Abstract
The effector CD8+ T cells recognize major histocompatibility complex (MHC) class I binding altered self-peptides expressed in tumour cells. Although the requirement for CD4+ T helper type 1 (Th1) cells in regulating CD8+ T cells has been documented, their target epitopes and functional impact in antitumour responses remain unclear. We examined whether a potent immunogenic peptide of Mycobacterium tuberculosis eliciting Th1 immunity contributes to the generation of CD8+ T cells and to protective antitumour immune responses to unrelated tumour-specific antigens. Peptide-25, a major Th epitope of Ag85B from M. tuberculosis preferentially induced CD4+ Th1 cells in C57BL/6 mice and had an augmenting effect on Th1 generation for coimmunized unrelated antigenic peptides. Coimmunization of mice with Peptide-25 and ovalbumin (OVA) or Peptide-25 and B16 melanoma peptide [tyrosinase-related protein-2 (TRP-2)] for MHC class I led to a profound increase in CD8+ T cells specific for OVA and TRP-2 peptides, respectively. This heightened response depended on Peptide-25-specific CD4+ T cells and interferon-γ-producing T cells. In tumour protection assays, immunization with Peptide-25 and OVA resulted in the enhancement of CD8+ cytotoxic cell generation specific for OVA and the growth inhibition of EL-4 thymoma expressing OVA peptide leading to the tumour rejection. These phenomena were not achieved by immunization with OVA alone. Peptide-25-reactive Th1 cells counteractivated dendritic cells in the presence of Peptide-25 leading them to activate and present OVA peptide to CD8+ cytotoxic T cells.
Keywords: antigen presentation, cytotoxic T cells, peptide, T helper 1 cells, tumour immunity
Introduction
The identification of tumour antigens has renewed interest in immunotherapy for cancer. There is a body of evidence that tumour-specific T cells recognize tumour-associated antigens on the cancer cells and play an essential role in inhibiting tumour growth and eradicating cancer cells.1–3 CD8+ cytotoxic T lymphocytes (CTL) from specifically immunized mice are capable of destroying tumour target cells in vitro4 and adoptive transfer of CD8+ T cells from immunized donors confers resistance to tumour transplants on naive mice.5–7 As CD8+ CTL can lyse tumour cells directly and destroy large tumour masses in vivo, much attention has focused on the role of CD8+ T cells in the immunotherapy of cancer. Over the past two decades, a wide range of peptides derived from tumour cells of mice and humans that bind major histocompatibility complex (MHC) class I and are recognized by CD8+ T cells has been defined.1,8,9 However, in both clinical and animal studies, therapeutic strategies focused on the use of CD8+ T cells and MHC class I-restricted tumour antigens have not been effective in eliminating cancer cells.
There has been a recent reappraisal of the role and importance of CD4+ T helper (Th) cells in antitumour responses, because CD4+ Th cells are required for generating and maintaining potent antitumour immunity.5,6,10 The role of CD8+ and CD4+ T cells in tumour systems has been the object of intense interest. A major obstacle for the development of optimal cancer vaccines is the lack of effective methods for identifying MHC class II-restricted tumour antigens that can stimulate CD4+ T cells.11,12 Identification of such antigens would provide new opportunities for developing effective CD8+ CTL and would improve our understanding of the mechanisms by which CD4+ T cells regulate the host immune system.
A variety of tumour-derived antigens have been defined by immunoglobulin G (IgG) antibodies in sera taken from tumour bearers with serological identification of antigens by recombinant expression cloning (SEREX).13–16 The SEREX repertoire can be considered a reflection of the CD4+ T-cell repertoire. Shiku and his colleagues reported that coimmunization of mice with plasmids encoding these SEREX-defined wild-type antigens and mutated mitogen-activated protein kinase 2 (mERK2; containing tumour-specific CTL epitope 9m of CMS5) led to a profound increase in CD8+ T cells specific for mERK2.13 This heightened response depends on CD4+ T cells and on the copresentation of SEREX-defined wild-type antigens and the CTL epitope. Their results indicate the essential role of CD4+ T cells in mediating the increased CD8+ T-cell response and tumour inhibition induced by coimmunization with SEREX-defined antigens.
We have reported that immunization of Mycobacterium tuberculosis-primed mice with purified protein derivative (PPD)-modified attenuated X5563 myeloma cells induces an X5563-specific CD8+ CTL response and antitumour immunity.17–19 We infer from these results that M. tuberculosis-derived proteins or peptides may enhance the CD8+ CTL response and antitumour immunity by coimmunization with tumour antigen or neo-tumour antigen. Ag85B, one of the major proteins secreted by M. tuberculosis, elicits a strong Th1 response in vitro in T cells from both PPD-positive asymptomatic human subjects and Ag85B-primed cells of C57BL/6 (I-Ab) mice. Peptide-25 (amino acids 240–254) of Ag85B, which is the most potent antigen species yet purified for both humans and mice, is a major Th1 cell epitope of Ag85B. Active immunization of C57BL/6 mice with Peptide-25 induces the differentiation of CD4+ T-cell receptor (TCR) Vβ11+ T cells that produce interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)20–23.
We investigated whether Th1-inducible Peptide-25 intensifies the CD8+ CTL response to unrelated tumour-specific antigens through stimulation of a CD4+ Th1 cell response leading to the induction of antitumour immunity that is effective in eliminating cancer cells. We also discuss the possible mechanisms of Peptide-25-induced enhancement of the CD8+ CTL response.
Materials and methods
Mice
C57BL/6 mice were purchased from Charles River Japan (Tokyo, Japan). Peptide-25-reactive TCR transgenic (Tg) (P25 TCR-Tg) mice were generated and maintained as described previously.24 IFN-γ deficient (IFN-γ–/–) mice25 were kindly provided by Dr Y. Iwakura (Institute of Medical Science, University of Tokyo, Tokyo, Japan). Ovalbumin (OVA)-specific TCR-Tg (OT-1) mice were kindly provided by Dr T. Hirano (Osaka University, Suita, Japan). These mice were housed in the animal facility at the Institute of Medical Science, University of Tokyo, under specific pathogen-free conditions, and were used at 8–12 weeks of age.
Antigens and reagents
Peptide-25 (FQDAYNAAGGHNAVF), Peptide-9 (DWYSPACGKAGCQTY), and Peptide-18 (AGGYKAADMWGPSSD) of Ag85B were synthesized by Funakoshi Co., Ltd (Tokyo, Japan). Purified chicken OVA was purchased from Sigma-Aldrich, Co. (St Louis, MO). MHC class I-binding OVA Peptide (SIINFEKL) and B16 melanoma peptide tyrosinase-related protein-2 (TRP-2) (VYDFFVWL)26 were also synthesized by Funakoshi Co., Ltd.
Culture medium
RPMI-1640 (Gibco BRL, Grand Island, NY) supplemented with 10% (v/v) heat-inactivated fetal calf serum (Sigma-Aldrich, Co.), 50 μm 2-mercaptoethanol, 100 IU/ml penicillin G and 50 μg/ml streptomycin was used as the complete medium for cultures throughout the present experiment.
Cell lines
The murine thymoma line, EL-4 (H-2Kb) was purchased from the American Type Culture Collection (Rockville, MD). EL-4 transfectant of the OVA gene (E.G7 cells) was kindly provided by Dr H. Udono (Nagasaki University School of Medicine, Nagasaki, Japan) and the B16 melanoma cell line was kindly provided by Dr H. Tahara (Institute of Medical Science, University of Tokyo, Tokyo, Japan).
