Abstract
CD200 (OX2) is a membrane glycoprotein that interacts with a structurally related receptor (CD200R) involved in the regulation of macrophage function. The interaction is of low affinity (KD ∼ 1 μm) but can be detected using CD200 displayed in a multivalent form on beads or with dimeric fusion proteins consisting of the extracellular region of CD200 and immunoglobulin Fc regions. We prepared putative pentamers and trimers of mouse CD200 with sequences from cartilage oligomeric matrix protein (COMP) and surfactant protein D (SP-D), respectively. The COMP protein gave high-avidity binding and was a valuable tool for showing the interaction whilst the SP-D protein gave weak binding. In vivo experiments showed that an agonistic CD200R monoclonal antibody caused some amelioration in a model of experimental autoimmune encephalomyelitis but the COMP protein was cleared rapidly and had minimal effect. Pentameric constructs also allowed detection of the rat CD48/CD2 interaction, which is of much lower affinity (KD ∼ 70 μm). These reagents may have an advantage over Fc-bearing hybrid molecules for probing cell surface proteins without side-effects due to the Fc regions. The CD200-COMP gave strong signals in protein microarrays, suggesting that such reagents may be valuable in high throughput detection of weak interactions.
Keywords: CD200, microarray, myeloid, pentamer, receptor
Introduction
The surface of leucocytes contains many proteins that are involved in the fine control of the immune system by transmitting signals from the environment. Interactions of leucocyte surface proteins with proteins on other cells are generally of low affinity (KD=1–100 μm) when measured in the monomeric state.1,2 These membrane proteins often contain immunoglobulin-related domains such as CD200 that interacts with the CD200 receptor (CD200R) with a KD=1 μm3,4 and rodent CD48 that interacts with CD2 and CD244 with KD=70 μm and KD=16 μm, respectively.5,6 From a technical point this makes new interactions hard to identify because monomeric binding reagents will dissociate in a matter of seconds and ligands have yet to be identified for many surface proteins. This problem may be overcome by increasing the avidity of the binding reagent and this is commonly achieved by making the recombinant protein dimeric by engineering an immunoglobulin Fc region onto the extracellular region of the surface protein.7 Another method is to produce a monomeric recombinant protein and render it multimeric by coupling it to beads. This method was successful in the identification of the receptors for CD200 (OX2)3 and CD244.6 The receptor is expressed at high levels on myeloid cells where it can be recognized using soluble CD200-Fc fusion proteins; it is also expressed on some T cells.4 The CD200-deficient mouse had observable alterations in the behaviour of myeloid cells in tissues that normally expressed CD200. These included an increase in number and state of activation of macrophages in several tissues and a profound increase in susceptibility to autoimmune disease models affecting the brain and joints.8 These results indicated that CD200–CD200R interactions are involved in the control of myeloid cellular function.8 The broad tissue distribution of CD200 and changes in its level of expression provide a mechanism for locally regulating myeloid cellular activity at appropriate sites such as inflamed tissues.9,10 CD200-Fc fusion proteins have been shown to provide beneficial immunomodulatory effects in models of arthritis and allograft rejection.11,12 CD200 and a viral homologue found in herpesvirus 8, when expressed at the cell surface, gave inhibition of pro-inflammatory cytokines by activated macrophages.13
We tested different ways of making recombinant proteins multivalent by fusion to sequences designed to give pentamers and trimers and compared these to Fc fusion proteins. We show that the pentameric cartilage oligomeric matrix proteins (COMP) are particularly good reagents for detecting weak interactions typical of membrane protein interactions.
Materials and methods
Monoclonal antibodies
The following monoclonal antibodies (mAbs) were used: YTA 3.1.2, rat anti-mouse CD4;14,15 OX68, mouse anti-rat CD4d3+4;16 OX108, rat anti-mouse CD200R;4 OX102, mouse anti-rat CD200R;3 OX110, rat anti-mouse CD200;4 and OX21, mouse anti-human C3b inactivator.17
Construction, expression and purification of soluble recombinant proteins
