Abstract
OX40/OX40 ligand (OX40L) interactions have been shown to exert potent costimulatory effects on T-cell activation. OX40 expression is transiently up-regulated on T cells following T-cell receptor engagement, while OX40L is expressed on antigen-presenting cells following activation. Although expression of the OX40L by T cells has been reported, the requirements for induction of OX40L on T cells have not been studied in detail. Here, we demonstrate that the OX40L can be induced on murine CD4+ and CD8+ T cells after 6 days of culture under T helper type 1 (Th1) conditions, but not under Th2 conditions. Induction of OX40L expression required a high concentration of interleukin-12 (IL-12), was not seen in the presence of interferon-γ, and was dependent on signal transducer and activator of transcription type 4 (STAT4). Notably, induction of OX40L on T cells was only seen at very low concentrations of antigen or anti-CD3. T-cell-expressed OX40L was fully capable of delivering a potent costimulatory signal that enhanced the proliferation of CD4+ T cells as well as promoted their differentiation to Th2 cells. OX40L expression could also be induced on CD4+ T cells in vivo following immunization with low-dose antigen and an IL-12 inducer. OX40/OX40L interactions between antigen-specific T cells may occur in T-cell zones in lymph node and spleen when OX40L expression has diminished on APC. Costimulation by T-cell-expressed OX40L may result in deviation of a Th1 response to a Th2 response under conditions where T cells are exposed to low concentrations of foreign or autoantigens in the presence of high concentrations of IL-12.
Keywords: cell surface molecules, costimulation, interleukin-12, rodent, T cells
Introduction
Activation of T cells during an immune response requires two signals. Signal one is delivered through the binding of the T-cell receptor (TCR) to the antigen–major histocompatibility complex (MHC), while signal two is delivered by costimulatory molecules on antigen-presenting cells (APC). The best-characterized costimulatory molecules on APCs are CD80 and CD86, which bind CD28 on T cells.1 Many members of the tumour necrosis factor receptor (TNFR)–TNF super-family also exhibit costimulatory function. The most highly studied is OX40 (CD134) and its ligand, OX40L (CD134L). OX40 is not expressed by naive T cells, but is transiently induced following TCR engagement with peak expression on day 2–3, followed by down-regulation on day 5.2 However, antigen-primed CD4+ T cells can rapidly up-regulate OX40 expression upon re-encounter with antigen. Similarly, the OX40L is not detectable on resting APC, but its expression can be up-regulated following 2–3 days of activation of B cells via the B-cell receptor3,4 or following the activation of dendritic cells,5 Langerhans cells, macrophages5 and endothelial cells4–6 by engagement of CD40 or with lipopolysaccharide.
The OX40/OX40L interaction has been shown to exert significant costimulatory effects on T-cell proliferation, survival and cytokine secretion.7 Following immunization of OX40-deficient (–/–) mice, antigen-specific T cells proliferate normally 2–3 days after TCR ligation, but reduced proliferation is seen by days 5–6; lower frequencies of antigen-specific effector T cells are detected late in the primary response and fewer memory T cells develop.4,8 The OX40/OX40L interaction may also regulate the differentiation of CD4+ T cells into T helper 1 (Th1) or Th2 cells as several reports have suggested that OX40/OX40L interactions can drive naive T cells to up-regulate interleukin-4 (IL-4) production and therefore promote their development into Th2 effector cells.9,10 The most prominent effect of OX40 engagement is the induction of Bcl-xL and Bcl-2 expression leading to prolonged T-cell survival.11
Although the OX40L is mainly expressed by activated APC, it was also detected on human T cells infected with human T-cell leukaemia virus type 1,12 long-term human cytotoxic T lymphocyte clones13 and activated murine T cells.12,14,15 However, the requirements for induction of OX40L expression on either human or mouse T cells have not been studied in detail. Here, we show that a fully functional OX40L can be induced both in vitro and in vivo on mouse T cells when they are primed in the presence of IL-12 in combination with a weak TCR stimulatory signal. The significance of OX40/OX40L interactions between T cells in amplifying signals that are initiated by APC will be discussed.
Materials and methods
Mice
13A TCR transgenic mice specific for myelin oligodendrocyte glycoprotein (35–55) (MOG35–55) were previously described.16 These mice were bred and maintained in the NIAID animal facility. C57BL/6 and BALB/c mice were purchased from NCI/Frederick animal facility (Frederick, MD). Mice deficient –/– for signal transducer and activator of transcription type 4 (STAT4) were purchased from the Jackson Laboratories (Bar Harbor, ME). B6.SJL CD45.1 mice, TCR transgenic OT-I/RAG–/– mice specific for ovalbumin (257−264) (OVA257–264) and TCR transgenic OT-II mice specific for OVA323−339 were obtained from Taconic Farms (Germantown, NY). All mice were 2–4 months old when used in these studies.
