Abstract
Interleukin (IL)-23 is a heterodimeric cytokine consisting of a novel p19 molecule and the p40 subunit of IL-12. Since secreted p40 can act as an antagonist for IL-12, we investigated whether p40 also inhibited IL-23-mediated immunological functions. p40 did not induce interferon (IFN)-γ or IL-17 production from splenocytes but impaired IL-23-induced cytokine production by competitive binding to the IL-23 receptors. Furthermore, a mixed population of murine colon carcinoma Colon 26 cells transduced with the p40 gene and those transduced with the IL-23 gene developed tumours in syngenic mice, whereas the IL-23-expressing Colon 26 cells were completely rejected. p40 also suppressed IFN-γ production of antigen-stimulated splenocytes and IL-23-mediated cytotoxic T-lymphocyte activities in the mice that rejected Colon 26 cells expressing IL-23. p40 can thereby antagonize IL-23 and is a possible therapeutic agent for suppression of IL-23 functions.
Keywords: anergy, suppression, tolerance, cytokines, interleukins, T cells
Introduction
The recently discovered cytokine interleukin (IL)-23 is a covalently linked heterodimeric cytokine that consists of a novel p19 subunit, which is structurally related to the p35 subunit of IL-12, and the p40 subunit of IL-12.1 IL-23 is primarily secreted from activated dendritic cells and macrophages, although p19 mRNA is expressed in various tissues, and induces interferon (IFN)-γ production and proliferation of phytohaemagglutinin (PHA) T-cell blasts.1 Although some of the immunological activities of IL-23 are similar to those of IL-12, IL-23 has distinct functions in that it induces production of a proinflammatory cytokine, IL-17, from activated T cells and promotes antigen presentation of splenic CD8+ dendritic cells, which have not to date been reported as IL-12 activities.2,3 A receptor for IL-23 is composed of IL-23R, a novel receptor subunit related to IL-12Rβ2, and the IL-12Rβ1 subunit of the IL-12 receptor.4 IL-12Rβ2 is selectively expressed on T helper type 1 (Th1) but not Th2 cells, while IL-12Rβ1 is consistently expressed on both cell types.5,6 IL-23R is expressed on memory and activated but not naïve CD4+ T cells; thus, the responses of CD4+ T cells to IL-23 or IL-12 are attributable to differential expression of IL-23R or IL-12Rβ2.4
Identification of IL-23 has resulted in revision of the in vivo roles of IL-12 in the pathogenesis of experimental autoimmune encephalomyelitis (EAE). Previous studies suggested that IL-12 was a key mediator for EAE because administration of the neutralizing antibodies for IL-12 to animals with this autoimmune disease ameliorated symptoms.7,8 In contrast, several reports suggested a controversial role for IL-12 in the pathogenesis of EAE; p35- and IL-12Rβ2-deficient mice still remained susceptible to EAE, whereas p40- and IL-12Rβ1-deficient mice did not develop EAE.9–11 A recent report showed that p19-deficient mice did not develop EAE, demonstrating that IL-23 rather than IL-12 was involved in the pathogenesis of EAE.12
p40, the common subunit of IL-23 and IL-12, is experimentally secreted when neither p19 nor p35 is expressed, and can bind to IL-12Rβ1, acting as an antagonist for IL-12;13–16 thus, the production of p40 in vivo can be a novel self-regulatory process to suppress IL-12 functions. IL-12Rβ1 is an essential signalling component of both IL-23 and IL-12 receptors; in fact, memory T-cell functions were impaired in individuals with genetic IL-12Rβ1 deficiency as a result of reduced responsiveness to both IL-23 and IL-12.17–19 We therefore presumed that the secreted p40 also impaired IL-23-mediated responses, and examined the inhibitory effects of p40 in vitro and in vivo.
Materials and methods
Animals and cells
C57BL/6 and BALB/c mice (6- to 8-week-old females) were purchased from Japan SLC (Hamamatsu, Japan). Murine colon carcinoma Colon 26 cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS). COS-7 cells and murine sarcoma Meth A cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated FCS.