Recombinant chimeric proteins were constructed as a succession of interchangeable modules, in the following order (5′ to 3′): (1) an XbaI/SalI module containing the extracellular domain of mouse CD200 (mCD200); (2) a SalI/EcoRI module containing domains 3 and 4 of mouse CD4 (mCD4d3+4); (3) an 11-amino-acid linker sequence (NSGGGSGGGTG) followed by the rat COMP (rCOMP) oligomerization domain [residues 26–72 (GenBank 297438)] or a six-amino-acid linker sequence (NSGGGS) followed by the murine surfactant protein-D (mSP-D) oligomerization domain [amino acids 223–249 (GenBank 1129061)] in an EcoRI/BamHI module, and a stop codon. DNA encoding for the two immunoglobulin superfamily (IgSF) domains that comprise the extracellular region of mCD200 was previously amplified by polymerase chain reaction by Gavin Wright as described in ref. 18, introducing an XbaI site (5′) and a SalI restriction site (3′). The mCD200 extracellular fragment was inserted via the 5′XbaI site and the 3′SalI site into a modified Bluescript (BS KS+) vector containing a rat CD4 leader and rat CD4d3+4 (CD4Ld3+4RI19), thus replacing the XbaI–SalI fragment encoding the CD4L. The SalI–EcoRI fragment encoding the rCD4d3+4 was replaced with DNA encoding mCD4d3+4. The join at the SalI junction (underlined) was 5′-(mCD200)aaagggtcgacagc(mCD4d3+4). The resulting construct was digested with EcoRI and BamHI and ligated with appropriately digested DNA encoding for the rCOMP or the mSP-D peptide. The 141-base-pair DNA fragment coding for the assembly domain of rCOMP (amino acids 26–72) was amplified using cDNA of the N-terminal region of rat COMP (kindly provided by V. Effimov and J. Engel, Biozentrum, Basel), introducing an EcoRI (5′) and a BamHI (3′) restriction site. The 81-base-pair DNA fragment coding for the mSP-D assembly domain or ‘neck region’ (amino acids 223–249)20 was constructed to introduce the linker peptide NSGGGS and the EcoRI and BamHI sites and to destroy an internal XbaI restriction site. An XbaI–BamHI fragment encoding (XbaI)-mCD200-(SalI)-mCD4d3+4-(EcoRI)-rCOMP/mSP-D-(BamHI) was transferred to a modified pEF-BOS vector, pEF-BOS-XB.21 A control COMP lacked the extracellular CD200 region but contained the mCD4d3+4. Constructs were tested by automated DNA sequencing, before subcloning XbaI–BamHI fragments into the pEE14 expression vector (Lonza Biologics, Slough, UK) at the XbaI and BclI sites. The mCD200-COMP and mCD200-SP-D proteins and Control-COMP and Control-SP-D were expressed in Chinese hamster ovary cells with the glutamine synthetase selectable marker and purified by affinity chromatography with mouse CD4 mAb as for rat CD4.22 The yield of purified COMP and SP-D proteins was between 0·5 and 1 mg/l. The proteins were purified further by gel filtration on a Superdex S-200 (10/30 HR) column (Pharmacia, Uppsala, Sweden). In analytical runs (Figs 2a, b) 500 μg of each protein was loaded onto a (10/30 HR) column and eluted with Hepes buffered salt solution at a flow rate of 0·5 ml/min. The column was standardized with the following molecular weight markers; thyroglobulin, 669 000; ferritin, 440 000; catalase, 232 000; aldolase, 158 000; albumin, 68 000; ovalbumin, 43 000; chymotrypsinogen, 25 000.
Figure 2.
Analysis of the multivalent CD200 proteins by gel filtration and SDS—PAGE. (a) Gel filtration of purified mCD200-COMP and Control-COMP and (b) mCD200-SP-D and Control-SP-D proteins on Superdex 200 (10/30 HR). The elution positions of molecular mass standards are also shown together with the void volume Vo. (c) SDS—PAGE analysis of purified soluble recombinant proteins on gradient (4—12%) SDS gels under non-reducing and reducing conditions stained with Coomassie Blue. Migration positions of protein standards are shown.
Soluble biotinylated mCD200 was produced by ligating DNA encoding for the extracellular regions of mCD20018 in frame using XbaI/SalI digestion to mCD4d3+4 and a biotin ligase (BirA) biotinylation sequence at the C terminus of the protein (in vector pEF-BOS-XB6,21). The protein was expressed in HEK293T cells, enzymatically biotinylated, and used to generate multivalent binding reagents (mCD200-beads) using avidin-coated fluorescent beads (Spherotech Inc. Libertyville, IL) as described previously.6 The soluble biotinylated form of mouse CD200R (mCD200R) was produced as described elsewhere3 and the CD48-COMP version was constructed as above (although this protein contained rCD4d3+4 rather than mCD4d3+4). The mouse CD200-immunoglobulin was prepared as described previously.4 On gel filtration this eluted as a single homogeneous peak with molecular weight of approximately 150 000 consistent with a dimeric structure. Levels of aggregates were negligible and unlikely to contribute to the binding observed.
Determination of binding properties of fusion proteins to CD200R by surface plasmon resonance
The interactions were followed using a BIAcore™ 2000 at 25° as described previously.3 Mouse CD200RCD4d3+4 chimeric protein and rat CD147CD4d3+4 (control, see ref. 23) dissolved in 10 mm sodium acetate (pH 4·5) were coupled directly in individual flow cells to a CM5 research grade chip (BIAcore AB), using amine coupling at 50 μg/ml. Serially diluted monomeric mCD200-COMP, mCD200-SP-D, mCD200-immunoglobulin purified soluble chimeric proteins and OX110 mAb were injected at the indicated active concentrations over the flow cells. The concentration of each protein was determined by absorbance at 280 nm. Molar extinction coefficients were calculated using the protparam tool from the Expasy website (http://www.expasy.ch) giving values of mCD200-COMP: 57 040 M−1 cm−1 (MW 367 000), Control-COMP: 24 040 M−1 cm−1 (MW 164 500), mCD200-SP-D: 58 800 M−1 cm−1 (MW 208 000), Control-SP-D: 25 320 M−1 cm−1 (MW 96 000). The Koff dissociation rate constant was determined by fitting a first-order exponential decay curve to normalized data over the dissociation phase.