Reagents
Peptides corresponding to residues 35–55 of MOG (MEVGWYRSPFSRVVHLYRNGK), and residues 257–264 and 323–339 (SIINFEKL and ISQAVHAAHAEINEAGR, respectively) of OVA were synthesized and purified by the Peptide Synthesis Facility, Laboratory of Molecular Structure (NIAID, NIH, Bethesda, MD). CpG #1826 (5′-TCC ATG ACG TTC CTG ACG TT-3′) for induction of Th1 responses was provided by Dr Bob Seder, VRC, NIH. Human recombinant IL-2 (rIL-2) and mouse IL-12 were purchased from Peprotech (Rocky Hill, NJ). Anti-CD4-Cy, anti-IL-4-phycoerythrin (PE), anti-CD8-fluorescein isothiocyanate (FITC), anti Vβ4-FITC, anti-Vβ5.1/5.2-FITC, streptavidin-FITC, streptavidin-PE, anti-interferon-γ (IFN-γ) -APC, anti-IFN-γ (XMG1·2), anti-IL-12 (C17·8), anti-OX40L, biotin anti-OX40L, biotin anti-CD45.1, anti-CD45.2-FITC, anti-CD3, and anti-CD28 were purchased from Pharmingen (San Diego, CA). Both rIL-4 and anti-IL-4 (11B.11) were provided by Dr William Paul, NIAID, NIH.
Cell purification
CD4+ and CD8+ T cells were purified from pooled spleen and lymph nodes. A cell suspension was prepared and erythrocytes were removed using ACK lysis buffer. Cells were washed in phosphate-buffered saline (PBS) and incubated at 4° for 15 min in buffer containing PBS, 0·5% bovine serum albumin, 2 mm ethylenediaminetetraacetic acid and anti-CD4 or anti-CD8 microbeads (Miltenyi Biotec, Auburn, CA). Cells were then washed, resuspended in the same buffer, and positively selected on the Automacs (Miltenyi Biotec); cell purity was 85–90%. For the generation of CD4+ OX40L+ cells, CD4+ T cells (2·5 × 105/ml) were stimulated with irradiated (3000 rads) syngeneic T-depleted spleen cells (TdS, 106/ml) and the corresponding peptide or soluble anti-CD3 with IL-12 (10 ng/ml) and anti-IL-4 (10 μg/ml) for 3 days in RPMI-1640 medium supplemented with l-glutamine, β-mercaptoethanol, antibiotics and 5% fetal calf serum as previously described.17 Subsequently, the cells were maintained in vitro in medium containing IL-2 (100 U/ml). Where indicated, IL-2 was added during the first 3 days as well. The same culture conditions were used for the generation of CD8+ OX40L+ T cells except that the TdS were pulsed with peptide for 1 hr at 37° before adding them to the culture. TdS were prepared by first lysing the erythrocytes with ACK lysis buffer, followed by incubation with anti-Thy-1·2 microbeads (Miltenyi Biotec). Cells were then washed, resuspended in the same buffer and negatively selected on the Automacs (Miltenyi Biotec). To enrich for OX40L+ cells, 1 week after priming under Th1 conditions and with low antigen dose, cells were incubated with biotin anti-OX40L for 20 min, washed, stained with streptavidin-PE for an additional 20 min, and then washed again followed by incubation with anti-PE microbeads (Miltenyi Biotec) for 15 min. Following one additional wash, the cells were run through magnetic antibody cell sorting (autoMACS) using a positive-selection-sensitive program to achieve highly purified OX40L+ cells (> 90%). To obtain a pure OX40L– population, the negative fraction was collected and run under the deplete-sensitive program. The level of purity (< 5% OX40L+) was determined by fluorescence-activated cell sorter analysis. As a negative control, Th2 polarization was performed in the presence of IL-4 (10 ng/ml), anti-IFN-γ (10 µg/ml) and anti-IL-12 (10 µg/ml).
Microarray protocol
CD4+ T cells from the 13A mice were stimulated with MOG peptide (10 ng/ml) in the presence of TdS from a normal C57BL/6 mouse and anti-IL-4 (10 μg/ml) or IL-4 (10 μg/ml) and IL-12 (10 ng/ml) for 3 days. The culture was then split and stimulated with IL-2 (50 U/ml) and 7 days later RNA was isolated for the microarray. In two experiments, the level of OX40L mRNA was elevated 10-fold when the cells were cultured in the presence of IL-12; no significant enhancement of other mRNAs was observed.
Intracellular staining
The cells were stimulated with plate-bound anti-CD3 and anti-CD28 (5 μg/ml of each) for 4 hr in the presence of monensin (2 μm/ml). The quantification of intracellular cytokines was performed as previously described.16
T-cell proliferation
TCR transgenic T cells (5 × 104) were cultured in microtitre wells in the presence of irradiated TdS (3000 rads; 2 × 105/well) and the corresponding antigen as previously described.16 The data are expressed as mean counts per minute (c.p.m.) [3H]thymidine (TdR) incorporation of triplicate cultures or as Δc.p.m. (i.e. c.p.m. with antigen − c.p.m. in the absence of antigen). CD4+ T cells (5 × 104/well) from B6 mice were stimulated for 3 or 5 days with plate-bound anti-CD3 alone or in the presence of 4% paraformaldehyde-fixed OT-II OX40L+ or OX40L– CD4+ T cells. [3H]TdR was added to the cultures for the last 8 hr. To determine the role of OX40L+ T cells on Th1/Th2 differentiation, CD4+ CD62Lhi cells from CD45.1 mice were cultured (2·5 × 105/ml) in a 24-well plate with plate-bound anti-CD3 and anti-CD28 (1 μg/ml of each) alone or in the presence of fixed OT-II OX40L+ or OX40L– CD4+ T cells. After 3 days, the cells were washed, re-cultured in the presence of IL-2 for a further 4 days, and then tested for cytokine production.