Immunization
Mice were immunized by subcutaneous injection on the abdomen with OVA (10 μg/mouse) emulsified in incomplete Freund's adjuvant (IFA), Peptide-25, or its related peptide (10 μg/mouse) in IFA or a mixture of OVA (10 μg/mouse) and Peptide-25 (10 μg/mouse) in IFA as described previously.21 In some experiments, mice were immunized with OVA (10 μg/mouse) in IFA on the left-hand side of the abdomen and with Peptide-25 (10 μg/mouse) in IFA on the right-hand side of the abdomen. We also immunized mice with MHC class I-binding TRP-2 peptide (10 μg/mouse) in place of OVA.
In vivo and in vitro T-cell depletion
CD4+ T cells were depleted in vivo by the administration of 0·3 mg monoclonal antibodies (mAbs) against CD4 (GK1.5) on days −13, −12, −11, −6, −5, −4, +1, +2 and +3 relative to immunization. Fluorescence-activated cell sorter (FACS) analysis of blood mononuclear cells from GK1.5-treated mice at the time of immunization confirmed the effectiveness of the CD4+ T-cell depletion. In vitro T-cell depletion was achieved by the incubation of spleen cells with either the IgM subclass of mAb against CD4 or CD8 and guinea-pig complement. FACS analysis of the treated spleen cells confirmed the effectiveness of the depletion.
In vitro CTL induction and CD8+cytotoxic T-cell assay
In vitro CTL induction and CD8+ CTL assay were carried out according to previously described methods17,18 with slight modification. Ten days after immunization with OVA in IFA or OVA and Peptide-25 in IFA, spleen cells (1 × 107) were cultured in vitro with γ-irradiated (20 000 rad) E.G7 cells (8 × 105). Spleen cells from TRP-2-immunized mice were stimulated in vitro with TRP-2 (10 μg/ml). After 5 days in culture, the CTL activity of the resulting effector cells was assayed. Target cells (E.G7, EL-4, and B16 melanoma cells) were labelled with 51Cr (Perkin Elmer Life Science, Boston, MA) at 37° for 40 min. After washing, 51Cr-labelled target cells (1 × 104) were incubated with effector cells at various effector cell to target cell ratios. Release of 51Cr was measured in the supernatants that were harvested after 4 hr incubation. Maximum release was measured by resuspending the target cells in lysis buffer containing 0·1% Triton-X-100. Spontaneous release was obtained from target cells incubated with medium alone and was less than 10% of maximum 51Cr release. The percentage specific lysis was calculated according to the following formula, where c.p.m. represents counts per minute: percentage specific lysis=[(c.p.m.experimental release − c.p.m.spontaneous release)/(c.p.m.maximum release − c.p.m.spontaneous release)] × 100.
A dose–response curve of effector cells was established in all experiments and the number of lytic units (LU) was calculated as previously described.19 In these calculations 1 LU was arbitrarily defined as the number of spleen cells required to achieve 50% lysis of 1×104 51 Cr-labelled target cells during a 4-hr incubation.
Tumour challenge experiments
Three groups of 12 mice were immunized by subcutaneous injection of the abdomen with OVA (10 μg/mouse) in IFA, Peptide-25 (10 μg/mouse) in IFA, or a mixture of OVA (10 μg/mouse) and Peptide-25 (10 μg/mouse) in IFA. Twelve mice were injected with IFA without any protein or peptide to act as a control group. Ten days after the immunization, all mice were challenged by subcutaneous injection with E.G7 (5 × 105 cells/mouse) on their backs. In some experiments, B16 melanoma cells (5 × 105 cells/mouse) were transplanted in TRP-2-immunized mice. Tumour size was assessed using a microcaliper a 2-day to 3-day intervals and was expressed as the square of the smallest diameter of the tumour multiplied by its largest diameter. The survival of the mice was also monitored periodically.
Frequency analysis of OVA-specific CTL
The frequency of OVA-specific CTL in spleen cells after immunization was measured using OVA peptide-loaded H-2Kb:Ig protein (BD Biosciences Pharmingen, San Diego, CA) according to the manufacturer's instructions. Spleen cells prepared from mice 10 days after immunization, were stained with 4 μg OVA peptide-loaded H-2Kb:Ig protein and incubated for 60 min at 4°. After washing, cells were stained with anti-mouse IgG1 (A85-1)–phycoerythrin (PE; BD Biosciences Pharmingen) and anti-CD8 (53-6.7)–fluorescein isothiocyanate (FITC; BD Biosciences Pharmingen) and incubated for 30 min at 4°. After washing, cells were analysed using FACSCalibur (Becton Dickinson, Mountain View, CA).
Assay for dendritic cell activation
Immature dendritic cells (DCs) were propagated in vitro by culturing CD11c+ bone marrow cells with granulocyte–macrophage colony-stimulating factor (GM-CSF) (20 ng/ml) and interleukin-3 (IL-3) (20 ng/ml) for 6 days. To assess the expression of surface molecules and IL-12 production of DCs after Peptide-25 treatment, the cells obtained (5 × 105) were cocultured with Peptide-25 (10 μg/ml) in the presence of CD4+ T cells (5 × 105) from P25 TCR-Tg mice for 48 hr. The expression of surface molecules on DCs was analysed by FACS. The IL-12 production was assessed by enzyme-linked immunosorbent assay (ELISA). To assess the antigen-presenting activity of DCs after Peptide-25 treatment, the cells obtained (5 × 105) were cocultured with CD4+ T cells (5 × 105) from P25 TCR-Tg mice and 5-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled CD8+ T cells (5 × 105) from OT-1 mice for 96 hr in the presence of Peptide-25 (10 μg/ml) and OVA (10 μg/ml). After the culture, cell division cycles were determined by FACS analysis.27
Assay for cytokine production by intracellular cytokine staining and ELISA
For assessment of cytokine production of spleen cells from OVA- or TRP-2-immunized mice, spleen cells (1 × 106/ml) prepared from mice 10 days after immunization, were stimulated with 10 μg/ml OVA or 10 μg/ml TRP-2. After the stimulation, IFN-γ- and IL-4-producing cells were examined by intracellular staining according to previously described methods.22,24 In brief, 2 μm of monensin (BD Biosciences Pharmingen) was added for the last 4 hr of the culture. The cells were harvested and stained with 7-amino-actinomycin D and anti-CD4 (GK1·5)- or anti-CD8- allophycocyanin (BD Biosciences Pharmingen). Then, the cells were washed in 0·05% azide−1%FCS–phosphate-buffered saline, fixed with 1·6% formaldehyde, made permeable with 0·1% saponin and stained with anti-IFN-γ-FITC (XMG1.2) (BD Biosciences Pharmingen) and anti-IL-4-PE (11B11) (BD Biosciences Pharmingen) or isotype control antibodies. Stained cells were gated on live CD4+ or CD8+ cells and analysed by FACSCalibur. The amounts of IFN-γ and IL-4 in the culture supernatant after OVA or TRP-2 stimulation in vitro were quantified by ELISA following the manufacturer's instructions. The mAbs specific for mouse IFN-γ and IL-4 that were used for capture and detection of cytokines were purchased from BD Biosciences Pharmingen. ELISA of IL-12p40 was conducted using a murine IL-12 p40 OptEIA™ ELISA kit (BD Biosciences Pharmingen).
