Abstract
Surfactant protein D (SP-D) is a pattern-recognition molecule of the innate immune system that recognizes various microbial surface-specific carbohydrate and lipid patterns. In vitro data has suggested that this binding may lead to increased microbial association with macrophages and dendritic cells. The aim of the present in vivo study was to study the expression of porcine SP-D (pSP-D) in the lung during different pulmonary bacterial infections, and the effect of the routes of infection on this expression was elucidated. Furthermore, the aim was to study the in vivo spatial relationship among pSP-D, pathogens, phagocytic cells and dendritic cells. Lung tissue was collected from experimental and natural bronchopneumonias caused by Actinobacillus pleuropneumoniae or Staphylococcus aureus, and from embolic and diffuse interstitial pneumonia, caused by Staph. aureus or Arcanobacterium pyogenes and Streptococcus suis serotype 2, respectively. By comparing normal and diseased lung tissue from the same lungs, increased diffuse pSP-D immunoreactivity was seen in the surfactant in both acute and chronic bronchopneumonias, while such increased expression of pSP-D was generally not present in the interstitial pneumonias. Co-localization of pSP-D, alveolar macrophages and bacteria was demonstrated, and pSP-D showed a patchy distribution on the membranes of alveolar macrophages. SP-D immunoreactivity was intracellular in dendritic cells. The dendritic cells were identified by their morphology, the absence of macrophage marker immunoreactivity and the presence of dendritic cell marker immunoreactivity. Increased expression of pSP-D in the surfactant coincided with presence of pSP-D-positive dendritic cells in bronchus-associated lymphoid tissue (BALT), indicating a possible transport of pSP-D through the specialized M cells overlying (BALT). In conclusion, we have shown that pSP-D expression in the lung surfactant is induced by bacterial infection by an aerogenous route rather than by a haematogenous route, and that the protein interacts specifically with alveolar macrophages and with dendritic cells in microbial-induced BALT. The function of the interaction between pSP-D and dendritic cells in BALT remain unclear, but pSP-D could represent a link between the innate and adaptive immune system, facilitating the bacterial antigen presentation by dendritic cells in BALT.
Keywords: collectins, dendritic cells, immunolocalization, pig, porcine surfactant protein D
Introduction
Surfactant protein D (SP-D) is a collectin believed to play an important role in innate immunity and thus in the first line of the host defence against invading micro-organisms.1–4 The collectins are a family of collagenous, calcium-dependent carbohydrate-binding proteins, including mannan-binding lectin (MBL), the lung surfactant proteins A (SP-A) and D (SP-D), collectin-liver 1 (Cl-L1) and collectin-placenta 1 (Cl-P1); also included are the bovine collectins conglutinin, collectin-43 (Cl-43) and collectin-46 (Cl-46).1–5
SP-D appears to be a multifunctional protein. In in vitro studies it binds selectively to carbohydrates and lipids on microbial surfaces and mediates agglutination, neutralization, opsonization or direct lysis of the micro-organisms.2,6 SP-D enhances the in vitro production of superoxide by alveolar macrophages7 and inhibits T-lymphocyte proliferation.8 In both in vitro and in vivo studies, SP-D has been reported to bind to, and mediate the clearance of, apoptotic inflammatory cells by alveolar macrophages.9–11 Studies with SP-D knockout mice have also indicated a role of SP-D in the regulation of surfactant lipid homeostasis.12
Furthermore, Brinker and colleagues13 found that human SP-D binds to immature bone marrow-derived dendritic cells (DC) in vitro in a dose-, carbohydrate- and calcium-dependent manner, enhancing the phagocytic uptake of Escherichia coli and the antigen presentation to T cells.
The pSP-D cDNA sequence has been determined,14 the pSP-D protein has been purified and characterized,15,16 and the interaction between pSP-D and influenza A virus described.17,18 Furthermore, pSP-D was found to be immunolocalized in normal tissues (predominantly in Clara cells and in serous cells of the bronchial submucosal glands) and, to a lesser extent, in alveolar type II cells, in epithelial cells of the intestinal glands (crypts of Lieberkühn) in the duodenum, jejunum and ileum, and in serous cells of the dorsolateral lacrimal gland.15
DCs are the most potent antigen-presenting cells known.19 DCs exist in an immature state at mucosal surfaces, including the lungs, where they constantly survey the environment for the presence of foreign antigen.20 Porcine bronchus-associated lymphoid tissue (BALT), induced by Mycoplasma hyopneumoniae infection of the lungs, is known to be composed of DCs, macrophages, T and B lymphocytes, and IgG+ and IgA+ plasma cells.21 Little is known about the structure and function of BALT, but it is believed to equal that of the intestinal Peyer's patches. In the Peyer's patches antigen is taken up by specialized epithelial microfold (M) cells overlying the Peyer's patches and then transported through the cells, by transcytosis, to the underlying DCs and lymphocytes, enabling their interaction and induction of the acquired immune response. Pneumonia in pigs is considered to be the most serious disease problem in modern swine production.22 Entrance of the micro-organisms into the lungs occurs either by the aerogeneous route, which causes bronchopneumonia, or by the haematogenous route, causing either a focal (induced by emboli) or a diffuse interstitial (induced by septicaemia) pneumonia.23 In this study, tissue samples were collected and investigated from pigs that were either experimentally or naturally infected with common bacterial pathogens causing bronchopneumonia, embolic pneumonia or diffuse interstitial pneumonia. The aim was to elucidate the effect of infection and route of infection on the immunohistochemical expression of pSP-D. Furthermore, in light of the in vitro study of SP-D and DC by Brinker and colleagues,13 the aim was to study the spatial relationship among pSP-D, pathogens, phagocytic cells and DC in vivo.
Materials and methods
Experimental pulmonary infection
Actinobacillus pleuropneumoniae serotype 5b. Three, 12-week-old Landrace-Yorkshire crossbred pigs were infected, via aerosol, with A. pleuropneumoniae serotype 5, as previously described.24 Briefly, the pigs were pretreated with nebulized 1% acetic acid (pH 3·2) for 20 min, then infected with a nebulized broth containing 9·6 × 106 (one pig) or 38 × 106 (two pigs) colony-forming units (CFU) of A. pleuropneumoniae serotype 5. Two age-matched pigs of similar breed, pretreated with acetic acid only, served as uninfected controls. All animals were killed on day 1 postchallenge. Samples of pulmonary lesions and normal lung tissue from infected animals, lung tissue from the controls and tracheobronchial lymph nodes from all animals, were collected for bacteriology and for histology, as described below.