Expression of cytokines and cytokine production assay
Murine p19, p35 and p40 genes were generated by reverse transcriptase–polymerase chain reaction (RT-PCR).20,21 The p19 and p35 genes were linked to the p40 gene using an internal ribosomal entry site (IRES) to construct an expression vector with pcDNA3 (Invitrogen, Carlsbad, CA): for IL-12, p35-IRES-p40; for IL-23, p19-IRES-p40; and for p40, IRES-p40. COS-7 cells were transfected with these vectors using Lipofectin reagent (Invitrogen) and cell-free supernatants were collected after 48 hr. The amounts of p40 in the supernatants were measured with an enzyme-linked immunosorbent assay (ELISA) kit to detect p40 (Biosource, Camarillo, CA). Splenocytes from C57BL/6 mice were stimulated with 5 µg/ml concanavaline A (Con A) for 48 hr and then incubated for 24 hr with various amounts of supernatants from the transfected COS-7 cells. The amounts of IFN-γ and IL-17 were measured with respective ELISA kits (R & D Systems, Minneapolis, MN).
RT-PCR for each subunit of cytokines
First-strand cDNA was amplified for 30 cycles with the following primers and conditions: for the p19 gene, 5′-CACAGAGCCAGCCAGATCTGAGAAGC-3′ (as a 5′-primer) and 5′-CCATGGGAACCTGGGCATCCTTAAGC-3′ (as a 3′-primer), and an annealing temperature of 60°; for the p35 gene, 5′-ACCTGCTGAAGACCACAGATG-3′ (5′-primer) and 5′-TTTCACTCTGTAAGGGTCTGC-3′ (3′-primer), and an annealing temperature of 57°; for the p40 gene, 5′-CCAGAGACATGGAGTCATAG-3′ (5′-primer) and 5′-GGGTCTGGTTTGATGATGTC-3′ (3′-primer), and an annealing temperature of 60°; for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, 5′-ACCACAGTCCATGCCATCAC-3′ (5′-primer) and 5′-TCCACCACCCTGTTGCTGTA-3′ (3′-primer), and an annealing temperature of 60°.
Transduction of tumour cells and animal experiments
Colon 26 cells were retrovirally transduced with LXSN vectors bearing p35-IRES-p40,20 p19-IRES-p4021 or p40 DNA. G418 (600 µg/ml; Invitrogen)-resistant Colon 26 cells were selected and were stained with fluoresecin isothiocyanate-conjugated anti-H-2Kd (BD PharMingen, San Diego, CA), anti-H-2Dd (BD PharMingen) or anti-H-2Ld (e-Bioscience, San Diego, CA) monoclonal antibodies. The expression profiles were analysed with FACScan and Cell Quest software (Beckton Dickinson, Mountain View, CA). For animal experiments, cells (1 × 106) were inoculated subcutaneously into BALB/c mice and the tumour volume was calculated according to the formula 1/2 × length × width2.
IFN-γ production and cytolytic assay in splenocytes
Mice were inoculated with tumour cells (1 × 106) and killed on day 24. Splenocytes were stimulated with 60 Gy-irradiated Colon 26 cells for 24 hr and were measured for IFN-γ production. For the cytolytic assay, splenocytes of the mice that had rejected IL-23-producing Colon 26 cells were stimulated with mitomycin C (60 µg/ml; Sigma, St Louis, MO)-treated cells for 5 days. The mitomycin C treatment inhibits cell division but not cytokine secretion up to 7 days. Cytolytic activities against Colon 26 and Meth A cells were measured with the standard 6-hr 51Cr release assay at various effector:target ratios.