ELISA for anti-OVA antibody titration
For assessment of anti-OVA IgG1 and IgG2a levels, serum was collected from the immunized mice at 10 days after immunization and added to the OVA-coated plate. Biotinylated goat anti-mouse IgG1 antibody (Southern Biotechnology Associates, Inc., Birmingham, AL) or biotinylated goat anti-mouse IgG2a (5.7.2) was applied and detection was performed using streptavidin-peroxidase (Zymed Laboratories Inc., San Francisco, CA).
Results
Peptide-25 enhances the generation of OVA-specific CD8+ CTL response
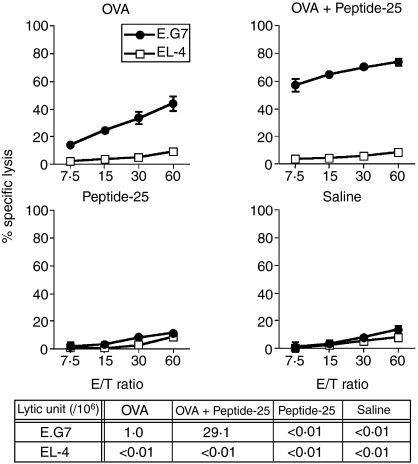
Peptide-25, a 15-mer peptide of Ag85B is a major T-cell epitope recognized by CD4+ I-Ab-restricted Th1 cells specific for Ag85B of M. tuberculosis.20,21 Immunization of C57BL/6 mice with Peptide-25 induced the generation of IFN-γ- and TNF-α-producing Th1 cells that preferentially express TCRVβ11.21 As CD4+ Th1 cells can augment the CD8+ CTL response28,29 we examined whether immunization with a mixture of OVA and Peptide-25 can enhance the generation of an OVA-specific CTL response compared to OVA immunization. Three groups of mice were immunized with OVA in IFA, Peptide-25 in IFA, or a mixture of OVA and Peptide-25 in IFA. As a control, a group of mice was treated with IFA. Ten days after the immunization, spleen cells from each group of mice were stimulated in vitro for 5 days with heavily irradiated E.G7 as stimulator cells that express OVA linked to the MHC class I molecule. The responding cells recovered after the culture were subjected to OVA-specific CTL assay as effector cells. The CTL activity was assessed on a 4-hr 51Cr-release assay using 51Cr-labelled E.G7 or EL-4. Results clearly revealed that the spleen cells from OVA-immunized mice mounted a significant CTL response to E.G7, but not to EL-4 upon in vitro stimulation with irradiated E.G7 (Fig. 1). Interestingly, a robust OVA-specific CTL response was induced in the culture of the spleen cells from mice immunized with a mixture of OVA and Peptide-25 in IFA upon E.G7 stimulation. The enhancement of the OVA-specific CTL response by coimmunization of OVA with Peptide-25 was quantitatively confirmed by calculating the lytic unit. Spleen cells from Peptide-25-immunized mice or from IFA-treated mice did not mount a significant CTL response to E.G7 upon E.G7 re-stimulation in vitro, indicating that Peptide-25 immunization does not induce a polyclonal CTL response. We confirmed that an enhanced OVA-specific CTL response was observed after coimmunization with Peptide-25 and MHC class I-binding OVA peptide (data not shown). The OVA-specific CTL activity in effector cells was abrogated completely by the depletion of CD8+ T cells using anti-CD8 mAb plus complement treatment before CTL assay, while the CTL activity remained the same in the treatment of spleen cells with anti-CD4 mAb plus complement (data not shown).
Figure 1.
Enhanced induction of OVA-specific CD8+ CTL response in spleen by coimmunization with OVA and Peptide-25. Three groups of mice were immunized with OVA (10 μg) in IFA, OVA (10 μg) and Peptide-25 (10 μg) in IFA or Peptide-25 (10 μg) in IFA subcutaneously. Spleen cells from each group of mice 10 days after the immunization were subjected to OVA-specific CTL assay. CTL assay was conducted by incubating sensitized cells with 51Cr-labelled E.G7 or 51Cr-labelled EL-4 cells of various effector to target ratios at 37° for 4 hr. The values represent the geometric means and standard deviation of percentage specific lysis in triplicate cultures. Background lysis of the target cells was less than 10% of maximum 51Cr-release. The number of LU was calculated from the dose–response curve obtained for each group. A representative result of a series of five experiments is shown.
To examine the enhancing effect of I-Ab-binding peptides other than Peptide-25 on OVA-specific CTL generation, three groups of mice were immunized with OVA in IFA, a mixture of OVA and Peptide-25 in IFA, or a mixture of OVA and Peptide-9 of Ag85B in IFA. As a control, a group of mice was immunized with OVA and Peptide-18 (non-I-Ab-binding peptide of Ag85B) in IFA. The generation of an OVA-specific CTL response in spleen cells was assessed 10 days after the immunization. While immunization with OVA and Peptide-25 induced a potent OVA-specific CTL response, the CTL response observed in spleens from mice immunized with OVA and Peptide-9 was much less, if present at all (data not shown). Immunization with a mixture of OVA and Peptide-18 did not show enhancement of the OVA-specific CTL response.
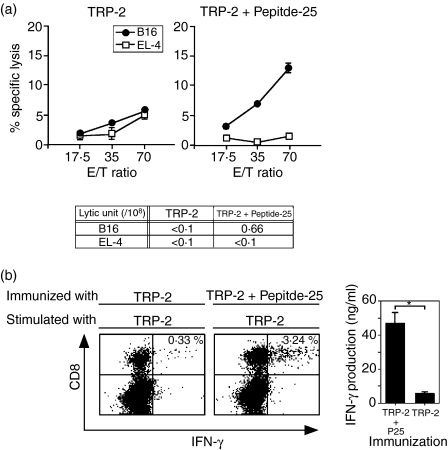
In separate experiments, we analysed the augmenting effect of Peptide-25 on the CTL response specific for TRP-2 peptide, which is an MHC class I-binding peptide of murine melanoma. We immunized C57BL/6 mice with TRP-2 in IFA or a mixture of TRP-2 and Peptide-25 in IFA twice with a 10-day interval. Spleen cells from each group of mice were stimulated in vitro with TRP-2 for 5 days and TRP-2-specific CTL assay was conducted. Co-immunization with TRP-2 and Peptide-25 induced a significant CD8+ CTL response in T cells to TRP-2 and IFN-γ production, while TRP-2 immunization was ineffective (Fig. 2a,b).
Figure 2.
Enhancing effect of Peptide-25 on TRP-2-specific CD8+ CTL response by coimmunization with TRP-2. (a) Two groups of mice were immunized subcutaneously with TRP-2 (10 μg) in IFA or TRP-2 (10 μg) and Peptide-25 (P25) (10 μg) in IFA. Spleen cells from each group of mice were subjected to TRP-2-specific CTL assay 10 days after the immunization. CTL assay was conducted by incubating sensitized cells with 51Cr-labelled B16 melanoma or 51Cr-labelled EL-4 cells of various effector to target ratios at 37° for 4 hr. The values represent the geometric means and standard deviation of percentage specific lysis in triplicate cultures. Background lysis of the target cells was less than 9% of maximum 51Cr-release. The number of LU was calculated from the dose–response curve obtained with each group. A representative result of a series of three experiments is shown. (b) Ten days after immunization, spleen cells were stimulated with TRP-2 (10 μg/ml) for 2 days. Intracellular staining of IFN-γ was carried out on the recovered cells and they were examined by FACSCalibur. The percentages of IFN-γ-producing CD8+ cells are presented in the upper right region. IFN-γ production of spleen cells in the culture supernatants was quantified by ELISA. The values represent the mean and standard deviation of the triplicate cultures. *P < 0·01 by Student's t-test.
