Abstract
Here we review the evidence for immune cells expressing multiple components of the serotonergic and dopaminergic systems that are more commonly associated with the central nervous system (CNS). We discuss where and how peripheral encounters with these biogenic monoamines occur and posit reasons as to why the immune system would wish to deploy these pathways. A full taxonomy of serotonergic and dopaminergic constituents and their workings in component cells of the immune system should facilitate the formulation of novel therapeutic approaches in diseases characterized by immune dysfunction and potentially provide a range of surrogate peripheral markers for registering and monitoring disturbances within the CNS.
Keywords: central nervous system, dopamine, lymphocytes, serotonin, transporters
Immune encounters with serotonin
Location
Serotonin (5-hydroxytryptamine/5-HT), though conventionally considered a neurotransmitter, is produced primarily by the enterochromaffin cells of the gut.1 A recent study in rhesus macaques revealed that both CD3+ (T cells) and CD20+ (B cells) lymphocytes sit proximal to 5-HT-containing enteroendocrine cells.2 The 5-HT released at these sites on mechanical (or psychological/emotional) stress is taken up via an active transport mechanism into a number of cell types with platelets providing an especially rich reservoir of serotonin in the circulation. Subsequent release of platelet-stored 5-HT can be rapid, triggered for example by platelet-activating factor, thrombin, complement component fragments C3a and C5a, and immunoglobulin E-containing immune complexes. At sites of inflammation and platelet activation, local concentrations of 5-HT could greatly exceed the relatively low amounts found free in serum. Both primary and secondary lymphoid organs are innervated with nerve fibres,3 as is skin,4 while even under non-pathological conditions both CD20+ B cells and activated T-cell ‘blasts’ can apparently cross the blood–brain barrier.5 Thus both in the periphery and the central nervous system (CNS) immune cells have the potential to be exposed directly to 5-HT flow (summarized schematically in Fig. 1). Though much play is made of mast cells as an important source of 5-HT in rodents, serotonin is normally absent from human mast cells, being found only when they are associated with discrete pathologies such as in the stroma of carcinoid tumours and in mastocytosis.
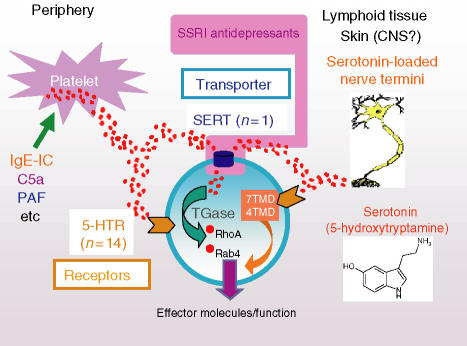
Figure 1.
5-HT stored in platelets or loaded into nerve fibres/cells within the circulation, skin, primary/secondary lymphoid tissues and even possibly the CNS is released on appropriate immune/inflammatory (stress/emotional?) activation. Immune cells exposed to 5-HT flow respond via receptors (up to 14 potentially, majority of which are 7-transmembrane domain (TMD) G-coupled proteins; 5-HT3 is a 4TMD cation ion channel) and/or the serotonin transporter, serotonin transporter (SERT; also a target for the SSRI antidepressants that block 5-HT uptake). Novel SERT-mediated signalling pathway recently described by Walther and colleagues in platelets (15) may also operate in immune cells where direct SERT-driven change has been shown (11, 13): transported 5-HT via transglutaminase (TGase) used to ‘serotonylate’ small GTPases such as RhoA and Rab4, both of which impact lymphocyte (and other immune cell) effector function.
Receptors
There are numerous reports of 5-HT modulating immune cell function. Of the 14 currently known receptor subtypes for 5-HT (see Table 1 for nomenclature and molecular characteristics), transcripts for eight have been found in rat immune tissues.6 Particularly for the promotion of T-cell proliferation, a major target for serotonin action appears to be the 5-HT1A receptor, a property conserved between mammals and fish. B lymphocytes also carry this receptor subtype (among others) and, as with T cells, display NF-κB-dependent up-regulation of both transcripts and protein for the 5-HT1A receptor. At least for rodent splenic B cells, this receptor subtype seems to be involved in the 5-HT-promoted augmentation of mitogenic responses to both lipopolysaccharide and dextran sulphate (reviewed in ref. 6). Interestingly, serotonin, as well as the selective 5-HT1A receptor agonist R-DPAT, were shown recently to increase cell survival and S-phase transition in mouse splenocytes stimulated by T- or B-cell mitogens: these processes being accompanied by translocation of NF-κB to the nucleus.7 These observations in toto point to a potential 5-HT1A receptor/NF-κB-dependent amplification loop in lymphocyte responses to serotonin. Extracellular signal regulated kinase (ERK) phosphorylation is another downstream signalling consequence of ligating 5-HT1A receptors in peripheral blood mononuclear cells.8 In addition to its activity on lymphocytes, the 5-HT1A receptor has been implicated in up-regulating phagocytic function in mouse peritoneal macrophages.9
Table 1.