Flow cytometry
Freshly isolated rat peritoneal macrophages were incubated with fusion proteins or rat mAb for 60 min at 4°, washed and then stained with a fluorescence-conjugated secondary mAb, fluorescein isothiocyanate (FITC) goat anti-mouse immunoglobulin adsorbed against rat immunoglobulins (Serotec Ltd, Oxon) or FITC anti-mouse CD4 (Pharmingen, Heidelberg, Germany). Macrophages were detected with a CD11b:R-phycoerythrin-conjugated mAb (Serotec, Ltd, Oxon). Fluorescence-labelled cells were detected by flow cytometry with a Becton Dickinson FACSCalibur. Jurkat cells transfected with rat CD224 were labelled with CD48-COMP and detected with OX68 mAb and FITC-labelled anti-mouse immunoglobulin G (IgG).
Experimental autoimmune encephalomyelitis (EAE) model
Myelin oligodendrocyte glycoprotein peptide 35–55 (MOG 35–55; MEVGWYRSPFSROVHLYRNGK) corresponding to the mouse sequence was synthesized by QCB Inc., Division of BioSource International (Hopkinton, MA), and purified by high-performance liquid chromatography. To induce active EAE, female C57BL/6 mice, at 6–8 weeks of age, were immunized subcutaneously in the back with 100 μg of MOG 35–55 peptide in 0·1 ml phosphate-buffered saline (PBS) and 0·1 ml complete Freund's adjuvant (Difco Laboratories, Detroit, MI) containing 0·4 mg Mycobacterium tuberculosis (H37Ra; Difco Laboratories) and intraperitoneally injected with 500 ng pertussis toxin (List Biological Laboratories Inc., Campbell, CA) on the day of immunization and 48 hr later. Individual mice were examined daily for clinical signs of neurological deficit and scored on a 0–5 scale as follows:25 0, no disease; 1, limp tail; 2, partial hind limb paralysis; 3, total hind limb paralysis; 4, total hind and front limb paralysis; 5, moribund or dead animal. Mice were humanely killed at disease score 4. Nine days following peptide injection, close to the time when symptoms first appear, mice (n=5) were injected intraperitoneally with either 250 μg of anti-CD200R mAb (OX110) or 100 μg of mCD200-COMP protein or 100 μg of control COMP protein. All treatment reagents were diluted in 0·5 ml PBS. Mice in the control group (n=11) received 0·5 ml PBS only. In some experiments a control mAb was given but it showed no difference from PBS controls. An enzyme-linked immunosorbent assay (ELISA) for mouse CD4 assayed COMP protein levels in serum.
Protein microarrays
Preparation of protein microarrays, detection and data extrapolation and analysis were performed as described previously.26 Briefly, a QArrayMini microarray printer (Genetix Ltd, New Milton, UK) was used to apply the protein solutions onto epoxy-coated microscope slides using 300 μm solid-tipped tungsten pins (Genetix Ltd). YTA 3.1.2 and OX68 mAb were labelled with Alexa Fluor 555 and Alexa Fluor 647 amine-reactive dyes, respectively, using the Molecular Probes Monoclonal Antibody Labelling Kits (Cat. No. A-20186 and A-20187) and according to the manufacturer's instructions (Molecular Probes, Invitrogen Ltd, Paisley, UK). Labelling of microarrays was carried out in hybridization chambers (Corning Incorporated, Sunderland, UK). LifterSlips (Erie Scientific, Portsmouth, NH) were placed gently over the arrays and the binding reagent (25–70 μl of mCD200-CD4d3+4 streptavidin–FITC beads or mCD200-COMP) was introduced with a micropipette. The arrays were scanned using a GenePix4000B microarray scanner (Axon Laboratories, Palo Alto, CA) with 532 nm and 635 nm lasers and the genepix Pro 5·0 (Axon Laboratories) software. Extraction of spot intensity and data analysis were performed using genepix pro 5·0 (Axon Laboratories) and scanarray express (Perkin Elmer) software.