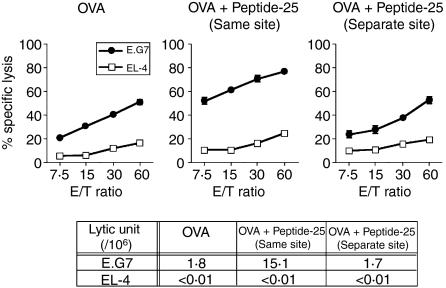
Co-immunization of a mixture of OVA and Peptide-25 at the same site is required for the enhanced CD8+ CTL response
We examined whether enhanced OVA-specific CTL generation by coimmunization with Peptide-25 can be induced when OVA and Peptide-25 are immunized separately. A group of mice was immunized with a mixture of OVA and Peptide-25 in IFA subcutaneously at the same site on the right-hand side of the abdomen. A group of mice was immunized with OVA in IFA and Peptide-25 in IFA separately (left and right sides of the abdomen, respectively). The CTL assay was conducted using spleen cells from each group of mice 10 days after the immunization. As shown in Fig. 3, the enhancement of the OVA-specific CTL response by Peptide-25 was observed only when a mixture of OVA and Peptide-25 in IFA was immunized at the same site. These results suggest that OVA and Peptide-25 need to be presented by the same antigen-presenting cells (APCs) for antigen processing to occur.
Figure 3.
Enhanced OVA-specific CD8+ T-cell response induced by coimmunization with OVA and Peptide-25 at the same site. A group of mice was immunized with OVA (10 μg) and Peptide-25 (10 μg) in IFA at the same site subcutaneously. Another group of mice was immunized with OVA (10 μg) in IFA and Peptide-25 (10 μg) in IFA at two distant sites (separate sites). As a control, we also immunized a group of mice with OVA (10 μg) in IFA. Spleen cells from each group of mice 10 days after the immunization were subjected to OVA-specific CTL assay. CTL assay was conducted by incubating sensitized cells with 51Cr-labelled E.G7 or 51Cr-labelled EL-4 cells at various effector to target ratios at 37° for 4 hr. The values represent the geometric means and standard deviation of percentage specific lysis in triplicate cultures. Background lysis of the target cells was less than 10% of maximum 51Cr-release. The number of LU was calculated from the dose–response curve obtained with each group. A representative result of a series of three experiments is shown.
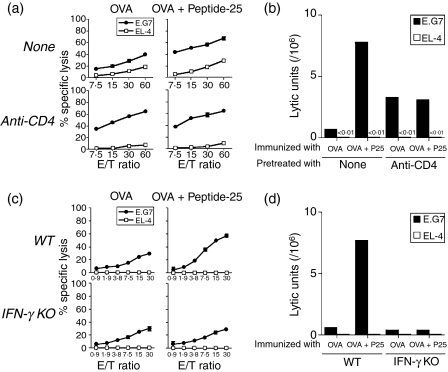
The enhancement of the OVA-specific cytolytic T-cell response by Peptide-25 depends on CD4+ T cells and IFN-γ
To understand the efficacy of Peptide-25 to enhance OVA-specific cytotoxic activity, we examined whether IFN-γ-producing CD4+ T cells contribute to the above enhancing effect of Peptide-25 on OVA-specific CTL generation. First, we depleted CD4+ T cells in vivo by administering anti-CD4 mAb (GK1.5) to two groups of mice as described in the Materials and methods. As a control, rat IgG was administered in place of anti-CD4 to another two groups of mice. All groups of mice were immunized with OVA in IFA or with a mixture of OVA and Peptide-25 in IFA. As shown in Fig. 4(a,b), an enhanced CTL response specific for OVA, mounted in a culture of spleen cells taken from mice immunized with a mixture of OVA and Peptide-25 in IFA, was abrogated by the anti-CD4 mAb treatment before immunization, and the level of CTL response, measured as LU, was similar to that mounted in spleen cells from OVA-immunized mice. Unexpectedly, an enhanced OVA-specific CTL response, mounted in a culture of spleen cells from OVA-immunized mice, was observed when anti-CD4 mAb mice had been treated before OVA immunization.
Figure 4.
Role of CD4+ T cells and IFN-γ in the enhancing effect of Peptide-25 on CTL response. (a,b) Two groups of wild-type mice were injected with anti-CD4 mAb (GK1.5) on days −13, −12, −11, −6, −5, −4, +1, +2 and +3 relative to immunization. Each group of mice was immunized with OVA (10 μg) in IFA or a mixture of OVA (10 μg) and Peptide-25 (10 μg) in IFA on Day 0. As controls, two other groups of mice had been treated with normal rat IgG in place of GK1.5 and immunized with OVA in IFA or a mixture of OVA and Peptide-25 in IFA. Spleen cells from each group of mice were subjected to in vitro OVA-specific CTL assay 10 days after the immunization. (c,d). Either wild-type or IFN-γ–/– mice with C57BL/6 background were immunized with OVA (10 μg) in IFA or a mixture of OVA (10 μg) and Peptide-25 (P25) (10 μg) in IFA. Spleen cells from each group of mice were subjected to OVA-specific CTL assay 10 days after the immunization. CTL assay (a,c) and LU calculations (b,d) were conducted as described in Figure 1.
To examine the roles of IFN-γ-producing cells in the CTL response, we immunized both wild-type and IFN-γ–/– mice with a mixture of OVA and Peptide-25 in IFA. Spleen cells from each group of mice were then subjected to the in vitro CTL assay specific for OVA. The results revealed that an enhanced CTL response specific for OVA was mounted in spleen cells from wild-type mice immunized with a mixture of OVA and Peptide-25 in IFA, while the enhancement was not observed in spleen cells from IFN-γ–/– mice (Fig. 4c,d). These results indicate that Peptide-25-reactive IFN-γ-producing T cells contribute to the enhancing effect of Peptide-25 on OVA-specific CTL generation.
Co-immunization with a mixture of OVA and Peptide-25 increases proportions of OVA-specific CTL precursors
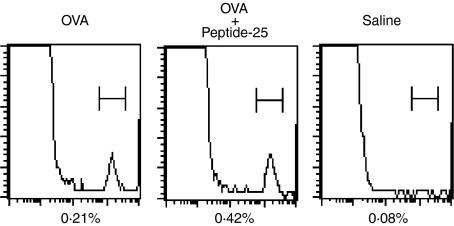
To examine the cellular mechanisms of the enhancing effect of coimmunization of mice with a mixture of OVA and Peptide-25 on CTL generation following in vitro OVA stimulation, we examined the frequencies of OVA-specific CTL precursors. Spleen cells from mice were immunized with either OVA in IFA or a mixture of OVA and Peptide-25 in IFA 10 days before the experiments. The cells were then stained with OVA peptide-loaded H-2Kb:Ig protein and anti-mouse IgG1–PE and analysed using FACSCalibur. The results revealed that frequencies for OVA-specific CTL precursors in spleen cells (0·42%) from mice immunized with a mixture of OVA and Peptide-25 were about two-fold higher than those in spleen cells (0·21%) from OVA-immunized mice (Fig. 5).
Figure 5.
Frequency analysis of OVA-specific CTL. Two groups of mice were immunized with either OVA in IFA or OVA and Peptide-25 in IFA. Spleen cells from each group of mice were prepared 10 days after the immunization and stained with 4 μg of OVA peptide (SIINFEKL)-loaded H-2Kb:Ig protein and incubated for 60 min at 4°. After washing with staining buffer, cells were resuspended in 100 μl staining buffer containing appropriately diluted anti-mouse IgG1-PE and anti-CD8-FITC. After washing twice with staining buffer, cells were analysed using FACSCalibur.
