Abstract
CD8+ T-cell responses are critical in the immunological control of tumours and infectious diseases. To prime CD8+ T cells against these cell-associated antigens, exogenous antigens must be cross-presented by professional antigen-presenting cells (APCs). While cross-presentation of soluble antigens by dendritic cells is detectable in vivo, the efficiency is low, limiting the clinical utility of protein-based vaccinations. To enhance the efficiency of presentation, we generated nanoparticles from a biodegradable polymer, poly(d,l-lactide-co-glycolide) (PLGA), to deliver antigen into the major histocompatibility complex (MHC) class I antigen presentation pathway. In primary mouse bone marrow-derived dendritic cells (BMDCs), the MHC class I presentation of PLGA-encapsulated ovalbumin (OVA) stimulated T cell interleukin-2 secretion at 1000-fold lower concentration than soluble antigen and 10-fold lower than antigen-coated latex beads. The microparticles also served as an intracellular antigen reservoir, leading to sustained MHC class I presentation of OVA for 72 hr, decreasing by only 20% after 96 hr, a time at which the presentation of soluble and latex bead-associated antigens was undetectable. Cytosol extraction demonstrated that antigen delivery via PLGA particles increased the amount of protein that escaped from endosomes into the cytoplasm, thereby increasing the access of exogenous antigen to the classic MHC class I loading pathway. These data indicate that the unique properties of PLGA particle-mediated antigen delivery dramatically enhance and sustain exogenous antigen presentation by MHC class I, potentially facilitating the clinical use of these particles in vaccination.
Keywords: dendritic cells, antigen presentation/processing, vaccination
Introduction
For effective control of tumours and pathogens by the immune system, neoplastic and infected cells must be targeted and destroyed by cytotoxic T lymphocytes (CTLs). While major histocompatibility complex (MHC) class I molecules conventionally present endogenous cytosolic antigens, an alternative pathway, termed cross-presentation, allows the presentation of peptides derived from exogenous antigens in the context of MHC class I. 1–3 As tumour antigens and pathogen-derived proteins are often not endogenously produced by antigen-presenting cells (APCs), this exogenous pathway is crucial for the generation of CD8+ CTL responses against these cell-associated antigens.4,5 Enhancement of the targeting of exogenous antigens to the cross-presentation pathway may help develop effective vaccines against tumours, parasites, intracellular bacteria and viruses.6,7
While dendritic cells (DCs) efficiently target internalized proteins to the MHC class I presentation pathway, all endocytic cells, including macrophages and in some circumstances B cells, can present low levels of exogenous antigens, making antigen internalization a prerequisite for cross-presentation. The mechanism of antigen internalization, however, significantly influences the efficiency of cross-presentation; soluble antigens internalized by macropinocytosis are poorly presented, while particulate antigens, which enter cells via phagocytosis, are presented more efficiently by MHC class I molecules.8 Consequently, more potent CTL responses are generated by particulate antigen delivery systems than those following the loading of soluble antigens.9
Previously, lipid particles, polymeric microparticles, live recombinant vectors, and virus-like particles have been utilized as artificial particulates to facilitate antigen delivery. Biodegradable nano/microparticles generated from poly(d,l-lactide-coglycolide) (PLGA) have recently attracted substantial attention because of their clinically proven biocompatibility.10,11 This material has been extensively tested in clinical applications and is currently used as a suture material and as part of other drug delivery formulations12,13 and has recently been proposed as a potential antigen delivery vehicle for DC-based vaccines against pathogens and cancer.14 In addition to facilitating the uptake of encapsulated materials, PLGA particles also potentially protect nucleic acids, peptides, and protein antigens contained within from extracellular degradation, increasing delivery efficiency.15 As phagocytic substrate size can also affect the magnitude of cross-presentation16 the ability to adjust PLGA particle size makes this delivery method a promising candidate technique for vaccinations. Anti-pathogen CD8+ CTL responses have been successfully generated by direct delivery of PLGA/antigenic peptide complexes by various routes; including oral, nasal and subcutaneous delivery 17–20 suggesting that PLGA particles can be taken up in vivo and cross-presented by resident APC. Because ex vivo generated dendritic cells are a logical cellular adjuvant in vaccination strategies, recent studies have also focused on the uptake 21–23 and potential cross-presentation of peptide antigens encapsulated in PLGA microspheres by DC. 24–26
However, a systematic attempt to follow the intracellular distribution, degradation, and cross-presentation of native protein antigens delivered by PLGA particles has not been undertaken, and the mechanism responsible for PLGA-mediated delivery of exogenous antigen to the MHC class I pathway of DC is poorly understood. Such an understanding is critical if these reagents are to be utilized as antigen delivery vehicles for DC-based therapies dependent on the loading of whole protein antigens, such as immunotherapy against solid tumours. 27–29 An antigen delivery study which traces the localization of PLGA-associated antigens and directly compares cross-presentation efficiency of these reagents verses other antigen forms presently favoured to load DC, including soluble antigen or non-biodegradable solid microparticles30 would contribute significantly to our understanding of PLGA-mediated cross-presentation. It would also aid in determining whether these reagents can significantly improve CD8+ responses to exogenous protein antigens, a key to improving the potency of many DC-based cancer applications including therapeutic vaccination or adoptive transfer of amplified antitumour T cells. Using ovalbumin (OVA) as a model antigen, we report that in both primary mouse bone marrow-derived DCs (BMDCs) and the human dendritic cell-like cell line (KG-1), antigens delivered by PLGA particles are internalized, processed and presented by the MHC class I pathway by a mechanism not previously observed for soluble or non-biodegradable particulate forms. We provide biochemical evidence that antigens delivered by PLGA particles are able to escape endosomal degradation and reach the cytoplasm at a significantly higher level than other antigen forms, and that these antigens are presented on MHC class I more efficiently and for significantly longer durations. Our results suggest that microparticle-associated antigens may circumvent the need for cross-presentation; instead, they enter the classical class I antigen presentation pathway directly following endocytosis through endosomal escape mediated by the PLGA particles.
Materials and methods
Cell lines
The human dendritic-like cell line KG-1,31 KG1.Kb and PeCr2.Kb, which both stably express mouse MHC class I H2-Kb32 and the B-cell lines C1R.Kb33 and T2.Kb34 were cultured in Iscove's modified Dulbecco's medium medium supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen, Grand Island, NY) at 37° in a 5% CO2/air incubator. Mouse BMDCs were generated from female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) as described.35 Immature DC clusters, obtained at day 4, were dislodged and replated for antigen presentation studies.