Streptococcus suis serotype 2. Formalin-fixed lung tissue, and the corresponding tracheobronchial lymph nodes, from four 6-week-old Landrace-Yorkshire crossbred pigs, systemically infected with Strep. suis, and from two uninfected control pigs of similar age and breed, were retrieved from a previous study of the pathogenesis of Strep. suis infection, as described by Madsen and colleagues.25 Briefly, septicaemia was induced in four pigs by aerogenous infection, and with the tonsils as the portal of entry, by using a nebulized 5-hr broth culture of Strep. suis serotype 2 (strain P321/6) containing 1010 CFU/ml. Infected animals were killed, or died before possible treatment, on day 2 (two pigs), day 3 (one pig) or day 4 (one pig) postchallenge. Lung tissue and tracheobronchial lymph nodes from all animals, as well as spleen and blood, were tested for bacteria to verify the presence (infected) or absence (uninfected) of Strep. suis septicaemia.
Natural pulmonary infections
Tissue samples for histology were collected from slaughter pigs (≈ 5 months of age) with pneumonia, at a Danish abattoir. Samples were taken within 2 hr after slaughter. From each animal, tissue samples were taken from two pulmonary lesions, normal lung tissue, two tracheobronchial lymph nodes (some showing reactive hyperplasia) and from the spleen. Bacteriology was performed on pulmonary lesions at two or three locations from each animal, and only animals with identical monocultures were selected for further study. These criteria generated 16 pigs, and additional information from these pigs, regarding gross pathological findings, were recorded when available.
Uninfected controls
As described above, four pigs served as uninfected controls for the experimental A. pleuropneumoniae pneumonia and the experimental Strep. suis septicaemia. Pulmonary tissue and tracheobronchial lymph nodes from two normal, five-month-old slaughter pigs were collected for histology, as described below, and used as age-matched uninfected controls for the natural pulmonary infections.
Bacteriology
The surface covering the pulmonary lesions was decontaminated by searing or by brief immersion of tissue in boiling water. By using a sterile scalpel blade, the lesion was incised and material removed. Cultures of this material were made on blood agar (Blood Agar Base, CM55; Oxoid, Basingstoke, Hampshire, UK), containing sterile bovine blood (5%) and bearing colonies of a nicotinamide adenine dinucleotide (NAD)-producing strain of Acinetobacter calcoacticus to enable V-factor (NAD)-dependent bacteria to grow. Parallel cultures were incubated in a candle jar and anarobically. Bacterial isolates were identified by standard methods for phenotypical characterization.26,27
Gross pathology and histochemistry
Lungs were examined macroscopically, and lesions were characterized when present. Tissue samples were fixed in 10% neutral-buffered formalin for 24–36 hr, paraffin wax-embedded, sectioned (2–5 µm) and mounted on conventional slides for histochemistry and fluorescence in situ hybridization (FISH), and on Superfrost®Plus slides (Merck Eurolab, Albertslund, Denmark) for immunohistochemistry (IHC) and immunohistofluorescence (IHF). The slides were initially heat-treated for 15 min at 60°, then taken through xylene and a series of graded alcohol preparations (99–70% v/v ethanol), tap water and finally washed three times (5 min each wash) in Tris-buffered saline [TBS; 7 mm Tris(hydroxymethyl)-aminomethan, 23 mm Tris–HCl, 0·15 m NaCl, pH 7·6]. Slides were stained with haematoxylin and eosin (H & E), according to standard procedures. For detecting Gram-positive and Gram-negative bacteria simultaneously, a modified version of the conventional Gram-stain28 was used by expanding the completed, conventional stain by subsequent treatments with 0·03% (v/v) 1% acetic acid in 37% formaldehyde (90 seconds), acetone (3 seconds), 0·05% (w/v) picric acid in acetone (3 seconds), acetone (3 seconds) and 50% (v/v) acetone in xylene (5 seconds). Sections with visible bacteria (as judged by H & E staining) taken from lungs with monoculture of Staphylococcus aureus or A. pleuropneumoniae, were included in each test round as positive staining controls.
FISH
For the specific detection of A. pleuropneumoniae, sections were examined by FISH using a fluorescein isothiocyanate (FITC)-labelled oligonucleotide probe (Ap185; MWGbiotech AG, Ebersberg, Germany), with the sequence 5′-CCCACCCTTTAATCCATAG-3′, targeting 16S rRNA of A. pleuropneumoniae, as previously described.29–31 In each test round, sections from lung tissue containing A. pleuropneumoniae, as verified by bacteriology and FISH, were included as positive staining controls.
IHC and IHF
Experiments were carried out to determine optimal antigen retrieval, dilution of primary antibodies and choice of detection system, and the antibodies used, their specificity and further information are listed in Table 1.
Table 1. Primary antibodies used in immunohistochemical and fluorescence-localization studies.
| Ref.* | Detection† | Ag retrieval‡ | Dilution | Source§ | Specificity | Antigen¶ | Antibody |
|---|---|---|---|---|---|---|---|
| Monoclonal Ab | |||||||
| 15 | PV+ | T-EG TA | 1 : 100 | DFVF | pSP-D | pSP-D | 1.7 |
| Ab-bio/strep-Cy3 | T-EGTA | 1 : 100 | DFVF | pSP-D | pSP-D | 1.7 | |
| 31 | PV+ | Protease | 1 : 100 | Serotec | Macrophages, monocytes, neutrophils | hL1 | MAC387 |
| 21 | APAAP | Protease | 1 : 20 | Serotec | DCs, macrophage, B lymphocyte, epithelium | hMHC-II | MCA1335 |
| 32 | APAAP | Protease | 1 : 40 | DAKO | Epithelium | h cytokeratin | M3515 |
| Polyclonal Ab | |||||||
| 21 | PV+ | None | 1 : 500 | DAKO | DCs, neural tissue, chondrocyte, endothelium | bS-100 | Z0311 |
| 33 | EV | None | 1 : 8000 | SSI | Strep. suis type I | Strep. suis type II | 22282,2A |
References for previously described reactivity in pig.