Co-immunization of mice with a mixture of OVA and Peptide-25 can suppress E.G7 growth, leading to tumour rejection in vivo
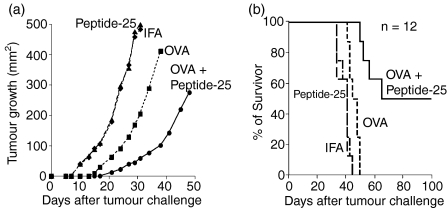
We examined whether the immunization of C57BL/6 mice with a mixture of OVA and Peptide-25 was effective as a prophylactic intervention into the growth of E.G7. Three groups of 12 mice each were immunized with OVA in IFA, Peptide-25 in IFA, or a mixture of OVA and Peptide-25 in IFA. As a control, a fourth group of 12 mice was injected with IFA. Ten days after the immunization, we transplanted viable E.G7 tumour cells onto the backs of the mice and monitored tumour growth (Fig. 6a) and survival (Fig. 6b) after tumour challenge. As shown in the figures, the transplanted E.G7 established tumour masses within days of the tumour challenge in the abdomen of both the control group and the Peptide-25-immunized group of mice, leading to the death of all animals by day 30. Tumour growth in mice immunized with OVA in IFA was slightly delayed compared with that in the control mice however, all mice died by day 40 after the tumour challenge. Interestingly, mice immunized with a mixture of OVA and Peptide-25 in IFA showed significantly delayed tumour progression. Among these 12 mice, the E.G7 tumours of six of them were eradicated by Day 60 after the tumour challenge and the lifetimes of the mice were continued, tumour-free, for up to 100 days. By contrast, all mice in the other groups died within 40 days after the tumour challenge. These results indicate that coimmunization of Peptide-25 with OVA enhances not only an OVA-specific CTL response in vitro but also induces a potent antitumour immunity against OVA-expressing tumour cells in vivo.
Figure 6.
Enhancement of antitumour immunity by Peptide-25. 51 (a) Suppression of E.G7 growth by augmented induction of E.G7-specific immunity. Three groups of mice were immunized with OVA (10 μg) in IFA, OVA (10 μg) and Peptide-25 (10 μg) in IFA or Peptide-25 (10 μg) in IFA subcutaneously. As a control, a group of mice was injected with IFA. All groups of mice were challenged with 5 × 105 viable E.G7 cells subcutaneously 10 days after the immunization. Growth of E.G7 tumour was monitored by measuring its size periodically (2- to 3-day intervals) and expressed as mm2. (b) Survival of E.G7-bearing mice. The percentages of survivors in the respective groups shown in (a) are displayed.
The enhancement of the Th1 response to OVA by coimmunization with Peptide-25
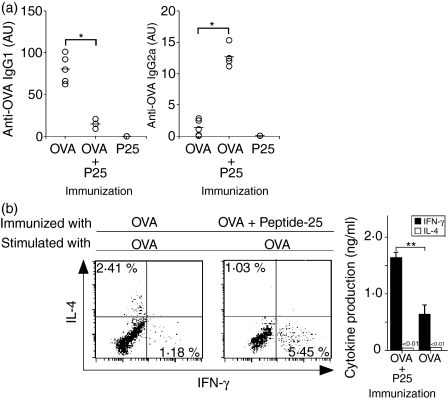
To understand the mechanisms of the enhancing effect of Peptide-25 on the OVA-specific CTL response, we examined the Th1 generation specific for OVA when C57BL/6 mice were immunized with OVA in IFA, Peptide-25 in IFA, or a mixture of OVA and Peptide-25 in IFA. First, we measured anti-OVA antibody in the serum of each group of mice by ELISA 10 days after immunization. As shown in Fig. 7(a), OVA-immunized mice produced predominantly anti-OVA IgG1 antibody and produced lower levels of the anti-OVA IgG2a antibody. In contrast, mice immunized with a mixture of OVA and Peptide-25 produced predominantly anti-OVA IgG2a antibody. We could not detect any anti-OVA antibody in sera from Peptide-25-immunized mice (Fig. 7a).
Figure 7.
Enhancement of OVA-specific Th1 response by coimmunization of C57BL/6 mice with OVA and Peptide-25. Two different groups of mice were immunized with OVA (10 μg) in IFA, OVA (10 μg) and Peptide-25 (P25) (10 μg) in IFA, or Peptide-25 (10 μg) in IFA subcutaneously. (a) Serum anti-OVA IgG1 and IgG2a were titrated by ELISA 10 days after the immunization. Each open circle represents the results of an individual mouse. The horizontal bar represents the mean value of six mice. *P < 0·01 by Student's t-test. (b) Ten days after immunization, spleen cells were stimulated in vitro with OVA (10 μg/ml) for 4 days. Intracellular staining of IL-4 and IFN-γ was carried out to the recovered cells. Cells stained were gated on live CD4+ cells and examined by FACSCalibur. The percentages of IL-4- and IFN-γ-producing CD4+ T cells are presented in the upper left and lower right regions, respectively. IL-4 and IFN-γ produced in the culture supernatants were titrated by ELISA. The values represent the mean and standard deviation of the triplicate cultures. **P < 0·05 by Student's t-test.
To evaluate the cytokine-producing profiles in CD4+ T cells in the spleen, spleen cells from each group of immunized mice were stimulated in vitro with OVA for 48 hr, and the proportions of IFN-γ- and IL-4-producing cells were examined by intracellular cytokine staining. Cells stained were gated on live CD4+ cells and analysed by FACS. The results revealed that we could detect IFN-γ-producing CD4+ T cells but not IL-4-producing cells when spleen cells from mice immunized with a mixture of OVA and Peptide-25 were stimulated with OVA (Fig. 7b). In contrast, OVA stimulation of spleen cells from OVA-immunized mice could become both IFN-γ- and IL-4-producing cells. We confirmed the enhancement of OVA-induced IFN-γ production by ELISA (Fig. 7b). Co-immunization of OVA with Peptide-9 slightly enhanced the proportions of IFN-γ-producing cells, while coimmunization with Peptide-18 did not (data not shown). We did not observe differences in proportion of CD4+ CD25+ T cells between Peptide-25-immunized and Peptide-9-immunized T cells. These results suggest that the weak Th1-inducing ability of Peptide-9 correlates with a weak ability to enhance OVA-specific CTL generation when coimmunized with OVA.
The involvement of Peptide-25-reactive T cells in the induction of an OVA-reactive Th1 response was examined by using the rat anti-mouse clonotypic TCR mAb KN7 that can recognize TCR expressed on the Peptide-25-reactive Th1 clone of C57BL/6 mice. Enhanced induction of an OVA-specific IFN-γ-producing T-cell response in mice immunized with a mixture of OVA and Peptide-25 was impaired when we injected KN7 intraperitoneally 1 day before the immunization. In contrast, the control group that received rat IgG injection in place of KN7 did not show this suppressive effect (data not shown).