Molecular, functional and pharmacological information on the human proteins that recognize 5-HT
| 5-HT1A | 5-HT1B | 5-HT1D | 5-ht1E | 5-ht1F | |
| Structure | 7TMD | 7TMD | 7TMD | 7TMD | 7TMD |
| Transduction | Gi/o | Gi/o | Gi/o | Gi/o | Gi/o |
| Selective agonist | 8-OHDPAT | none | none | none | LY334370 |
| Selective antagonist | WAY100635 | SB236057 | BRL15572 | none | none |
| 5-HT2A | 5-HT2B | 5-HT2C | 5-HT3 | 5-HT4 | |
| Structure | 7TMD | 7TMD | 7TMD | 4TMD1 | 7TMD |
| Transduction | Gq/11 | Gq/11 | Gq/11 | Integral cation channel | GS |
| Selective agonist | none | none | none | none | BIMU8 |
| Selective antagonist | MDL100907 | none | SB242084 | Ondansetron | SB204070 |
| 5-ht5A | 5-ht5B | 5-ht6 | 5-HT7 | 5-HTT | |
| Structure | 7TMD | 7TMD2 | 7TMD | 7TMD | 12TMD |
| Transduction | unknown | unknown | GS | GS | Transport |
| Selective agonist | none | none | none | none | 5-HT3 |
| Selective antagonist | none | none | SB271046 | SB656104 | Paroxetine4 |
In accordance with IUPHAR recommendations, receptors in lower case appellation are classified as gene products because of the absence of a defined response in native tissue.
The 5-HT3 receptor is comprised of five proteins which form a ligand-gated cation channel. To date, five different 5-HT3 receptor subunits have been identified (5-HT3A, 5-HT3B, 5-HT3C, 5-HT3D and 5-HT3E) although only the 5-HT3A and 5-HT3B have been studied in detail. To date, no pharmacological tools are able to discriminate between the 5-HT binding sites of the expressed isoforms (presence of the 5-HT3A subunit appears essential to form a functional receptor).
Expression of the 5-ht5B gene in humans is doubtful because of the presence of a stop codon in the gene sequence which would result in a heavily truncated, presumably non-functional, protein. A number of additional ‘orphan’ 5-HT receptors (e.g. 5-HT1P) have also been described although no corresponding gene sequences have been identified. TMD, transmembrane domain.
5-HT is the transported substrate of the 5-HTT.
Paroxetine blocks the transporter.
A recent detailed study highlighted the expression of 5-HT receptors, the signalling pathway they engage, and the biological activity of 5-HT on human dendritic cells (DC). Immature DC preferentially expressed transcripts for the 5-HT1B, 5-HT1E and 5-HT2B receptors, while mature DC mostly expressed 5-HT4 and 5-HT7 receptors. The use of isotype-selective receptor agonists showed that 5-HT stimulated 5-HT3-dependent Ca2+ influx in both immature and mature DC whereas 5-HT1 and 5-HT2 receptor stimulation induced intracellular Ca2+ in immature DC only. Activation of 5-HT4 and 5-HT7 induced cAMP elevation in mature DC while enhancing the release of interleukin-1β (IL-1β) and IL-8 and reducing the secretion of IL-12 and tumour necrosis factor-α.10
Transporter
Cell lines generated from B lymphocytes transformed with Epstein–Barr virus have provided a rich and productive resource for genotyping polymorphisms in the promoter region and intron 2 of the serotonin transporter (sert). Yet a functional role for lymphoid SERT has, until recently, been essentially ignored. We recently reviewed in detail the evidence for lymphocytes expressing functional SERT.1 This was prompted by our finding of SERT protein and active 5-HT uptake in the constituent cells of Burkitt's lymphoma, a highly aggressive tumour characterized by an extraordinarily high mitotic index and phenotypically resembling B cells found in the germinal centres of secondary lymphoid tissues. The consequence of 5-HT entering these cells was the promotion of apoptosis, a process reversible by transporter blockers [such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine] but not 5-HT receptor antagonists.11 Interestingly, at higher concentrations, the SSRI are themselves pro-apoptotic for the lymphoma cells, offering the potential for a deliverable therapy in Burkitt's lymphoma.12 We have since found appreciable levels of SERT protein in a diverse range of B-cell malignancies representing stages of differentiation arrest from pre-B (B-cell precursor acute lymphoblastic leukaemia) through to plasma cells (multiple myeloma). Though 5-HT could affect a number of the malignancies (as registered by cell-cycle arrest), an apoptotic outcome appeared to be specific to Burkitt's lymphoma cells, reflecting their ready propensity to enter this pathway as a result of negligible expression of the Bcl-2 survival protein.13 SERT may additionally offer a target to amphetamine analogues, such as methylenedioxymethamphetamine (MDMA), in these cells. The impact of MDMA – also known by its street name, ‘Ecstasy’– on immune function was recently reviewed by Connor.14
