Abstract
The presence of interleukin-4 (IL-4) during the generation of dendritic cells (DC) from precursor cells results in measurable increases of IL-12 in supernatants but IL-4 secretion has not been reported. However, DC have IL-4 receptors and are able to make IL-4. We therefore sought evidence for autocrine effects of IL-4 on DC. IL-4 gene expression was low in DC generated from bone-marrow stem cells in the presence of granulocyte–macrophage colony-stimulating factor but was up-regulated by exposure of the developing DC to IL-4. Exposure to IL-4 also induced intracellular IL-4 production in DC. The intracellular IL-4 induced in the presence of IL-4 was increased following further DC maturation with tumour necrosis factor-α. By contrast, in supernatants of DC, IL-4 was rarely detected and only at late culture periods. However, after exposure of DC to IL-4, cell-bound IL-4 was detected transiently, which suggested binding and internalization of the cytokine. Binding via IL-4 receptor-α was indicated from phosphorylation of the signal transducer and activator of transcription (STAT) protein 6, which is known to mediate IL-4 function. Cytokine persisting within the supernatants of the cells may therefore be unrepresentative of the actual production and function of IL-4 in the cells; IL-4 may be produced in DC in response to exposure to IL-4 but may then be lost from the supernatants during cell binding and activation of the cells.
Keywords: autocrine effects, cytokines, dendritic cells, immunity type 1 and type 2
Introduction
Early in primary immune responses dendritic cells (DC) are the major cells contributing interleukin-12 (IL-12) production and levels of IL-12 produced in DC are thought to influence the development of T helper 1 (Th1) responses.1 DC can bias responses towards Th1 or Th2. The terms DC1 and DC2, respectively, for cells stimulating these types of immunity were initially coined. This distinction between cells types was thought to depend on the balance between IL-12 and IL-10 production within the DC.2 Some reports suggest that the myeloid and lymphoid subpopulations of DC may be associated with either Th1 or Th2 cytokine profiles.3–5 However, individual populations of DC may produce multiple cytokines and switch between cytokine profiles. Such changes in cytokine profiles in DC may be based on their exposure during development to cytokines,6,7 or to antigens.8–19
DC not only produce IL-12 during development but also express IL-12 receptors.7,20 Exposure to IL-12 causes activation through the IL-12 receptor via a nuclear factor-κB pathway that results in the production of more IL-12,7 up-regulation of the costimulatory molecule CD80 and an increased capacity to stimulate T-cell proliferation.6 Similarly, there is an autocrine loop promoting production of IL-10.21 DC can be switched from producing IL-12 to producing IL-4 after exposure to the immunosuppressive murine retrovirus, Rauscher leukaemia virus.8 Exposure of DC to fungal hyphae can also induce IL-4 production in DC.13 Many studies have examined the cytokines produced by lymphocytes following stimulation by DC. For example, so-called ‘exhausted’ DC, which are produced following an extended culture period, induce a Th2-biased T-cell stimulation22 and switching between DC stimulating Th1-type and Th2-type lymphocyte responses has been reported.23,24 A temporal progression of cytokine profiles in DC used to stimulate lymphocytes, moving possibly from an early Th1-promoting to a later Th2-promoting profile, may underlie these reported knock-on effects in lymphocyte stimulation. However, there may also be a direct relationship between the presence of IL-4 during the maturation of the DC and the production of IL-4 in T cells activated by these DC.25
The influence of cytokines in the DC growth medium may be profound. The effect on later lymphocyte stimulation of the IL-4 used in combination with granulocyte–macrophage colony-stimulating factor (GM-CSF) for the purification of so-called ‘immature’ DC is not clear, although IL-12 is increased in the supernatants of DC by this treatment and it may down-regulate IL-10 production.24,26,27 Here we examined whether IL-4 acting via IL-4 receptors expressed on developing DC might influence the IL-4 production within the DC themselves. We postulated that exposure to IL-4 during maturation might favour Th2 cytokine production in DC, promoting the later, Th2-biased stimulation of lymphocytes. IL-4 produced in DC exposed to retrovirus or to fungal hyphae was not reported in the supernatants of DC by enzyme-linked immunosorbent assay (ELISA); it was seen when intracellular protein was measured using flow cytometry8 or enzyme-linked immunoSPOT (ELISPOT) assays.13 It therefore seemed possible that DC exposed to IL-4 might be induced to produce IL-4 that remained difficult to detect because it was not measurable as secreted cytokine. Our studies indicate that IL-4 does promote the production of IL-4 within DC but the IL-4 was rarely detected in a secreted form because it binds to and activates the DC in an autocrine fashion. Such influences of the cytokine milieu during maturation of antigen-presenting DC may then contribute to the reported changes in lymphocyte activity stimulated by these DC. The use of IL-4 for the generation of DC to be used therapeutically may therefore be inappropriate if the aim is to induce long-term Th1 responses preferentially in lymphocytes.