PV+, PowerVision+ kit (DPVB+110AEC) (Immunovision Technologies, Springdale, AR); Ab-bio/strep-Cy3(115-065-146/016-160-084) (Jackson ImmunoResearch, West Grove, PA); APAAP, alkaline phosphatase anti-alkaline phosphatase (D0651) (DakoCytomation, Glostrup, Denmark); EV, EnVision/AP (K4018) (DakoCytomation); detection was carried out according to the manufacturers'.
Antigen retrieval. T-EGTA, microwave oven in Tris-EGTA buffer, 2 ×5 min (700 Watts), cool 15 min; Protease, a 5-min incubation in Tris-buffered saline (TBS) containing 0.018% protease, followed by a 5-min wash in cold TBS, and a 5-min wash in running tap water.
DAKO, DakoCytomation, Glostrup, Denmark; DFVF, Danish Institute for Food and Veterinary Research, Copenhagen, Denmark; Serotec, Serotec Ltd., Oxford, UK; SSI, Statens Serum Institut, Copenhagen, Denmark.
b, bovine; h, human; p, porcine.
DC, dendritic cell; MHC-II, major histocompatibility complex II; pSP-D, porcine surfactant protein D; Strep. suis, Streptococcus suis.
The primary antibodies were all incubated overnight at 4°, but other steps were generally carried out at room temperature. Between each step, except after the blocking of non-specific protein binding, the sections were washed twice, for 5 min each wash, in TBS. The different detection systems were applied according to the instructions of the manufacturers. Fast Red (catalogue no. 4210; Kem-En-Tec, Copenhagen, Denmark) was generally used as chromogen, except that amino-ethyl-carbazole (AEC) was applied when using the PV+ detection system [PowerVision+ kit (DPVB +110AEC); Immunovision Technologies, Springdale, AR]. Finally, the tissue was counterstained with Mayer's haematoxylin and mounted with glycerol-gelatine.
Sections of normal porcine lung tissue, with previously identified pSP-D immunoreactivity,15 were included as positive staining controls in each test round of pSP-D staining (i.e. only rounds with immunoreactivity in control slides were regarded as valid). The specificity of the monoclonal antibody (mAb) 1·7 for purified pSP-D and pSP-D in bronchoalveolar lavage fluid has previously been shown by Western blot and N-terminal sequencing.15 Negative-staining controls of all tissues were generated by substituting the primary mAbs, all of which were of the IgG1 isotype, with an irrelevant monoclonal IgG1 (DAKOCytomation, no. X0931; Glostrup, Denmark) of identical concentration, and by substituting the primary polyclonal antibodies with normal rabbit immunoglobulins (DAKOCytomation, no. X0903) of similar concentration.
In addition to the specific detection of antigens, the extent of pSP-D immunoreactivity in the surfactant was also evaluated. Thus, comparison of pSP-D immunoreactivity in surfactant in pulmonary lesions was made to the baseline reactivity in normal lung tissue from the same animal (Table 2); in the Strep. suis infection with interstitial pneumonia, comparison was made with the uninfected controls.
Table 2. Effect of infection and route of infection on porcine surfactant protein D (pSP-D) immunoreactivity in the lungs.
| Route of infection | Type of pneumonia | Age of lesions | Infection type | No. of animals | Bacteria | pSP-D upregulation* | pSP-D present in DCs in BALT |
|---|---|---|---|---|---|---|---|
| Aerogeneous | Bronchopneu. | Acute | Experimental | 3 | A. pleuropn. | + | + |
| Chronic | Natural | 7 | A. pleuropn. | + | + | ||
| 1 | Staph. aureus | + | + | ||||
| Haematogenous | Embolic | Chronic | Natural | 2 | Staph. aureus | + | − |
| 3 | Staph. aureus | − | − | ||||
| 3 | Ar. pyogenes | − | − | ||||
| Interstitial† | Acute | Experimental | 4 | Strep. suis | − | ||
| Uninfected controls | 6 | − | − |
The extent of the pSP-D immunoreactivity in the surfactant in pulmonary lesions was compared to the extent in normal lung tissue from the same animal, or to the extent in uninfected controls, to determine the degree of up-regulation.
Systemic infection induced aerogeneously with the tonsils as entrance portal.25
A. pleuropneumoniae, Actinobacillus pleuropneumoniae; Ar. pyogenes, Arcanobacterium pyogenes; BALT, bronchus-associated lymphoid tissue; Bronchopneu., Bronchopneumonia; DCs, dendritic cells; Staph. aureus, Staphylococcus aureus; Strep. suis, Streptococcus suis.
Serial sections were stained for the detection of pSP-D immunoreactivity (IHC or IHF) and either bacteria (Gram-stain or FISH) or antigens (IHC) identifying phagocytic cells (Table 1). This enabled evaluation of the spatial relationhip among pSP-D, pathogens and phagocytic cells. The slides were examined by using either an Olympus BX 51 light microscope (Olympus, Ballerup, Denmark) or an zAxioplan 2 epifluorescence microscope (Carl Zeiss, Oberkochen, Germany), equipped for epifluorescence with a 75-W Xenon lamp, and filter sets 09 (Carl Zeiss) and XF34 (Omega Optical, Brattleboro, VT) were used to visualize fluorescein and CY3, respectively. A double filter set – XF53 (Omega Optical) – was used for the simultaneous detection of red and green fluorescence (FISH and IHF).
Results
Bacteriology
Bacteriology on the pulmonary lesions in the pigs experimentally infected with A. pleuropneumoniae confirmed infection, whereas uninfected animals were sterile. Strep.suis serotype 2 was identified in the lungs, tracheobronchial lymph nodes, spleen and blood from all infected animals, verifying the presence of septicaemia, and uninfected animals were sterile.25 The bacteria identified in monoculture in naturally infected pigs represented the Gram-positive Staph. aureus and Arcanobacteriumpyogenes and the Gram-negative A. pleuropneumoniae (Table 2).