Normal B cells also express SERT though in their basal state its levels are low. On stimulation, however, they rapidly acquire readily detectable SERT protein with characteristics similar, if not identical, to the 5-HT transporter found in brain. We are currently undertaking a full molecular comparison of lymphoid and neuronal SERT.13
Why should lymphocytes express the uptake machinery for 5-HT? One possible answer could find analogy to the mechanisms and consequences of 5-HT transport in the brain. Thus at local inflammatory sites of high 5-HT production, activated lymphocytes expressing functional SERT could serve to limit/regulate the amount of bioactive indoleamine available for external receptor-triggering; at the same time, any sequestered 5-HT protected from cellular monoaminoxidases (which are undoubtedly present in lymphocytes) could be stored for later release on appropriate stimulation at a site distal to the initial exposure. In addition, evidence has been presented for SERT acting not merely as a transport protein but as a signal transducer in its own right when contacting substrate. Moreover, SERT-delivered 5-HT was recently shown to affect signal transduction directly by a novel modification, the so-called ‘serotonylation’ of small GTPases (see Fig. 1).15 Thus, SERT appears to be potentially equipped to modify a lymphocyte's functional behaviour in a number of distinct ways. The precise unravelling of these is likely to be an area of intense research, providing fascinating insights into the way serotonin affects immune responses. Moreover, SERT is not restricted to lymphoid cells within the immune system: macrophages for example express both transcripts and protein with the appropriate pharmacology for the transporter.16
Immune encounters with dopamine
Location
As for serotonin, there is evidence that the innervation of lymphoid tissues can be dopaminergic in nature, particularly during psychological stress, thus providing a source of this catecholamine for immune cells in an appropriate locale when released on sympathetic activation.17 However, in addition to this potential paracrine flow is the recognition that immune cells themselves have a capacity for dopamine production whereupon the dopamine could be utilized either in paracrine or autocrine mode. Catecholamines are present in lymphocytes, macrophages and neutrophils and at least in the case of the first two, dopamine is actively synthesized in the cells from tyrosine via the intermediary l-dopa (reviewed in ref. 1).
Receptors
A comprehensive description of dopamine receptor expression in the immune system was undertaken by McKenna and colleagues who used flow cytometry and subtype-specific antibodies to study the distribution among peripheral blood leucocytes.18 Of the D1-like receptor family only D5 was detected, and of the D2-like receptor family all three receptor subtypes were found. T lymphocytes and monocytes had low expression of dopamine receptors, whereas neutrophils and eosinophils had moderate expression. B cells and natural killer cells showed higher and more consistent expression. Dopamine receptors D3 and D5 were found in most individuals whereas expression of D2 and D4 appeared more variable. Dopamine, via a receptor-dependent mechanism, also appears to modify directly the activity of regulatory T cells (Treg).19 The catecholamine was shown to reduce both the suppressive and trafficking activities of Treg through type 1 receptors (D1 and D5) which were abundantly expressed by these regulatory cells. Evidence was presented for dopamine having a negative impact on D1-like receptor-dependent ERK1/2 phosphorylation in the Treg population.