Materials and methods
Animals
Female BALB/c and CBA mice between 4 and 6 weeks old were obtained from Harlow Co. (Bicester, UK) and housed in the Specific Pathogen Free Unit at Northwick Park Institute for Medical Research. BALB/c IL-4 knockout mice were a gift from Dr Mueller (Department of Immunology, St Mary's Hospital Medical School, London, UK).
Isolation of dendritic cells from bone marrow
Dendritic cells were derived from bone marrow precursors as previously described.6,8,28 Briefly, bone marrow cells from mouse femurs were flushed into complete medium and washed once. The single cell suspension was overlaid onto Lympholyte M (Cedarlane, Ontario, Canada) and spun at 1200 g for 30 min, at room temperature. The mononuclear cells were isolated from the interface and resuspended at a concentration of 1 × 106 cells/ml in complete culture medium supplemented with GM-CSF (100 U/ml) with or without IL-4 (1–20 ng/ml). At day 3 of culture, the non-adherent cells were either overlaid onto 2 ml of metrizamide (5 ml, analytical grade 13·7% w/v; Nygaard, Oslo, Norway; and centrifuged at 600 g for 10 min at room temperature to separate DC), or replaced in the original tissue culture flask with complete medium supplemented with GM-GSF with or without tumour necrosis factor-α (TNF-α; 50 U/ml). After 5–13 days in culture, the non-adherent cells were centrifuged on metrizamide, as described above. Interface cells were counted in Trypan blue; their viability was over 95% and using light scatter and phenotype they were found to be < 95% DC, as previously described.6,8
Primary proliferative responses
Varying numbers of DC (500, 1000, 2000 DC/well) generated in the presence of different cytokines were cultured with 25 × 103 to 100 × 103 allogeneic lymph node T cells in triplicate 20-μl hanging drops in Terasaki plates. Plates were inverted and cultured for 3 or 4 days over sterile saline in plastic boxes at 37°. Each hanging drop then received 1 μl [3H]thymidine (2 Ci/mm equivalent to 1 μg thymidine/ml; Amersham International, Amersham, UK) and after 2 hr at 37° they were blotted onto filter discs, washed with saline, trichloroacetic acid (5%) and methanol and counted in a scintillation counter.29
Flow cytometry
For surface labelling, the DC were incubated on ice with antibodies to mouse I-ak[mouse immunoglobulin G2ab (IgG2ab)] or CD11b (rat IgG2b) that were directly conjugated to fluorescein isothiocyanate (FITC). Phycoerythrin (PE)-conjugated antibodies employed were CD80 (hamster IgG) and CD40 (rat IgG2a). Biotinylated antibodies were CD11c (hamster IgG), CD86 (rat IgG), IL-4 (rat IgG1), IL-10 (IgM) and IL-12 (rat IgG2a). Pharmingen (San Diego, CA) supplied all the antibodies. Streptavidin-peridinine chlorophyll protein (PerCP; Becton Dickinson, Mountain View, CA) was used to label the biotinylated antibodies. Two per cent fetal calf serum (FCS) in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline with 1 mm ethylene diaminetetraacetic acid and 0·02% sodium azide) was used to prevent non-specific antibody binding. FITC-conjugated and biotinylated monoclonal antibodies were added to the DC and the samples were left on ice for 30 min. The cells were then washed twice in FACS buffer and streptavidin-PerCP was added. After 20 min these samples were washed twice in FACS buffer and 500 μl of 1% paraformaldehyde was added for overnight fixation. Fluorescence profiles were generated on a flow cytometer (Becton Dickinson). Histogram analysis was produced by Verity's Winlist (version 4·0) software package and enhanced normalized subtraction was used to determine the percentage of positive cells. Viable DC were selected after gating on forward and side scatter with dead cells excluded by propidium iodide staining.
