high background spot formation as the cytokine-secretion kinetics of ex vivo-isolated PEC indicated a low production of IFN-γ at this time-point.3 Indeed, background levels proved to be very low. Co-culture with Gal-peptide-pulsed DCs resulted in a 26·6-fold increase in the number of IFN-γ-producing T cells, giving a frequency of 1 : 128. The difference in the frequency of responding cells after a specific (Gal-peptide) or a nonspecific (MCMV-peptide) stimulus was highly significant (P = 0·0004) (Fig. 2).
Figure 2.
In vitro Gal-specific interferon-γ (IFN-γ) production by T cells isolated from memory peritoneal exudate cells (mPEC). mPEC were isolated from memory DBA/2 mice that had been primed at a non-tumorigenic site, the ear pinna (i.e.), and challenged (intraperitoneally) with ESbL-Gal 1·5 months earlier, and T cells were enriched by using Thy-1.2 magnetic antibody cell sorting (MACS) beads. The T cells were then co-cultured with antigen-pulsed dendritic cells (DC) for 24 hr, and IFN-γ-producing cells were enumerated in an enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). All samples were measured in triplicate. **Significantly higher (P < 0·005) than the control groups; Gal, β-galactosidase peptide 876–884; MCMV, MCMV pp89 peptide 168–176.
Anti-ESbL-Gal long-term protection
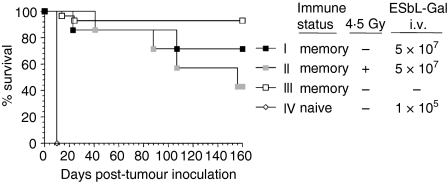
Memory nude mice received a high-dose i.v. tumour challenge of live ESbL-Gal tumour cells more than 2 months after their first exposure. A total of 66% of the animals were able to reject a tumour dose of 5 × 107 cells (Fig. 3, group I) – a 500-fold excess compared to the primary challenge which they had received – and survived for longer than 8 months. Naïve nude mice (group IV) died within 10 days following injection of 1 × 105 tumour cells. Although untreated memory animals (group III) had a better survival than tumour-challenged mice, this difference was not significant (P = 0·0999). The cells involved in long-term immune protection are memory cells by definition, as tumour challenge occurred more than 2 months after the initial effector phase had subsided.
Figure 3.
Anti-ESbL-Gal-specific memory T cells are long-lived and radio-resistant. Two months post-adoptive immunotherapy (ADI), BALB/c nu/nu mice (see Table 1, group I) received a tumour challenge of 5 × 107 ESbL-Gal intravenously (i.v.), either without pretreatment (group I, n = 6) or following whole-body γ-irradiation of 4·5 Gy (group II, n = 6). Positive control animals remained without tumour challenge (group III, n = 10), while naïve BALB/c nu/nu inoculated with 1 × 105 ESbL-Gal i.v. served as negative controls (group IV, n = 5).
Radio-resistance of long-term immunological memory
Memory nude mice that were whole-body irradiated 1 day before receiving the high-dose tumour challenge of 5 × 107 cells (Fig. 3, group II) had a survival similar to that of non-irradiated recipients (group I) during the first 100 days. Thereafter, preirradiated animals exhibited a slightly higher death rate, but the difference from non-irradiated animals was not significant (P = 0·34), suggesting that long-term T-cell memory is largely radio-resistant.
Localization of Gal-specific memory CD8+ T cells
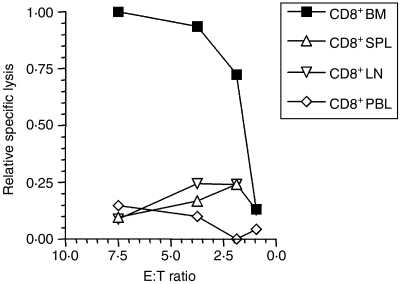
In order to determine in which lymphoid organ the Gal-reactive memory CTL predominantly reside, CD8+ T cells were isolated from bone marrow, spleen, lymph nodes and peripheral blood of memory nude mice 3 months after ADI treatment, and tested in a 4-hr 51Cr-release assay against P815-Gal (Fig. 4). Ex vivo Gal-specific CD8+ T-cell reactivity was only detectable in cells isolated from the bone marrow.
Figure 4.