An intriguing study, recently reported by Ilani and co-workers, indicates the involvement of D3 receptors in a novel paradigm: the neurotransmitter-mediated brain regulation of peripheral T lymphocytes.20 Their study appeared to be spurred, at least in part, by the conflictingly low concentrations of dopamine found in the periphery compared to the relatively high amounts seemingly needed to exert discernible effects on immune cells. They resolved this paradox by demonstrating that: (1) only in-vitro-activated human T-cell ‘blasts’ and not resting T lymphocytes respond to D3 receptor stimulation; (2) peripheral T cells from rats injected with l-DOPA/carbidopa demonstrated properties identical to those of human T-blasts exposed to dopamine; (3) perturbations seen in the model systems were recaptured by peripheral T cells from patients with schizophrenia, postulating here that activated T blasts will have been exposed to increased brain levels of dopamine associated with the disease. The authors propose a ‘brain-to-T cells’ pathway whereby activated T-blasts that are known to cross the ‘blood–brain barrier’ are exposed and respond to dopaminergic flow before re-entering the circulation and transmitting knowledge/information of the encounter – via, for example, cytokine release – to peripheral T (and perhaps other immune) cells excluded from the CNS.
Transporter
We recently reviewed the published evidence for lymphocytes expressing a functioning uptake system for dopamine.1 As for sert, lymphocytes offer a readily accessible resource for genotyping polymorphisms in the dopamine transporter (DAT). Correspondingly, Mill and colleagues demonstrated and quantified DAT transcripts in cerebellum, temporal lobe and lymphocytes using quantitative real-time reverse transcription–polymerase chain reaction when investigating the impact on expression of individual variations in the 10-repeat allele of a variable number tandem repeat (VNTR) in the 3′-untranslated region of the transporter.21 Though also shown to contain immunoreactive DAT protein, no specific function for the transporter in lymphocytes has so far been proffered. Nevertheless, the same arguments offered above as to the teleology of SERT's presence in immune cells could be applied equally to DAT and an exploration of these postulates should prove a fertile area of future research activity.
Overview/synthesis of dopamine's encounters with immune cells
We have attempted in Table 2 to summarize the literature that is available regarding dopamine's impact on immune cells and/or the functioning of an immune response. It is evident that the bulk of reported effects are inhibitory rather than stimulatory. We wish to suggest that this could reflect in large part the generally accepted ‘toxicity’ displayed by the catecholamine against a variety of cells, both immune and non-immune. An overview of possible mechanisms that lead to this outcome is presented pictorially in Fig. 2. It needs to be determined which (combinations) of these are operative in specific immune cell responses to dopamine and related compounds.
Table 2.
Immunomodulatory effects of dopamine
| Concentration | Organism/Cell type | Effect | Proposed mechanism | Ref. |
|---|---|---|---|---|
| Immunosuppressive | ||||
| Dop 250 pm | Human T cells (CD4+/CD8+) activated with IL-2/anti-CD3 | ∼ 50% decrease in thymidine uptake | Activity at D1-like receptors. Effects reversed by D1-like receptor antagonist SCH23390 | 24 |
| Dop 10 nm | Human B and T cells | Apoptosis | Bcl-2/Bax and Fas/FasL alterations | 25 |
| Dop 30 nm | Human T cells activated with anti-CD3 | ↓ thymidine uptake;↓ cytokine release | D2 and D3 receptors (reversed by receptor antagonism) | 26 |
| Dop 100 nm–100 μM | Murine splenic B cells stimulated with anti-μ | Inhibition of proliferation (EC50 ∼ 10 μm) | 27 | |
| Dop 10 μm–100 μm | Murine splenic lymphocytes stimulated with Con A/LPS | Dose-dependent inhibition of thymidine uptake | 28 | |
| Continuous Dop infusion | Murine splenic T cells (in vivo) | ↓ numbers of IFN-γ- producing cells | D2 receptor. Effects reversed by D2R antagonist | 29 |