Results
Design and production of the trimeric and pentameric CD200 fusion proteins
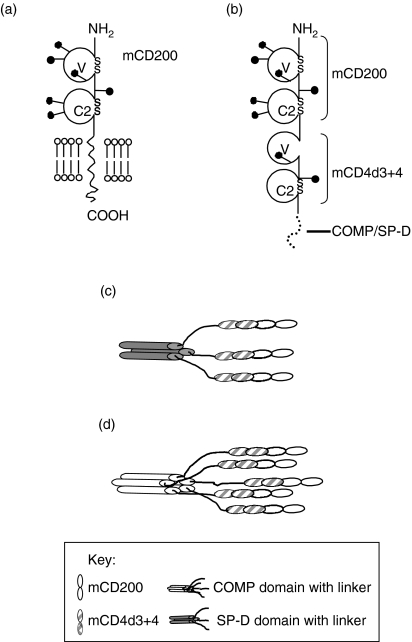
Constructs were designed with the two extracellular immunoglobulin-like domains of mouse CD200 (mCD200) and with domains 3 and 4 of mouse CD4 (Fig. 1). The equivalent domains from rat CD4 have been widely used as an effective, well-expressed tag6,16 but in this case mouse CD4 was chosen so the new reagents could be tested in vivo in mice being less immunogenic than rat CD4. The monovalent reagent CD200CD4d3+4 binds very weakly to CD200R with a half-life in the order of seconds.3 To increase CD200-binding avidity, sequences were engineered at the C terminus of the constructs to cause multimerization (Fig. 1). Two constructs were made with additional peptides from the coiled coil regions of mouse lung SP-D, known to induce protein trimerization,27,28 and from the rat COMP that forms pentamers.29 The rat COMP sequence for the assembly domain responsible for pentamerization was 97% identical to the mouse COMP. The constructs were expressed in Chinese hamster ovary cells, assayed by ELISA and purified by antibody affinity chromatography using a mAb recognizing mouse CD4d3+4. The proteins were purified further by gel filtration to exclude larger protein aggregates that are known to influence binding measurements (Fig. 2a, b). The fractionated CD200 multimers were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) (Fig. 2c). On gel filtration the mCD200-COMP and Control-COMP run with apparent MW of around 500 000 and 300 000 that are consistent with the predicted MW of 370 000 and 170 000, respectively, of the pentamers; allowing for glycosylation and the fact that these proteins are likely to be asymmetric and run with larger apparent MW, mCD200-SP-D and Control-SP-D ran with apparent MW of around 160 000 and 50 000, which were smaller than the predicted MW of 208 000 and 96 000, respectively. This suggested that this material might not be trimeric. Little material was eluted at the void volume, suggesting that there were few large aggregates. The SDS–PAGE analysis gave bands of the expected sizes under reducing conditions. Under non-reducing conditions the mCD200-COMP and Control-COMP ran with much larger MW, consistent with the presence of higher multimers that only dissociated on reduction of disulphide bonds present in the COMP sequence.
Figure 1.
Design of multivalent recombinant CD200 proteins. (a) Schematic representation of the domain organization of mouse CD200 and (b) of mCD200-mCD4d3+4-COMP/SP-D soluble chimeric constructs. The filled lollipop symbols represent the approximate sites of potential N-linked glycosylation, the immunoglobulin-like domains are labelled and the C-terminal location of the COMP or SP-D peptide is indicated. (c) and (d) Schematic depiction of putative mCD200-SP-D trimeric protein and mCD200-COMP pentameric proteins, respectively.
Analysis of recombinant CD200 binding to CD200R by surface plasmon resonance
Different concentrations of gel-purified recombinant multivalent CD200 proteins were passed over mCD200RCD4d3+4 that had been directly immobilized on the BIAcore™ chip (Fig. 3). The pentameric CD200 gave good binding over a large range of concentrations down to around 5·5 nm (Fig. 3a). The dissociation rate was extremely slow and thus a value could not be determined. The SP-D chimeric protein bound to CD200R to a lesser degree, with a fast dissociation rate (Koff ∼ 0·06 second−1; t½ =11·5 seconds) compatible with the gel filtration data and a lack of stable trimers (Fig. 2b). The binding of COMP and SP-D chimeras to CD200R was compared to a high-affinity mAb, OX110 and a dimeric CD200-Fc fusion protein. Figure 3(c) shows that the mAb gave the best binding but both the mAb and pentamer had equivalent and extremely low dissociation rates. As expected from Fig. 3(b), the SP-D chimera gave poor binding. The Fc fusion protein gave binding similar to the pentamer but with a faster dissociation rate. Saturation was not obtained for the different reagents but the highest levels of binding were obtained with the mAb (Fig. 3c). The signal from surface plasmon resonance is proportional to mass so the COMP protein would be expected to give a bigger signal than the mAb, but the opposite was found. However binding of the COMP protein presumably involves up to five binding sites and there may be steric hindrance because of the orientation of the CD200R on the chip leading to fewer protein molecules being bound compared to the divalent antibody.
Figure 3.
Binding of multivalent mCD200 proteins to mCD200R analysed by surface plasmon resonance in a BIAcoreTM. (a) Various concentrations of pentavalent CD200-COMP were injected through flow cells with mCD200R-CD4d3+4 (6500 RU, solid trace) or 1500 RU of negative control immobilized on the surface. The results for each concentration are shown as an overlay plot. (b) Overlay plot of mCD200-SP-D binding to immobilized soluble mCD200R-CD4d3+4 (12 000 RU). (c) Comparison of binding of COMP (pentamer), SP-D and dimeric (Fc Fusion protein) CD200 recombinant proteins and OX110 mAb at 0·2 μm to immobilized CD200R-CD4d3+4 (12 000 RU). The mAb and COMP bind with high affinity with barely discernable dissociation rates, the dimer gives reasonable binding and moderate dissociation whilst the mCD200-SP-D gives low binding and dissociates rapidly. The half-lives calculated from the curves are shown on the right. Experiments were carried out at 25° and the flow rate was 10 μl/min. Bars indicate the period of injection of proteins. The background signal for each protein passing over a chip with an irrelevant protein was subtracted from each trace.