Results
p40 did not induce cytokine production in splenocytes
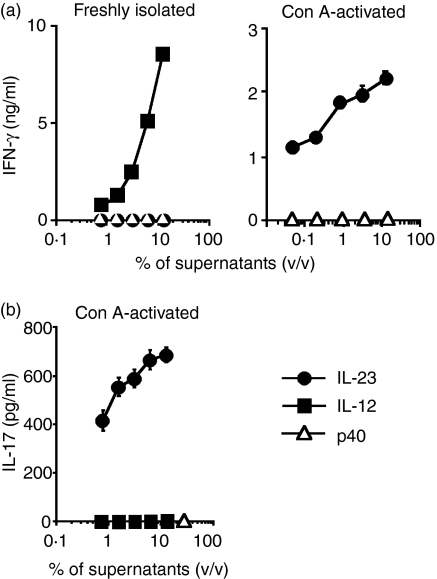
COS-7 cells were transfected with p35-IRES-p40, p19-IRES-p40 or IRES-p40 DNA (COS-7/IL-12, COS-7/IL-23 or COS-7/p40, respectively) and the amounts of p40-containing proteins secreted from the transfected cells were measured (Table 1). These transfectants, but not untransfected COS-7 cells, secreted the p40-containing proteins, and the supernatants of respective transfectants were used as IL-12, IL-23 and p40. We examined whether these molecules induced IFN-γ production in freshly isolated and Con A-activated splenocytes (Fig. 1a). Freshly isolated splenocytes produced IFN-γ in response to supernatants of COS-7/IL-12 but not COS-7/IL-23 cells; however, Con A-activated splenocytes produced IFN-γ when the cells were stimulated either with the supernatants of COS-7/IL-12 (6043 ± 391 pg/ml, mean ± standard deviation (SD), produced under the culture with 12·5% of the supernatants) or with those of COS-7/IL-23. The ability of splenocytes to respond to IL-23 is attributable to Con A-mediated induction of IL-23R expression.3 Con A-activated splenocytes also produced IL-17 when the cells were stimulated with the supernatants of COS-7/IL-23 but not those of COS-7/IL-12 (Fig. 1b). In either type of splenocyte, supernatants of COS-7/p40 cells did not induce IFN-γ or IL-17 production, showing that secreted p40 was not agonistic to IFN-γ or IL-17 production.
Table 1. Production of p40-containing proteins in COS-7 cells.
| Transfectants | p40-containing protein1 (ng/ml) |
|---|---|
| COS-7/IL-12 | 2·48 ± 0·03 |
| COS-7/IL-23 | 7·16 ± 0·85 |
| COS-7/p40 | 4·88 ± 0·14 |
Amounts of p40-containing proteins secreted in the supernatants were expressed as corresponding amounts of interleukin (IL)-12 used as standard in a p40-specific enzyme-linked immunosorbent assay (ELISA) kit. Data represent mean ± standard deviation (n = 3).
Figure 1.
Cytokine production induced by interleukin (IL)-12 and IL-23 but not p40. Freshly isolated or concanavaline A (Con A)-activated splenocytes of C57BL/6 mice were cultured with various concentrations of supernatants from COS-7/IL-12 (closed squares), COS-7/IL-23 (closed circles) or COS-7/p40 (open triangles) cells. The amounts of interferon (IFN)-γ(a) and IL-17(b) were measured in the supernatants of splenocytes. Data represent mean ± standard deviation (SD) of triplicate samples. v/v, volume/volume.
p40 inhibited IL-23-mediated cytokine production
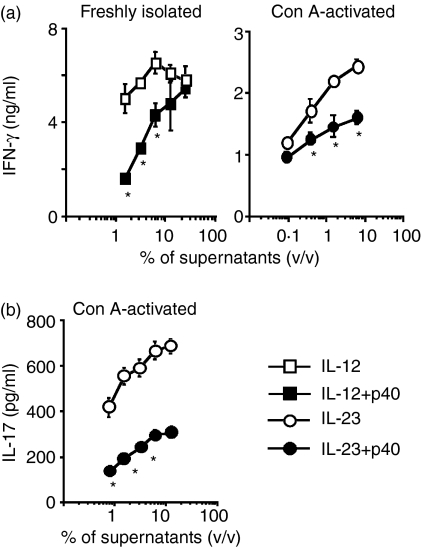
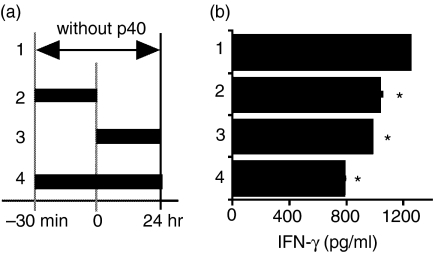
We examined whether p40 inhibited IFN-γ production induced by IL-23 as well as IL-12 (Fig. 2a). IL-12- and IL-23-mediated IFN-γ production was decreased when freshly isolated and Con A-activated splenocytes were cultured with supernatants of COS-7/p40 cells. IL-23-mediated IL-17 production was also decreased when Con A-activated splenocytes were cultured with supernatants of COS-7/p40 cells (Fig. 2b). We then examined whether the timing of p40 treatment affected the level of IL-23-mediated IFN-γ production. Treatment of Con A-activated splenocytes with supernatants of COS-7/p40 cells prior to IL-23 decreased IFN-γ production to a similar level to that observed in the cells simultaneously treated with p40 and IL-23 (Fig. 3). IFN-γ production was further suppressed when the splenocytes were incubated with supernatants of COS-7/p40 cells before and during the stimulation with IL-23.