Localization of Gal-specific memory CD8+ T cells. ESbL-Gal-inoculated BALB/c nu/nu mice received adoptive immunotherapy (ADI), as described. Three months later, CD8+ cells were isolated from bone marrow (BM), spleen (SPL), lymph nodes (LN) and peripheral blood (PBL), and tested in a 4-hr 51Cr-release assay for Gal-specific cytotoxic activity using P815-Gal as target cells. Specific lysis of 10·4% was obtained with BM-derived CD8+ T cells at an effector-to-target ratio (E:T) of 7·5, which is notable, considering that the E:T ratio is very low and that the assay was performed directly ex vivo. Relative percentage lysis was chosen as a means to illustrate more clearly the differences between the ex vivo cytolytic capacity of CD8+ T cells from BM, SPL, LN and PBL.
Requirement of Ag for the maintenance of detectable frequencies of Gal-specific CD8+ T cells ex vivo
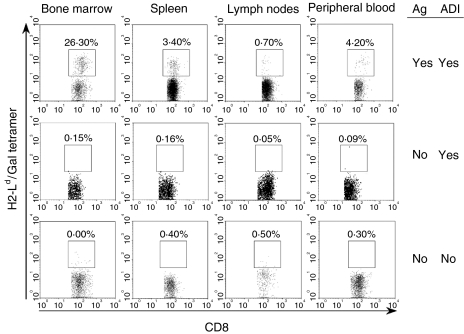
H2-Ld/Gal multimer staining of CD8+ T cells isolated from bone marrow, spleen, lymph nodes or peripheral blood of memory nude mice (6·5 months) revealed a remarkably high frequency (26·3%) of Gal876−884-specific cells in the bone marrow compared to a frequency of 0·7–4·2% in the other organs tested (Fig. 5, upper panel), supporting the cytotoxicity data illustrated in Fig. 4. Bone marrow resident tumour dormancy has been observed in Eb-Gal-inoculated DBA/2 mice with a frequency of 1–100 tumour cells per 106 bone marrow cells.18 A similar situation might exist in the present model, and such a continuous presence of TAA might play a role in the selective long-term maintenance of Ag-specific T-cell memory in the bone marrow compartment. In order to test this hypothesis, iPEC were transferred to naïve nude mice in a ‘parking experiment’. After 5 months (Fig. 5, middle panel), the frequencies of H2-Ld/Gal multimer-binding cells were in the same range as those detected in organs from naïve aged BALB/c nu/nu mice (Fig. 5, lower panel), indicating that tumour dormancy was indeed implicated in maintaining high frequencies of Gal-specific cells in the standard ADI protocol.
Figure 5.
Persistence of antigen is required for the maintenance of Gal-specific CD8+ memory T cells at detectable frequencies. The presence of live (propidium iodide negative) CD8+ T cells, specific for the immunodominant Gal-peptide (amino acids 876–884), was analysed by tetramer staining and flow cytometric analysis. Three groups of BALB/c nu/nu mice were compared, as follows: mice having received adoptive immunotherapy (ADI) following a tumour challenge with ESbL-Gal according to the standard protocol (analysed 6·5 months later) (upper panel); mice having received ADI without antigenic stimulation (‘parking experiment’, analysed 5 months later) (middle panel); and aged naïve BALB/c nu/nu mice having received neither ADI nor an antigenic stimulus (negative control) (lower panel).
Persistence of Gal-specific reactivity in the absence of Ag
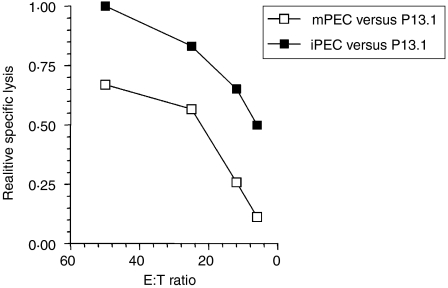
Even though Gal-specific CD8+ T cells could not be detected with H2-Ld/Gal multimers after ‘parking’ Gal-specific iPEC in Ag-free nude mice, the possibility remained that low levels of Ag-specific T cells persisted, and that these retained the capacity to expand and react upon an antigenic stimulus. mPEC were isolated from Ag-free mice (‘parking experiment’) 3 days after i.p. stimulation with 1 × 107 irradiation-inactivated ESbL-Gal and compared with DBA/2-derived iPEC for their ex vivo Gal-specific cytolytic activity against P815-Gal (Fig. 6). Although iPEC demonstrated a more powerful response, mPEC demonstrated Gal-specific cytotoxicity.
Figure 6.