Gross pathology and histochemistry
Acute fibrinous bronchopneumonia and pleuritis (acute pleuropneumoniae) (n = 3) was found in all the experimental, aerogeneous, A. pleuropneumoniae infections, while the natural pulmonary infections included chronic bronchopneumonia (n = 8) caused by A. pleuropneumoniae or Staph. aureus (Table 2). The chronic bronchopneumonias were mainly fibrinonecrotic pleuropneumoniaes, with sequestration and fibrotic incapsulation of the necrotic tissue in some of the lesions. The natural pulmonary infections also included chronic embolic apostematous pneumonia (n = 8) caused either by Staph. aureus or Ar. pyogenes (Table 2). Generally, these chronic abscesses consisted of a purulent centre, with or without bacterial colonies, encircled by a broad fibrous capsule. Tail bite wounds were found in five out of eight animals, and one of these five additionally had chronic osteomyelitis. The Strep. suis-infected lungs all showed acute diffuse interstitial pneumonia (n = 4) with mononuclear cell infiltrates dominated by macrophages.
IHC and IHF
Expression of pSP-D antigen related to lumina and epithelia of the lungs.
By comparing normal and diseased lung tissue, increased pSP-D immunoreactivity was seen in the surfactant in acute and chronic bronchopneumonias caused by either A. pleuropneumoniae or Staph. aureus (n = 11); moderately increased reactivity was seen in two of the chronic embolic pneumonias caused by Staph. aureus (Table 2).
The increased expression of pSP-D in the surfactant was seen as a strong and diffuse pSP-D immunoreactivity lining most alveoli (Fig. 1a,b). In these areas, enlarged and strongly pSP-D immunoreactive alveolar type II cells were frequently found (Fig. 1b), distinguishable from alveolar macrophages by their cytokeratin immunoreactivity (Fig. 1b, insert). In areas with damaged alveoli and subsequent epithelialization, the hyperplastic alveolar type II cells generally showed intense intracellular pSP-D immunoreactivity (data not shown). In the chronic lesions, immunoreactivity was confined to the periphery, and pSP-D was not found centrally (Fig. 1a).
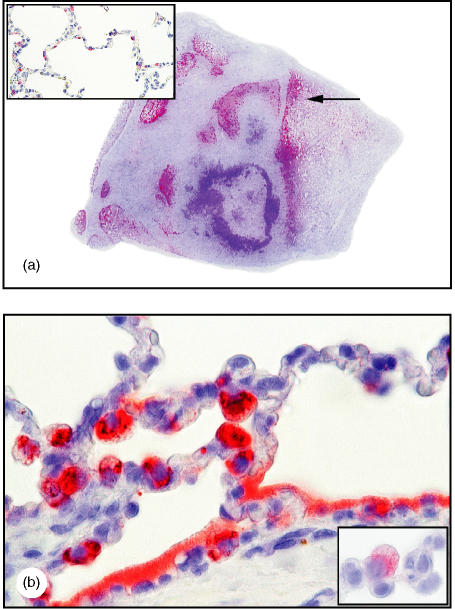
Figure 1.
Enhanced porcine surfactant protein D (pSP-D) immunoreactivity in chronic Actinobacillus pleuropneumoniae bronchopneumonia. (a) Strong pSP-D expression in lung tissue, with no pSP-D in fibrotic and necrotic areas (approximate magnification ×2). [The arrow indicates the area represented in (b). ] (b) Strong pSP-D immunoreactivity, diffuse in the surfactant lining alveoli and in hypertrophic alveolar type II cells, and distinguishable from alveolar macrophages by cytokeratin immunoreactivity (lower insert shown in panel b) (magnification ×250, oil). For comparison, in normal lung tissue pSP-D immunoreactivity is present in non-hypertrophic alveolar type II cells but not in the alveoli (upper insert) (magnification ×100). Detection was performed in the sections shown in panels (a) and (b) by using amino-ethyl-carbazole (AEC) and in the insert of (b) by using Fast red. All sections were counterstained with Mayers's haematoxylin.
The remaining chronic embolic pneumonias caused by Staph. aureus or Ar. pyogenes (n = 6), and all interstitial pneumonias caused by Strep. suis (n = 4), showed no increased expression of pSP-D. This was found by the general absence of pSP-D from the pulmonary surfactant, which was also the case in normal lung tissue, whether taken from diseased animals or uninfected controls (data not shown).
In the Staph. aureus and Ar. pyogenes pneumonias, bacteria were identified by histochemistry, with bacterial microcolonies frequently located centrally in the lesions. In A. pleuropneumoniae bronchopneumonias, bacteria were identified, by FISH, as microcolonies related to the lumina of the lungs. Immunohistochemistry showed that Strep. suis was confined to the intravascular space.
In both acute and chronic bronchopneumonias, serial sections showed a co-localization among pSP-D, macrophages/neutrophils and A. pleuropneumoniae or Staph. aureus(Fig. 2a –d).
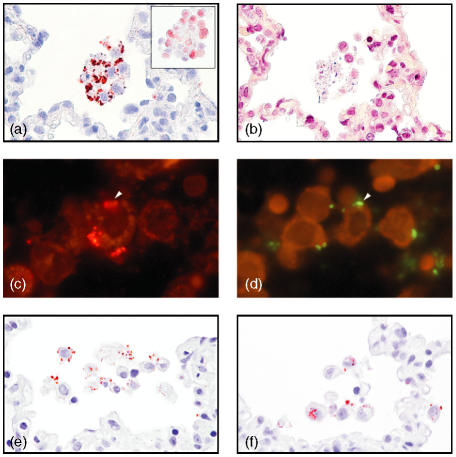
Figure 2.