Flow cytometry for measuring intracellular cytokines
Monensin at a concentration of 3 μm was added to 5 × 105 to 5 × 106 DC to ensure intracellular cytokine retention. The DC/monensin preparation was incubated for 6 hr at 37°. The cells were then washed twice in FACS buffer containing 2% FCS. Cytoperm A (Serotec, Oxford, UK) was added and the cells were kept at room temperature for 15 min for fixing and then washed again in FACS buffer with 2% FCS. Cells were then exposed to permeabilizing agent, Cytoperm B (Serotec), at room temperature for 15 min. The cells were then labelled at room temperature for intracellular cytokines IL-4, IL-10 and IL-12. Cells were washed twice in FACS buffer and then fixed in 1% paraformaldehyde for overnight fixation.
Western blot analysis
Bone-marrow-derived DC were cultured in various doses of IL-4 for 3 days then re-stimulated with the same dose of IL-4 for 15 min in complete medium at 37°. The cells were then washed with cold phosphate-buffered saline. Cells were lysed in Triton-X buffer containing a protein inhibitor cocktail (Sigma, Poole, Dorset, UK) for 30 min on ice and centrifuged to remove cell debris. The total protein content of the lysates was determined by the Bio-Rad protein assay. Lysates were run on an 8% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gel (200 V, 40 min) and proteins were transferred onto a nitrocellulose membrane using a semidry transfer blot system. Non-fat dry milk (0·5% Marvel) was used to prevent non-specific binding and membranes were probed with antibody overnight at 4°. Polyclonal anti-Stat 6 (M20) was purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA and anti-phospho-Stat 6 was from Upstate Biotechnology, Cambridge, UK. Bands were visualized using alkaline phosphatase staining.
RNA isolation and cDNA synthesis
Total ribonucleic acid (RNA) was isolated using TRIzol Reagent (Life Technologies, Paisley, UK) according to the manufacturer's protocol. Each reaction tube contained total RNA (2 μg) and was reverse transcribed using a Superscript™ Preamplification System. First-strand copy deoxyribonucleic acid (cDNA) synthesis was carried out either in the presence of reverse transcriptase (+ RT) cDNA or without the enzyme (– RT control) according to the manufacturer's protocols.
Reverse transcription–polymerase chain reaction (RT-PCR) (conventional)
The cDNA (20 μl) was amplified as previously described using the following primers:30 β-actin sense GGACTCCTATGTGGGTGACGAGG, antisense GGGAGAGCATAGCCCTCGTAGAT; and IL-4 sense GAGCCATATCCACGGATGCGACAA, antisense CATGGTGGCTCAGTACTACGAGTA.
RT-PCR (Taqman)
Taqman probes and primers for the quantitative detection of the target messenger RNA (mRNA) were designed using computer software Primer Express (PE Biosystems, Warrington, Cheshire, UK). A Taqman PCR core reagent kit was used to prepare a Master Mix for each target gene according to the manufacturer's protocols. The reaction was carried out on an ABI PRISM 7700 sequence detector programmed for the initial stem of 2 min at 50° and 10 min at 95°, followed by 40 thermal cycles of 15 seconds at 95° and 1 min at 60°. Each measurement was performed in duplicate.
IL-4 surface receptor binding assay
DC were cultured for 5 days in a combination of cytokines: GM-CSF only, GM-CSF + IL-4 (continuous), GM-CSF (continuous) + IL-4 + TNF-α (IL-4 for first 3 days, TNF-α for last 2 days). Cells at 1 × 106/ml were incubated in medium containing 20 ng/ml IL-4 at 37° for various times after which the cells were chilled to 4° for 30 min. Cells were washed with ice-cold FACS buffer to remove unbound ligand and then incubated with anti-IL-4 antibody (10 μg/ml) for 30 min at 4°. Free antibody was removed by washing twice in FACS buffer and cells were fixed in 1% paraformaldehyde.