| Dop/Dop agonist 500 nm−1 mm | Human peripheral lymphocytes | Apoptosis | β-adrenergic receptor engagement at least partially responsible for effect | 30 |
| 6-OHDA50–250 μm | Human (male) peripheral blood cells | Apoptosis | Oxidative stress (H2O2 production). Reversed by antioxidants | 31 |
| Dop > 100 μm | Macrophage cell line RAW264.7 | ↓ proliferation,↑ apoptosis | Oxidative stress. Effects reversed by ascorbic acid | 32 |
| Dop 1 mm | Murine thymocytes | Apoptosis | Oxidative stress. Effects reversed by dithiothreitol | 33 |
| l-DOPA 1–100 μm | Human lymphocytes or murine splenocytes stimulated with LPS/ConA | ↓ thymidine uptake | Oxidative stress | 34 |
| Dop or l-DOPA10 or 100 μm | Human PBMC stimulated with T-cell (ConA) or B-cell (pokeweed) mitogens | ↓ thymidine uptake↓ IFN-γ release↓ number of IgG/IgM-secreting cells | 35 | |
| Dop 10 μm | Murine splenic and thymic cells stimulated with T-cell (ConA) or B-cell (LPS) mitogens | ↓ thymidine uptake, EC50 ∼ 10 μm | Could not be reversed with D2-like receptor antagonist | 36 |
| Dop 100 μm−1 mm | EBV-transformed B-lymphocytes | Impaired mitochondrial function | Suggested to be result of oxidative stress | 37 |
| Dopamine (in response to physical stress) | Mouse (in vivo) | Impaired Th1/Th2 cytokines and impaired NK response | D2 receptors (effects reversed with D2-like receptor antagonists) | 38 |
| Immunostimulatory | ||||
| Dop < 1 μm | Human T cells | Activation | Signalling via D2 and D3 receptors | 39 |
| Dop or l-DOPA infusions | Murine splenic T cells (in vivo) | Enhanced response of ConA/anti-CD3 stimulated splenocytes | Signalling through D2 receptors. Effects reversed by D2 receptor antagonist | 29 |
| Dop/Dop-R agonists | Murine splenocytes with ConA or LPS –in vivo and in vitro | Enhanced proliferation | Receptor-mediated | 40 |
| Dop levels ↓by lesions to dop. neurons | Mouse | Impaired proliferation of splenic lymphocytes; depressed NK activity | Inferred that dopaminergic pathways necessary for immune function | 41 |
| Dop receptor agonists | Mouse model (in vivo) | Enhanced immune responses | 42 | |
| 100 nmD2/D3 agonist | Human CD4+ or CD8+ T-cells | CD4+ cells shift to TH1.CD8+ cells triggered to produce IFN-γ | Signalling through D3 receptor (effects reversed by D3 antagonist U-maleate) | 20 |
Dop, dopamine; ConA, concanavalin A; IFN-γ, interferon-γ; LPS, lipopolysaccharide; NK, natural killer; PBMC, peripheral blood mononuclear cells; Th1, T helper type 1.
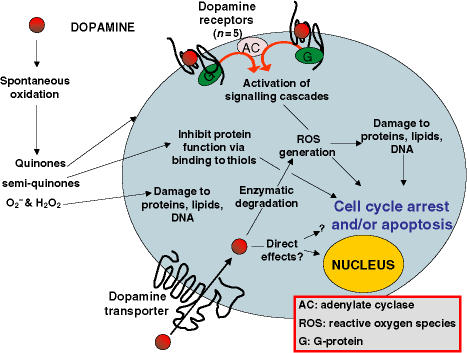
Figure 2.
Specific – receptor-mediated: Binding of the monoamine to dopamine receptors alters cAMP levels through modulation of adenylate cyclase (AC) – an enzyme to which these receptors couple (via stimulatory or inhibitory G-proteins). Activation of D1-like and D2-like receptors may inhibit T-lymphocyte activity (26) possibly via increases in cAMP levels (24). Co-activation of D1 and D2 dopamine receptors may initiate a unique signalling pathway, not activated by either D1 or D2 receptors alone (43). Alternatively, receptor ligation may initiate a signalling pathway that leads to oxidative stress (44). Specific – transporter-mediated: Dopamine can be actively internalised by cells that express the dopamine transporter (DAT). Intracellular dopamine may bind directly to cellular components such as GTPases to alter their activity (15) or enter the nucleus (45) and bind to nuclear elements (46) to alter transcriptional activity. Cellular dopamine can also chelate iron leading to cell cycle arrest (47). Inside the cell, dopamine can be metabolised by enzymes with the generation of reactive oxygen species (ROS) which may damage cellular components leading to cell-cycle arrest or death. Non-specific mechanisms: Extra-cellular dopamine can undergo auto-oxidation to generate quinones, semi-quinones and hydrogen peroxide (48). Quinones/semi-quinones may bind to thiol groups on cell-surface proteins and inhibit their activity. Hydrogen peroxide generation from dopamine is toxic to immunocytes (31, 37) possibly through the generation of superoxide and hydroxyl radicals which can damage lipids, proteins or DNA, culminating in cell cycle arrest or death.