Figure 2.
Decreases in interleukin (IL)-12- and IL-23-induced cytokine production mediated by p40. Freshly isolated and Con A-activated splenocytes of C57BL/6 mice were cultured for 24 hr with various concentrations of supernatants from COS-7/IL-12 (squares) or COS-7/IL-23 (circles) cells in the presence of 20% (volume/volume) of either COS-7/p40 (closed symbols) or COS-7 (open symbols) cells. The amounts of interferon (IFN)-γ (a) and IL-17 (b) in the supernatants of splenocytes are shown as mean ± standard deviation (SD) of triplicate samples. Asterisks indicate statistical significance [repeated-measures analysis of variance (anova); P < 0·01]. v/v, volume/volume.
Figure 3.
Transient treatment with p40 inhibited interleukin (IL)-23-dependent interferon (IFN)-γ production. (a) A schema of p40-treated periods for splenocyte culture. (b) Con A-activated splenocytes of C57BL/6 mice were stimulated for 24 hr with supernatants of COS-7/IL-23 (2% of total volume) and COS-7/p40 (20%) cells. Data represent mean ± standard deviation (SD) of triplicate samples. Asterisks indicate statistical significance compared with splenocytes cultured with IL-23 (Student t-test, P < 0·05).
p40 abrogated IL-12- and IL-23-mediated antitumour effects
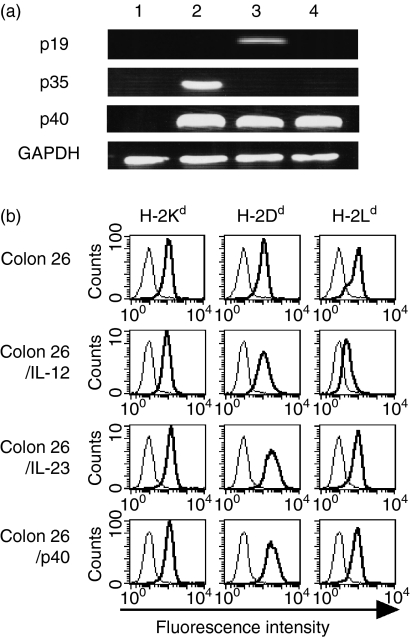
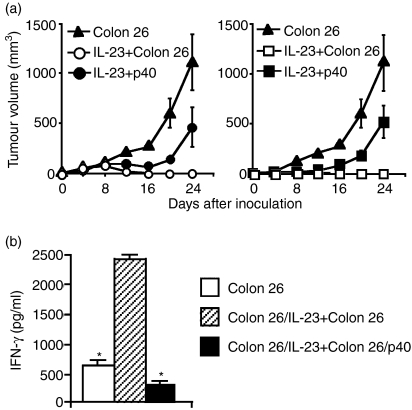
We previously reported that Colon 26 cells expressing IL-12 or IL-23 (Colon 26/IL-12 or Colon 26/IL-23) were rejected in syngeneic mice by stimulation of systemic immunity.20,21 Here, we retrovirally transduced Colon 26 cells with the p40 gene (Colon 26/p40) and examined whether local production of p40 impaired IL-12- and IL-23-induced antitumour effects in vivo. Expression of the cytokine subunit genes in the transduced cells was confirmed with RT-PCR (Fig. 4a). The proliferation rate in vitro of Colon 26/p40 cells was identical to that of Colon 26, Colon 26/IL-12 and Colon 26/IL-23 cells (data not shown). The expression levels of H-2Kd and H-2Dd of Colon 26/p40 cells remained the same as those of Colon 26, Colon 26/IL-12 and Colon 26/IL-23 cells (Fig. 4b). The level of H-2Ld expression of Colon 26/p40 cells was the same as those of Colon 26 and Colon 26/IL-23 cells but higher than that of Colon 26/IL-12 cells. We confirmed that the growth of Colon 26/p40 tumours developed in syngenic mice remained the same as that of Colon 26 tumours, as previously described.21
Figure 4.