Co-localization of porcine surfactant protein D (pSP-D), alveolar macrophages and bacteria. (a) In Staphylococcus aureus pneumonia, pSP-D was found to be localized in a patchy distribution on the membranes of alveolar macrophages, which show no signs of apoptosis/necrosis (magnification ×250, oil). The alveolar macrophages are identified by morphology and by hL1 immunoreactivity (insert) (magnification ×250, oil). (b) In a serial section, Gram-positive bacteria localized near these alveolar macrophages (magnification × 250, oil). (c) Immunofluorescent pSP-D and (d) Actinobacillus pleuropneumoniae detected by fluorescence in situ hybridization (FISH) localized in serial sections on the same alveolar macrophage (arrowhead) (approximate magnification ×400, oil). pSP-D immunoreactivity as round structures (e) extracellular and (f) intracellular in alveolar macrophages in areas with acute infection (magnification ×250, oil). Detection in panels (a), (e) and (f) was carried out by using amino-ethyl-carbazole (AEC), in (b) by using a modified Gram stain, in (c) by using Cy3, and in (d) by using fluorescein isothiocyanate (FITC)-labelled oligonucleotide probe. (a) (e) and (f) were counterstained with Mayers's hematoxylin.
Occasionally, in areas with acute inflammation, whether from acute or chronic lesions, pSP-D immunoreactivity was found extracellularly as round structures (Fig. 2e) and intracellularly in alveolar macrophages (Fig. 2f), with bacteria detected in serial sections (data not shown).
Furthermore, pSP-D immunoreactivity was generally found intracellularly in the non-ciliated bronchiolar epithelial cells (Clara cells), often located in their domeshaped apex (Fig. 3a, arrowheads), and in the bronchial submucosal glands (data not shown).
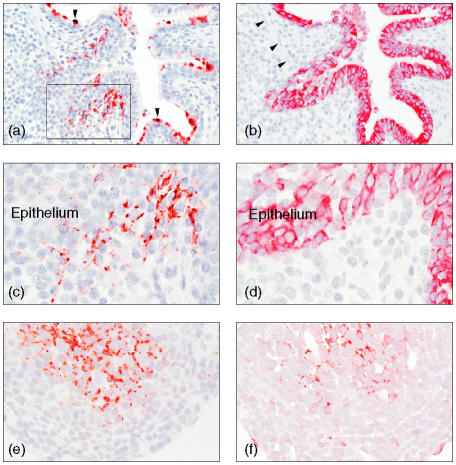
Figure 3.
Porcine surfactant protein D (SP-D) intracellularly in dendritic cells localized subepithelially in bronchus-associated lymphoid tissue (BALT) in a chronic Actinobacillus pleuropneumoniae bronchopneumonia. (a) pSP-D in dendritic cells in BALT and in the dome-shaped apex of Clara cells (arrowheads) (magnification ×100). [The boxed area is shown at a higher magnification in panel (c)]. (b) In a serial section is a bronchiolus with cytokeratin-immunoreactive bronchiolar epithelial cells and cytokeratin-negative lymphocyte-like cells in the epithelium overlying BALT. This epithelium lacks goblet and ciliated cells, and extends through the muscularis (arrowheads). (c) Close-up of pSP-D-positive dendritic cells and weak pSP-D immunoreactivity seen in the epithelium (magnification ×250, oil). (d) Close-up of cytokeratin-negative cells in the epithelium (magnification ×250, oil). (e) pSP-D intracellularly in dendritic cells localized centrally in BALT. (f) In a serial section S-100 protein-positive cells, indicating dendritic cells central in BALT (magnification ×250, oil). The sections shown in (a), (c), (e) and (f) were stained with amino-ethyl-carbazole (AEC) and the sections shown in (b) and (d) were stained with Fast red. All sections were counterstained with Mayers's haematoxylin.
Expression of pSP-D antigen in BALT
In pulmonary tissue showing increased expression of pSP-D in the surfactant, pSP-D was also found intracellularly in stellate cells with long cytoplasmatic processes. These cells were located in BALT, either peripherally, under the epithelium (Fig. 3a,c), or centrally (Fig. 3e). The majority of the BALTs were located at the bronchioli and only a few at the bronchi. pSP-D-positive stellate cells were found exclusively in BALT associated with bronchioli. In serial sections, these stellate cells did not immunolabel for hL1 antigen, a marker for macrophages, monocytes and neutrophils (data not shown), but a diffuse cytoplasmatic S-100 antigen immunolabeling, a marker for DCs, was present (Fig. 3f), and the absence of cytokeratin immunoreactivity verified the non-epithelial origin of these cells (Fig. 3a–d). The expression of major histocompatibility complex (MHC) class II molecules in stellate cells was found both in the centre and periphery of BALT, but no evidence of MHC class II molecule expression was present in the pSP-D-positive stellate cells in BALT, as evaluated by serial sections. Numerous pan-cytokeratin-negative lymphocyte-like cells were often found in the epithelium overlying the pSP-D-positive stellate cells in BALT (Fig. 3b, d); furthermore, this overlying epithelium often had a folded appearance and extended through the muscularis (Fig. 3b, arrowheads). Weak pSP-D immunoreactivity was occasionally found in the epithelium overlying the pSP-D-positive stellate cells in BALT (Fig. 3c).
BALT was generally absent or infrequent in normal lung tissue and in tissue with embolic pneumonia, and frequent in pigs with A. pleuropneumoniae bronchopneumonia (data not shown). pSP-D immunoreactivity was detected neither in the tracheobronchial lymph nodes, whether taken from uninfected or infected lungs, nor in the spleen samples.