Closing remarks
The cumulative data for immune cells expressing multiple molecular components that equip them to respond to both serotonin and dopamine are overwhelming. The precise roles of the receptors and transporters for these biogenic monoamines within the immune system are at present, however, less clear. Nonetheless, their eventual disclosure should provide fresh insights and offer new paradigms to the interconnections operating within the neuro–immune axis. Excitingly, this in turn will indicate novel therapeutic targets on immune cells for which pharmacologically well-characterized drugs are already available, a concept recently reviewed by two of us in some detail.1 Finally, the constituent cells of the circulating immune system provide a readily accessible source, and resource, of material that can potentially be exploited as surrogates to probe for markers of CNS-related disturbances. The examples of sert and dat polymorphisms have already been cited, the latter in relation to attention deficit disorder for example, the former in a wide variety of psychiatric disturbances including obsessive–compulsive disorder and major depression. Recently, the dopamine D3 receptor has begun to attract special interest with respect to the ‘peripheral marker hypothesis’ with mRNA levels reported as being disturbed in individuals with the personality trait of ‘persistence’, in cigarette smokers, and those suffering from schizophrenia;22 migraine sufferers show increased D5 receptor expression.23 A full taxonomy of the precise complement of serotonergic and dopaminergic components expressed by distinct immune cell subsets, together with the details of their regulation, will greatly facilitate and accelerate both the therapeutic and diagnostic promise being indicated by the intriguing, but limited, studies described so far.
Acknowledgments
We wish to thank the Leukaemia Research Fund for supporting our research in this field.
References
- 1.Gordon J, Barnes NM. Lymphocytes transport serotonin and dopamine: agony or ecstasy? Trends Immunol. 2003;24:438–43. doi: 10.1016/s1471-4906(03)00176-5. 10.1016/S1471-4906(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 2.Yang G-B, Lackner AA. Proximity between 5-HT secreting enteroendocrine cells and lymphocytes in the gut mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role for enterochromaffin cell 5-HT in mucosal immunity. J Neuroimmunol. 2004;146:46–9. doi: 10.1016/j.jneuroim.2003.10.044. 10.1016/j.jneuroim.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Felten D, Felten S, Bellinger D, Lorton D. Noradrenergic and peptidergic innervation of secondary lymphoid organs: role in experimental rheumatoid arthritis. Eur J Clin Invest. 1992;22:37–41. [PubMed] [Google Scholar]
- 4.Beresford L, Orange O, Bell EB, Miyan JA. Nerve fibres are required to evoke a contact sensitivity response in mice. Immunology. 2004;111:118–25. doi: 10.1111/j.1365-2567.2003.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain. 2003;126:1058–67. doi: 10.1093/brain/awg118. [DOI] [PubMed] [Google Scholar]
- 6.Serafeim A, Gordon J. The immune system gets nervous. Curr Opin Pharmacol. 2001;1:398–403. doi: 10.1016/s1471-4892(01)00069-8. 10.1016/S1471-4892(01)00069-8. [DOI] [PubMed] [Google Scholar]
- 7.Abdouh M, Albert PR, Drobetsky E, Filep JG, Kouassi E. 5-HT1A-mediated promotion of mitogen-activated T and B cell survival and proliferation is associated with increased translocation of NF-κB to the nucleus. Brain Behav Immun. 2004;18:24–34. doi: 10.1016/s0889-1591(03)00088-6. 10.1016/S0889-1591(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 8.Cloez-Tayarani I, Kayyali US, Fanburg BL, Cavaillon J-M. 5-HT activates ERK MAP kinase in cultured-human peripheral blood mononuclear cells via 5-HT1A receptors. Life Sci. 2004;76:429–43. doi: 10.1016/j.lfs.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Freire-Garabal M, Nunez MJ, Balboa J, et al. Serotonin upregulates the activity of phagocytosis through 5-HT1A receptors. Br J Pharmacol. 2003;139:457–63. doi: 10.1038/sj.bjp.0705188. 10.1038/sj.bjp.0705188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idzko M, Panther E, Stratz C, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172:6011–19. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 11.Serafeim A, Grafton G, Chamba A, Gregory CD, Blakely RD, Bowery NG, Barnes NM, Gordon J. 5-Hydroxytryptamine drives apoptosis in biopsy-like Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood. 2002;99:2545–53. doi: 10.1182/blood.v99.7.2545. [DOI] [PubMed] [Google Scholar]