In vivo induction of OX40L
CD4+ T cells (5 × 106) from OT-II mice were transferred into naive CD45.1 recipients. One day later, the mice were immunized subcutaneously with CpG (50 μg/mouse) and various doses of OVA323−339 emulsified in incomplete Freund's adjuvant (IFA). Nine days later draining lymph nodes were excised and lymph node cells stained for OX40L expression.
Results
OX40L is expressed on 13A CD4+ Th1 cells
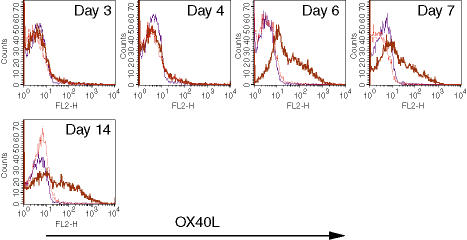
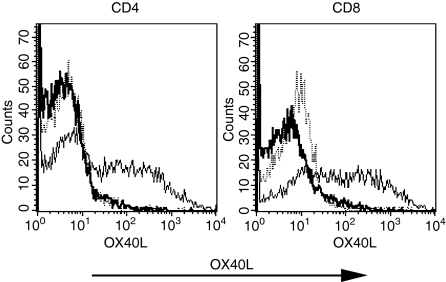
We have previously described that CD4+ T cells from 13A mice are susceptible to activation-induced cell death upon priming under Th1, but not Th2, conditions.16 In an attempt to determine the molecular basis for that susceptibility, a restricted microarray specific to apoptosis-related molecules was performed on Th1-differentiated 13A CD4+ T cells. Unexpectedly, OX40L mRNA was selectively expressed in 13A cells cultured under Th1 conditions (data not shown). To confirm these results at the protein level, 13A CD4+ T cells were primed with MOG35−55 (10 μg/ml) under Th1 or Th2 conditions and stained for OX40L expression at different time-points. OX40L was detected on the membrane surface only after 6 days of culture, but expression was maintained up to 14 days (Fig. 1). In general, we did not observe cells that expressed both OX40 and OX40L as the OX40L could only be detected after 6 days of priming at a time when OX40 expression had markedly declined.
Figure 1.
Expression of OX40L on 13A CD4+ Th1 cells. 13A CD4+ T cells were primed for 3 days with MOG35−55 (10 μg/ml) under Th1 (IL-4, anti-IFN-γ, anti-IL-12) or Th2 (IL-12, anti-IL-4) conditions, stained for OX40L expression, or split in IL-2, and then tested on the indicated days for the expression of OX40L (thin line, isotype control; dotted line, Th2 conditions; thick line, Th1 conditions).
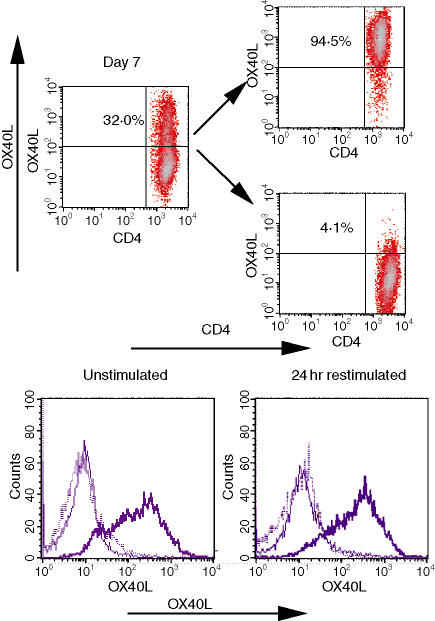
As the OX40L was only detected on a subpopulation of the cells, we next tested whether expression of the OX40L was a stable characteristic or whether it could also be induced on the OX40L– cells. Thus, 7 days after priming under Th1 conditions, the cells were separated into OX40L– and OX40L+ populations (Fig. 2, top), cultured for an additional 7 days, and then re-stained for OX40L before and 24 hr after challenge with antigen (Fig. 2, bottom). The percentage of OX40L-expressing cells in the OX40L+ population was only marginally reduced (85%) and was not increased following antigen stimulation (88%). The OX40L– population remained negative and expression was not enhanced by antigen stimulation. These results indicate that expression of OX40L on a subset of 13A CD4+ Th1 cells is induced on day 6 after priming and is sustained for a prolonged time on this subpopulation.
Figure 2.
OX40L is stably expressed on a subpopulation of activated 13A Th1 cells. Seven days after priming under Th1 conditions, 13A CD4+ Th1 cells were separated based on their expression of the OX40L (top) and maintained in IL-2. Seven days after separation, OX40L– and OX40L+ cells were left untreated or restimulated with MOG35−55 (10 μg/ml) for 24 hr followed by staining for OX40L (bottom panels: thin line, isotype control; thick line, OX40L+; dotted line, OX40L–). Results are on gated CD4+ Vβ4+ cells.