Persistence of Gal-specific reactivity in the absence of antigen. Gal-specific cytolytic activity of memory T cells from antigen-free memory nude mice (‘parking experiment’), after recruitment and reactivation in the peritoneal cavity. Immune peritoneal exudate cells (iPEC) were produced by intraperitoneal (i.p.) injection of 1 × 107 irradiation-inactivated ESbL-Gal into ESbL-Gal-primed DBA/2, while memory peritoneal exudate cells (mPEC) were produced by an equivalent i.p. restimulation of antigen-free BALB/c nu/nu mice having been adoptively transferred with Gal-primed iPEC 3 months earlier (‘parking experiment’). iPEC and mPEC were isolated by peritoneal lavage and tested for Gal-specific cytotoxic activity in a 4-hr 51Cr-release assay using P815-Gal as target cells. At an effector-to-target ratio (E:T) of 50, 16·4% and 11·0% specific lysis were obtained with iPEC and mPEC, respectively. Relative specific lysis was chosen as a means to illustrate more clearly the differences between the ex vivo cytolytic capacity of iPEC and mPEC.
Rapid turnover of memory T cells in the bone marrow
In order to determine whether the striking enrichment of Gal-specific memory T cells in the bone marrow was solely caused by the TAA persistence in this compartment, turnover rates of both naïve and memory T cells were analysed and compared between lymphoid compartments of naïve DBA/2 mice. Twenty-four hours after having supplemented the drinking water with BrdU, spleen, lymph nodes and bone marrow were isolated, and naïve (CD44int/lo) and memory (CD44hi) T cells were analysed for BrdU uptake, indicating cellular proliferation (Table 2). Memory T cells consistently exhibited a higher proliferation rate than naïve cells, and bone marrow-resident cells had a significantly higher proliferation rate than their spleen or lymph node resident counterparts. The highest turnover rate was detected in memory T cells from the bone marrow, with a mean BrdU uptake of 41·6%.
Table 2.
In vivo T-cell turnover
| % BrdU+ cells | ||
|---|---|---|
| CD3+ CD44hi | CD3+ CD44int/lo | |
| BM-T | 41·6 ± 10·3* | 12·8 ± 5·8 |
| LN-T† | 3·4 ± 0·8* | 0·2 ± 0·1 |
| SPL-T‡ | 8·7 ± 2·9* | 0·9 ± 0·6 |
Twenty-four hours after supplementing the drinking water with bromodeoxyuridine (BrdU) (0·8 mg/ml), DBA/2 mice were killed and the bone marrow (BM), lymph nodes (LN) and spleen (SPL) were isolated. Single-cell suspensions were prepared and stained against CD3, CD44 and BrdU. The means and standard deviation (SD) (n = 3) of BrdU+ memory (CD44hi) or naïve (CD44int/lo) T-cell (T) frequencies are shown.
Significant difference (P < 0·05) between memory and naïve T cells.
Significant difference (P < 0·05) between BM-T and LN-T memory cells.
Significant difference (P < 0·05) between BM-T and SPL-T memory cells.
Phenotype of BM-derived tumour cells from tumour dormancy animals
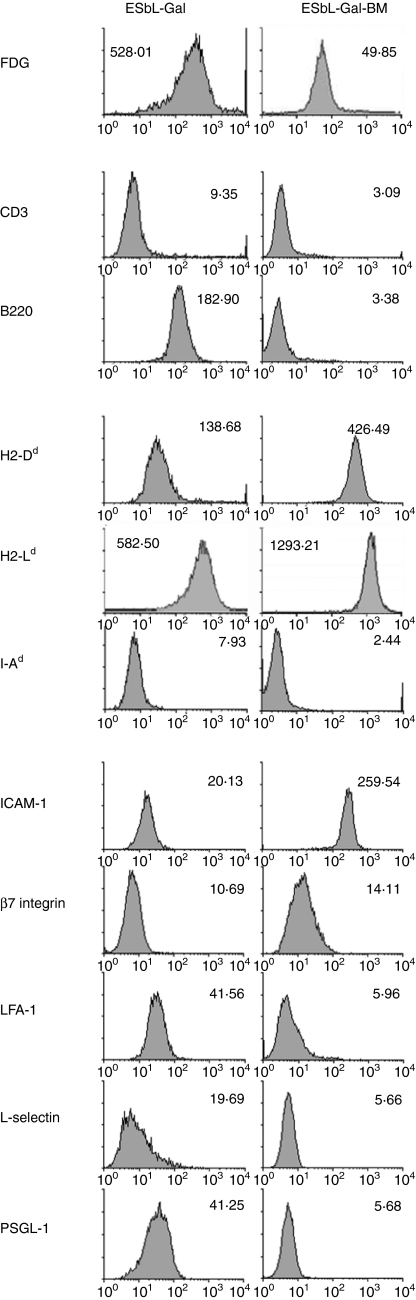
As tumour dormancy in the bone marrow appeared to play an important role in the long-term maintenance, and boosting of Gal-specific CD8+ T-cell memory and tumour-dormant cells had previously been demonstrated to be prevented from outgrowth by active host control involving CD8+ T cells,19 we were interested in obtaining an insight into the mechanisms allowing the co-existence of Ag-expressing tumour cells and Ag-reactive CD8+ T cells in the same anatomical compartment. To achieve this, we established a tumour cell line (ESbL-Gal-BM) from bone marrow-derived tumour-dormant cells, which had a reduced but stable Gal-expression, as revealed by FDG (Fig. 7) and X-Gal staining (data not shown).