Discussion
The localization of pSP-D, in non-ciliated bronchiolar epithelial cells (Clara cells) and submucosal glands, was similar to the pSP-D immunoreactivity previously described in normal lung tissue.15 Comparing normal and diseased lung tissue from the same animals, pSP-D was found to be expressed excessively in the surfactant in both acute and chronic bronchopneumonic lesions, in particular those caused by A. pleuropneumoniae. Embolic pneumonias caused by Staph. aureus and Ar. pyogenes, and systemic haematogenous infections (septicaemia) with Strep. suis (present intravascularly), generally showed no change in the pSP-D expression in the surfactant. The moderately increased reactivity in two chronic apostematous pneumonias caused by Staph. aureus may be a result of the spread of bacteria from the embolic process to lumina of the surrounding lung tissue causing a secondary bronchopneumonia, or these chronic abscesses may be a result of an aerogenous infection rather than a haematogenous infection.32,33 In rat bronchoalveolar lavage fluid, both SP-D and SP-A increase during the early progression of acute aerogeneous inflammation as a result of the intratracheal aerosolization of endotoxin (lipopolysaccharide) from Gram-negative bacteria.34 Our findings show that aerogenous infection with A. pleuropneumoniae or Staph. aureus, but not pneumonias caused by haematogeneously intravascular dissemination of Staph. aureus, Ar. pyogenes or Strep. suis, results in the up-regulation of pSP-D in the surfactant. It has recently been shown that such a response may be specific for certain bacterial species – in sheep, where SP-D mRNA expression was not increased during Mannheimia haemolytica infecton,35 and in humans, where different bacterial species induced different SP-D responses in vivo, as determined by using an enzyme-linked immunosorbent assay (ELISA).36 In this study, the difference in bacterial species appears to much be less important than the route of infection, but further studies, analysing a greater number of bacterial species, must be used to clarify this point. The enlarged and macrophage marker-negative alveolar cells, found in alveoli with increased pSP-D expression in the surfactant, represent hypertrophic alveolar type II cells with strong pSP-D immunoreactivity. Furthermore, strongly pSP-D immunoreactive hyperplastic alveolar type II cells were found in areas with epithelialization, which is a replacement of damaged alveolar epithelium by alveolar type II cells.23 The intense pSP-D immunoreactivity in these hypertrophic and hyperplastic alveolar type II cells indicated an increased pSP-D synthesis and, thus, up-regulation of the protein in these cells, although increased surfactant uptake could be a possibility. The absence of pSP-D in the centre of chronic lesions can be explained by the destruction of pSP-D-producing cells in these areas and the presence, also in these areas, of necrotic cells, fibrotic tissue and inflammatory cells.
The finding, in the present study, of pSP-D on leucocyte membranes in a localized, patchy distribution, instead of the more diffuse distribution seen in the surfactant, strongly indicates a specific interaction between leucocytes and pSP-D. SP-D has been shown to bind specifically to alveolar macrophages by an unknown receptor, which is different from the receptor C1q that binds the complement factor.37 The direct binding of SP-D to alveolar macrophages, without the presence of microbial ligands, has been shown previously37,38 and it is likely that pSP-D binds to, and ‘arms’, alveolar macrophages that express the appropriate receptor. Another putative function was indicated in a recent in vitro study, where mouse SP-D bound apoptotic neutrophils in a localized, patchy pattern, promoting the phagocytosis of such cells.9 In the present study, leucocytes with the pSP-D bound in a patchy pattern, but showed no signs of apoptosis. SP-D has previously been shown to bind in vitro to Staph. aureus,39 and the present study showed an in vivo co-localization of Staph. aureus or A. pleuropneumoniae, pSP-D and phagocytes. This co-localization of pSP-D, phagocytes and bacteria, and the occasional intracellular presence of pSP-D in macrophages, may indicate an opsonic effect of pSP-D, possibly through the calreticulin–CD91 complex, although the possibility of a non-specific pinocytosis of pSP-D by the phagocytes should not be ruled out.
BALT is only constitutive in some species, as it is always present in normal rabbit lungs and generally absent in normal human lungs.40 In pigs, BALT has been shown to be present in 33% of the normal pigs studied.40
Pigs infected with A. pleuropneumoniae41 or M. hyopneumoniae21 showed an increased number and size of nodular BALT, and in pigs, like in humans, BALT is induced by microbial stimulation.42 In the present study, BALT was generally absent or infrequent in normal lung tissue and in tissue with embolic pneumonia, and common in pigs with A. pleuropneumoniae-induced bronchopneumonia, indicating microbially induced stimulation of BALT.
In the rabbit and rat, a specialized epithelium is seen overlying BALT; this epithelium is characterized by a lack of goblet cells and the presence of non-ciliated M cells, in close association with intraepithelial lymphocytes.43 M cells are thought to be able to proliferate when BALT is stimulated.44 In pigs not microbially stimulated, precursors of M cells have been found covering BALT.45 In the present study, the epithelium overlying BALT with pSP-D-positive DCs also showed signs of specialization by lacking goblet cells, ciliated cells and the pSP-D-immunoreactive Clara cells. Furthermore, numerous pan-cytokeratin-negative lymphocyte-like cells were present in this epithelium, which often extended through the lamina muscularis, possibly as a result of M-cell proliferation.
The pulmonary M cells are believed to be related to the M cells associated with the intestinal Peyer's patches. They transport luminal antigens to the gut-associated lymphoid tissue (GALT), facilitating the antigen uptake and presentation by the antigen-processing cells to lymphocytes, and thereby playing an important part in inducing the adaptive immune response.20 In the present study, weak pSP-D immunoreactivity, distinct from the reactivity in Clara cells, was frequently found in the epithelium overlying BALT with pSP-D-positive DCs. It is tempting to speculate that this weak pSP-D immunoreactivity represents pSP-D crossing the epithelium by trancytosis in M cells, although crossing through intraepithelial cytoplasmatic processes of DCs could be a possibility.
The porcine BALT, in infected lungs, is composed of macrophages, DCs, T- and B lymphocytes, and IgG+ and IgA+ plasma cells.21 DCs exhibit a unique stellate morphology. In the lungs, skin and lymphoid organs, DCs have long (> 10 µm), delicate processes extending in many directions from the cell body.46 The morphology of the pSP-D-positive stellate cells in BALT, the absence in serial sections of macrophage marker reactivity and the presence of DC marker (S-100) reactivity, strongly indicated that these pSP-D-positive stellate cells in BALT were DCs. Whenever BALT was present, these pSP-D-positive DCs in BALT, and increased pSP-D expression in the surfactant, coincided, and the cells were either located peripherally in BALT immediately under the epithelium, or in the centre. MHC class II-positive stellate cells, probably representing DCs, were found in the centre and periphery of BALT in infected lung tissue, which confirms the results from a previous study, in pigs, using the same antibody.21 In the present study, MHC class II immunoreactivity was not found serial to the pSP-D positive DCs in the BALT, which might be a result of the lack of membrane-expressed MHC class II molecules, as seen in immature DCs or in follicular dendritic cells (FDCs). Whether FDCs, which in the lymph nodes play a role in the development of memory B cells, are present in porcine BALT, as in rabbit BALT,20 is unknown. Brinker and colleagues13 found that human SP-D binds to immature bone marrow-derived DCs in vitro in a dose-, carbohydrate- and calcium-dependent manner, enhancing the phagocytic uptake of E. coli and the antigen presentation of a bacterial antigen model to T cells. The pSP-D-positive, MHC class II-negative DCs, found in the present study, could represent such immature DCs. Furthermore, as pSP-D was not found in the tracheobronchial lymph nodes, these DCs might, after maturation, be responsible for a local antigen presentation in the BALT, rather than migrating to the regional lymph nodes. Little is, however, known about the function of BALT and the nature and function of DCs in BALT, and further research is needed to understand the presence of pSP-D in these cells.