Fabrication of PLGA microparticles
PLGA particles were fabricated using a modified double-emulsion method.36 OVA (Sigma, St. Louis, MO), bovine serum albumin (BSA; Sigma), or fluoroscin isothiocyanate (FITC)–OVA (Molecular Probes, Eugene, OR) was dissolved in 200 µl phosphate-buffered saline (PBS) at 1% or 5% (w/v). The aqueous protein solution was added to 10% (w/v) PLGA (Birmingham Polymers, Inc., Birmingham, AL) methylene chloride solution, and the resulting solution was sonicated to produce a homogeneous emulsion. One percent polyvinyl alcohol (PVA, MW = 30 000–70 000, Sigma) was added to the emulsion and vortexed; this double emulsion was poured into a beaker containing aqueous 0·3% PVA stirring at high speed. The resulting particles were collected by centrifugation, rapidly frozen and lyophilized. Particle size and morphology were determined by scanning electron microscopy (SEM), and the number of particles per given mass or volume was determined by SEM or counting on a standard haemocytometer. Equivalent antigen concentrations were used for all presentation studies. Concentrations of antigen encapsulated in PLGA particles were determined by first dissolving the particles in a NaOH/sodium dodecyl sulphate solution and then subjecting these solutions to standard 2 bicinchoninic acid (BCA) absorbance assays as previously described.37 Protein concentrations identical to those contained within particles were also conjugated to the surface of 1·0 µm carboxylated latex beads according to the manufacturer's instructions (Polysciences, Inc., Warrington, PA) or to the surface of blank PLGA particles. Particle size and quantity were approximately equivalent in all assays. This was accomplished by determining the number of PLGA particles necessary to deliver a given antigen dose and then matching that quantity with an equal number of latex or blank PLGA beads to which antigen had been passively adsorbed.
Uptake study
Mouse BMDCs were incubated in the presence of PLGA/FITC–OVA, latex beads/FITC–OVA, or soluble FITC–OVA for 3 hr at 37°. Cells were washed once in cold PBS and separated from particles using an ISOLYMPH® gradient (Gallard-Schlesinger, Carle Place, NY) by centrifugation at 400 g for 20 min at 20°. At various timepoints (0, 24 and 48 hr), cells were harvested to assay antigen uptake. After quenching unincorporated FITC signal with 0·4% trypan blue, uptake was quantified as mean cell-associated fluorescence intensity measured by flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA) and analysed with WinMDI 2.8 (Joseph Trotter, Scripps Institute, La Jolla, CA).
Class I antigen presentation of KG1.Kb by flow cytometry
KG1.Kb, C1R.Kb, PeCr2.Kb, and T2.Kb were incubated with PLGA/OVA microparticles, latex bead-conjugated OVA, empty microparticles coincubated with soluble OVA, and soluble OVA alone for 16 hr. Following extensive washing, cells were stained with Alexa 647-conjugated 25-D1.16 monoclonal antibody (mAb, the kind gift of Dr J. Yewdell, National Institutes of Health, Bethesda, MD), which recognizes the SIINFEKL peptide (OVA257−264) in association with Kb.38 Antigen presentation was quantified by flow cytometry. Cell lines incubated with PLGA/BSA microparticles, latex bead-conjugated BSA, and soluble BSA served as negative controls.
Immunofluorescence and confocal microscopy
For duration studies, bone marrow-derived immature mouse DCs cells were fixed in 3·7% formaldehyde at the indicated times after incubation with PLGA/FITC–OVA, latex bead-conjugated FITC–OVA, or soluble FITC–OVA. Cells were then prepared for indirect immunofluorescence as previously described39 and were visualized using differential interference contrast microscopy on an Axiophot 2 fluorescence microscope (Zeiss, Thornwood, NY). Digital images were acquired with a CCD camera (Princeton Instruments, Trenton, NJ). For studies confirming endosomal escape, FITC–OVA PLGA particles were incubated with BM-derived DCs overnight and washed extensively following a 30 minute exposure to 50 nm LysoTracker Red (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Confocal images were acquired from live cells utilizing a Leica DM IRE2 confocal microscope outfitted with a heated stage. Single plane images of fluorescent markers and DIC outlines of the cells were acquired to insure particle internalization and three-dimensional images of whole cells were reconstructed utilizing Leica software.
MHC class I antigen presentation by T hybridoma assay
MHC class I antigen presentation was also evaluated by B3Z T hybridoma cells that produce IL-2 upon recognition of OVA peptide (SIINFEKL) in association with class I molecules.40 Immature mouse BMDCs were incubated with varying concentrations of PLGA/OVA particles, latex bead-conjugated OVA, or soluble OVA for 3 hrs, followed by removal of excess antigen and addition of 30 ng/ml lipopolysaccharide (LPS) for 18 hr to induce DC maturation. After the indicated incubation time (T0 was immediately following removal of excess antigen), B3Z T cells were added at an effector: APC ratio of 1: 1. Interleukin-2 (IL-2) content of the culture supernatants, isolated after 6 hr incubation, was assayed by IL-2 enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, Palo Alto, CA) according to the manufacturer's instructions.
Cytosol extraction
Cells were loaded with soluble FITC–OVA, latex bead-conjugated FITC–OVA, or PLGA/FITC–OVA for 18 hr at 37°. Following extensive washing, cytosol extracts were generated according to the method described by Yang et al.41 The fluorescence contained within the cytosol was determined by fluorometry.
Results
PLGA microparticles enhance exogenous antigen cross-presentation by DCs
To test the effect of PLGA encapsulation on antigen internalization and presentation, we encapsulated OVA or BSA into PLGA particles using the double-emulsion method. PLGA microparticles containing OVA were similar in terms of morphology and size to those containing BSA; particles average size ranged from 500 nm to 1 µm with a spherical morphology (Supplemental Fig. 1).
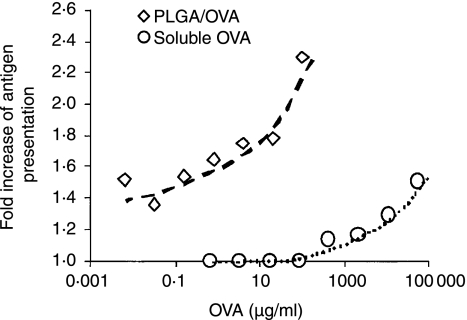
Figure 1.