Results
Generation of DC
As previously described,6,8 purified DC expressed CD11c, major histocompatibility complex (MHC) class II, costimulatory molecules CD80, CD86 and CD40, stimulated primary allogeneic mixed leukocyte reactions and showed morphology under light and electron microscopy that was characteristic of DC. Cells for molecular studies were used after 6 or more days of culture because pure DC were obtained on the second metrizamide gradient after this culture period. Changes induced in the DC populations by exposure to different doses of IL-4 during the first 3 days of culture were assessed.
Phenotype and function of IL-4-treated DC
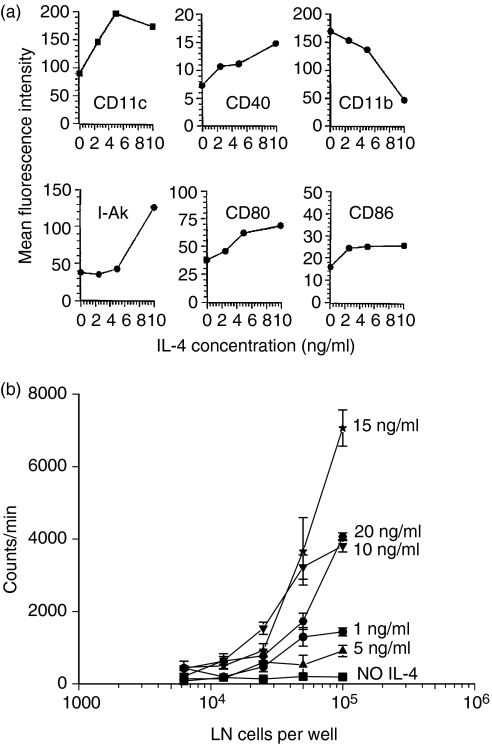
DC generated in the presence of IL-4 showed an increase in levels of CD11c and MHC class II expression and a small increase in the expression of the costimulatory molecules CD40, CD80 and CD86. A down-regulation of CD11b was seen (Fig. 1a). Figure 1(b) shows the effects of responder cell concentration in culture in a mixed leukocyte reaction31 and the effects of exposure of the stimulating DC to IL-4 at doses of 1–20 ng/ml. DC exposed to low doses (1 and 5 ng/ml) during development stimulated low levels of primary allogeneic T-cell proliferation. Stimulation was usually optimal after treatment with 10–15 ng of IL-4 given during the first 3 days of culture (e.g. Fig. 1a). With high doses of IL-4 (> 15 ng/ml) there was a reduction in the capacity of these cells to stimulate T-cell proliferation. At 20 ng/ml, the IL-4 also killed a proportion of the DC as previously reported.5
Figure 1.
Effects of IL-4 on phenotype and stimulatory function of dendritic cells. (a) Bone marrow-derived dendritic cells were grown in GM-CSF and different doses of IL-4 for 8 days and surface phenotype was studied by flow cytometry. The changes in mean fluorescence intensity in response to IL-4 are shown. Increased CD11c and IAk and reduced CD11b were the most marked changes.
IL-4 gene expression
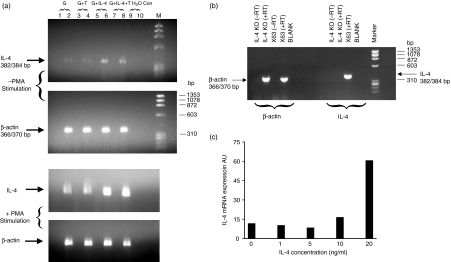
The expression of IL-4 mRNA in DC derived in the presence of different cytokines in the growth medium was examined. Some cultures were stimulated with phorbol ester. Using RT-PCR, IL-4 gene expression was detected in only one of three experiments in non-stimulated cells but was identified in all the phorbol 12-myristate 13-acetate (PMA)-stimulated samples. The mRNA levels were greatest in the cells grown in the presence of IL-4 (Fig. 2a). The IL-4-transfected cell line X63 was used as a positive control for IL-4 mRNA whereas PMA stimulation of cells from bone-marrow-derived DC from IL-4 knockout mice and cultured in GM-CSF + IL-4 for 8 days acted as a negative control. Although the IL-4 and β-actin PCR products are similar in size, they can be differentiated on a 2% agarose gel (Fig. 2b). The presence of IL-4 gene expression in non-stimulated DC was confirmed using a Taqman technique, which also permitted quantification of the IL-4 gene expression. IL-4 mRNA was normalized to 18s ribosomal RNA; mRNA levels were greatest in the cells grown in the presence of the higher doses of IL-4.