Figure 7.
Cell-surface phenotype of parental tumour cells (ESbL-Gal) compared to a tumour dormancy-derived cell line (ESbL-Gal-BM). Cells were taken from cultures during the exponential growth phase and incubated with the Gal substrate, fluorescein di-β-d-galactopyranoside (FDG), or with monoclonal antibodies (mAb)s to the indicated molecules. ICAM-1, intercellular adhesion molecule-1; LFA-1, lymphocyte function-associated antigen-1; PSGL-1, P-selectin glycoprotein ligand-1.
A comparison of the cell-surface expression of lymphocyte lineage markers and molecules involved in antigen-presentation and cell adhesion between ESbL-Gal-BM and the parental T-lymphoma, ESbL-Gal (Fig. 7), revealed a general down-regulation of the majority of molecules analysed, with the exception of both major histocompatibility complex (MHC) class I molecules tested (namely H2-Dd and H2-Ld) and intercellular adhesion molecule-1 (ICAM-1), which they expressed at higher levels than the parental cell line. Interestingly, these are molecules involved in the formation of an immunological synapse with CTL, leading to tumour cell death. Whether the observed differences are caused by in vivo selection, active down-regulation or an altered phenotype as a result of epigenetic or genetic mechanisms, is presently unknown.
Discussion
TAA-specific CD8+ T-cell memory could be adoptively transferred to tumour-inoculated T-cell-deficient nude mice, where it proved to be long-lasting and highly effective in rejecting a high-dose tumour challenge administered 2 months after ADI.
Early reports from mouse models suggested that T-cell memory was short lived in the absence of antigen.27 However, more recent evidence suggests that both CD4+ and CD8+ memory T cells can be sustained in the absence of both antigen and MHC molecules.28–31 Contradicting this hypothesis are the findings in human immunodeficiency virus (HIV)-infected individuals, where the frequency of HIV-specific CTL and T-helper lymphocytes present in peripheral blood decrease over time, following highly active antiretroviral therapy (HAART), suggesting that antigen may be required to maintain high levels of memory T cells.32,33 The persistence of infectious agents in the case of tuberculosis (TB), hepatitis B virus (HBV), hepatitis C virus (HCV), HIV, Epstein–Barr virus (EBV), cytomegalovirus (CMV), varicella zoster virus (VCV) and other herpes viruses may be acting as natural boosters of immunity. In addition, it is now thought that the measles virus may persist in a crippled form in the host34 which may explain why, in the classical epidemiological studies of the Faroe Islands, protective memory was maintained for a very long time in the previously infected survivors.35 While CD4+ T-cell memory can be nourished by the low-level persistence of Ag in specialized depots, such as follicular dendritic cells (FDCs),36–38 this mechanism does not work for CD8+ T cells.
The model system used in the present study allows for the analysis of antitumour T-cell memory both in vivo and ex vivo and makes use of tumour syngeneic non-transgenic T cells, which are subjected to the same selective pressures as would be encountered under physiological conditions. ADI recipients are whole-body irradiated before treatment, resulting in an increased therapeutic efficiency.5,16 It has previously been shown that irradiation leads to increased permeability of blood vessels39 and causes a severe depletion of immune organs,16 thus increasing extravasation of injected cells and their expansion within the space and niches created in immune organs. In lymphopenic conditions, T cells expand to re-establish homeostasis by a process dependent on self-MHC recognition and the availability of proliferation and survival-promoting cytokines such as interleukin (IL)-7 and IL-15.40,41 We here document that whole-body irradiation does not abolish antitumour protective T-cell memory. The memory T-cell pool consists of effector memory and central memory cells,42 which differ in their recirculation patterns and activation state. While the former are effector-cell like in that they respond rapidly to antigenic stimulus, the latter need to first mature into fully reactive CTL. It might well be that in regard to radio-sensitivity, effector memory and central memory cells also resemble effector (resistant) and naïve T cells (susceptible),39,43 respectively, but this will need to be elucidated in separate studies.