PLGA microparticles enhance exogenous antigen cross-presentation by human DC-like KG-1.Kb cells. KG-1.Kb cells were cocultured with PLGA/OVA particles (open diamonds) or soluble OVA (open circles) at the concentrations indicated for 16 hr. The amount of particles added was calculated to yield concentrations of OVA equivalent to that of soluble OVA. Following extensive washing, cells were stained with Alexa 647-conjugated 25-D1·16 mAb, which recognizes the SIINFEKL peptide (OVA257−264) in association with Kb. The cross-presentation of exogenous antigen was detected as a shift in the fluorescence intensity of samples incubated with OVA from the level seen when incubated with an irrelevant protein (BSA).
We first sought to determine the effect of antigen encapsulation into PLGA particles on the cross-presentation efficiency of exogenous antigens. KG1.Kb, a human dendritic-like cell line that stably expresses the mouse MHC class I molecule H2-Kb, can cross-present low levels of the SIINFEKL peptide (OVA257−264) in association with MHC class I (Kb) which can be detected using 25-D1.16, a SIINFEKL-Kb complex specific mAb.32 The cross-presentation of exogenous antigen was detected as a shift in the fluorescence intensity of samples incubated with OVA from the level seen when incubated with an irrelevant protein (BSA). As 25-D1.16 staining reached a plateau with increasing PLGA/OVA concentration, potentially caused by saturation of the phagocytic machinery, we titrated the amount of antigen necessary to observe a shift in 25-D1.16 staining. The shift in 25-D1.16 staining of KG-1.Kb cells incubated with OVA-containing particles was significantly greater than that observed following incubation with soluble OVA (Fig. 1). When encapsulated into PLGA particles, 1000-fold lower OVA concentrations were able to generate a similar level of class I presentation as soluble OVA. This increase is similar to the levels of enhancement previously reported for antigens solely conjugated on the surface of solid microspheres8 indicating the efficiency of release from PLGA microspheres, which are only partially degraded at this point, is very high. To confirm that antigen encapsulation in the PLGA matrix, and not characteristics of the PLGA polymer itself, was responsible for the observed increases in cross-presentation, a comparison of PLGA encapsulated OVA versus OVA adsorbed to the surface of an otherwise identical particle was carried out. As shown in Supplemental Fig. 2, the requirement for large quantities of soluble OVA antigen (10 mg/ml - left panel) to initiate cross-presentation in the KG-1 DC line is overcome by the encapsulation of the antigen in PLGA particles (100-fold less antigen - middle panel). The same quantity of antigen passively adsorbed to the surface of blank PLGA particles (right panel) is not cross-presented, indicating that PLGA encapsulation fundamentally alters antigen delivery.
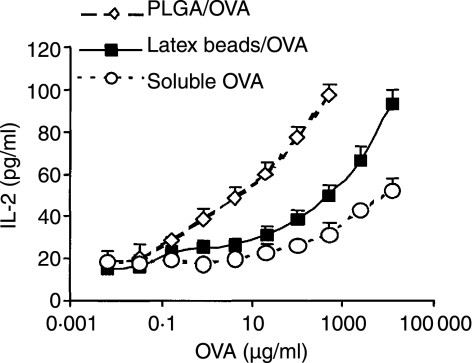
Figure 2.
Microparticle-encapsulated antigens generate potent CD8+ T cell responses in vitro. Immature BMDCs from C57BL/6 mice were incubated with varying concentrations of OVA-containing PLGA particles (open diamonds), OVA-coated latex beads (solid squares), and soluble OVA (open circles) for 3 hr followed by particle separation from cells to remove excess antigen. 30 ng/ml LPS was then added for an additional 18 hr to induce DC maturation. After maturation, B3Z T cells were added at an effector:APC ratio of 1: 1. The IL-2 content of culture supernatants, isolated after 6 hr incubation, was assayed by IL-2 ELISA. Results show the mean and SD of triplicate measurements, and are representative of two different experiments with DCs from different mice.
Microparticle-encapsulated antigens generate potent T-cell responses in vitro
To examine antigen-specific T-cell activation by antigen-loaded DCs, we tested the ability of microparticle-treated DCs to stimulate a CD8+ T-hybridoma cell line, B3Z, which secretes IL-2 in response to stimulation with the SIINFEKL-Kb complex. Mouse Kb BMDCs were treated for 3 hr with either PLGA/OVA or soluble OVA, then induced to mature with LPS. Encapsulation of OVA into PLGA microparticles dramatically shifted the dose–response curve for T-cell stimulation, and required 1000-fold less antigen to achieve the same level of presentation as soluble OVA (Fig. 2). To determine if the higher efficiency of presentation by antigen-loaded microparticles was simply attributed to the presence of a phagocytic substrate, we also examined the presentation of latex bead-associated OVA. Cells treated with OVA-containing PLGA particles displayed enhanced B3Z stimulation over the levels seen after treatment with OVA-coated latex beads (Fig. 2). Thus, the presentation of antigens encapsulated into PLGA particles was significantly more efficient than other particulate antigen forms, suggesting that PLGA microparticles may access the MHC class I presentation pathway in a unique way. Control empty PLGA particles coated with antigen behaved similarly to OVA-treated latex beads (data not shown), indicating antigen encapsulation in PLGA was critical to increased efficiency of antigen presentation.