Expression of cytokine subunits and major histocompatibility complex (MHC) class I molecules of parent and transduced Colon 26 cells. (a) Expression of the p19, p35, p40 and GAPDH genes in transduced cells: lane 1, Colon 26; lane 2, Colon 26/IL-12; lane 3, Colon 26/IL-23; lane 4, Colon 26/p40 cells. (b) Expression of MHC class I molecules. Parent and transduced Colon 26 cells were stained with anti-H-2Kd, anti-H-2Dd, anti-H-2Ld(thick line) or control antibodies (thin line).
We mixed equal numbers of Colon 26/p40 cells with either Colon 26/IL-12 or Colon 26/IL-23 cells and then inoculated the mixtures into syngeneic mice. More than half of the mice inoculated with the mixture of Colon 26/p40 cells and either Colon 26/IL-12 or Colon 26/IL-23 cells developed tumours, whereas all the mice inoculated with the mixture of Colon 26 and either Colon 26/IL-12 or Colon 26/IL-23 cells rejected the tumours (Table 2). The tumour growth of the mixtures consisting of Colon 26/p40 cells and the IL-12 or IL-23 producers, when compared with that of Colon 26 cells only, was retarded in the initial phase but the growth rate remained the same thereafter (Fig. 5a). We further investigated IFN-γ production in splenocytes of the tumour-bearing mice (Fig. 5b). Splenocytes of naïve mice did not produce any INF-γ when cultured with Colon 26 cells (data not shown), but those of Colon 26 tumour-bearing mice produced small amounts of IFN-γ. IFN-γ production increased in the splenocytes of mice inoculated with the mixture of Colon 26/IL-23 and Colon 26 cells, while production in the splenocytes of mice inoculated with Colon 26/IL-23 and Colon 26/p40 cells was completely suppressed.
Table 2. Tumour development in mice inoculated with p40 producers.
| Inoculated cells (day 0) | Tumorigenesis4 (day 30) |
|---|---|
| Colon 261 | 6/6 |
| Colon 26/p401 | 6/6 |
| Colon 26/IL-23 + Colon 262 | 0/6 |
| Colon 26/IL-23 + Colon 26/p402 | 4/6 |
| Colon 26/IL-12 + Colon 263 | 0/6 |
| Colon 26/IL-12 + Colon 26/p403 | 4/6 |
Cells (1×106) were inoculated subcutaneously into BALB/c mice.
Colon 26/IL-23 cells (5×105) were mixed with Colon 26 or Colon 26/p40 cells (5 × 105) and the mixtures (1 × 106) were then inoculated subcutaneously into BALB/c mice.
Colon 26/IL-12 cells (5 × 105) were mixed with Colon 26 or Colon 26/p40 cells (5 × 105) and the mixtures (1 ×106) were then inoculated subcutaneously into BALB/c mice.
The number of tumour-bearing mice/the number of mice tested.
Figure 5.
p40-producing tumours prevented interleukin (IL)-23- and IL-12-mediated antitumour immunity in vivo. (a) Tumour growth of mixtures of transduced cells in BALB/c mice. Cytokine-producing cells (5 × 105) were mixed with either Colon 26 or Colon 26/p40 cells (5 × 105) and the cells (1 × 106 in total) were inoculated subcutaneously into the flank (n = 6): symbols represent Colon 26 (closed triangle), and the mixtures of Colon 26/IL-23 with Colon 26 (open circle), Colon 26/IL-23 with Colon 26/p40 (closed circle), Colon 26/IL-12 with Colon 26 (open square) and Colon 26/IL-12 with Colon 26/p40 (closed square). Data represent mean ± standard deviation (SD) for tumour-bearing mice. (b) Interferon (IFN)-γ production in the splenocytes of mice inoculated with Colon 26 cells (open bar), mixtures of Colon 26/IL-23 and Colon 26 cells (hatched bar) or mixtures of Colon 26/IL-23 and Colon 26/p40 cells (closed bar). On day 24, splenocytes were cultured for 24 hr with irradiated Colon 26 cells. Data represent mean ± SD of triplicate samples. Asterisks indicate statistical significance compared with splenocytes of mice inoculated with mixtures of Colon 26/IL-23 and Colon 26 cells (Student t-test, P < 0·05).