Adoptive transfer of iPEC to naïve nude mice showed that the maintenance of functional long-term CD8+ T-cell memory is independent of Ag-persistence, although the continuous presence of Ag results in the boosting of Ag-specific T-cell numbers. In the absence of Ag (‘parking experiment’), the frequency of Gal876−884-specific CD8+ T cells was below the detection limit with Ld/Gal multimers and flow cytometry, while in the presence of bone marrow resident dormant tumour cells, high frequencies of TAA-specific CD8+ T cells were readily detectable. Even though the MHC-identical donor (DBA/2) and host (BALB/c nu/nu) mouse strains differ at minor histocompatibility (minor H) loci, it is unlikely that these genetic differences influenced the results, because the adoptive therapy described involves the treatment of a DBA/2-derived tumour cell line with DBA/2-derived immune cells. The minor H differences did not appear to cause expansion of TAA-specific memory T cells in the ‘parking experiment’. Furthermore, ADI-recipient BALB/c nu/nu mice did not exhibit graft-versus-host reactions (weight loss, wasting), and host-versus-graft reactions did not appear to pose a problem in terms of donor immune cell survival in immunocompromised nude hosts.
It has been reported that different types of memory T cells compete for bone marrow seeding.44 Antigenic stimulation of the TAA-specific CD8+ T cells could occur either via direct interaction with tumour cells expressing the Ld-restricted, immunodominant Gal-epitope, TPHPARIGL, or via interaction with professional antigen-presenting cells that cross-present the relevant TAA epitope. Both interactions would induce proliferation of Gal-specific T cells, boosting the antigen-specific memory T-cell pool at the cost of other memory T cells, as homeostatic regulation45,46 limits the expansion of the total T-cell pool, as well as the size of lymphoid organs. TAA stimulation might thus confer clonal dominance to Gal-specific CD8+ memory T cells through antigen-driven selection. It is then conceivable that such clonal dominance is lost following antigen removal, which would explain our observation that antigen reactivity is maintained, even when the frequency of Ag-specific T cells is below the detection level.
Our observation that the highest frequency of TAA-specific cells was found in the bone marrow correlates not only with tumour dormancy in this compartment,18,19 but also with previous reports of memory T-cell enrichment in the bone marrow of experimental animals44 and cancer patients47,48 and the involvement of DC : T-cell interactions in the bone marrow in CD4+ T-cell memory maintenance.48,49 In addition to the continuous presence of TAA as a result of tumour dormancy, the bone marrow microenvironment also has other characteristics that might favour the enrichment of memory T cells in this compartment, including its unique structural organization with its fine network of arterioles and sinuses that provides an optimal blood supply.50 Significantly higher frequencies of CD8+ T-cell blasts were observed in the bone marrow microenvironment than in spleen or lymph nodes, indicating that bone marrow-resident CD8+ T cells are in a different basic activation state than those found in the periphery,51 and it was found that memory, but not naïve, T cells preferentially home to the bone marrow.44 The bone marrow stroma produces vital growth factors, and stromal cell-derived type I interferons,52 as well as IL-7 and IL-15,40,41 have been implicated in the long-term maintenance of memory T cells.
Interestingly, the phenotype of bone marrow resident memory T cells differed between tumour-inoculated and tumour-free hosts (Y.M., personal observation): while in Ag+ hosts the majority of CD8+ T cells was CD45RBlo/neg CD62L– CD44– (cycling cells), in Ag– hosts they were mainly CD45Rbhi CD62L– CD44+ (resting cells).
Consistently, bone marrow resident T cells, both naïve and memory, of naïve mice were found to have higher turnover rates than corresponding cells in the spleen. This indicates that even in the absence of tumour dormancy in the bone marrow, which confers a selective advantage to TAA-specific T cells in this compartment, the bone marrow is implicated in homeostatic T-cell proliferation. The delicate balance between proliferating ‘dormant’ tumour cells19 with proliferating TAA-specific T cells is reminiscent of observations in asymptomatic HIV patients, where high virus replication and high CD4+ T-cell proliferation and death co-exist.
Longevity of memory T cells could not be directly evaluated by telomere length analysis53,54 because this is extremely problematic to perform with murine tissues.55 Instead we evaluated their capacity to expand and survive following multiple transfers to T-cell-deficient nude recipients. Memory T cells from such mice could be boosted and recruited to the peritoneal cavity by i.p. tumour vaccination. mPEC isolated by peritoneal lavage were then transferred from one group of ESbL-Gal tumour cell-inoculated nude mice to the next, leaving them to ‘rest’ for at least 1 month in every host. At every transfer, the cells prevented premature death of the host animals after tumour challenge. mPEC isolated from the tertiary hosts were found to be as efficient as iPEC from DBA/2 mice in prolonging the survival of ESbL-Gal cell inoculated nude mice (data not shown).