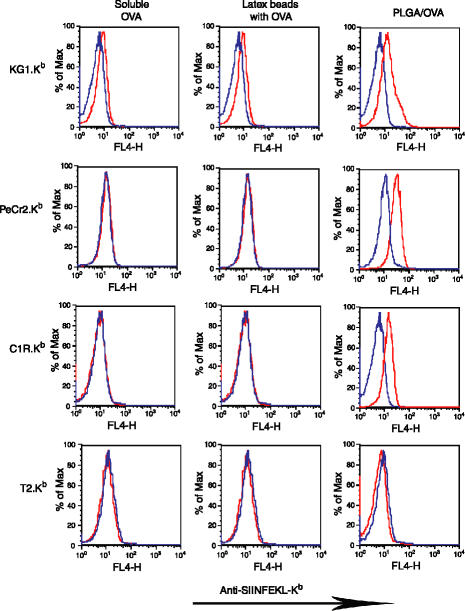
PLGA/OVA particle-loaded B cells are able to cross-present OVA
To determine whether the increases in MHC class I presentation associated with PLGA/OVA particle delivery are attributed to the escape of PLGA particle-associated antigen from the phagosome to the cytosol, we examined the class I presentation of PLGA/OVA particles in endocytic but non-cross-presenting B cells. B cells do not present soluble exogenous antigen in the context of MHC class I unless antigen is directly introduced into the cytosol by lipofection or delivered via linking to specific cell ligands.42,43 Two B-cell lines, PeCr2 and C1R, stably transfected with Kb to yield PeCr2.Kb and C1R.Kb, were loaded with PLGA/OVA particles overnight and compared to soluble OVA and OVA-coated latex beads to determine if any could facilitate cross-presentation. MHC class I expression was determined by the appearance of the SIINFEKL-Kb complex at the cell surface by flow cytometry. As shown in Fig. 3, the cross-priming competent DC line KG1.Kb could cross-present low levels of soluble or latex bead-associated OVA as expected; however, presentation was significantly enhanced by inclusion of OVA into microparticles. But, while neither B-cell line could present soluble or latex bead-associated OVA on MHC class I under these conditions, both were able to present antigen in association with MHC class I if antigen was delivered by PLGA particles, at levels comparable to the DC line. These results suggest that, in B cells, exogenous antigen delivered by PLGA particles must directly access the cytosol to reach the MHC class I antigen processing and presentation pathway, circumventing the conventional cross-presentation mechanism.
Figure 3.
Cross-presentation by two B-cell lines (PeCr2.Kb and C1R.Kb) and TAP-deficient T2.Kb cells in comparison to human DC-like KG-1.Kb cells. Indicated cell lines were cocultured with latex bead/OVA, PLGA/OVA particles, soluble OVA, or control latex bead/BSA, PLGA/BSA particles, or soluble BSA for 16 hr. Following extensive washing, cells were stained with Alexa 647-conjugated 25-D1·16 mAb, which recognizes the SIINFEKL peptide (OVA257−264) in association with Kb. Blue histograms represent signal from BSA and red for signal from OVA.
OVA encapsulated into PLGA particles more efficiently escapes from the endocytic compartment to the cytosol
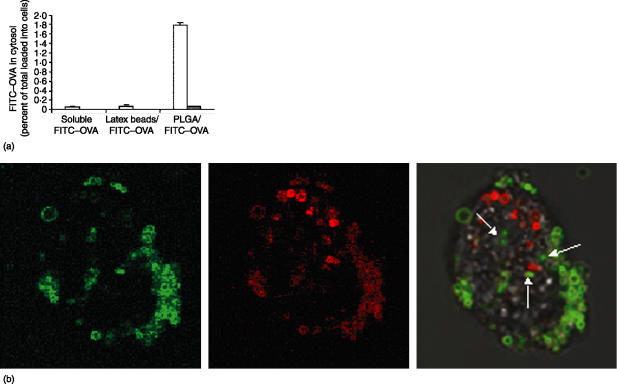
Our results in B cells indicate that antigens delivered by PLGA particles are able to exit the phagocytic compartment, enter the cytosol and gain access the MHC class I pathway. To biochemically confirm this, we quantitated the entry of labelled antigens into the cytoplasm following internalization by the C1R B-cell and KG-1 DC lines (Fig. 4a). Both cell types were incubated for 18 hr with soluble FITC–OVA, latex bead-associated FITC–OVA, and PLGA/FITC–OVA. We then extracted the cytosol from these cells and measured the associated fluorescence by fluorometry. The amount of antigen in the cytosol was normalized based on the amount of antigen initially added to cells. Cytosolic antigen was extremely low in C1R B cells incubated with either soluble FITC–OVA or latex bead-associated FITC–OVA; small, but significant, levels could be detected in KG-1 cells. When antigen was given as PLGA microparticles, however, the cytosolic antigen increased greater than thirty-fold in both C1R and KG-1 (Fig. 4a). In both cell types, the increases in exogenous antigen appearing in the cytosol mirrored increases in the levels of cross-presentation previously observed for the three antigen forms (Fig. 3). This result confirms that encapsulation of antigen into PLGA particles greatly enhances the delivery of antigen to the cytosol, leading to improved class I antigen presentation in both competent and non-competent cross-presenting cells.
Figure 4.
(a) Endosomal escape of FITC–OVA after being phagocytosed as soluble form, coated onto latex beads, or encapsulated in PLGA particles. KG-1 cells (open bars) or C1R B cells (closed bars) were incubated with indicated antigen forms for 16 hr, followed by cytosol extraction for fluorimetry assay. Percent in cytosol was calculated from total antigen initially added to cells. (b) Confocal images of murine BM-derived DC showing endosomal escape of antigen. Left panel shows FITC–OVA PLGA particles, middle panel shows LysoTracker Red stained endosomal/lysosomal compartments, right panel is overlay which includes a superimposed DIC image to outline the cell membrane. Arrows in overlay panel show non-colocalized FITC microparticles.
To determine whether endosomal escape of PLGA encapsulated antigen could be confirmed microscopically, additional experiments to visualize PLGA particles no longer associated with the endo-lysosomal compartment were also undertaken. Live murine BM-derived DC were loaded with PLGA FITC–OVA microspheres, washed extensively and stained for endolysosomal localization with the acidotropic dye, LysoTracker Red. LysoTracker Red selectively labels late endosomes and lysosomes in live cells44 and has been used extensively to track the fate of phagocytosed or endocytosed particles or bacteria.45 As shown in Fig. 4(b), while a large number of fluorescent particles are contained within discreet lysosomal vesicles in a confocal section of a labeled cell (colocalized FITC and LysoTracker Red signal – panel 3), a number of FITC(+) particles also appear to have escaped the endolysosome and no longer colocalize (arrows – panel 3). The result was confirmed in 3-D confocal reconstructions of whole cells which had incorporated FITC–OVA particles (Supplemental Fig. 3), again showing particles no longer associated with labeled lysosomes which presumably had escaped to the cytosol.
Taken together, this combination of biochemical, microscopic, and T-cell stimulatory data strongly suggest that PLGA encapsulation leads to cytoplasmic delivery of antigen in quantities not possible when antigen is in soluble form or simply conjugated to the surface of other artificial particulates.