In the present in vivo study we found that the expression of pSP-D in the lung increased during aerogenous pulmonary infections. Furthermore, a spatial relationship among pSP-D, bacterial pathogens and phagocytic cells was demonstrated, as was pSP-D in DCs in BALT. These findings point to pSP-D up-regulation during infection, with possible antigen opsonization. Furthermore, indications of an uptake and transport of pSP-D by M cells to the underlying DCs was found, and it is tempting to speculate that pSP-D facilitates the antigen presentation in DCs to lymphocytes in BALT, thereby acting as a link between the innate and adaptive immune system.
Acknowledgments
This work was kindly supported by the Danish Research Agency (Ministry of Science Technology and Innovation, grant no. 23-00-0282). We thank the employees at Danish Crown (Ringsted, Denmark), and are grateful to Dennis Brok, Lisbeth Kioerboe, Betina Andersen and Hanne H. Moeller for excellent technical assistance.
Abbreviations
- BALT
bronchus-associated lymphoid tissue
- CFU
colony-forming units
- CL-43
collectin-43
- CL-46
collectin-46
- CL-L1
collectin-liver 1
- CL-P1
collectin-placenta 1
- DC
dendritic cell
- mAb
monoclonal antibody
- FDCs
follicular dendritic cells
- FISH
fluorescence in situ hybridization
- FITC
fluorescein isothiocyanate
- IHC
immunohistochemistry
- IHF
immunohistofluorescence
- M cells
microfold cells
- MBL
mannan-binding lectin
- MHC
major histocompatibility complex
- NAD
nicotinamide adenine dinucleotide
- pSP-D
porcine surfactant protein D
- SP-A
surfactant protein A
- TBS
Tris-buffered saline
References
- 1.Crouch EC. Surfactant protein-D and pulmonary host defense. Respir Res. 2000;1:93–108. doi: 10.1186/rr19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 3.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–12. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JR. Host defense functions of pulmonary surfactant. Biol Neonate. 2004;85:326–32. doi: 10.1159/000078172. [DOI] [PubMed] [Google Scholar]
- 5.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–54. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Iwaarden JF, Shimizu H, Van Golde PH, Voelker DR, van Golde LM. Rat surfactant protein D enhances the production of oxygen radicals by rat alveolar macrophages. Biochem J. 1992;286:5–8. doi: 10.1042/bj2860005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borron P, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161:4599–603. [PubMed] [Google Scholar]
- 9.Vandivier RW, Ogden CA, Fadok VA, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–86. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 10.Schagat TL, Wofford JA, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol. 2001;166:2727–33. doi: 10.4049/jimmunol.166.4.2727. [DOI] [PubMed] [Google Scholar]
- 11.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KB. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol. 2002;169:2892–9. doi: 10.4049/jimmunol.169.6.2892. [DOI] [PubMed] [Google Scholar]
- 12.Ikegami M, Whitsett JA, Jobe A, Ross G, Fisher J, Korfhagen T. Surfactant metabolism in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol. 2000;279:L468–76. doi: 10.1152/ajplung.2000.279.3.L468. [DOI] [PubMed] [Google Scholar]
- 13.Brinker KG, Martin E, Borron P, Mostaghel E, Doyle C, Harding CV, Wright JR. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1453–63. doi: 10.1152/ajplung.2001.281.6.L1453. [DOI] [PubMed] [Google Scholar]
- 14.van Eijk M, Haagsman HP, Skinner T, Archibald A, Reid KB, Lawson PR, Archibold A. Porcine lung surfactant protein D. complementary DNA cloning, chromosomal localization, and tissue distribution. J Immunol. 2000;164:1442–50. doi: 10.4049/jimmunol.164.3.1442. [DOI] [PubMed] [Google Scholar]
- 15.Soerensen CM, Nielsen OL, Willis A, Heegaard PMH, Holmskov U. Purification, characterization and immunolocalization of porcine surfactant protein D. Immunology. 2005;114:72–82. doi: 10.1111/j.1365-2567.2004.01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Eijk M, van de Lest CH, Batenburg JJ, Vaandrager AB, Meschi J, Hartshorn KL, van Golde LM, Haagsman HP. Porcine surfactant protein D is N-glycosylated in its carbohydrate recognition domain and is assembled into differently charged oligomers. Am J Respir Cell Mol Biol. 2002;26:739–47. doi: 10.1165/ajrcmb.26.6.4520. [DOI] [PubMed] [Google Scholar]
- 17.van Eijk M, White MR, Batenburg JJ, Vaandrager AB, van Golde LM, Haagsman HP, Hartshorn KL. Interactions of influenza A virus with sialic acids present on porcine surfactant protein D. Am J Respir Cell Mol Biol. 2003;30:871–9. doi: 10.1165/rcmb.2003-0355OC. [DOI] [PubMed] [Google Scholar]
- 18.van Eijk M, White MR, Crouch EC, Batenburg JJ, Vaandrager AB, van Golde LM, Haagsman HP, Hartshorn KL. Porcine pulmonary collectins show distinct interactions with influenza A viruses: role of the N-linked oligosaccharides in the carbohydrate recognition domain. J Immunol. 2003;171:1431–40. doi: 10.4049/jimmunol.171.3.1431. [DOI] [PubMed] [Google Scholar]
- 19.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med. 1997;186:665–72. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janeway CA, Jr, Travers P, Walport M. Immuno Biology. New York: Garland Publishing; 2001. [Google Scholar]
- 21.Sarradell J, Andrada M, Ramirez AS, et al. A morphologic and immunohistochemical study of the bronchus-associated lymphoid tissue of pigs naturally infected with Mycoplasma hyopneumoniae. Vet Pathol. 2003;40:395–404. doi: 10.1354/vp.40-4-395. [DOI] [PubMed] [Google Scholar]