Figure 2.
IL-4 gene expression in dendritic cells. (a) Reverse transcriptase (RT)-PCR analysis of IL-4 mRNA was performed on DC grown in GM-CSF, GM-CSF + TNF (G + T), GM-CSF + IL-4 (10 ng/ml) or GM-CSF + IL-4 + TNF for 8 days. Cells were cultured with or without PMA (10 ng/ml) and ionomycin (1 μg/ml) for 2 hr and cell lysates were subjected to RT-PCR. Lanes 2, 4, 6 and 8 were RT positive. Lanes 1, 3, 5 and 7 were RT negative and β-actin primers were used as controls. mRNA for IL-4 but not β-actin was up-regulated upon PMA stimulation. All samples showed IL-4 gene expression but this was greater in cells exposed to IL-4.
Intracellular cytokine production
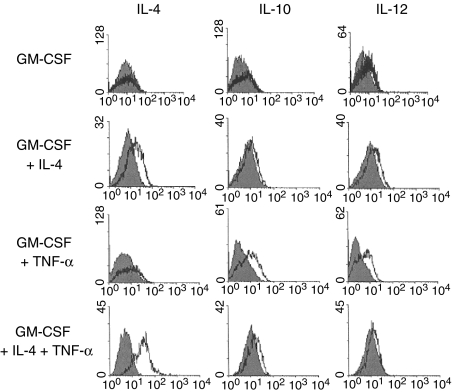
Figure 3 shows the intracellular cytokine production for IL-4, IL-10 and IL-12. Previous studies had already established the conditions for the use of monensin to block export of cytokine and had shown that this concentration of monensin did not, itself, change the cellular labelling. In addition, blocking experiments using excess IL-4, IL-10 or IL-12 showed the specificity of the intracellular labelling with each of these antibodies as previously reported.32 DC developing from bone marrow stem cells in the presence of GM-CSF only, showed little evidence of cytokine production but a hint of IL-10 and IL-12 was seen (Fig. 3). When DC were matured further with TNF-α a high proportion of the cells produced IL-10 and IL-12. On the basis of the high proportion of IL-12 and IL-10-producing cells in some experiments, individual cells must be producing both cytokines as previously described.8 When GM-CSF was supplemented with IL-4 during DC development, IL-4 production was seen in the cells. When TNF-α was given in addition to GM-CSF, there was IL-10 and IL-12 production but little detectable IL-4 (Fig. 3). However, when TNF-α plus IL-4 was used, a high proportion of cells was shown to produce IL-4. In parallel, lower levels of IL-10 and IL-12 production were indicated (Fig. 3). Little or no staining was detected for IL-4 with a directly conjugated antibody (not shown) but staining was only evident with an amplification step provided by using a biotinylated antibody followed by a streptavidin-conjugated fluorochrome. Previous experiments had shown a variation in the levels of IL-12 production in bone-marrow-derived DC and that IL-12 also inhibits the production of IL-48 so some variability in IL-4 production was anticipated. IL-4 was detected in five of 12 experiments and the results in Fig. 3 are representative of the five positive experiments. The percentage of IL-4-positive cells ranged from 18% to 70% (mean 40 ± 9·2). Since the identification of cytokines depended on the presence of monensin to block the IL-4 export from the Golgi, the cytokine was being produced within the DC rather than being acquired from the cytokine added earlier in the culture period. As a further check on the specificity of the IL-4 labelling, IL-4 knockout mice were studied. DC derived from knockout mouse bone marrow cultured with GM-CSF plus 5 or 10 ng/ml IL-4 showed no labelling for IL-4 whereas DC from normal animals studied in parallel showed 18–24% IL-4-positive cells.