Peptide-25-reactive T cells can activate DCs through Peptide-25 leading to enhanced induction of OVA-presenting activity
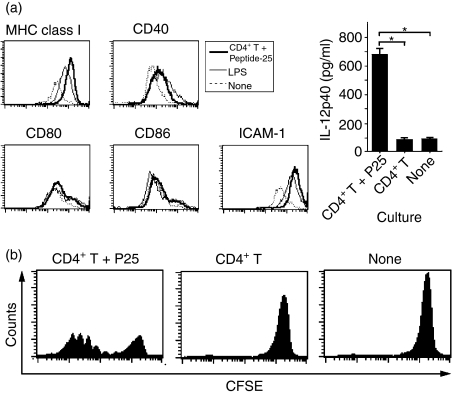
The enhancement of an OVA-specific CTL response by coimmunization of Peptide-25 with OVA may be because the Peptide-25 directly or indirectly affects the OVA-presenting activity of APCs. To investigate this issue, we first evaluated the antigen-presenting activity of DCs. We propagated immature DCs in vitro by culturing bone marrow cells with GM-CSF and IL-3 for 6 days. They were stimulated for 48 hr with Peptide-25 in the presence of splenic CD4+ T cells from P25 TCR-Tg mice. As a control, DCs were also stimulated with lipopolysaccharide. The expression of surface markers such as CD40, CD80, CD86, MHC class I antigen and intracellular adhesion molecule-1 (ICAM-1) was analysed using FACSCalibur. The results revealed that Peptide-25 stimulation alone did not alter the expression of the above surface markers on DCs (data not shown). When we cocultured DCs with CD4+ T cells from P25 TCR-Tg mice together with Peptide-25, the expressions of MHC class I and ICAM-1 were enhanced to a similar extent to that found under lipopolysaccharide stimulation and this led to the induction of IL-12p40 production (Fig. 8a). Such DCs showed more effective OVA presentation to CD8+ T cells from OT-1 mice and enhanced OT-1 cell divisions (Fig. 8b). These results suggest that Peptide-25-reactive CD4+ T cells directly activate DCs in the presence of Peptide-25, leading to effective OVA cross-presentation for the activation of CD8+ T cells.
Figure 8.
Activation of DCs by culturing with Peptide-25 in the presence of CD4+ T cells from P25 TCR-Tg mice. (a) Immature DCs were propagated by culturing bone marrow cells with GM-CSF (20 ng/ml) and IL-3 (20 ng/ml) for 6 days. The cells recovered (5 × 105) were cultured with lipopolysaccharide (LPS; 5 μg/ml), Peptide-25 together with CD4+ T cells (5 × 105) from P25 TCR-Tg mice or left untreated for 48 hr. The expression of surface markers was assessed by FACS analysis. IL-12p40 in the cultured supernatant was assessed by ELISA. (b) CFSE-labelled CD8+ T cells (5 × 105) from OT-1 mice were cultured with immature DCs (5 × 105) and OVA (10 μg/ml) for 4 days. The cells were cocultured with CD4+ T cells (5 × 105) from P25 TCR-Tg mice and Peptide-25 (P25) (10 μg/ml) (left panel) or CD4+ T cells from P25 TCR-Tg mice (middle panel). Subsequently, cell division of the CD8+ T cells was monitored by FACSCalibur.
Discussion
Antitumour immune responses involve complex interactions among various immunocompetent cells. CD8+ CTLs are major effector cells capable of direct tumour destruction both in vivo and in vitro, and they recognize MHC class I binding peptides derived from molecules with altered expression in tumour cells.28–30 Although the need for CD4+ Th cells in regulating CD8+ T cells has been documented, their target epitopes and functional impact in antitumour responses remain unclear.
There has been a recent reappraisal of the role and importance of CD4+ Th cells in antitumour responses. CD4+ Th cells are considered to contribute to the activation of CD8+ CTLs through the expression of the CD40 ligand and production of cytokines, such as IL-2 and IFN-γ that are essential for the proliferation and maturation of CD8+ CTL precursors. CD4+ Th cells recognize MHC class II binding peptides on APCs and their interaction may result not only in the activation and priming of CD4+ Th cells, but also in the activation of APCs themselves.31–33 Consequent to these mutual activations, APCs prime and activate CD8+ CTLs specific for the tumour antigen or peptides. This scenario of cellular interaction assumes that CD4+ Th cells and CD8+ CTLs may not necessarily be in direct or close association. CD4+ T cells are also implicated in the activation of tumoricidal macrophages that are involved in tumour clearance. Cytokines produced by CD4+ T cells can recruit and activate macrophages and eosinophils, linking the T-cell response with the innate immune response.
In this study using OVA as neo-tumour antigen in a mouse model, we investigated the contribution of the Th1 epitope within the 15 amino acid residues of the Ag85B protein. We then examined the significance of the defined Th1 epitope regarding CD8+ CTL generation and tumour eradication. We demonstrated that the coimmunization of Peptide-25 and the OVA CTL epitope resulted in a marked increase in the OVA-specific CD8+ CTL response (Fig. 1). The selection of immunization site for Peptide-25 and OVA is important and both should be immunized at the same site (Fig. 3). The augmenting effect of Peptide-25 on CD8+ CTL generation was cancelled by in vivo administration of the anti-CD4 mAb and was diminished in IFN-γ–/– mice (Fig. 4), indicating that the CD4+ T cells and IFN-γ-producing T cells are required. It remains elusive why the enhancement of OVA-specific CTL activity was abrogated when IFN-γ–/– mice were immunized with the mixture of Peptide-25 and OVA. As we reported, the generation of Peptide-25-reactive CD4+ T cells significantly decreases in IFN-γ–/– mice as compared with wild-type mice. Furthermore, Peptide-25 stimulation of Peptide-25-immunized cells induces IL-2 production that is also impaired in IFN-γ–/– cells.22 IFN-γ-dependent IL-2 production may be indispensable to enhance the OVA-specific CD8+ CTL generation and expansion in this particular system. Whilst we favour the possibility that impaired production of IFN-γ by CD4+ T cells contributes to the reduction of CTL activity seen in mice primed with Peptide-25 and OVA we cannot exclude the possibility that ablation of IFN-γ production by CD8+ T cells also plays a role.
Another explanation is that IFN-γ may play an important role in a cell-to-cell contact between Peptide-25-specific CD4+ Th cells and APCs to enhance the induction of the OVA-specific CD8+ CTL response. APCs are capable of processing and presenting exogenous antigens along with MHC class I molecules to CD8+ T cells, which is termed antigen cross-presentation. The antigenic peptide generation for cross-presentation appears to be dependent on both the ubiquitin–proteasome system and the transporter associated with antigen processing.34–37 Imai and his colleagues recently demonstrated that exogenously added OVA is accumulated in microsomal fractions, including the endoplasmic reticulum and late endosomes followed by retrograde transport to the cytoplasm through the Sec61 transporter complexes.38 IFN-γ may facilitate these processes of OVA cross-presentation by APCs leading to enhancement of the induction of the OVA-specific CD8+ CTL response.
Unexpectedly, our result showed that anti-CD4 mAb treatment before OVA immunization in mice enhanced the OVA-specific CTL response. Although we do not have concrete evidence, anti-CD4 mAb treatment may eliminate OVA-specific CD4+ T cells that negatively regulate OVA-specific CD8+ CTL generation.
Among I-Ab-binding peptides, Peptide-25 showed the most potent effect that correlated with potency for the in vivo induction of Th1 generation to each peptide. These results indicate that a Th1 epitope, regardless of the difference in its molecular origin, exhibits a helper activity for in vivo CD8+ CTL generation. It is unclear why I-Ab binding Peptide-9 does not augment the induction of the OVA-specific CTL response. Peptide-9 showed lower immunogenicity for Th1 induction specific for its own compared with Peptide-25.22 We analysed the Foxp3 expression in Peptide-9-reactive T cells by FACS and found no significant increase in the Foxp3+ T regulatory cell population compared with Peptide-25-reactive T cells (data not shown). These results imply that the weak potency of Peptide-9 to augment the OVA-specific CTL response is well correlated with its weak Th1-inducing potency instead of a T regulatory cell induction.