- 12.Serafeim A, Holder MJ, Grafton G, et al. Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood. 2003;101:3212–19. doi: 10.1182/blood-2002-07-2044. [DOI] [PubMed] [Google Scholar]
- 13.Meredith EJ, Holder MJ, Chamba A, et al. FASEB J. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential anti-tumor target for psychotropics. in press. [DOI] [PubMed] [Google Scholar]
- 14.Connor TJ. Methylenedioxymethamphetamine (MDMA, ‘Ecstasy’): a stressor on the immune system. Immunology. 2004;111:357–67. doi: 10.1111/j.0019-2805.2004.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walther DJ, Peter JU, Winter S, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–62. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 16.Rudd ML, Nicolas AN, Brown BL, Fischer-Stenger K, Stewart JK. Peritoneal macrophages express the serotonin transporter. J Neuroimmunol. 2005;159:113–18. doi: 10.1016/j.jneuroim.2004.10.013. 10.1016/j.jneuroim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Bencsics A, Sershen H, Baranyi M, Hashim A, Lajtha A, Vizi ES. Dopamine, as well as norepinephrine, is a link between noradrenergic nerve terminals and splenocytes. Brain Res. 1997;761:236–43. doi: 10.1016/s0006-8993(97)00313-2. [DOI] [PubMed] [Google Scholar]
- 18.McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132:34–40. doi: 10.1016/s0165-5728(02)00280-1. 10.1016/S0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- 19.Kipnis J, Cardon M, Avidan H, Lewitus GM, Mordechay S, Rolls A, Shani Y, Schwartz M. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. J Neurosci. 2004;24:6133–43. doi: 10.1523/JNEUROSCI.0600-04.2004. 10.1523/JNEUROSCI.0600-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilani T, Strous R, Fuchs S. Dopaminergic regulation of immune cells via D3 dopamine receptor: a pathway mediated by activated T cells. FASEB J. 2004;18:1600–2. doi: 10.1096/fj.04-1652fje. [DOI] [PubMed] [Google Scholar]
- 21.Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3 UTR VNTR. Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–9. doi: 10.1002/ajmg.b.10948. 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 22.Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, Fuchs S. A peripheral marker for schizophrenia: increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci USA. 2001;98:625–8. doi: 10.1073/pnas.021535398. 10.1073/pnas.021535398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbanti P, Bronzetti E, Ricci A, Cerbo R, Fabbrini G, Buzzi M, Amenta F, Lenzi G. Increased density of dopamine D5 receptor in peripheral blood lymphocytes of migraineurs: a marker for migraine? Neuroscience Lett. 1996;207:73–6. doi: 10.1016/0304-3940(96)12491-5. [DOI] [PubMed] [Google Scholar]
- 24.Saha B, Mondal AC, Basu S, Dasgupta PS. Circulating dopamine level, in lung carcinoma patients inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors. Int Immunopharmacol. 2001;1:1363–74. doi: 10.1016/s1567-5769(01)00068-6. 10.1016/S1567-5769(01)00068-6. [DOI] [PubMed] [Google Scholar]
- 25.Bergquist J, Josefsson E, Tarkowski A, Ekman R, Ewing A. Measurements of catecholamine-mediated apoptosis of immunocompetent cells by capillary electrophoresis. Electrophoresis. 1997;18:1760–6. doi: 10.1002/elps.1150181009. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, Dasgupta P. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol. 2003;3:1019–26. doi: 10.1016/S1567-5769(03)00100-0. 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Wolfe S. Haloperidol and spiperone potentiate murine splenic B cell proliferation. Immunopharmacology. 1996;34:147–59. doi: 10.1016/0162-3109(96)00120-8. 10.1016/0162-3109(96)00120-8. [DOI] [PubMed] [Google Scholar]
- 28.Josefsson E, Bergquist J, Ekman R, Tarkowski A. Catecholamines are synthesized by mouse lymphocytes and regulate function of these cells by induction of apoptosis. Immunology. 1996;88:140–6. doi: 10.1046/j.1365-2567.1996.d01-653.x. 10.1046/j.1365-2567.1996.d01-653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr L, Tucker A, Fernandez-Botran R. In vivo administration of l-dopa or dopamine decreases the number of splenic IFN-gamma-producing cells. J Neuroimmunol. 2003;137:87–93. doi: 10.1016/s0165-5728(03)00047-x. [DOI] [PubMed] [Google Scholar]