p40 suppressed IL-23-mediated cytotoxic T lymphocyte (CTL) stimulation
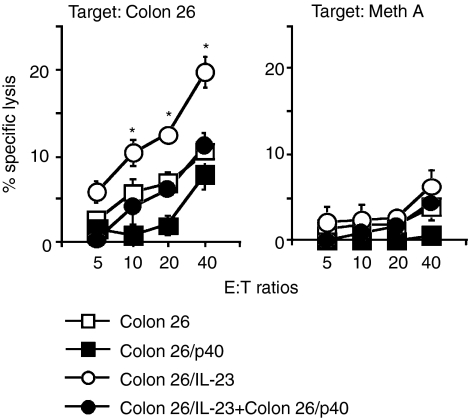
Since CD8+ T cells play a pivotal role in IL-23-dependent antitumour effects,21,22 we examined whether p40 suppressed IL-23-mediated CTL stimulation in vitro. Splenocytes of mice that had rejected Colon 26/IL-23 cells or those of naïve mice were stimulated with Colon 26/IL-23 and either Colon 26 or Colon 26/p40 cells, and were measured for their cytolytic activities against Colon 26 or irrelevant Meth A cells (Fig. 6). Splenocytes from naïve mice did not kill Colon 26 cells or Meth A cells, even if the cells were stimulated with Colon 26/IL-23 cells in vitro (data not shown). Stimulation in vitro with Colon 26/IL-23 cells enhanced the cytolytic activity of the splenocytes from Colon 26/IL-23-rejected mice when compared with the splenocytes stimulated with Colon 26 cells alone; however, the cytolytic activity of the splenocytes stimulated with a mixture of Colon 26/IL-23 and Colon 26/p40 cells was reduced to the level of that found with Colon 26 cells alone. The cytolytic activity of the splenocytes stimulated with Colon 26/p40 cells alone was not as great as that with Colon 26 cells. The inhibitory action of p40 was not observed in CTL activity against Meth A cells. We also found that stimulation in vitro with Colon 26/IL-12 cells enhanced cytolytic activity in the splenocytes from the mice that had rejected Colon 26/IL-23 cells and the presence of p40 inhibited the IL-12-mediated CTL stimulation in vitro (data not shown).
Figure 6.
p40 inhibited interleukin (IL)-23-dependent cytotoxic T lymphocyte (CTL) induction in vitro. Splenocytes from BALB/c mice that had rejected Colon 26/IL-23 cells were cultured with mitomycin C-treated Colon 26 (open squares) or Colon 26/p40 (closed squares) cells, or equal mixtures of populations of Colon 26/IL-23 with either Colon 26 (open circles) or Colon 26/p40 (closed circles) cells. Cytolytic activity against Colon 26 and Meth A cells was measured at various effector:target (E:T) ratios. Data represent mean ± standard deviation (SD) of triplicate samples. Asterisks indicate statistical significance compared with splenocytes cultured with a mixture of Colon 26 cells [repeated-measures analysis of variance (anova); P < 0·01].
Discussion
This is the first study, to our knowledge, to demonstrate that a secreted p40 was inhibitory to IL-23-mediated immune responses. Since competitive binding analyses showed that p40 prevented binding of IL-12 to its receptor,17,18 we hypothesized that p40 could also prevent IL-23 ligation to its receptors. The splenocytes treated with p40 before IL-23 ligation to the receptors produced smaller amounts of IFN-γ, suggesting that p40 occupied IL-23 receptor complexes and prevented the binding of IL-23 to its receptor. The modest inhibitory action of p40 on IL-23-mediated cytokine production could be dependent on the molar ratio of p40 to IL-12 or IL-23 contained in supernatants of cytokine-producing COS-7 cells and on the binding affinity of p40 to respective receptor complexes. Further investigations are required to determine the differential binding of p40 to respective receptors for IL-12 and IL-23.