Our data suggest that a small amount of persistent TAA produced by dormant tumour cells from the bone marrow contributes to the maintenance of TAA-specific long-term memory. There thus appears to be a delicate balance between TAA-reactive CD8+ T cells and dormant tumour cells that are susceptible to CTL-mediated lysis in this microenvironment. In comparison to the parental tumour cell line, the bone marrow-derived line had completely down-regulated the expression of certain adhesion molecules – lymphocyte function-associated antigen-1 (LFA-1), l-selectin, P-selectin glycoprotein ligand-1 (PSGL-1)– and cell lineage markers (CD3, B220). Further adhesion molecules and lineage markers analysed also exhibited a down-regulation to background levels on ESbL-Gal-BM (data not shown). In contrast, the two MHC class I molecules, H2-Ld (the restricting MHC molecule of the immunodominant Gal-peptide) and H2-Dd, as well as ICAM-1, were expressed at increased levels. The bone marrow-derived tumour cells were thus equipped to present endogenously derived Gal-peptides and could potentially stimulate tumour-reactive CD8+ memory T cells directly. Whereas the adhesion molecules expressed on ESbL-Gal might be necessary for its metastatic phenotype, the absence of such molecules on ESbL-Gal-BM might be related to its bone marrow resident phenotype, as well as representing a strategy of immune evasion. Interestingly, ESbL-Gal-BM were more sensitive to CTL-mediated cytotoxicity in vitro than the parental ESbL-Gal cells (Y.M., unpublished data), but, nevertheless, persisted for prolonged periods of time in the presence of tumour-reactive memory CTL.19 One explanation could be that the expression of only few adhesion molecules on tumour cells could complicate CTL–tumour cell interactions in situ, thus allowing for the co-existence of CTL-sensitive tumour cells and tumour reactive killers in the bone marrow microenvironment, whereas the close cell–cell contact during cytotoxicity assays owing to the experimental conditions could circumvent the need for interaction via specific adhesion molecules. It is then easy to imagine a situation where ‘dormant’ tumour cells residing in the bone marrow proliferate at a slow rate and are maintained at a constant population size by the active control of CD8+ T cells, which, in turn, are present at elevated frequencies as a result of regular antigenic stimulation.
In conclusion, we exploited a recently described novel model system to study the longevity of protective antitumour immunity in T-cell-deficient nude mice. TAA-reactive CD8+ T cells survived over a prolonged period of time in antigen-inexperienced hosts, although the presence of TAA-expressing dormant tumour cells was indispensable to boost specific T-cell frequencies to levels that are detectable by peptide–MHC multimers. The results corroborate previous data that indicate the bone marrow to be a special compartment for both tumour dormancy and antitumour T-cell memory. This may partly be a result of the persistence of TAA in this compartment, giving TAA-specific T cells a selective advantage over irrelevant clones, and partly a result of the special architecture and presence of T-cell survival cytokines, such as type I interferons, IL-7 and IL-15. Indeed, T-cell turnover was found to be significantly higher in the bone marrow than in other immune organs studied.
Acknowledgments
We thank A. Griesbach for excellent technical assistance with the in vivo experiments, the NIAID Tetramer Facility and NIH AIDS Research and Reference Reagent Program (Bethesda, MD) for providing the PE-conjugated H2-Ld/TPHPARIGL multimeric complexes, as well as P. Altevogt and G. Hämmerling (both at DKFZ, Heidelberg) for kindly providing monoclonal antibodies specific for adhesion and MHC molecules, respectively. This project was financed by the D. Hopp-Stiftung (Walldorf, Germany).