Both quantitative and qualitative changes may be involved in the Th effect of Peptide-25 in the CTL response. We think that a two-fold increase in the frequency of OVA-specific CD8+ T cells and an increase of the lytic unit are qualitatively correlated with the Th effect of Peptide-25 (Figs 1–4). In addition, Peptide-25 stimulation of spleen cells from Peptide-25-immunized wild-type mice induces secretion of IL-2 that is impaired in IFN-γ–/– mice.22 IL-2 produced by the Th1 cells may also enhance and expand the CTL maturation quantitatively. To evaluate qualitative changes more directly, we examined Granzyme B expression in OVA-reactive CD8+ T cells. Results revealed that we did not observe significant increases in Granzyme B-expressing cells in the OVA-stimulated CD8+ T cells in the presence of Peptide-25-primed Th cells compared with the precursors induced in the absence of the primed Th cells (data not shown). These results suggest that OVA-specific CD8+ T-cell precursors induced in the presence of primed Th cells may differ quantitatively from these induced in the absence of primed Th cells.
The basis for the strong ‘helper’ function of Peptide-25 in the CD8+ CTL response to E.G7 rejection is unknown. There are several possibilities to account for the enhancing effect of Peptide-25 on Th1 and CTL generation specific for OVA when Peptide-25 and OVA were coimmunized at the same site. Peptide-25 may directly activate APCs through unknown molecules resulting in effective cross-presentation of OVA molecules to both Th1 and CTL precursors. To address this issue, we propagated bone-marrow-derived DCs in vitro, stimulated them with Peptide-25 and analysed the surface expression of activation markers on DCs. The results revealed that expression of MHC class I, ICAM-1, CD40, CD80 and CD86 was not enhanced upon Peptide-25 stimulation (data not shown). We found that myeloid differentiating factor (MyD)88-deficient mice also showed enhanced CTL generation specific for OVA when coimmunized with Peptide-25 and OVA (data not shown). The MyD88-dependent signalling pathway may not be required for exerting helper activity by Peptide-25. On the whole, we do not support the hypothesis that Peptide-25 directly activates APCs.
As Peptide-25-reactive CD4+ Th cells recognize MHC class II binding cognate Peptide-25 on APCs and their interaction may result in the activation of APCs, consequent to these mutual activations, APCs prime and activate CD8+ CTLs specific for OVA peptides. The requirement for coimmunization of Peptide-25 and OVA in the same site suggests that the physical proximity of OVA and Peptide-25 is crucial for intracellular events in APCs. Cell-to-cell contact between Peptide-25-specific CD4+ Th1 cells and APCs may be required or the two antigens need to be processed and presented by the same APC to augment the OVA-specific CD8+ CTL response. To simplify the experimental system for further evaluation, we established an in vitro culture system using T cells from P25 TCR-Tg mice, CFSE-labelled T cells from OT-1 mice and bone-marrow-derived immature DCs propagated in vitro. As we reported, CD4+ T cells from P25 TCR-Tg mice preferentially develop IFN-γ-producing T cells upon Peptide-25 stimulation in the presence of I-Ab splenic APCs under neutral conditions.24 Surface expression of the MHC class I molecule, ICAM-1, CD40, CD80 and CD86 on DCs as well as IL-12p40 production was enhanced when DCs were cultured with CD4+ T cells from P25 TCR-Tg mice and Peptide-25 (Fig. 8a). Furthermore, enhanced cell divisions of CFSE-labelled OT-1 T cells were observed when the cells were stimulated with OVA in the presence of DCs, Peptide-25 and CD4+ T cells from P25 TCR-Tg mice (Fig. 8b). These results support the notion that CD4+ Th1 cell-derived factor may augment antigen processing by APCs leading to the enhancement of the cross-priming of the antigenic peptide for CD8+ T cells.
A number of approaches to augment CD4+ T-cell help have been investigated.2,10,13,39 One involves modifying the immunizing antigen itself by, for instance, haptenizing the antigen40 or linking heterologous immunogenic peptides directly to the antigen.17,41 The second involves coimmunization with tumour antigens and molecules with strong helper determinants.42,43 The third, the discovery of a range of molecular signals, such as the CD40 ligand and other costimulatory signals31–33 involved in the helper function of CD4+ T cells, provides other ways to augment the CD8+ T-cell response. Finally, broadly expressed wild-type molecules in murine tumour cells eliciting humoral immunity contribute to the generation of CD8+ T cells and protective antitumour immune responses to unrelated tumour-specific antigen.13
It is important to ask whether the enhancing effect of Peptide-25 on CD8+ CTL responses is beneficial, detrimental, or insignificant to the tumour-bearing host. It is likely that the phenomena that we have described in the mouse of a heightened CD8+ CTL response to tumour antigens by corecognition of Peptide-25 has its counterpart in humans, occurring as a consequence of the simultaneous uptake of complex antigenic mixtures from disintegrating tumour cells by APCs. As coimmunization with Peptide-25 and tumour antigens also results in heightened resistance to tumour challenge in the mouse, this approach may be an attractive strategy for human cancer immunotherapy. In fact, there are ongoing clinical trials utilizing either heterologous helper antigens, such as keyhole limpet haemocyanin or tumour-derived helper antigens to augment antitumour immune responses by CD8+ CTLs directed against tumour-derived CTL epitopes.44,45 As Ag85B and Peptide-25 are stimulatory to human CD4+ T cells from PPD+ healthy donors in a certain proportion of the Japanese population, Ag85B or peptide-25 may be applicable to augment antitumour immune responses by CD8+ CTLs against tumour cells. Along with the scenario, we re-examined the effect of preimmunization with M. tuberculosis or Peptide-25 on the antitumour CTL response and found that preimmunization of mice with heat-killed M. tuberculosis or Peptide-25 followed by immunization with a mixture of OVA and Peptide-25 produced a significant increase in the number of CD8+ CTL s (data not shown).
While it is obvious that CD8+ T cells recognize MHC class I binding peptides derived from tumour target cells based on the subsequent tumour-specific destruction, peptide derivation from tumour cells may not be an absolute requirement for CD4+ T cells because they do not directly interact with tumour cells that mostly lack MHC class II expression. Co-immunization of mice with TRP-2 of B16 melanoma and Peptide-25 in IFA enhances CTL generation specific for TRP-2 (Fig. 2). However, mice that had been immunized with a mixture of TRP-2 and Peptide-25 in IFA failed to eradicate B16 melanoma, a less immunogenic tumour, although B16 melanoma growth was reduced to a certain extent (data not shown), suggesting that enhancing the effect of Peptide-25 in the TRP-2-specific CTL may not be potent enough to eradicate a less immunogenic TRP-2-expressing tumour. We need another regimen together with Peptide-25 immunization that activates effector cells leading to the eradication of tumour cells with weak immunogenicity. This notion has become extremely important in the design of future vaccines aimed at the efficient activation of both T-cell populations involved in antitumour immune responses.