Inoculation of mice with mixtures of Colon 26/p40 cells and Colon 26/IL-12 or Colon 26/IL-23 cells revealed that p40 impaired IL-12- and IL-23-mediated antitumour immunity in vivo. We also demonstrated that IL-23 directly enhanced T cell-mediated cytotoxic activity and that p40 inhibited IL-23-mediated CTL stimulation in vitro. Previous studies implied that CD8+ T cells could be one of the direct targets of IL-23, as IL-23 induced CD8+ T cell-mediated antitumour immunity.21,22 The present study also showed that CD8+ T cells were activated by IL-23: the cytotoxic activity of splenocytes from mice primed with Colon 26/IL-23 cells increased when they were subsequently stimulated with Colon 26/IL-23 cells rather than with Colon 26 cells. This enhanced cytolytic activity was not observed in the presence of p40, and p40 also impaired the cytolytic activity induced in splenocytes stimulated with Colon 26 cells alone. We presume that macrophage and dendritic cells in the spleen produced IL-12 and/or IL-23 in the secondary CTL stimulation with Colon 26 cells and the cytotoxic activity was inhibited by p40. We did not observe any toxicity of p40 against the splenocytes in our experimental systems (data not shown).
Other studies have reported contradictory findings that the administration of p40 to p35–/– and p40–/– mice, which were susceptible to Mycobacterium and Salmonella infection, increased resistance to the infection.23,24 However, we did not find any evidence for such agonistic effects of p40 in the present assay systems. Moreover, the forced expression of the p40 gene suppressed Th1-mediated immune responses and consequently prevented allograft rejection in recipient animals and ameliorated the development of autoimmune diseases.25–27 Since p40 activates macrophages and microglia to produce tumour necrosis factor-α,28 further studies are needed to clarify the agonistic effects of p40 on macrophages.
Recent studies suggested that IL-23 was a key mediator for not only Th1-mediated immune responses but also systemic inflammation. IL-23 induced IL-17, a proinflammatory cytokine involved in the pathogenesis of collagen-induced arthritis.2,29 Transgenic mice that aberrantly expressed either the p19 or the p40 gene showed inflammation in multiple organs because of excess amounts of IL-23.30,31 The expression of IL-23 was elevated in skin lesions of patients with psoriasis.32 These studies collectively suggest that IL-23 is a candidate target molecule for the treatment of relevant inflammatory diseases; p40 could therefore be of use in diseases with long-lasting inflammation through its inhibitory action on IL-23.
In summary, we demonstrated that p40 inhibited IL-23-dependent cytokine production and CTL stimulation in vitro, and IL-23-mediated antitumour effects in vivo. The present study implies a potential therapeutic use of p40 as an immunosuppressant for autoimmune diseases and as an anti-inflammatory agent for treatment of patients with severe systemic inflammatory diseases. p40 may circumvent non-specific immune suppression caused by current immunosuppressive medicines, which often results in adverse reactions.