Induction of OX40L expression on 13A CD4+ T cells is IL-12-dependent, but IFN-γ-independent
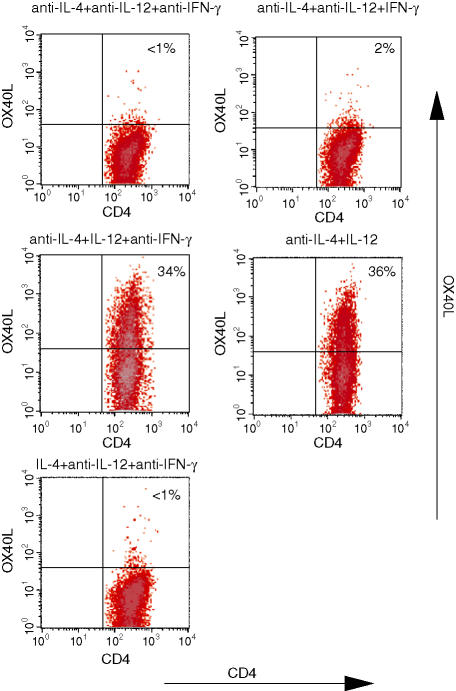
Both IL-12 and IFN-γ play key roles in the differentiation of CD4+ T cells to the Th1 phenotype.18 To determine which of these cytokines is essential for the expression of OX40L, CD4+ T cells from 13A mice were primed with MOG35−55 (10 μg/ml) under various differentiation conditions and then stained for the presence of OX40L. After priming under null conditions (anti-IL-4, anti-IL-12, anti-IFN-γ) or Th2 (IL-4, anti-IL-12, anti-IFN-γ), the OX40L was undetectable on the cell surface (Fig. 3). High levels of OX40L could be induced in the presence of IL-12 and anti-IFN-γ (Fig. 3), but no significant induction of the OX40L was observed when the cells were primed in the presence of IFN-γ and in the absence of and IL-12 (Fig. 3).
Figure 3.
OX40L expression on 13A CD4+ T cells is IL-12 dependent. 13A CD4+ T cells were primed for 3 days with MOG35−55 (10 μg/ml) under the indicated culture conditions, rested for 4 more days in IL-2, and then tested for the expression of OX40L. Results are on gated CD4+ Vβ4+ cells.
Expression of OX40L is controlled by the strength of stimulation
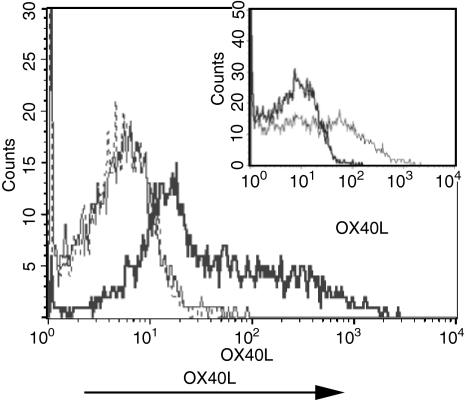
To determine if induction of the OX40L on 13A T cells was only seen following antigen stimulation, we stimulated 13A cells under Th1 conditions with different concentrations of soluble anti-CD3 in the presence of APC. Expression of the OX40L could only be detected when the cells were primed with 0·005 μg/ml of anti-CD3 under Th1 conditions (Fig. 4), but not in the presence of higher concentrations (0·05 or 0·5 μg/ml). The level of OX40L induced with this concentration of anti-CD3 was similar to that seen following stimulation with MOG35−55 (Fig. 4, insert). These results suggest that in addition to a requirement for IL-12, induction of expression of OX40L on 13A CD4+ T cells is inversely correlated with the strength of TCR stimulation.
Figure 4.
Expression of the OX40L is inversely correlated with the strength of TCR stimulation. 13A CD4+ T cells were primed for 3 days with 0·5 μg/ml (dashed line), 0·05 μg/ml (thin line), or 0·005 μg/ml (thick line) of soluble anti-CD3 under Th1 differentiation conditions, rested for 4 more days in IL-2, and then tested for the expression of OX40L. Insert illustrates the level of OX40L expression on 13A Th1 cells primed for a similar length of time with MOG35−55 (10 μg/ml, thin line). Results are on gated CD4+ Vβ4+ cells.
OX40L is inducible on both CD4+ and CD8+ T cells
To determine whether the requirements for induction of OX40L expression on 13A T cells would also apply to other T-cell populations, we stimulated CD4+ T cells from OT-II mice with increasing concentrations of their cognate antigen (OVA323−339) under Th1 conditions (IL-12, 10 ng/ml) for 7 days, and then measured surface expression of OX40L (Table 1). In parallel, we compared the concentrations of antigen needed for induction of CD69 expression and proliferation. Significant expression of the OX40L (48·6%) could only be detected when the OT-II cells were primed in the presence of 0.001 μg/ml of peptide, while much lower levels were seen when the peptide concentration was increased by just one log(0.01 μg/ml, 14·5%). OX40L expression could not be detected at the higher concentrations of peptide that induced optimal proliferative responses. In addition, expression of the OX40L was seen at concentrations of antigen that failed to induce significant expression of CD69 after 18 hr of priming.
Table 1.
Expression of OX40L on OT-II CD4+ Th1 cells
| IL-12 (ng/ml) | Antigen (µg/ml)a | %CD69+/ MFIb | Δc.p.m.c | OX40L+d |
|---|---|---|---|---|
| Effect of antigen concentration | ||||
| 10 | 10−3 | 4/50 | 650 | 49 |
| 10 | 10−2 | 25/78 | 9398 | 15 |
| 10 | 10−1 | 83/182 | 75 526 | 2 |
| 10 | 10° | 93/234 | 85 660 | 1 |
| 10 | 101 | 96/247 | 122 291 | 1 |
| 10 | 102 | 97/246 | 156 377 | 0 |
| Effect of IL-12 concentration | ||||
| 0 | 10−2 | < 1 | ||
| 10−3 | 10−2 | < 1 | ||
| 10−2 | 10−2 | < 1 | ||
| 10−1 | 10−2 | 3 | ||
| 10° | 10−2 | 9 | ||
| 101 | 10−2 | 26 | ||
Concentration of OVA 323−329;
%CD69 + cells from gated CD4+ Vβ5+ cells after 18 hr of stimulation;
c.p.m. 72 hr after priming;
%OX40L + cells from gated CD4+ Vβ5+ cells 7 days after stimulation.