PLGA/OVA particle-induced MHC class I antigen presentation is TAP-dependent
Internalized exogenous antigens can be loaded onto MHC class I molecules either by an endosomal or cytosolic pathway. The cytosolic pathway has been suggested as the primary pathway for efficient class I antigen presentation.46 The cytosolic pathway involves the transport of internalized proteins into the cytosol and subsequent proteasomal proteolysis. The resulting peptides must be reintroduced into the ER or phagosomal lumen by the transporters associated with antigen processing (TAP), a dimeric ATP-dependent peptide channel, and loaded onto nascent MHC class I molecules through the action of a TAP-associated loading complex. In contrast, the endosomal pathway is TAP-independent (reviewed in 47). Preliminary studies of PLGA-mediated antigen delivery to DC derived from TAP-deficient mice indicated that PLGA-encapsulated antigen could be presented through both TAP-dependent and TAP-independent pathways, depending on antigen dose and DC loading conditions.48 To determine the TAP dependency of the MHC class I antigen presentation of our particular microparticles and loading conditions, we examined the presentation of PLGA-encapsulated OVA by T2.Kb, a TAP-deficient human B cell line stably expressing Kb (Fig. 3, bottom row). In contrast to the robust presentation by the TAP-expressing C1R.Kb and PeCr2.Kb lines, T2.Kb was unable to present PLGA/OVA-derived SIINFEKL on MHC class I molecules. This result suggests that the loading of MHC class I with PLGA/OVA-derived SIINFEKL uses the classic, TAP-dependent cytosolic pathway. This result is consistent with our previous results, which indicated that increases in cross-presentation efficiency are attributed to endosomal escape of antigen encapsulated in PLGA/OVA particles.
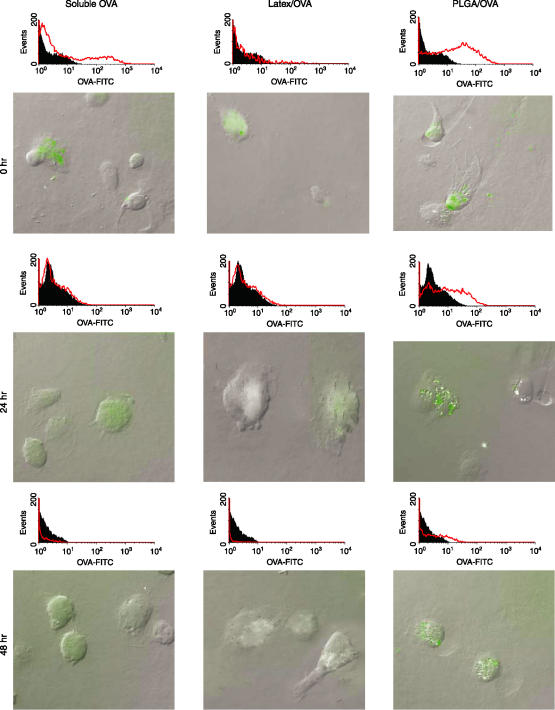
PLGA particles sustain the level of antigen inside DC
Another unique feature of PLGA particles is the slow release of antigen in a continuous, sustained fashion.37 We examined whether this feature could maintain antigen levels within DC for periods exceeding other antigen forms, thus prolonging class I antigen presentation. BM-derived murine immature DCs were pulsed with fluorescently labelled soluble, latex bead-associated and PLGA-encapsulated OVA for 3 hr and washed extensively to remove unincorporated antigen. At indicated time points postloading, cells were fixed and observed microscopically or by flow cytometry to determine if measurable antigen was still present (Fig. 5). Immediately following loading, substantial FITC–OVA was readily detectable in cells incubated with each substrate (Fig. 5– top panels). At 24 hr and 48 hr after internalization, however, the fluorescence in cells treated with either soluble (Fig. 5, left) or latex bead-associated FITC–OVA (Fig. 5, middle) were reduced (24 hr) and virtually undetectable (48 hr). In contrast, DCs treated with PLGA/FITC–OVA retained substantial fluorescence that persisted for 48 hr after internalization (Fig. 5, right).
Figure 5.
PLGA particles sustain the level of antigen in DCs. Mouse BMDCs were cultured in mGM-CSF and mIL-4 for 4 days, and then were exposed to PLGA/FITC–OVA, soluble FITC–OVA, and latex beads/FITC–OVA for three hrs. At various timepoints (0, 24 and 48 hr), cells were harvested to assay antigen internalization and degradation by flow cytometry and fluorescence microscopy.
PLGA microparticles prolong cross-presentation by DC
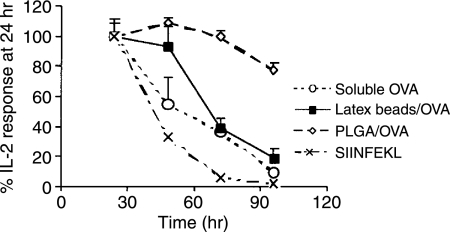
Since sustained levels of OVA antigen were observed intracellularly following PLGA-mediated antigen delivery to DC, we also assessed the effect of this prolonged antigen presence on the duration of cross-presentation. A time course of MHC class I antigen presentation was evaluated after exposure of mouse BMDCs to PLGA/OVA or soluble and latex bead-associated OVA (Fig. 6). Presentation was again assessed using IL-2 secretion by SIINFEKL-Kb-specific B3Z T cells. The presentation of SIINFEKL by Kb when antigen was loaded as either soluble OVA, OVA-coated latex beads, or control synthetic SIINFEKL peptide diminished rapidly with time. The magnitude of antigen presentation was reduced to 50% of the initial responses at 41, 53, and 67 hr for peptide, soluble OVA, and OVA-coated latex beads, respectively. The presentation of PLGA/OVA-derived SIINFEKL by Kb+ DCs, however, remained constant for more than 72 hr, decreasing by only 20% at 96 hr. Similar results were observed at lower concentrations of OVA (25 µg/ml, data not shown). These results indicate that PLGA particles serve as an intracellular antigen reservoir capable of providing a steady and prolonged source of protein antigen for APC, increasing both the efficiency and duration of MHC class I cross-presentation.
Figure 6.
PLGA particles prolong cross-presentation by C57BL/6 mouse BMDCs. DCs were incubated with soluble OVA (100 µg/ml), PLGA particles containing 100 µg/ml OVA (PLGA/OVA), latex beads containing 100 µg/ml OVA (latex beads/OVA), or SIINFEKL peptide (1 µm), matured and then cocultured with B3Z T cells at a 1: 1 ratio. At various time points, supernatants were assayed for IL-2 production. Data shows mean ± SD (n = 3).