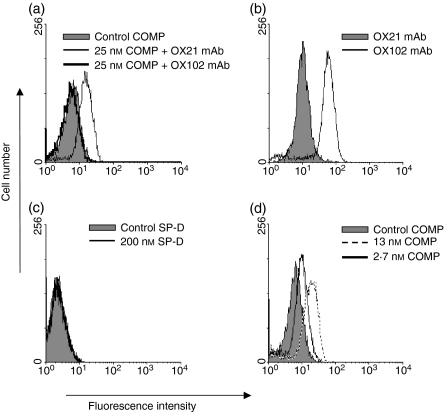
Pentameric CD200 recombinant protein gives strong labelling of cells
The above experiments indicated that the various constructs could bind polyvalently to the CD200R on the BIAcore chip. It is likely that the full valency of the binding reagents cannot be achieved on the chip because of steric hindrance effects30 but might be obtained on cells. Rat peritoneal cells were tested as they express moderate levels of CD200R known to bind murine CD200; furthermore, OX102 mAb can block this interaction (ref. 3 and unpublished data). Figure 4(a) shows that the mCD200-COMP binds to peritoneal macrophages and this binding can be completely blocked by prior incubation of the cells with CD200R mAb (OX102) but not control mAb (OX21). The labelling pattern was similar to that of the CD200R mAb (Fig. 4b). No labelling was obtained with the mCD200-SP-D construct (Fig. 4c), compatible with the fast dissociation observed with the BIAcore. Titration of the mCD200-COMP showed that labelling decreases with dilution but is still detectable at 2·7 nM (Fig. 4d) paralleling the sensitivity expected on the BIAcore (Fig. 3). Note that the cell labelling experiment was performed at 4° whilst the BIAcore experiment was carried out at 25° so differences in labelling might be the result of both temperature and steric differences.
Figure 4.
Figure4 mCD200-COMP but not mCD200-SP-D binds rat CD200R on peritoneal macrophages. (a) Flow cytometry profiles showing specific binding of mCD200-COMP protein (thin line) but no binding of Control-COMP (solid fill) to rat peritoneal macrophages. The binding is specific as it can be blocked by prior incubation of the rat peritoneal cells with the mAb OX102 that recognizes rat CD200R (thick line). (b) Comparable binding to the CD200R was obtained with the OX102 mAb (line) compared with an isotype-matched negative control mAb (OX21, solid fill). (c) No binding was obtained with the trimer mCD200-SPD protein (solid line) compared to control SPD protein (solid fill). (d) Specific binding was still obtained with the pentamers at lower concentrations of 13 and 2·7 nm. Rat peritoneal exudate cells were incubated for 1 hr at 4º with the multivalent CD200 reagents, followed by detection with mCD4-FITC-conjugated mAb (a) (c) (d), or in (b) with FITC-labelled anti-mouse immunoglobulin antibody. Cells shown in the histograms were gated on the CD11b/c+ macrophage population.
CD200 pentamers are less efficacious than mAb in ameliorating EAE
Studies from knockouts and in vitro experiments indicate that CD200 engagement of CD200R gives a down-regulatory signal to macrophages.8,13 CD200R-Fc fusion proteins exacerbated inflammatory disease models8 whereas CD200-Fc fusion proteins inhibited similar diseases.12 The possibility that a non-blocking CD200R mAb might ameliorate disease models through an agonistic signal was investigated by testing the OX110 mAb in EAE. Administration of OX110 mAb just as symptoms arose gave about 2 days delay in the progress of the disease but similar levels of mCD200-COMP protein gave no significant effect (data not shown). This may be because the protein was cleared too rapidly to have functional effects. Blood samples taken at intervals after injection showed that the Control-COMP was detectable at 12 hr in all mice but was lost by 36 hr, the mCD200-COMP was detected in 50% of the mice at 12 hr but was lost by 24 hr (data not shown). Thus the COMP was cleared from the circulation much more rapidly than mAbs whose half-lives are typically greater than 10 days.31
Stability of pentamer binding to cells
Functional effects of surface-bound reagents depend on binding capacity and stability and on potential internalization of the complex. The stability of the mCD200-COMP binding to cells was compared to that of a high-affinity OX102 mAb (Fig. 5). At 4°, there was little change in the level of binding with up to 4 hr of washing whereas the labelling with mCD200-COMP slowly decreased but was still detectable after 4 hr. At 37° in the presence of sodium azide, mAb binding was unaffected whereas mCD200-COMP decreased but was still detectable after 4 hr. At 37° in the absence of sodium azide, mAb binding was unaffected whereas the mCD200-COMP was cleared after 1 hr. Thus the higher multimerization as a result of the COMP probably causes internalization and loss of the mCD200-COMP, limiting its usefulness in long-term assays.
Figure 5.
Stability of mCD200-COMP and CD200R mAb to cell surface expressed CD200R. Rat macrophages were incubated for t = 1, t = 2, t = 3, or t = 4 hr, at either 4° or 37°, in the presence or absence of NaN3, with (a) 0·2 μm of mCD200-COMP (line) or Control-COMP (solid fill); (b) CD200R mAb (OX102, line) or isotype-matched negative control mAb (OX21, solid fill). Cells were washed extensively between each indicated time-point before incubation with (a) FITC-labelled anti-mouse CD4-conjugated secondary antibody or (b) FITC anti-mouse immunoglobulin. Cells shown in the labelling were gated on the CD11b/c+ population.