- 30.Cioca DP, Watanabe N, Isobe M. Apoptosis of peripheral blood lymphocytes is induced by catecholamines. Jap Heart J. 2000;41:385–98. doi: 10.1536/jhj.41.385. 10.1536/jhj.41.385. [DOI] [PubMed] [Google Scholar]
- 31.Del Rio MJ, Velez-Pardo C. Monoamine neurotoxins-induced apoptosis in lymphocytes by a common oxidative stress mechanism: involvement of hydrogen peroxide (H2O2), caspase-3, and nuclear factor kappa-B (NF-κB), p53, c-Jun transcription factors. Biochem Pharmacol. 2002;63:677–88. doi: 10.1016/s0006-2952(01)00907-8. 10.1016/S0006-2952(01)00907-8. [DOI] [PubMed] [Google Scholar]
- 32.Brown SW, Meyers RT, Brennan KM, et al. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- 33.Offen D, Ziv I, Gorodin S, Barzilai A, Malik Z, Melamed E. Dopamine-induced programmed cell death in mouse thymocytes. Biochim Biophys Acta. 1995;1268:171–7. doi: 10.1016/0167-4889(95)00075-4. 10.1016/0167-4889(95)00075-4. [DOI] [PubMed] [Google Scholar]
- 34.Slominski A, Goodman-Snitkoff G. Dopa inhibits induced proliferative activity of murine and human lymphocytes. Anticancer Res. 1992;12:753–6. [PubMed] [Google Scholar]
- 35.Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci United States America. 1994;91:12912–16. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook-Mills J, Cohen R, Perlman R, Chambers D. Inhibition of lymphocyte activation by catecholamines: evidence for a non-classical mechanism of catecholamine action. Immunology. 1995;85:544–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Elkashef AM, Al-Barazi H, Venable D, Baker I, Hill J, Apud J, Wyatt RJ. Dopamine effect on the mitochondrial potential in B lymphocytes of schizophrenic patients and normal controls. Prog Neuro-Psychopharmacol Biol Psychiatr. 2002;26:145–8. doi: 10.1016/s0278-5846(01)00239-1. 10.1016/S0278-5846(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 38.Fierová A, Starec M, Kuldová M, Ková H, Páv M, Vannucci L, Pospíil M. Effects of D2-dopamine and -adrenoceptor antagonists in stress induced changes on immune responsiveness of mice. J Neuroimmunol. 2002;130:55–65. doi: 10.1016/s0165-5728(02)00211-4. 10.1016/S0165-5728(02)00211-4. [DOI] [PubMed] [Google Scholar]
- 39.Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function. European J Immunol. 2001;31:3504–12. doi: 10.1002/1521-4141(200112)31:12<3504::aid-immu3504>3.0.co;2-f. 10.1002/1521-4141(200112)31:12<3504::AID-IMMU3504>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Tsao C, Lin Y, Cheng J. Effect of dopamine on immune cell proliferation in mice. Life Sci. 1997;61:361–71. doi: 10.1016/s0024-3205(97)00962-4. [DOI] [PubMed] [Google Scholar]
- 41.Deleplanque B, Vitiello S, Le Moal M, Neveu P. Modulation of immune reactivity by unilateral striatal and mesolimbic dopaminergic lesions. Neurosci Lett. 1994;166:216–20. doi: 10.1016/0304-3940(94)90489-8. 10.1016/0304-3940(94)90489-8. [DOI] [PubMed] [Google Scholar]
- 42.Idova G, Cheido M, Zhukova E, Devoino L. Stimulation of the immune response during activation of the dopaminergic system in mice with opposite types of behavior. Neurosci Behav Physiol. 2004;34:417–21. doi: 10.1023/b:neab.0000018755.99063.05. 10.1023/B:NEAB.0000018755.99063.05. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, So C, Rashid A, Varghese G, Cheng R, Lança A, O'Dowd B, George SR. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–8. doi: 10.1074/jbc.M401923200. 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Rusnak M, Luedtke R, Sidhu A. D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J Biol Chem. 2004;279:39317–30. doi: 10.1074/jbc.M403891200. [DOI] [PubMed] [Google Scholar]
- 45.Bergquist. Catecholaminergic suppression of immunocompetent cells. Immunol Today. 1998;19:562–7. doi: 10.1016/s0167-5699(98)01367-x. [DOI] [PubMed] [Google Scholar]
- 46.Buu NT. Uptake of 1-methyl-4-phenylpyridinium and dopamine in the mouse brain cell nuclei. J Neurochem. 1993;61:1557–60. doi: 10.1111/j.1471-4159.1993.tb13656.x. [DOI] [PubMed] [Google Scholar]
- 47.Pifl C, Kattinger A, Reither H, Hornykiewicz O. Cellular effects of dopamine – beyond oxidative mechanisms. Parkinsonism Related Disorders. 2002;8:433–7. doi: 10.1016/s1353-8020(02)00028-7. 10.1016/S1353-8020(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 48.Graham D, Tiffany S, Bell WJ, Gutknecht W. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978;14:644–53. [PubMed] [Google Scholar]