The studies reported thus far have used a single concentration of IL-12 (10 ng/ml) for induction of Th1 responses. To determine the optimal concentration needed for induction of OX40L, we stimulated OT-II cells with the optimal concentration of peptide, but in the presence of varying concentrations of IL-12. Although a marginal increase (3·2%) in OX40L+ cells could be detected in the presence of 0·1 ng/ml of IL-12, significant levels of OX40L expression were only detected at the highest concentration (10 ng/ml) of IL-12 tested (Table 1). Very similar results were obtained when CD8+ T cells from OT-I mice were stimulated with varying concentrations of cognate peptide and with different concentrations of IL-12 (Table 2). Induction of significant OX40L expression was only seen with low concentrations (5.0 or 0.5 pg/ml) of peptide and high concentrations of IL-12 (1–10 ng/ml).
Table 2.
Expression of OX40L on OT-I CD8+ T cells
| IL-12 (ng/ml) | Antigen (μg/ml)a | %OX40L+b |
|---|---|---|
| Effect of antigen concentration | ||
| 10 | 5 × 10−4 | 49 |
| 10 | 5 × 10−3 | 45 |
| 10 | 5 × 10−2 | 4 |
| 10 | 5 × 10−1 | < 1 |
| 10 | 5 × 10° | < 1 |
| Effect of IL-12 concentration | ||
| 0 | 5 × 10−3 | 1 |
| 10−3 | 5 × 10−3 | 2 |
| 10−2 | 5 × 10−3 | 3 |
| 10−1 | 5 × 10−3 | 12 |
| 10° | 5 × 10−3 | 37 |
| 101 | 5 × 10−3 | 54 |
Concentration of OVA 257−264;
%OX40L + cells of CD8+ cells 7 days after priming.
Induction of OX40L expression on T cells is STAT4-dependent
It is well documented that IL-12 mediates its function through activation of STAT4. To determine whether Stat4 activation was required for induction of expression of OX40L on T cells, we stimulated purified CD4+ and CD8+ T cells from STAT4-deficient (–/–) mice with a low dose of anti-CD3 (0·005 μg/ml) and IL-12 (10 ng/ml) and 10 days later stained the cells for OX40L. The OX40L was induced on both CD4+ (40%) and CD8+ (52%) T cells from wild-type BALB/c mice, but could not be detected on stimulated CD4+ or CD8+ T cells from STAT4–/– mice (Fig. 5).
Figure 5.
Induction of OX40L expression is mediated through activation of STAT4. CD4+ and CD8+ T cells were purified from wild-type and STAT4–/– mice, primed for 3 days under Th1 conditions [IL-2 (100 U/ml), IL-12 (10 ng/ml) and soluble anti-CD3 (0·005 μg/ml)], maintained in IL-2 for 7 more days, and then stained for OX40L (dotted line, isotype control; thin line, wild-type mice; thick line, STAT4–/– mice).
CD4+ T cells express a functional OX40L molecule
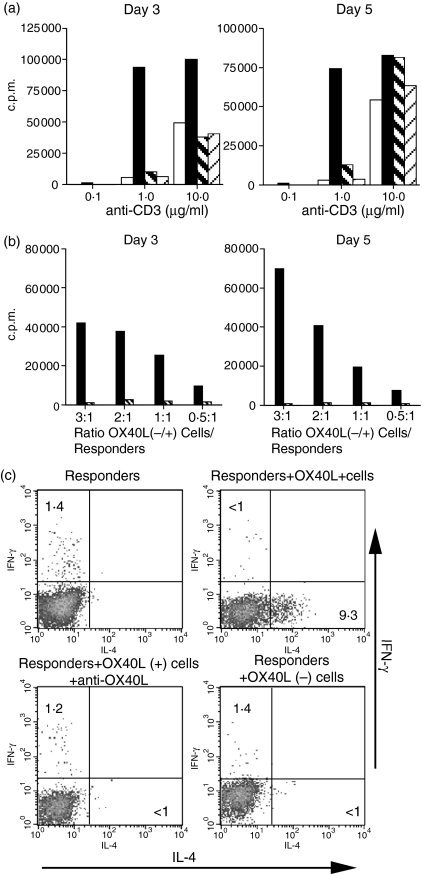
It has been previously demonstrated that OX40L expressed on APC acts as a costimulatory molecule and induces Th2 responses by T cells.19 We therefore wanted to determine whether OX40L expressed on CD4+ T cells would have similar properties. To avoid any influence of OX40L expressed on APC, we stimulated CD4+ T cells from B6 mice with plate-bound anti-CD3 in the presence of fixed OX40L+ or OX40L– T cells that had been generated as described above from OT-II mice stimulated with low-dose antigen and IL-12. Following either 3 days or 5 days of stimulation, the addition of OX40L+, but not OX40L–, T cells resulted in a markedly enhanced proliferative response, particularly at the low dose of anti-CD3 (Fig. 6a). The enhanced response seen in the presence of the OX40L+ cells was completely inhibited by anti-OX40L. The costimulatory signal delivered was significant even at the lowest ratio (0·5 : 1) of costimulator : responder cells studied (Fig. 6b).