Pentamers are efficient in recognizing other weak interactions
CD200 binds its receptor with a KD ∼ 1 μM but other interactions are considerably weaker such as that between CD48 and CD2 (KD ∼ 70 μM).5 COMP chimeras containing the extracellular region of rat CD48 and rCD4d3+4 were produced as for CD200. The recombinant proteins were purified and tested for binding on the BIAcore (Fig. 6a). The CD48-COMP gave good binding to levels below 5 nm but had a detectable dissociation rate compared to the mCD200-COMP. In cell-binding assays CD48-COMP gave good binding to Jurkat T-cell lines transfected with rat CD2.24 Thus the CD48-COMP could bind CD2 in both BIAcore and cell-binding experiments, demonstrating that interactions with a KD close to 100 μM can be detected with these pentameric fusion proteins that may have general applicability.
Figure 6.
Binding of pentameric CD48-COMP protein to rat CD2. (a) BIAcore analysis showing that varying concentrations of CD48-COMP interacted with CD2 immobilized on the BIAcore at 25°. The bar shows the period in which protein was passed over the chip. The results shown are an overlay of the traces of pentamer at the concentrations indicated with backgrounds subtracted. (b) CD48-COMP binding to Jurkat cells transfected with CD2. The binding can be blocked by prior treatment of the cells with CD2 mAb showing the specificity of CD48-COMP binding.
Use of recombinant COMP proteins for the detection of low-affinity interactions in high-throughput assays
The finding that the COMP proteins detected protein interactions that were weak in their monomeric state suggested that these pentamers might be effective in high throughput assays for the identification of leucocyte interactions. Thus the mCD200-COMP protein was tested in a protein microarray assay. Serial dilutions of mouse CD200R protein were immobilized directly on epoxy-coated glass slides and tested with either mCD200-COMP protein or multivalent beads (schematic diagrams in Fig. 7a, b). The array was incubated with mCD200-COMP and binding detected with Alexa 555-labeled mouse CD4 mAb (green) whilst protein immobilized was detected with Alexa 647-labeled rat CD4 mAb (red). Specific binding of mCD200-COMP to CD200R was obtained relative to the minimal binding of a Control-COMP protein identified by red staining only, representing binding to the CD4 tag present in the immobilized recombinant proteins (Fig. 7c). The minimum amount of immobilized CD200R that could be detected was about 3 μg/ml (Fig. 7e). We had previously used beads coupled with recombinant monomeric protein (human CD200) to make avid binding reagents3,6,24 and had shown that these could also be used in protein microarrays to detect human CD200R.26 Here, we have used biotinylated mCD200 proteins coupled to avidin-coated FITC beads to probe immobilized mCD200R (Fig. 7d). These beads gave comparable levels of binding to the mCD200-COMP (Fig. 7f).
Figure 7.
Detection of immobilized CD200R proteins with multimeric ligands mCD200-COMP and mCD200-beads [schematic representation in panels (a) and (b), respectively]. Serial two-fold dilutions of purified, soluble, recombinant mCD200RCD4d3+4 hybrid protein, was arrayed onto epoxy-coated microscope slides as described previously.26 Each protein dilution series was arrayed in quadruplicates of two rows of six spots, ranging in concentration from 80 μg/ml (first spot) to 0·16μg/ml (spot no. 10), with control spotting buffer containing 0·5 mg/ml BSA in the last two spots. All arrays were performed in duplicate and a representative set of two replicates per dilution series is shown in (c) and (d) whereas diagrams (e) and (f) represent the mean fluorescence intensity ± SEM of both sets. (c) Each slide was incubated either for 1 hr at 4° with 20μg/ml of pentavalent mCD200-COMP protein or Control-COMP prior to incubation for 1 hr at 4° with anti-mCD4 mAb (YTA 3.1.2, detecting the mCD4d3+4 antigenic tag of the recombinant COMP proteins) labelled with Alexa 555 (indicated as green fluorescence at 532 nm) or control anti-rCD4 mAb (OX68, detecting the rCD4d3+4 antigenic tag of the recombinant mCD200R protein captured in the array), labelled with Alexa 647 (indicated as red fluorescence when measured at 635 nm). (d) Each slide was incubated for 1 hr at 4° with polyvalent mCD200 FITC-fluorescent beads or Control CD4d3+4 beads in combination with OX68-Alexa 647. Slides were scanned at 532 and 635 nm and images were merged. Quantitative measurements were made as described before26 and fluorescence units at 532 nm (green) are plotted against the amount of mCD200R protein arrayed.