References
- 1.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 2.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, Washabaugh MW. Memory T cells and vaccines. Vaccine. 2003;21:419–30. doi: 10.1016/s0264-410x(02)00407-3. 10.1016/S0264-410X(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 3.Mahnke YD, Schirrmacher V. Characteristics of a potent tumour vaccine-induced secondary anti-tumour T cell response. Int J Oncol. 2004;24:1427–34. [PubMed] [Google Scholar]
- 4.Schirrmacher V. Clinical trials of antitumor vaccination with an autologous tumour cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol Immunother. 2005;54:587–98. doi: 10.1007/s00262-004-0602-0. 10.1007/s00262-004-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schirrmacher V, Beckhove P, Krüger A, et al. Effective immune rejection of advanced metastasized cancer. Int J Oncol. 1995;6:505–21. doi: 10.3892/ijo.6.3.505. [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oehen S, Waldner H, Kundig TM, Hengartner H, Zinkernagel RM. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J Exp Med. 1992;176:1273–81. doi: 10.1084/jem.176.5.1273. 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–11. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 9.Sourdive DJ, Murali-Krishna K, Altman JD, et al. Conserved T cell receptor repertoire in primary and memory CD8 T cell responses to an acute viral infection. J Exp Med. 1998;188:71–82. doi: 10.1084/jem.188.1.71. 10.1084/jem.188.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin MY, Selin LK, Welsh RM. Evolution of the CD8 T-cell repertoire during infections. Microbes Infect. 2000;2:1025–39. doi: 10.1016/s1286-4579(00)01257-0. 10.1016/S1286-4579(00)01257-0. [DOI] [PubMed] [Google Scholar]
- 11.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–8. doi: 10.1126/science.283.5408.1745. 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 12.Ochsenbein AF, Karrer U, Klenerman P, et al. A comparison of T cell memory against the same antigen induced by virus versus intracellular bacteria. Proc Natl Acad Sci USA. 1999;96:9293–8. doi: 10.1073/pnas.96.16.9293. 10.1073/pnas.96.16.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schirrmacher V, Jurianz K, Griesbach A. Intra-pinna induction of specific antitumour immune T-cell functions: effect of ear resection after antigen application. Int J Oncol. 1997;11:227–33. doi: 10.3892/ijo.11.2.227. [DOI] [PubMed] [Google Scholar]
- 14.Schirrmacher V, Leidig S, Griesbach A. In situ activation of syngeneic tumour-specific cytotoxic T lymphocytes: intra-pinna immunization followed by restimulation in the peritoneal cavity. Cancer Immunol Immunother. 1991;33:299–306. doi: 10.1007/BF01756594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahnke YD, Schirrmacher V. T cell memory to cell-bound antigen. In: Witz IP, editor. 2nd International Conference on Tumour Microenvironment: Progression, Therapy and Prevention. Baden, Austria: Monduzzi Editore; 2002. pp. 181–3. [Google Scholar]
- 16.Mahnke YD, Schirrmacher V. A novel tumour model system for the study of long-term protective immunity and immune T cell memory. Cell Immunol. 2003;221:89–99. doi: 10.1016/s0008-8749(03)00062-5. 10.1016/S0008-8749(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 17.Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–7. doi: 10.1038/nm914. 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 18.Khazaie K, Prifti S, Beckhove P, Griesbach A, Russell S, Collins M, Schirrmacher V. Persistence of dormant tumour cells in the bone marrow of tumour cell-vaccinated mice correlates with long-term immunological protection. Proc Natl Acad Sci USA. 1994;91:7430–4. doi: 10.1073/pnas.91.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller M, Gounari F, Prifti S, Hacker HJ, Schirrmacher V, Khazaie K. EblacZ tumour dormancy in bone marrow and lymph nodes: active control of proliferating tumour cells by CD8+ immune T cells. Cancer Res. 1998;58:5439–46. [PubMed] [Google Scholar]
- 20.Carbone FR, Bevan MJ. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377–87. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krüger A, Umansky V, Rocha M, Hacker HJ, Schirrmacher V, Von Hoegen P. Pattern and load of spontaneous liver metastasis dependent on host immune status studied with a lacZ transduced lymphoma. Blood. 1994;84:3166–74. [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 23.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–99. doi: 10.1084/jem.180.6.2089. 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993;151:3971–80. [PubMed] [Google Scholar]