Figure 6.
OX40L expressed on CD4+ T cells acts as a costimulatory molecule and induces Th2 responses in naive CD4+ T cells. (a)CD4+ T cells from B6 mice were cultured with plate-bound anti-CD3 for 3 or 5 days alone or in the presence of fixed OX40L+ CD4+ T cells or OX40L– CD4+ T cells generated from OT-II mice at a ratio of 1 : 3 (responder : costimulator). 3H[TdR] uptake was measured at the end of the co culture (empty box, responders alone; black box, responders + OX40L+ cells; thick hatched box, responders + OX40L+ cells and anti-OX40L; thin hatched box, responders + OX40L– cells).(b)CD4+ T cells from B6 mice were cultured with plate-bound anti-CD3 (1 μg/ml) for 3 and 5 days alone or in the presence of fixed OX40L+ CD4+ T cells in the presence or absence of anti-OX40L at the indicated ratios (black box, responders + OX40L+ cells; thick hatched box, responders + OX40L+ cells and anti-OX40L). (c)Naive CD62LhiCD4+ T cells from Ly5.1 mice were primed for 3 days with plate-bound anti-CD3 and anti-CD28 (1 μg/ml of each) alone or in the presence of fixed OX40L+ or OX40L– cells, washed, and reseeded for four more days in IL-2 medium. The recovered cells were then restimulated for intracellular staining. Results are on gated Ly5.1 cells.
To determine whether T-cell-expressed OX40L was also capable of enhancing the differentiation of CD4+ T cells along the Th2 pathway, naive CD4+ CD62Lhi T cells were stimulated with plate-bound anti-CD3/CD28 in the presence or absence of fixed OX40L+ or OX40L– T cells. When the responder CD4+ T cells were stimulated alone or in the presence of OX40L– T cells, no cytokine-producing cells could be detected (Fig. 6c). However, the addition of fixed OX40L+ T cells resulted in the generation of cells producing IL-4, but not IFN-γ. The induction of IL-4-producing cells was solely the result of OX40L expression as blocking with anti-OX40L antibody completely inhibited the generation of IL-4-producing cells. Taken together, these results indicate that OX40L expressed on CD4+ T cells can function as a potent costimulatory molecule for both T-cell proliferation and for the induction of Th2 differentiation of naïve CD4+ T cells.
Induction of OX40L expression on CD4+ T cells in vivo
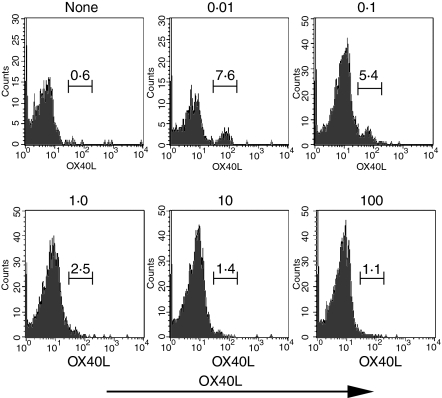
To determine whether OX40L could be induced on CD4+ T cells in vivo, we attempted to mimic the conditions we used in vitro. OT-II CD4+ T cells (Ly-5.2+) were transferred into normal C57BL/6 recipients (Ly-5.1+), and 1 day later the mice were immunized with varying doses of OVA323−339, emulsified in IFA containing CpG as an IL-12 inducer.20 Nine days post-priming the draining lymph nodes were evaluated for expression of OX40L on the transferred OT-II cells (Fig. 7). CD4+ OT-II T cells from control mice immunized with only CpG/IFA did not express detectable OX40L. In contrast, a distinctive subpopulation of OX40L+ cells could be detected only in the draining lymph nodes from animals immunized with the lowest dose (0·01 μg/mouse) of the OVA peptide. As seen in the in vitro studies, immunization with increasing doses of OVA peptide resulted in a decrease in the percentage of cells expressing OX40L. These results suggest that in vivo priming of CD4+ T cells under Th1 differentiation conditions by low antigen dose can lead to expression of OX40L on antigen-specific CD4+ T cells.
Figure 7.
In vivo induction of OX40L on CD4+ T cells. Purified CD4+ T cells from OT-II mice (Ly5.2) were transferred into naive Ly5.1 recipients. A day later, mice were immunized with CpG (50 μg/mouse) and OVA323−339 (in μg/mouse) both emulsified in IFA. Nine days after immunization, draining lymph node cells were stained for OX40L. Results are expressed as percentage of gated CD4+ Ly5.2+ cells. No significant staining was seen with an isotype-matched control antibody.
Discussion
During the course of studies designed to investigate the susceptibility of Th1 cells to activation-induced cell death16 we made the fortuitous finding that these cells expressed mRNA for OX40L and readily detectable cell surface OX40L. We therefore undertook a comprehensive analysis of the requirements for induction of OX40L expression on murine T cells. The OX40L could readily be induced on both CD4+ and CD8+ T cells, but only after a relatively prolonged (6-day) period of activation in vitro under Th1, but not Th2 or Th0, conditions. The presence of a high concentration of IL-12 was absolutely required and priming of T cells in the presence of IFN-γ, but in the absence of IL-12, failed to result in expression of the OX40L. The second requirement for induction of OX40L on T cells was that it was only seen at very low concentrations of antigen or anti-CD3. It should be noted that significant expression of the OX40L was induced at concentrations of antigen that failed to induce expression of CD69, one of the most sensitive indicators of TCR engagement. Although expression of the OX40L was induced on the 13A TCR transgenic cells with what appeared to be a high concentration of antigen (10 μg/ml), it should be noted that this concentration of antigen was the lowest concentration of antigen needed for induction of a T-cell proliferative response by these cells which our previous studies suggested expressed a very low-affinity TCR.16 Thus, a weak interaction of the TCR with antigen is probably required for induction of OX40L. Although the majority of our studies were performed in vitro, induction of OX40L could also be demonstrated following immunization of mice with very low doses of antigen in the presence of CpG, a known inducer of IL-12 production. We have not yet compared the functional properties of OX40L+ cells induced in vivo with those induced in vitro.