Discussion
Interactions between the proteins at the surfaces of leucocytes are normally of low affinity with fast dissociation rates.1,2,5 Therefore when purified recombinant proteins are used to study such interactions, they need to be made multivalent to obtain sufficient avidity to avoid dissociation during the course of the assay. Common methods involve using fusion proteins containing the extracellular region of the surface protein coupled to the Fc region of IgG to generate dimers or making tetramers with streptavidin, which has worked particularly well for major histocompatibility complex antigens.32 We describe the use of putative trimers using a sequence from the surfactant protein SP-D27 and pentamers using a sequence from the cartilage oligomeric matrix protein COMP that forms stable pentamers.29,33 The SP-D sequence did not give a good binding reagent in contrast to studies where the same SP-D sequence was used to make trimeric proteins with the globular regions of C1q B chain27 or when the complete SP-D sequence apart from the lectin domain was used to make dodecameric forms of CD40L.34 There might be steric hindrance at the join with the CD4 domain which was not seen with other domain types35 and additional linkers may be required to ensure stable folding. However the pentamers gave highly avid reagents valuable for the detection of weak surface interactions as shown with the CD200-CD200R and CD48-CD2 interactions that have KD in the range 1–100 μm. The binding of the mCD200-COMP to macrophages was specific and the COMP reagents may be preferable to Fc fusion protein in terms of giving low levels of non-specific binding to cells with high levels of Fc receptors such as macrophages.
The COMP sequence has been used before in studies on cadherins29 and more recently as a fusion protein with angiopoietin-1 to give a potent blocking reagent.36 The use of pentamers in vivo will be hampered by their rapid clearance judging from the current studies. They may be of value for more acute situations such as the angiopoietin interaction discussed above and as reagents to down modulate surface proteins (Fig. 6). However, the pentamers provide avid reagents that are capable of detecting very weak interactions with monomeric half lives of less than 1 second and with similar sensitivity to the multimeric bead assays and that may be of value in in vitro assays. We have shown that the COMP proteins are capable of detecting receptor ligand interactions in a protein microarray format.
Acknowledgments
The authors are grateful to Jürgen Engel and Andrea Tomschy for the COMP sequences and advice, Hans-Jurgen Hoppe and Ken Reid for the SP-D sequence, to Steve Cobbold for the YTA3.1.2 anti-mouse CD4 mAb, Steve Simmonds for help with the experiments on EAE, Holly Cherwinski for the CD200-Fc fusion protein and Nigel J Saunders for help with microarrays. This research was supported by the Medical Research Council and the Arthritis Research Council.
Abbreviations
- BirA
biotin ligase
- IgSF
immunoglobulin superfamily
References
- 1.van der Merwe PA, Barclay AN. Transient intercellular adhesion: the importance of weak protein–protein interactions. Trends Biochem Sci. 1994;19:354–8. doi: 10.1016/0968-0004(94)90109-0. 10.1016/0968-0004(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 2.van der Merwe PA, Barclay AN. Analysis of cell–adhesion molecule interactions using surface plasmon resonance. Curr Opin Immunol. 1996;8:257–61. doi: 10.1016/s0952-7915(96)80065-3. 10.1016/S0952-7915(96)80065-3. [DOI] [PubMed] [Google Scholar]
- 3.Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–42. doi: 10.1016/s1074-7613(00)00023-6. 10.1016/S1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 4.Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–46. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 5.van der Merwe PA, Brown MH, Davis SJ, Barclay AN. Affinity and kinetic analysis of the interaction of the cell adhesion molecules rat CD2 and CD48. EMBO J. 1993;12:4945–54. doi: 10.1002/j.1460-2075.1993.tb06188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the NK and T cell immunoglobulin superfamily surface protein is a ligand for CD48. J Exp Med. 1998;188:2083–90. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanslow WC, Anderson DM, Grabstein KH, Clark EA, Cosman D, Armitage RJ. Soluble forms of CD40 inhibit biologic responses of human B cells. J Immunol. 1992;149:655–60. [PubMed] [Google Scholar]
- 8.Hoek RM, Ruuls SR, Murphy CA, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–71. doi: 10.1126/science.290.5497.1768. 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C, Muller WA. Putting the brakes on innate immunity: a regulatory role for CD200? Nat Immunol. 2001;2:17–19. doi: 10.1038/83124. 10.1038/83124. [DOI] [PubMed] [Google Scholar]
- 10.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–90. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 11.Gorczynski RM, Chen Z, Yu K, Hu J. CD200 immunoadhesin suppresses collagen-induced arthritis in mice. Clin Immunol. 2001;101:328–34. doi: 10.1006/clim.2001.5117. 10.1006/clim.2001.5117. [DOI] [PubMed] [Google Scholar]
- 12.Gorczynski RM, Cattral MS, Chen Z, et al. An immunoadhesin incorporating the molecule OX-2 is a potent immunosuppressant that prolongs allo- and xenograft survival. J Immunol. 1999;163:1654–60. [PubMed] [Google Scholar]