Acknowledgments
The authors thank Drs A. D. Miller (Fred Hutchinson Cancer Research Center, Seattle, WA) for the LXSN vector, J. Hamuro (Ajinomoto, Tokyo, Japan) for Colon 26 cells, H. A. Young (National Cancer Institute Frederick, Frederick, MD) for helpful discussion, and Mr K. Shinagawa for technical assistance. This work was supported by grants-in-aid for scientific research from the Japan Society for the Promotion of Science and for Center of Excellence research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- anova
analysis of variance
- Con A
concanavaline A
- CTL
cytotoxic T lymphocyte
- EAE
experimental autoimmune encephalomyelitis
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IFN
interferon
- IL
interleukin
- IRES
internal ribosomal entry site
- MHC
major histocompatibility complex
- p19
p19 subunit of IL-23
- p35
p35 subunit of IL-12
- p40
p40 subunit of IL-12
- PHA
phytohaemaggulutinin
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SD
standard deviation
- Th
T helper
References
- 1.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 3.Belladonna ML, Renauld JC, Bianchi R, et al. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J Immunol. 2002;168:5448–54. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 4.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 5.Szabo SJ, Dieghe SA, Gubler U, Muphy KM. Regulation of the interleukin (IL)-12 Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–24. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–34. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–4. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 8.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181:381–6. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–10. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 10.Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-β2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–60. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 11.Zhang GX, Yu S, Gran B, et al. Role of IL-12 receptor β1 in regulation of T cell response by APC in experimental autoimmune encephalomyelitis. J Immunol. 2003;171:4485–92. doi: 10.4049/jimmunol.171.9.4485. [DOI] [PubMed] [Google Scholar]
- 12.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 13.Ling P, Gately MK, Gubler U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 14.Gillessen S, Carvajal D, Ling P, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–6. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 15.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–8. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 16.Piccotti JR, Chan SJ, Li K, Eichwald EJ, Bishop DK. Differential effects of IL-12 receptor blockade with IL-12 p40 homodimer on the induction of CD4+ and CD8+ IFN-γ producing cells. J Immunol. 1997;158:643–8. [PubMed] [Google Scholar]
- 17.Wu CY, Ferante J, Gately MK, Magram J. Characterization of IL-12 receptor β1 chain (IL-12Rβ1) deficient mice: IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–65. [PubMed] [Google Scholar]
- 18.Cleary AM, Tu W, Enright A, Giffon T, Dewaal-Malefyt R, Gutierrez K, Lewis DB. Impaired accumulation and function of memory CD4 T cells in human IL-12 receptor β1 deficiency. J Immunol. 2003;170:597–603. doi: 10.4049/jimmunol.170.1.597. [DOI] [PubMed] [Google Scholar]
- 19.Hoeve MA, de Bohr T, Langenberg DML, Sanal O, Verreck FA, Ottenhoff TH. IL-12 receptor deficiency revisited: IL-23-mediated signaling is also impaired in human genetic IL-12 receptor β1 deficiency. Eur J Immunol. 2003;33:3393–7. doi: 10.1002/eji.200324343. [DOI] [PubMed] [Google Scholar]
- 20.Tasaki K, Yoshida Y, Maeda T, et al. Protective immunity is induced in murine colon carcinoma cells by the expression of interleukin-12 or interleukin-18, which activate type 1 helper T cells. Cancer Gene Ther. 2000;7:247–54. doi: 10.1038/sj.cgt.7700094. [DOI] [PubMed] [Google Scholar]
- 21.Wang YQ, Ugai S, Shimozato O, et al. Induction of systemic immunity by expression of interleukin-23 in murine colon carcinoma cells. Int J Cancer. 2003;105:820–4. doi: 10.1002/ijc.11160. [DOI] [PubMed] [Google Scholar]
- 22.Lo CH, Lee SC, Wu PY, et al. Antitumor and antimetastatic activity of IL-23. J Immunol. 2003;171:600–7. doi: 10.4049/jimmunol.171.2.600. [DOI] [PubMed] [Google Scholar]
- 23.Hölscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, Brombacher F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–66. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann J, Bellmann S, Werner C, Schroder R, Schutze N, Alber G. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J Immunol. 2001;167:5304–15. doi: 10.4049/jimmunol.167.9.5304. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, Shimozato O, Hoshi K, Wakimoto H, Hamada H, Yagita H, Okumura K. Local production of the p40 subunit of interleukin 12 suppresses T-helper 1-mediated immune responses and prevents allogeneic myoblast rejection. Proc Natl Acad Sci USA. 1996;93:9085–9. doi: 10.1073/pnas.93.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima A, Seroogy CM, Sandora MR, et al. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J Clin Invest. 2001;107:1293–301. doi: 10.1172/JCI12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa GL, Sandora MR, Nakajima A, et al. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. J Immunol. 2001;167:2379–87. doi: 10.4049/jimmunol.167.4.2379. [DOI] [PubMed] [Google Scholar]
- 28.Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-α (TNF-α) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–28. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17 deficient mice. J Immunol. 2003;171:6176–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 30.Wiekowski MT, Leach MW, Evans EW, et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–70. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 31.Kopp T, Petra L, Concha BF, Kastelein RA, Kupper TS, Georg S. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J Immunol. 2003;170:5438–44. doi: 10.4049/jimmunol.170.11.5438. [DOI] [PubMed] [Google Scholar]
- 32.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapker M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–30. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]