The function of the OX40L in vitro has primarily been studied by using either cells transfected with the OX40L or with T cells from TCR transgenic mice that express the OX40L under control of the lck promoter.21 Under the conditions used in our studies, only a subpopulation of activated T cells expressed the OX40L and expression of the OX40L on this subpopulation was stable following induction. Fixed OX40L+, but not OX40L–, T cells were capable of delivering a potent costimulatory signal that enhanced the proliferative response of CD4+ T cells to a suboptimal dose of plate-bound anti-CD3. All of the costimulatory effects of the OX40L+ cells were blocked by anti-OX40L, thereby ruling out the possibility that other membrane molecules coexpressed by the OX40L+ population were responsible for the costimulatory activity.
Considerable controversy still exists with regard to the role of OX40/OX40L interactions in regulating Th1 or Th2 responses. OX40/OX4OL interactions are involved at some level in the generation of Th1 responses as blocking the OX40/OX40L interaction prevented the development of Th1 diseases such as experimental allergic encephalomyelitis22 or inflammatory bowel disease.23 OX40–/– or OX40L–/– mice are also relatively resistant to diseases mediated by Th1 cells. On the other hand, OX40–/– mice are resistant to the induction of experimental models of Th2-mediated lung inflammation24 anti-OX40L blocks Th2-mediated lung disease25 as well as inhibiting the progression of Leishmania major in BALB/c mice, most probably by suppressing Th2 responses.26 It has been proposed that in the absence of adjuvant, OX40/OX40L interaction is capable of inducing a Th2 response, while in the presence of adjuvants both Th1 and Th2 responses are augmented.21,27 It still remains possible that the major effect of OX40/OX40L interactions is to augment both Th1 and Th2 responses by promoting cell survival and memory T-cell generation. To determine the potential influence of OX40L expressed on T cells to Th1/Th2 differentiation, we added fixed OX40L+ T cells to cultures of naive CD4+ CD62Lhigh T cells that were subsequently stimulated with anti-CD3/CD28 in the absence of IL-12 or IL-4. When the primed cells were analysed for induction of cytokine production by intracellular staining, a significant number of T cells producing IL-4, but not IFN-γ, could be detected. Thus, in our in vitro model, OX40L expressed on T cells seemed to preferentially promote Th2 differentiation in vitro.
At present we can only speculate about the functional importance of the expression of OX40L on antigen-specific T cells. The extensive studies of Croft and co-workers8,11 have shown that OX40 does not act in the initial phases of the response, but acts following CD28-derived costimulatory signals to regulate T-cell turnover at the peak of the expansion phase and to regulate the subsequent survival and expansion of antigen-specific T cells. Costimulation via CD28 is also required for optimal induction of expression of OX40 on T cells and OX40L on APC. We could only detect OX40L on T cells after 6 days of culture in vitro at a time-point when expression of OX40L would have already been down-regulated on APC. Thus, OX40/OX40L interactions between antigen-specific T cells may promote expansion, survival or differentiation of T cells at a time when the expression of the OX40L and antigen/MHC class II complexes have diminished on APC. Interactions between T cells could occur in T-cell zones of lymph node or spleen where large numbers of antigen-specific T cells accumulate following their initial stimulation.
Although Baba et al.28 have demonstrated that intact, full-length OX40L can be transferred from APC to the surface of responder T cells, we do not believe that the OX40L observed on T cells in our studies was derived from the APC. First, the APC used in the studies of Baba et al.28 were OX40L transfectants expressing very high levels of the antigen. Secondly, the transferred OX40L was detected very early in the cocultures and was rapidly lost from the cells' surface. In contrast, the OX40L detected in our studies only appeared after 6 days of culture and was stably expressed for 14 days in the absence of APC. Lastly, induction of the OX40L on T cells was STAT4-dependent and there is no data to suggest that the OX40L expression on APC is regulated by STAT4.
Why would T cells preferentially express OX40L only when stimulated under Th1 conditions and in the presence of low concentrations of antigen? This is a difficult question to address, but our limited in vivo studies suggest that this finding is not merely secondary to manipulation of cells in vitro. One possibility is that the induction of OX40L on T cells under these conditions is a protective mechanism for deviation of an autoreactive immune response from a damaging Th1 response to a protective Th2 response when T cells are challenged by low concentrations of an autoantigen in the presence of high environmental concentrations of IL-12 induced by an infectious agent. Indeed, the development of some of the manifestations of autoimmunity (elevated IL-5, IL-13, polyclonal B-cell activation) observed in OX40L transgenic mice may represent a pathogenic manifestation of this normally protective response.21 Blocking OX40/OX40L interactions may represent an important therapeutic approach for the treatment of autoimmune disease,29 while the enhancement of OX40 signalling by agonistic antibodies or soluble OX40L has been shown to break tolerance,30 enhance T-cell-mediated immunity to tumours and overcome the suppressive effects of regulatory T cells.31 The potential contribution of OX40L expressed on T cells to OX40/OX40L interactions must be considered in the future design of these therapeutic protocols.