Figure 3.
Intracellular cytokine production. Dendritic cells were derived in GM-CSF or GM-CSF + TNF-α for 10 days and some cultures were supplemented with IL-4 (10 ng/ml). The cells were cultured for 6 hr with or without monensin that blocked cytokine release. Shaded histograms are cultures without monensin and the overlay shows those with monensin. The addition of IL-4 promoted IL-4 production.
IL-4 measurement by ELISA
As reported in our previous studies,8 both IL-12 and IL-10 were seen in the supernatants of DC developing from bone marrow stem cells on days 3–10. However, IL-4 was not found in the supernatants of DC at these times, even when high amounts of intracellular IL-4 were detected. At later times during culture (12–13 days) IL-4 was measured intermittently in supernatants from DC that had been exposed to 10 ng or more of IL-4 during maturation. The highest amount was seen using maturation with 10 ng/ml IL-4 for the first 3 days of culture. When the cells were washed and incubated in fresh medium, on day 13, <200 pg/ml was secreted by 105 cells in 24 hr. No IL-4 was detected in the supernatants of cells that had been matured in the absence of IL-4.
Surface binding of IL-4
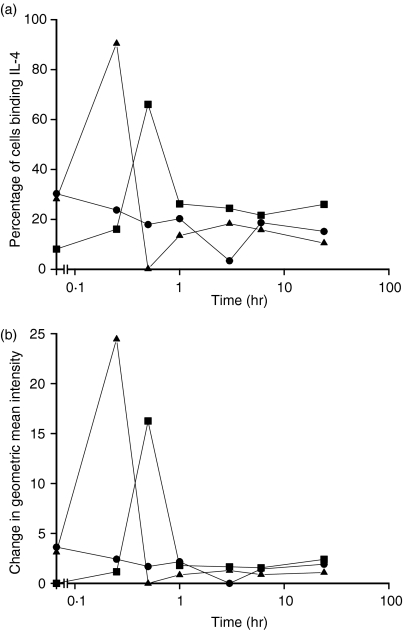
The IL-4 detected in the supernatants by ELISA did not correlate with that shown intracellularly. DC express IL-4 receptor and for other cell types binding and internalization of IL-4 had been described which influenced the amounts detectable in the supernatant.33–35 The binding and possible internalization of IL-4 was measured following exposure of DC to 20 ng/ml of IL-4 at 37° for different times and then probing for surface-bound IL-4 with specific antibodies by flow cytometry. Cells were grown initially in GM-CSF with or without IL-4 present for the first 3 days. On day 5, 20 ng/ml of IL-4 was then added for different periods of culture. There were rapidly increasing levels of bound IL-4 identified on the cell surface that then disappeared from the cell surface (Fig. 4a,Fig. 4b). These effects were reflected both in the numbers of cells which bound IL-4 (Fig. 4a) and in the level of binding (Fig. 4b).
Figure 4.
Fate of surface-bound IL-4 after incubation with IL-4 at 37°. DC cultured for 5 days in different combinations of cytokines were incubated in medium containing 20 ng/ml IL-4 at 37° for various times. The cells were cooled to 4° for 30 min. Surface-receptor-bound IL-4 was probed with an anti-IL-4 antibody. Data in
Activation of signal transducer and activator of transcription (STAT)-6
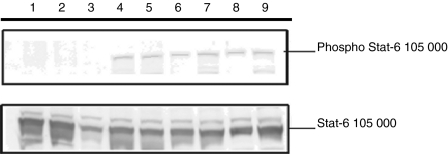
Cellular binding of IL-4 was observed and evidence for activation of the IL-4 receptor was therefore sought. Cellular activation by IL-4 uses the STAT-6 signalling pathway through engagement of the IL-4 receptor α (Rα). DC developing from bone marrow stem cells were exposed to IL-4 and phosphorylation of STAT-6 was examined. Fifteen minutes following exposure to IL-4 significant phosphorylation of STAT-6 was observed (Fig. 5), suggesting that activation of the cells was occurring through binding of IL-4 to the IL-4Rα.