Discussion
In this study, we demonstrated that antigen encapsulation into PLGA particles increased the efficiency of MHC class I antigen presentation in cells both normally competent for cross-presentation (DC) and those not (B cells), indicating that PLGA microspheres deliver antigen by a mechanism fundamentally different than that employed for soluble antigen or antigen conjugated to non-degradable beads. This dramatically improved T-cell stimulation in vitro; compared to free soluble antigen, inclusion of antigen into PLGA particles enhanced class I antigen presentation by greater than twofold and greater than 1000-fold less antigen was required to achieve the same magnitude of class I antigen presentation (Figs 1 and 2). PLGA particles were also superior to antigen-conjugated latex beads, indicating that the nature of the particle, not just the presence of a phagocytic substrate, is critical to cross-presentation efficiency (Figs 2–6). In addition, class I antigen presentation mediated by PLGA particles persisted much longer (> 4 days) than soluble antigen or antigen on non-degradable beads (Fig. 6) indicating this method of antigen delivery could significantly improve and prolong T-cell stimulation in vitro and in vivo.
A number of recent in vivo studies have indicated that PLGA particles loaded with antigenic peptides or pathogen-associated proteins are capable of inducing robust, specific CTL responses via various administration routes.17–20,49 It is unclear, however, how PLGA particles access the MHC class I antigen presentation pathway of the host APC, although it has been documented that particles are indeed taken up by resident DC or macrophages at the various administration sites.21 Our results indicate that PLGA particles may aid in both the uptake and cross-presentation of antigen by DC or macrophages in vivo, thus increasing T-cell stimulatory capacity and CTL responses. Our results also provide strong evidence that that the observed increases in cross-presentation efficiency are due to the superior ability of the microparticles to facilitate endosomal escape of antigen (Figs 3 and 4), an ability not observed in other antigen forms. This biochemical confirmation of endosomal escape bolsters previous reports utilizing fluorescent probes delivered by PLGA microspheres to peritoneal macrophages or smooth muscle cells,50,51 which indicated microscopically that these probes did not colocalize with the endo/phagosome. Furthermore, our results raise the possibility that this increased delivery of antigen to the cytoplasm of any endocytic cells may have in vivo implications, since any MHC class II (+) cell could potentially exhibit heightened cross-presentation capacity following PLGA-mediated antigen delivery; a condition favourable in macrophages and DC but undesirable in potentially tolerizing B cells.
Another unique feature of PLGA particles is their ability to release antigen in a sustained and pulsatile manner for weeks to several months.37 In our study this feature was exploited to provide a prolonged supply of intracellular antigens that lead to class I antigen presentation for an extended time period (more than 4 days, Fig. 6). The long duration of presentation is a major advantage of PLGA particle-based delivery over soluble antigen, antigenic peptide alone or other particulate delivery systems, which are associated with a short half-life of antigen presentation due to the rapid cellular turnover of MHC class I molecules and complexes on the cell surface and the lack of antigen replenishment from cellular stores. A recent study in a mouse model suggests the rapid turnover of externally loaded peptides critically limits the clinical use of DC-based tumour vaccines52 and methodologies that prolonged antigen presentation by various means has been shown to enhance T cell responses in vitro and in vivo. 53–56 This feature may be relevant to the use of PLGA particles in a variety of clinical applications, particularly vaccination strategies, as PLGA microspheres can be fabricated to incorporate specific release characteristics by adjusting the loading of protein, composition of PLGA, or size of particles. 57–59 Taken together, these results indicate it may be possible to fine-tune PLGA particle systems to provide optimal antigen presentation, a key to increasing the potency of vaccines against tumours and infectious agents.
One particularly promising clinical application of the nanoparticle technologies is in improving DC-based vaccination protocols for solid tumour immunotherapy. A major current stumbling block in such therapies is the efficient delivery of tumour-associated antigens to DC prior to their utilization in vaccination.7,60 Currently, several sources have been used to load DCs including whole cell lysates (soluble proteins), mRNA, eluted tumour peptides, dissociated apoptotic/necrotic cells, and tumour cells fused with DCs.61 The simplest and most clinically relevant means is to load DC with soluble proteins directly isolated from tumours. In this way, the entire spectrum of tumour-associated proteins are present, which is particularly useful in almost all solid tumours outside melanoma, where few, if any, tumour-specific proteins have been identified. However, the cross-presentation of soluble proteins is known to be less efficient than particulate forms in that high concentrations of proteins are normally required.32,62 An alternative is to encapsulate whole tumour antigens directly into PLGA microparticles. This technique can be done rapidly and under sterile conditions, and potentially maximizes both the spectrum of tumour antigens present and the conversion of these antigens to CTL-recognizable epitopes. Additional experiments to determine the practicality of this methodology are necessary, but success in developing this technology could significantly simplify the disparate DC loading strategies currently employed in the production of antitumour vaccines.
Acknowledgments
We would like to thank Juli Unternaehrer of Ira Mellman's laboratory (Yale University School of Medicine) for providing advice and reagents for dendritic cell generation.
This work was supported by NIH Minority Predoctoral Fellowship F31 AI10347 (A.L.A), Howard Hughes Medical Institute and NIH grant R37 AI23081 (P.C), R01 EB000487(NIH/EB) (H.S. & W.M.S) and a NIH YSDRCC Pilot grant (P30AR4194206A) and Yale Cancer Center Swebilius Foundation grant (D.H).
Abbreviations
- DCs
dendritic cells
- PLGA
poly(d,l-lactide-coglycolide)
- BMDCs
bone marrow-derived dendritic cells
- PVA
polyvinyl alcohol
- SEM
scanning electron microscopy
- ER
endoplasmic reticulum
Supplementary Material
The following supplementary material is available for this article online:
Figure S1. A representative scanning electron micrograph of PLGA particles loaded with OVA protein. Scale bar ¼ 500 nm.
Figure S2. Encapsulation of antigen in PLGA particles is necessary for efficient cross-presentation. The requirement for large quantities of soluble OVA antigen (10 mg/ml - left panel) to initiate cross-presentation in the KG-1 DC line is overcome by the encapsulation of the antigen in PLGA particles (100-fold less antigen - middle panel). The same quantity of antigen passively adsorbed to the surface of blank PLGA particles (right panel) is not crosspresented, indicating that PLGA encapsulation fundamentally alters antigen delivery.