- 13.Foster-Cuevas M, Wright GJ, Puklavec MJ, Brown MH, Barclay AN. Human herpesvirus 8, K14 protein mimics CD200 in down-regulating macrophage activation through CD200 receptor. J Virol. 2004;78:7667–76. doi: 10.1128/JVI.78.14.7667-7676.2004. 10.1128/JVI.78.14.7667-7676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–51. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 15.Qin S, Cobbold S, Tighe H, Benjamin R, Waldmann H. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur J Immunol. 1987;17:1159–65. doi: 10.1002/eji.1830170813. [DOI] [PubMed] [Google Scholar]
- 16.Brown MH, Barclay AN. Expression of immunoglobulin and scavenger receptor superfamily domains as chimeric proteins with domains 3 and 4 of CD4 for ligand analysis. Prot Eng. 1994;7:515–21. doi: 10.1093/protein/7.4.515. [DOI] [PubMed] [Google Scholar]
- 17.Hsiung L, Barclay AN, Brandon MR, Sim E, Porter RR. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982;203:293–8. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preston S, Wright GJ, Starr K, Barclay AN, Brown MH. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur J Immunol. 1997;27:1911–18. doi: 10.1002/eji.1830270814. [DOI] [PubMed] [Google Scholar]
- 19.Al-Shamkhani A, Mallett S, Brown MH, James W, Barclay AN. Affinity and kinetics of the interaction between soluble trimeric OX40 ligand, a member of the tumor necrosis factor superfamily, and its receptor OX40 on activated T cells. J Biol Chem. 1997;272:5275–82. doi: 10.1074/jbc.272.8.5275. 10.1074/jbc.272.8.5275. [DOI] [PubMed] [Google Scholar]
- 20.Motwani M, White RA, Guo N, Dowler LL, Tauber AI, Sastry KN. Mouse surfactant protein-D. cDNA cloning, characterization, and gene localization to chromosome 14. J Immunol. 1995;155:5671–7. [PubMed] [Google Scholar]
- 21.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucl Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis SJ, Ward HA, Puklavec MJ, Willis AC, Williams AF, Barclay AN. High level expression in Chinese hamster ovary cells of soluble forms of CD4 T lymphocyte glycoprotein including glycosylation variants. J Biol Chem. 1990;265:10410–18. [PubMed] [Google Scholar]
- 23.Hanna SM, Kirk P, Holt OJ, Puklavec MJ, Brown MH, Barclay AN. A novel form of the membrane protein CD147 that contains an extra Ig-like domain and interacts homophilically. BMC Biochem. 2003;4:17. doi: 10.1186/1471-2091-4-17. 10.1186/1471-2091-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MH, Preston S, Barclay AN. A sensitive assay for detecting low-affinity interactions at the cell surface reveals no additional ligands for the adhesion pair rat CD2 and CD48. Eur J Immunol. 1995;25:3222–8. doi: 10.1002/eji.1830251204. [DOI] [PubMed] [Google Scholar]
- 25.Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–43. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 26.Letarte M, Voulgaraki D, Hatherley D, Foster-Cuevas M, Saunders NJ, Barclay AN. Analysis of leukocyte membrane protein interactions using protein microarrays. BMC Biochem. 2005;6:2. doi: 10.1186/1471-2091-6-2. 10.1186/1471-2091-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishore U, Strong P, Perdikoulis MV, Reid KB. A recombinant homotrimer, composed of the alpha helical neck region of human surfactant protein D and C1q B chain globular domain, is an inhibitor of the classical complement pathway. J Immunol. 2001;166:559–65. doi: 10.4049/jimmunol.166.1.559. [DOI] [PubMed] [Google Scholar]
- 28.Hakansson K, Lim NK, Hoppe HJ, Reid KB. Crystal structure of the trimeric alpha-helical coiled-coil and the three lectin domains of human lung surfactant protein D. Structure Fold Des. 1999;7:255–64. doi: 10.1016/s0969-2126(99)80036-7. 10.1016/S0969-2126(99)80036-7. [DOI] [PubMed] [Google Scholar]
- 29.Tomschy A, Fauser C, Landwehr R, Engel J. Homophilic adhesion of E-cadherin occurs by a co-operative two-step interaction of N-terminal domains. EMBO J. 1996;15:3507–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson MW, Barclay AN, Singer MS, Rosen SD, van der Merwe PA. Affinity and kinetic analysis of l-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J Biol Chem. 1998;273:763–70. doi: 10.1074/jbc.273.2.763. 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- 31.Talbot PJ, Buchmeier MJ. Catabolism of homologous murine monoclonal hybridoma IgG antibodies in mice. Immunology. 1987;60:485–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. 10.1126/science.274.5284.94. [PubMed] [Google Scholar]
- 33.Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. The crystal structure of a five-stranded coiled coil in COMP. a prototype ion channel? Science. 1996;274:761–5. doi: 10.1126/science.274.5288.761. 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- 34.Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur J Immunol. 2001;31:3094–100. doi: 10.1002/1521-4141(2001010)31:10<3094::aid-immu3094>3.0.co;2-f. 10.1002/1521-4141(2001010)31:10<3094::AID-IMMU3094>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Head JF, Mealy TR, McCormack FX, Seaton BA. Crystal structure of trimeric carbohydrate recognition and neck domains of surfactant protein A. J Biol Chem. 2003;278:43254–60. doi: 10.1074/jbc.M305628200. 10.1074/jbc.M305628200. [DOI] [PubMed] [Google Scholar]
- 36.Cho CH, Kammerer RA, Lee HJ, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–52. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]