Abbreviations
- APC
antigen-presenting cell
- c.p.m.
counts per minute
- –/–
deficient
- IFA
incomplete Freund's adjuvant
- IL-12
interleukin-12
- MOG
myelin oligodendrocyte glycoprotein
- NIAI
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- OVA
ovalbumin
- OX40L
OX40 ligand
- PBS
phosphate-buffered saline
- TCR
T-cell receptor
- TdR
thymidine
- TdS
T-depleted spleen cells
- Th
T helper
- TNFR
tumour necrosis factor receptor
References
- 1.Lenschow DJ, Walunas TJ, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Gramaglia I, Weinberg AD, Lemon M, Croft M. OX40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–17. [PubMed] [Google Scholar]
- 3.Akiba H, Oshima H, Takeda K, et al. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–66. [PubMed] [Google Scholar]
- 4.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen presenting cell function in mice lacking expression of OX40L. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–48. [PubMed] [Google Scholar]
- 6.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–95. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft M. Co-stimulatory members of the TNFR family: keys to effective T cell immunity. Nat Rev Immunol. 2003;3:609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 8.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 9.Ohshima Y, Tang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naïve human CD4+ T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 10.Flynn S, Toellner KM, Rykundalia C, Goodall MP, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 12.Baum PR, Gayle RP, Ramsdell F, et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takasawa N, Ishii N, Higashimura N, Murata K, Tanaka Y, Nakamura M, Sasaki T, Sugamura K. 2001. Expression of gp34 (OX40 ligand) and OX40 on human T cell clones. Jpn J Cancer Res. 2001;92:377–82. doi: 10.1111/j.1349-7006.2001.tb01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pippig SD, Pena-Rossi C, Long J, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–9. [PubMed] [Google Scholar]
- 15.Kim MY, Bekiaris V, McConnell FM, Gaspal FMC, Raykundalia C, Lane PJL. OX40 signals during priming on dendritic cells inhibit CD4 T cell proliferation. IL-4 switches off OX40 signals enabling rapid proliferation of Th2 effectors. J Immunol. 2005;174:1433–7. doi: 10.4049/jimmunol.174.3.1433. [DOI] [PubMed] [Google Scholar]
- 16.Mendel I, Natarajan K, Ben-Nun A, Shevach EM. A novel protective model against experimental allergic encephalomyelitis in mice expressing a transgenic TCR-specific for myelin oligodendrocyte glycoprotein. J Neuroimmunol. 2004;149:10–21. doi: 10.1016/j.jneuroim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Mendel I, Shevach EM. The IL-10-producing competence of Th2 cells generated in vitro is IL-4 dependent. Eur J Immunol. 2002;32:3216–24. doi: 10.1002/1521-4141(200211)32:11<3216::AID-IMMU3216>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Smeltz RB, Chen J, Ehrhardt R, Shevach EM. Role of IFN-γ in Th2 differentiation. IFN-γ regulates IL-18Rα expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor β2 expression. J Immunol. 2002;168:6165–72. doi: 10.4049/jimmunol.168.12.6165. [DOI] [PubMed] [Google Scholar]
- 19.Murata K, Nose M, Ndhlovu LC, Sato T, Sugamura K, Ishii T. Constitutive OX40/OX4O ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–36. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- 20.Shah JA, Darrah PA, Ambrozak DR, et al. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J Exp Med. 2003;198:281–91. doi: 10.1084/jem.20030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii N, Ndhlovu LC, Murata K, Sato T, Kamanaka M, Sugamura K. OX40 (CD134) and OX40 ligand interaction plays an adjuvant role during in vivo Th2 responses. Eur J Immunol. 2003;33:2372–81. doi: 10.1002/eji.200324031. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX−40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–26. [PubMed] [Google Scholar]
- 23.Higgins LM, McDonald SA, Whittle N, Crockett L, Shields JG, MacDonald TT. Regulation of T cell activation in vitro and in vivo by targeting the OX40–OX40 ligand interaction: amelioration of ongoing inflammatory bowel diseases with OX40-IgG fusion protein, but not with OX40 ligand-IgG fusion protein. J Immunol. 1999;162:486–93. [PubMed] [Google Scholar]
- 24.Jember A, Zuberi GR, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the co-stimulatory receptor OX40. J Exp Med. 2001;193:387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salek-Arkadani S, Song J, Halteman BS, Jember AS, Akiba H, Yagita H, Croft M. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiba H, Miyahira Y, Atsuta M, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental Leishmaniasis 2000. J Exp Med. 2000;191:375–80. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33:861–9. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 28.Baba E, Takahashi Y, Lichtenfeld J, Tanaka R, Yoshida A, Sugamura K, Yamamoto N, Tanaka Y. Functional CD4 T cells after intercellular molecular transfer of OX40 ligand. J Immunol. 2001;167:875–83. doi: 10.4049/jimmunol.167.2.875. [DOI] [PubMed] [Google Scholar]
- 29.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX-40. Nat Rev Immunol. 2004;4:420–31. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 30.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–43. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 31.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40–OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–9. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]