Figure S3. PLGA encapsulated antigens escape the endosomal/lysosomal compartments. Murine BM-derived DCs loaded with PLGA/FITC-OVA particles were labelled and visualized as described in Fig. 4(b). Z-stack images were reconstructed to make a three-dimensional image that confirmed the escape of PLGA particles from endo/lysosomes to the cytosol.
This material is available as part of the online article from http://www.blackwell-synergy.com
References
- 1.Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo. Implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 2.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H-antigens with H-2 congenic cells which do not cross-react in cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heath WR, Belz GT, Behrens GMN, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang AYC, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone-marrow-derived cells in presenting MHC class I-restricted tumor-antigens. Science. 1994;264:961–5. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 5.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 6.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class-I major histocompatibility complex molecules. Science. 1990;249:918–21. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 7.Moron G, Dadaglio G, Leclerc C. New tools for antigen delivery to the MHC class I pathway. Trends Immunol. 2004;25:92–7. doi: 10.1016/j.it.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class-I MHC molecules. J Immunol. 1994;153:4925–33. [PubMed] [Google Scholar]
- 9.Rock KL, Clark K. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol. 1996;156:3721–6. [PubMed] [Google Scholar]
- 10.Foged C, Sundblad A, Hovgaard L. Targeting vaccines to dendritic cells. Pharm Res. 2002;19:229–38. doi: 10.1023/a:1014474414097. [DOI] [PubMed] [Google Scholar]
- 11.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Poly-α amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci USA. 2004;101:9534–9. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahoney MJ, Saltzman WM. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nat Biotech. 2001;19:934–9. doi: 10.1038/nbt1001-934. [DOI] [PubMed] [Google Scholar]
- 13.Maheshwari A, Han S, Mahato RI, Kim SW. Biodegradable polymer-based interleukin-12 gene delivery: Role of induced cytokines, tumor infiltrating cells and nitric oxide in anti-tumor activity. Gene Ther. 2002;9:1075–84. doi: 10.1038/sj.gt.3301766. [DOI] [PubMed] [Google Scholar]
- 14.Waeckerle-Men Y, Gander B, Groettrup M. Delivery of tumor antigens to dendritic cells using biodegradable microspheres. Methods Mol Med. 2005;109:35–46. doi: 10.1385/1-59259-862-5:035. [DOI] [PubMed] [Google Scholar]
- 15.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Del Rev. 2002;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 16.Raychaudhuri S, Rock KL. Fully mobilizing host defense: Building better vaccines. Nat Biotech. 1998;16:1025–31. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 17.Partidos CD, Vohra P, Jones D, Farrar G, Steward MW. CTL responses induced by a single immunization with peptide encapsulated in biodegradable microparticles. J Immunol Methods. 1997;206:143–51. doi: 10.1016/s0022-1759(97)00102-6. [DOI] [PubMed] [Google Scholar]
- 18.Partidos CD, Vohra P, Jones DH, Farrar G, Steward MW. Induction of cytotoxic T-cell responses following oral immunization with synthetic peptides encapsulated in PLG microparticles. J Cont Rel. 1999;62:325–32. doi: 10.1016/s0168-3659(99)00157-1. [DOI] [PubMed] [Google Scholar]
- 19.Partidos CD, Vohra P, Steward MW. Induction of measles virus-specific cytotoxic T-cell responses after intranasal immunization with synthetic peptides. Immunology. 1996;87:179–85. doi: 10.1046/j.1365-2567.1996.462527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nixon DF, Hioe C, Chen PD, et al. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine. 1996;14:1523–30. doi: 10.1016/s0264-410x(96)00099-0. [DOI] [PubMed] [Google Scholar]
- 21.Lutsiak ME, Robinson DR, Coester C, Kwon GS, Samuel J. Analysis of poly (d,l-lactic-co-glycolic acid) nanosphere uptake by human dendritic cells and macrophages in vitro. Pharm Res. 2002;19:1480–7. doi: 10.1023/a:1020452531828. [DOI] [PubMed] [Google Scholar]
- 22.Newman KD, Elamanchili P, Kwon GS, Samuel J. Uptake of poly (d,l-lactic-co-glycolic acid) microspheres by antigen-presenting cells in vivo. J Biomed Mater Res. 2002;60:480–6. doi: 10.1002/jbm.10019. [DOI] [PubMed] [Google Scholar]
- 23.Denis-Mize KS, Dupuis M, MacKichan ML, et al. Plasmid DNA adsorbed onto cationic microparticles mediates target gene expression and antigen presentation by dendritic cells. Gene Ther. 2000;7:2105–12. doi: 10.1038/sj.gt.3301347. [DOI] [PubMed] [Google Scholar]
- 24.Audran R, Peter K, Dannull J, Men Y, Scandella E, Groettrup M, Gander B, Corradin G. Encapsulation of peptides in biodegradable microspheres prolongs their MHC class-I presentation by dendritic cells and macrophages in vitro. Vaccine. 2003;21:1250–5. doi: 10.1016/s0264-410x(02)00521-2. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Pollock KG, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;21:849–55. doi: 10.1016/s0264-410x(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 26.Haining WN, Anderson DG, Little SR, et al. pH-triggered microparticles for peptide vaccination. J Immunol. 2004;173:2578–85. doi: 10.4049/jimmunol.173.4.2578. [DOI] [PubMed] [Google Scholar]
- 27.Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I. Vaccines for solid tumours. Lancet Oncol. 2004;5:681–9. doi: 10.1016/S1470-2045(04)01610-9. [DOI] [PubMed] [Google Scholar]
- 28.Stift A, Friedl J, Dubsky P, et al. Dendritic cell-based vaccination in solid cancer. J Clin Oncol. 2003;21:135–42. doi: 10.1200/JCO.2003.02.135. [DOI] [PubMed] [Google Scholar]
- 29.Geiger JD, Hutchinson RJ, Hohenkirk LF, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–9. [PubMed] [Google Scholar]
- 30.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity. therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 31.St Louis DC, Woodcock JB, Fransozo G, et al. Evidence for distinct intracellular signaling pathways in CD34 (+) progenitor to dendritic cell differentiation from a human cell line model. J Immunol. 1999;162:3237–48. [PubMed] [Google Scholar]