Acknowledgments
We thank Drs H. Udono (Nagasaki University School of Medicine, Nagasaki, Japan), Y. Iwakura (Institute of Medical Science, University of Tokyo, Tokyo Japan), and H. Tahara (Institute of Medical Science, University of Tokyo, Tokyo Japan) for providing E.G7 cells, IFN-γ–/– mice and B16 melanoma cells, respectively, and Drs S. Takaki (Institute of Medical Science, University of Tokyo, Tokyo Japan) and S. Taki (Shinshu University, School of Medicine, Matsumoto, Japan) for their valuable suggestions throughout this study. We are also indebted to our colleagues for their critical reading of the manuscript. This work was supported by Special Coordination Funds on ‘Molecular Analysis of the Immune System and Its Manipulation on Development, Activation and Regulation’ for Promoting Science and Technology (K.T.), by a grant for Advanced Research on Cancer and a grant for Scientific Research on Priority Areas from the Japanese Ministry of Education, Science, Sports and Culture, and by a grant from the Uehara Memorial Foundation.
References
- 1.Boon T, Cerottini JC, Van den Eynde B, Van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–76. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 3.Davis ID, Jefford M, Parente P, Cebon J. Rational approaches to human cancer immunotherapy. J Leukoc Biol. 2003;73:3–29. doi: 10.1189/jlb.0502261. [DOI] [PubMed] [Google Scholar]
- 4.Wagner H, Pfizenmaier K, Rollinghoff M. The role of the major histocompatibility gene complex in murine cytotoxic T cell responses. Adv Cancer Res. 1980;31:77–124. doi: 10.1016/s0065-230x(08)60657-0. [DOI] [PubMed] [Google Scholar]
- 5.North RJ. The murine antitumor immune response and its therapeutic manipulation. Adv Immunol. 1984;35:89–155. doi: 10.1016/s0065-2776(08)60575-1. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 7.Melief CJ. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res. 1992;58:143–75. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–7. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 9.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–94. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 12.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–6. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa H, Tanida K, Ikeda H, et al. Role of SEREX-defined immunogenic wild-type cellular molecules in the development of tumor-specific immunity. Proc Natl Acad Sci USA. 2001;98:14571–6. doi: 10.1073/pnas.251547298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin U, Tureci O, Schmitt H, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–13. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–16. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 16.Old LJ, Chen YT. New paths in human cancer serology. J Exp Med. 1998;187:1163–7. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takatsu K, Hamaoka T, Tominaga A, Kanamasa Y. Augmented induction of tumor-specific resistance by priming with Mycobacterium tuberculosis (TBC) and subsequent immunization with PPD-coupled syngeneic tumor cells. J Immunol. 1980;125:2367–73. [PubMed] [Google Scholar]
- 18.Takatsu K, Kikuchi Y, Takahashi T, Honjo T, Matsumoto M, Harada N, Yamaguchi N, Tominaga A. Interleukin 5, a T-cell-derived B-cell differentiation factor also induces cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1987;84:4234–8. doi: 10.1073/pnas.84.12.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaoka T, Takatsu K, Okuno K, Tsuchida T. Functional characterization of the killer-helper factor responsible for the induction of cytotoxic T lymphocytes from thymocytes, and evidence for the nature of this factor as distinct from T cell-replacing factor (TRF) in regard to B cell triggering. J Immunol. 1981;126:659–65. [PubMed] [Google Scholar]
- 20.Yanagisawa S, Koike M, Kariyone A, Nagai S, Takatsu K. Mapping of Vβ11+ helper T cell epitopes on mycobacterial antigen in mouse primed with Mycobacterium tuberculosis. Int Immunol. 1997;9:227–37. doi: 10.1093/intimm/9.2.227. [DOI] [PubMed] [Google Scholar]
- 21.Kariyone A, Higuchi K, Yamamoto S, et al. Identification of amino acid residues of the T-cell epitope of Mycobacterium tuberculosisα antigen critical for Vβ11+ Th1 cells. Infect Immun. 1999;67:4312–19. doi: 10.1128/iai.67.9.4312-4319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kariyone A, Tamura T, Kano H, Iwakura Y, Takeda K, Akira S, Takatsu K. Immunogenicity of Peptide-25 of Ag85B in Th1 development: role of IFN-γ. Int Immunol. 2003;15:1183–94. doi: 10.1093/intimm/dxg115. [DOI] [PubMed] [Google Scholar]
- 23.Takatsu K, Kariyone A. The immunogenic peptide for Th1 development. Int Immunopharmacol. 2003;3:783–800. doi: 10.1016/S1567-5769(02)00209-6. [DOI] [PubMed] [Google Scholar]
- 24.Tamura T, Ariga H, Kinashi T, et al. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int Immunol. 2004;16:1691–9. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- 25.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ–/– mice, but not in TNF-α–/– mice. Role for IFN-γ in activating apoptosis of hepatocytes. J Immunol. 1997;159:1418–28. [PubMed] [Google Scholar]
- 26.Khong HT, Rosenberg SA. Pre-existing immunity to tyrosinase-related protein (TRP) -2, a new TRP-2 isoform, and the NY-ESO-1 melanoma antigen in a patient with a dramatic response to immunotherapy. J Immunol. 2002;168:951–6. doi: 10.4049/jimmunol.168.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horikawa K, Kaku H, Nakajima H, et al. Essential role of Stat5 for IL-5-dependent IgH switch recombination in mouse B cells. J Immunol. 2001;167:5018–26. doi: 10.4049/jimmunol.167.9.5018. [DOI] [PubMed] [Google Scholar]
- 28.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–82. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchison NA, O'Malley C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur J Immunol. 1987;17:1579–83. doi: 10.1002/eji.1830171109. [DOI] [PubMed] [Google Scholar]
- 31.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 32.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 33.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 34.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990;249:918–21. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–8. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 36.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 37.Houde M, Bertholet S, Gagnon E, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–6. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 38.Imai J, Hasegawa H, Maruya M, Koyasu S, Yahara I. Exogenous antigens are processed through the endoplasmic reticulum-associated degradation (ERAD) in cross-presentation by dendritic cells. Int Immunol. 2005;17:45–53. doi: 10.1093/intimm/dxh184. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura T, Iwakabe K, Sekimoto M, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–27. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizushima Y, Fujiwara H, Takai Y, Shearer GM, Hamaoka T. Genetic control of hapten-reactive helper T-cell responses and its implications for the generation of augmented antitumor cytotoxic responses. J Natl Cancer Inst. 1985;74:1269–73. [PubMed] [Google Scholar]
- 41.Rice J, Elliott T, Buchan S, Stevenson FK. DNA fusion vaccine designed to induce cytotoxic T cell responses against defined peptide motifs: implications for cancer vaccines. J Immunol. 2001;167:1558–65. doi: 10.4049/jimmunol.167.3.1558. [DOI] [PubMed] [Google Scholar]
- 42.Romieu R, Baratin M, Kayibanda M, Lacabanne V, Ziol M, Guillet JG, Viguier M. Passive but not active CD8+ T cell-based immunotherapy interferes with liver tumor progression in a transgenic mouse model. J Immunol. 1998;161:5133–7. [PubMed] [Google Scholar]
- 43.Casares N, Lasarte JJ, de Cerio AL, Sarobe P, Ruiz M, Melero I, Prieto J, Borras-Cuesta F. Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated Th1 helper peptide elicits protective CTL immunity. Eur J Immunol. 2001;31:1780–9. doi: 10.1002/1521-4141(200106)31:6<1780::aid-immu1780>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Schuler-Thurner B, Schultz ES, Berger TG, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–88. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slingluff CL, Jr, Yamshchikov G, Neese P, et al. Phase I trial of a melanoma vaccine with gp100 (280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–24. [PubMed] [Google Scholar]