- 22.Christensen G, Sorensen V, Mousing J. Diseases of swine. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, editors. Diseases in Swine. 8. Oxford: Blackwell Science; 1999. pp. 913–40. [Google Scholar]
- 23.Dungworth DL. The respiratory system. In: Jubb KVF, Kennedy PC, Palmer N, editors. Pathology of Domestic Animals. 4. Vol. 2. San Diego: Academic Press, Inc.; 1993. pp. 539–698. [Google Scholar]
- 24.Jacobsen MJ, Nielsen JP, Nielsen R. Comparison of virulence of different Actinobacillus pleuropneumoniae serotypes and biotypes using an aerosol infection model. Vet Microbiol. 1996;49:159–68. doi: 10.1016/0378-1135(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 25.Madsen LW, Bak H, Nielsen B, Jensen HE, Aalbaek B, Riising HJ. Bacterial colonization and invasion in pigs experimentally exposed to Streptococcus suis serotype 2 in aerosol. J Vet Med Infect Dis Vet Public Health. 2002;49:211–5. doi: 10.1046/j.1439-0450.2002.00491.x. [DOI] [PubMed] [Google Scholar]
- 26.Krieg NR, Holt JG. Bergey's Manual for Systematic Bacteriology. Baltimore, USA: Wiliam and Wilkins; 1984. [Google Scholar]
- 27.Barrow GI, Feltham RKA. Cowan and Steel's Manual for the Identification of Medical Bacteria. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- 28.Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. New York: Churchill Livingstone; 1996. [Google Scholar]
- 29.Jensen TK, Boye M, Hagedorn-Olsen T, Riising HJ, Angen O. Actinobacillus pleuropneumoniae osteomyelitis in pigs demonstrated by fluorescent in situ hybridization. Vet Pathol. 1999;36:258–61. doi: 10.1354/vp.36-3-258. [DOI] [PubMed] [Google Scholar]
- 30.Fussing V, Paster BJ, Dewhirst FE, Poulsen LK. Differentiation of Actinobacillus pleuropneumoniae strains by sequence analysis of 16S rDNA and ribosomal intergenic regions, and development of a species specific oligonucleotide for in situ detection. Syst Appl Microbiol. 1998;21:408–18. doi: 10.1016/S0723-2020(98)80050-7. [DOI] [PubMed] [Google Scholar]
- 31.Sarli G, Mandrioli L, Laurenti M, Sidoli L, Cerati C, Rolla G, Marcato PS. Immunohistochemical characterisation of the lymph node reaction in pig post–weaning multisystemic wasting syndrome (PMWS) Vet Immunol Immunopathol. 2001;83:53–67. doi: 10.1016/s0165-2427(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 32.Liljegren CH, Aalbaek B, Nielsen OL, Jensen HE. Some new aspects of the pathology, pathogenesis, and aetiology of disseminated lung lesions in slaughter pigs. APMIS. 2003;111:531–8. doi: 10.1034/j.1600-0463.2003.1110501.x. [DOI] [PubMed] [Google Scholar]
- 33.Madsen LW, Svensmark B, Elvestad K, Jensen HE. Otitis interna is a frequent sequela to Streptococcus suis meningitis in pigs. Vet Pathol. 2001;38:190–5. doi: 10.1354/vp.38-2-190. [DOI] [PubMed] [Google Scholar]
- 34.McIntosh JC, Swyers AH, Fisher JH, Wright JR. Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 1996;15:509–19. doi: 10.1165/ajrcmb.15.4.8879185. [DOI] [PubMed] [Google Scholar]
- 35.Grubor B, Gallup JM, Ramìrez-Romero R, Baily TB, Crouch EC, Brogden KA, Ackermann MR. Surfactant protein D expression in normal and pneumonic ovine lung. Vet Immunol Immunopathol. 2004;101:235–42. doi: 10.1016/j.vetimm.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Leth-Larsen R, Nordenbaek C, Tornoe I, et al. Surfactant protein D (SP-D) serum levels in patients with community-acquired pneumonia small star, filled. Clin Immunol. 2003;108:29–37. doi: 10.1016/s1521-6616(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 37.Miyamura K, Leigh LE, Lu J, Hopkin J, Lopez BA, Reid KB. Surfactant protein D binding to alveolar macrophages. Biochem J. 1994;300:237–42. doi: 10.1042/bj3000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuan SF, Persson A, Parghi D, Crouch E. Lectin-mediated interactions of surfactant protein D with alveolar macrophages. Am J Respir Cell Mol Biol. 1994;10:430–6. doi: 10.1165/ajrcmb.10.4.8136158. [DOI] [PubMed] [Google Scholar]
- 39.van de Wetering JK, van Eijk M, van Golde LM, Hartung T, van Strijp JA, Batenburg JJ. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. J Infect Dis. 2001;184:1143–51. doi: 10.1086/323746. [DOI] [PubMed] [Google Scholar]
- 40.Pabst R, Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am J Respir Cell Mol Biol. 1990;3:131–5. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- 41.Delventhal S, Hensel A, Petzoldt K, Pabst R. Effects of microbial stimulation on the number, size and activity of bronchus-associated lymphoid tissue (BALT) structures in the pig. Int J Exp Pathol. 1992;73:351–7. [PMC free article] [PubMed] [Google Scholar]
- 42.Pabst R. Is BALT a major component of the human lung immune system? Immunol Today. 1992;13:119–22. doi: 10.1016/0167-5699(92)90106-H. [DOI] [PubMed] [Google Scholar]
- 43.Kroese FGM, Mage RG, Pescovitz MD. Handbook of Vertebrate Immunology. San Diego: Academic Press; 1998. [Google Scholar]
- 44.Ogra PL. Mucosal Immunology. San Diego: Academic Press; 1999. [Google Scholar]
- 45.Huang YT, Chu RM, Liu RS, Weng CN. Morphologic studies of intrapulmonary airway mucosa-associated lymphoid tissues in swine. Vet Immunol Immunopathol. 1990;25:13–22. doi: 10.1016/0165-2427(90)90106-3. [DOI] [PubMed] [Google Scholar]
- 46.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]