- 32.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170:4178–88. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 33.Alexander J, Payne JA, Murray R, Frelinger JA, Cresswell P. Differential transport requirements of HLA and H-2 class-I glycoproteins. Immunogenetics. 1989;29:380–8. doi: 10.1007/BF00375866. [DOI] [PubMed] [Google Scholar]
- 34.Anderson KS, Alexander J, Wei M, Cresswell P. Intracellular-transport of class-I MHC molecules in antigen-processing mutant-cell lines. J Immunol. 1993;151:3407–19. [PubMed] [Google Scholar]
- 35.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone-marrow cultures supplemented with granulocyte macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saltzman WM, Mak MW, Mahoney MJ, Duenas ET, Cleland JL. Intracranial delivery of recombinant nerve growth factor: Release kinetics and protein distribution for three delivery systems. Pharm Res. 1999;16:232–40. doi: 10.1023/a:1018824324275. [DOI] [PubMed] [Google Scholar]
- 37.Moynihan JS, Jones DH, Farrar GH, Howard CR. A novel microencapsulated peptide vaccine against hepatitis B. Vaccine. 2001;19:3292–300. doi: 10.1016/s0264-410x(00)00540-5. [DOI] [PubMed] [Google Scholar]
- 38.Porgador A, Yewdell JW, Deng YP, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–26. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 39.Hammond C, Denzin LK, Pan M, Griffith JM, Geuze HJ, Cresswell P. The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR-DM, and -DO molecules. J Immunol. 1998;161:3282–91. [PubMed] [Google Scholar]
- 40.Hosken NA, Bevan MJ. Defective presentation of endogenous antigen by a cell-line expressing class-I molecules. Science. 1990;248:367–70. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- 41.Yang JB, Beitz DC. A fluorometric assay for cholesterol reductase-activity. Anal Biochem. 1992;206:246–50. doi: 10.1016/0003-2697(92)90361-a. [DOI] [PubMed] [Google Scholar]
- 42.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CPG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172:1501–7. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 43.Ke Y, Kapp JA. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J Exp Med. 1996;184:1179–84. doi: 10.1084/jem.184.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 45.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 46.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–7. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 47.Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004;5:678–84. doi: 10.1038/ni1082. [DOI] [PubMed] [Google Scholar]
- 48.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I cross presentation in vivo. Immunity. 2004;21:155–65. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Moore A, McGuirk P, Adams S, Jones WC, McGee JP, O'Hagan DT, Mills KH. Immunization with a soluble recombinant HIV protein entrapped in biodegradable microparticles induces HIV-specific CD8+ cytotoxic T lymphocytes and CD4+ Th1 cells. Vaccine. 1995;13:1741–9. doi: 10.1016/0264-410x(95)00184-3. [DOI] [PubMed] [Google Scholar]
- 50.Newman KD, Kwon GS, Miller GG, Chlumecky V, Samuel J. Cytoplasmic delivery of a macromolecular fluorescent probe by poly (d,l-lactic-co-glycolic acid) microspheres. J Biomed Mat Res. 2000;50:591–7. doi: 10.1002/(sici)1097-4636(20000615)50:4<591::aid-jbm15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly (d,l-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002;16:1217–26. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 52.Ludewig B, McCoy K, Pericin M, et al. Rapid peptide turnover and inefficient presentation of exogenous antigen critically limit the activation of self-reactive CTL by dendritic cells. J Immunol. 2001;166:3678–87. doi: 10.4049/jimmunol.166.6.3678. [DOI] [PubMed] [Google Scholar]
- 53.Wang RF, Wang HY. Enhancement of antitumor immunity by prolonging antigen presentation on dendritic cells. Nat Biotech. 2002;20:149–54. doi: 10.1038/nbt0202-149. [DOI] [PubMed] [Google Scholar]
- 54.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen GP100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–48. [PubMed] [Google Scholar]
- 55.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, Margulies DH, Pardoll DM. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC–peptide–TCR complex. Immunity. 2000;13:529–38. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 57.Palinko-Biro E, Ronaszeki G, Merkle HP, Gander B. Release kinetics and immunogenicity of parvovirus microencapsulated in PLA/PLGA microspheres. Int J Pharmaceut. 2001;221:153–7. doi: 10.1016/s0378-5173(01)00679-2. [DOI] [PubMed] [Google Scholar]
- 58.Panyam J, Dali MA, Sahoo SK, Ma WX, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly (d,l-lactide-co-glycolide) nano- and microparticles. J Cont Rel. 2003;92:173–87. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 59.Morita T, Sakamura Y, Horikiri Y, Suzuki T, Yoshino H. Protein encapsulation into biodegradable microspheres by a novel s/o/w emulsion method using poly (ethylene glycol) as a protein micronization adjuvant. J Cont Rel. 2000;69:435–44. doi: 10.1016/s0168-3659(00)00326-6. [DOI] [PubMed] [Google Scholar]
- 60.Thumann P, Moc I, Humrich J, Berger TG, Schultz ES, Schuler G, Jenne L. Antigen loading of dendritic cells with whole tumor cell preparations. J Immunol Methods. 2003;277:1–16. doi: 10.1016/s0022-1759(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 61.Parmiani G, Pilla L, Castelli C, Rivoltini L. Vaccination of patients with solid tumours. Anal Oncol. 2003;14:817–24. doi: 10.1093/annonc/mdg246. [DOI] [PubMed] [Google Scholar]
- 62.Shen ZH, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A representative scanning electron micrograph of PLGA particles loaded with OVA protein. Scale bar ¼ 500 nm.
Figure S2. Encapsulation of antigen in PLGA particles is necessary for efficient cross-presentation. The requirement for large quantities of soluble OVA antigen (10 mg/ml - left panel) to initiate cross-presentation in the KG-1 DC line is overcome by the encapsulation of the antigen in PLGA particles (100-fold less antigen - middle panel). The same quantity of antigen passively adsorbed to the surface of blank PLGA particles (right panel) is not crosspresented, indicating that PLGA encapsulation fundamentally alters antigen delivery.
Figure S3. PLGA encapsulated antigens escape the endosomal/lysosomal compartments. Murine BM-derived DCs loaded with PLGA/FITC-OVA particles were labelled and visualized as described in Fig. 4(b). Z-stack images were reconstructed to make a three-dimensional image that confirmed the escape of PLGA particles from endo/lysosomes to the cytosol.