- 25.Reddehase MJ, Rothbard JB, Koszinowski UH. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989;337:651–3. doi: 10.1038/337651a0. 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- 26.Jurianz K, Von Hoegen P, Schirrmacher V. Superiority of the ear pinna over a subcutaneous tumour inoculation site for induction of a Th1-type cytokine response. Cancer Immunol Immunother. 1998;45:327–33. doi: 10.1007/s002620050450. 10.1007/s002620050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–74. doi: 10.1084/jem.174.5.969. 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–21. doi: 10.1084/jem.179.1.317. 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–62. doi: 10.1126/science.276.5321.2057. 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 30.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–81. doi: 10.1126/science.286.5443.1377. 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 31.Swain SL. CD4 T-cell memory can persist in the absence of class II. Philos Trans R Soc Lond B Biol Sci. 2000;355:407–11. doi: 10.1098/rstb.2000.0581. 10.1098/rstb.2000.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, Walker BD. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–8. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–25. doi: 10.1038/8400. 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 34.Katayama Y, Hotta H, Nishimura A, Tatsuno Y, Homma M. Detection of measles virus nucleoprotein mRNA in autopsied brain tissues. J Gen Virol. 1995;76(Part 12):3201–4. doi: 10.1099/0022-1317-76-12-3201. [DOI] [PubMed] [Google Scholar]
- 35.Panum PL. Observations made during the epidemic of measles on the Faroe Islands in the year 1846. Med Classics. 1939;3:39–868. [Google Scholar]
- 36.Van Essen D, Dullforce P, Brocker T, Gray D. Cellular interactions involved in Th cell memory. J Immunol. 2000;165:3640–6. doi: 10.4049/jimmunol.165.7.3640. [DOI] [PubMed] [Google Scholar]
- 37.Kelsoe G. Remembrance of things past. Nat Immunol. 2000;1:375–6. doi: 10.1038/80811. 10.1038/80811. [DOI] [PubMed] [Google Scholar]
- 38.Qin D, Wu J, Vora KA, Ravetch JV, Szakal AK, Manser T, Tew JG. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J Immunol. 2000;164:6268–75. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 39.Schirrmacher V, Zangemeister-Wittke U. γ-irradiation suppresses T cell mediated protective immunity against a metastatic tumour in the afferent phase of the immune response but enhances it in the efferent phase if given before immune cell transfer. Int J Oncol. 1994;4:335–46. doi: 10.3892/ijo.4.2.335. [DOI] [PubMed] [Google Scholar]
- 40.Baccala R, Theofilopoulos AN. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 2005;26:5–8. doi: 10.1016/j.it.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Alpdogan Ö, van den Brink MRM. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 43.Igietseme JU, Smith K, Simmons A, Rayford PL. Effect of gamma-irradiation on the effector function of T lymphocytes in microbial control. Int J Radiat Biol. 1995;67:557–64. doi: 10.1080/09553009514550671. [DOI] [PubMed] [Google Scholar]
- 44.Di Rosa F, Santoni A. Memory T-cell competition for bone marrow seeding. Immunology. 2003;108:296–304. doi: 10.1046/j.1365-2567.2003.01593.x. 10.1046/j.1365-2567.2003.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 46.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–9. doi: 10.1038/90950. 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 47.Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G, Diel IJ, Schirrmacher V. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer. 2001;92:96–105. 10.1002/1097-0215(200102)9999:9999<::AID-IJC1152>3.3.CO;2-H. [PubMed] [Google Scholar]
- 48.Feuerer M, Beckhove P, Bai L, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–8. doi: 10.1038/86523. 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 49.Feuerer M, Beckhove P, Mahnke Y, Hommel M, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow microenvironment facilitating dendritic cell:CD4 T cell interactions and maintenance of CD4 memory. Int J Oncol. 2004;25:867–76. [PubMed] [Google Scholar]
- 50.Ohtani O, Gannon B, Ohtsuka A, Murakami T. The microvasculature of bone and especially of bone marrow as studied by scanning electron microscopy of vascular casts – a review. Scanning Electron Microsc. 1982;(Part 1):427–34. [PubMed] [Google Scholar]
- 51.Di Rosa F, Santoni A. Bone marrow CD8 T cells are in a different activation state than those in lymphoid periphery. Eur J Immunol. 2002;32:1873–80. doi: 10.1002/1521-4141(200207)32:7<1873::AID-IMMU1873>3.0.CO;2-P. 10.1002/1521-4141(200207)32:7<1873::AID-IMMU1873>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 52.Akbar AN, Lord JM, Salmon M. IFN-alpha and IFN-beta: a link between immune memory and chronic inflammation. Immunol Today. 2000;21:337–42. doi: 10.1016/s0167-5699(00)01652-2. 10.1016/S0167-5699(00)01652-2. [DOI] [PubMed] [Google Scholar]
- 53.Harley CB, Villeponteau B. Telomeres and telomerase in aging and cancer. Curr Opin Genet Dev. 1995;5:249–55. doi: 10.1016/0959-437x(95)80016-6. 10.1016/0959-437X(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 54.Akbar AN, Beverley PC, Salmon M. Will telomere erosion lead to a loss of T-cell memory? Nat Rev Immunol. 2004;4:737–43. doi: 10.1038/nri1440. 10.1038/nri1440. [DOI] [PubMed] [Google Scholar]
- 55.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci USA. 1995;92:4818–22. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]