Figure 5.
Induction of STAT-6 phosphorylation by IL-4. Dendritic cells derived in GM-CSF plus a range of IL-4 concentrations (0–20 ng/ml) were re-stimulated with IL-4 20 min prior to harvesting the cells. Cell lysates were prepared, normalized for protein level and resolved by 10% SDS–PAGE followed by Western immunoblotting and staining with anti-STAT-6 or anti-phospho-STAT-6 antibodies. Lanes 4–9 show cells re-stimulated with 0·5, 2, 5, 10, 20 and 40 ng/ml of IL-4, respectively, and lanes 1–3, which were negative for phosphorylated product, were controls without re-stimulation with IL-4 from cells grown in GM-CSF, GM-CSF + TNF-α or GM-CSF plus 10 ng/ml of IL-4.
Discussion
Production of IL-4 within DC was stimulated by exposure of DC to IL-4 during their development from bone marrow stem cells. IL-4 gene expression was increased and intracellular protein was detected. Thus, exposure to IL-4, even during the early stages of development of DC, may influence the production of cytokines in those cells and lead to the production of this Th2-polarizing cytokine within the DC. The change from IL-12 to IL-4 production previously reported in lymphocytes that had been stimulated by DC grown in the presence of IL-422 may be explained by these changes in the cytokines in the DC themselves that are caused by their early cytokine milieu. The immunoregulatory effects and IL-4 production by DC engineered to express IL-4, despite differential changes in type 1 cytokines, may be promoted by the autocrine promotion of IL-4 production in DC in the amelioration of inflammatory arthritis.36 It will also be of interest to check whether parasite-induced switches from type 1 to type 2 responses in T cells could also be mediated at the level of changes in IL-4 expression in the DC.37,38
In the previous studies of IL-4 production in DC, the protein was identified within the cells using either intracellular cytokine staining by flow cytometry8 or ELISPOT assays.13 Thus, the absence of IL-4 in the supernatants of DC after stimulation of a type 2, parasite-induced response may not necessarily indicate that the DC themselves are not contributing IL-4.38 However, when we took DC derived in different cytokine milieu and looked for IL-4 mRNA, there was only occasional evidence that this was present. To check the capacity of DC to express the mRNA for IL-4 we therefore added the non-specific stimulus of PMA to the cells. Stimulation with PMA induced IL-4 gene expression, showing the capacity of these cells to produce the message for this cytokine. Higher IL-4 mRNA levels were seen in cells exposed to IL-4. These results suggested that the IL-4 gene expression may be low in the DC, or alternatively that our studies may not have looked at the appropriate times in culture. Low levels of IL-4 mRNA were detected in DC without the addition of the non-specific stimulant when the more sensitive Taqman technology was applied, and up-regulation on exposure to IL-4 was observed. Low levels of IL-4 gene expression may thus be present in DC derived in different cytokine milieus but up-regulation can occur on stimulation with IL-4. There may be other cytokines by which up-regulation of IL-4 production can be stimulated because addition of IL-3 to the growth medium also increased the levels of IL-4 mRNA (data not shown). There was no evidence of T-cell contamination in these cultures of metrizamide-separated cells.
The earlier studies of IL-12 have shown an IL-12 autocrine/paracrine pathway whereby IL-12 elicited in DC promotes the production of further IL-12 and consequent changes in stimulatory ability of the DC. The current work shows that a similar autocrine/paracrine loop exists for the production and promotion of IL-4. In our study IL-12 p40 levels were reduced in the presence of IL-4 as indicated by the level of intracellular cytokine staining although, in agreement with published work, the IL-4 treatment resulted in measurement of more secreted IL-12 (not shown). These discrepancies between intracellular labelling and secreted protein could result from different timing of the changes in mRNA production and secretion of the protein or reflect, in part, changes in the binding of IL-12 to receptors on the surface of the cells. There was variability in the levels of expression of cytokines between experiments and this variability for IL-4, where detection of the protein required an amplification step, meant that the protein was not detected in half the experiments. Expression may be sensitive to small differences in culture conditions. IL-12 treatment of mice resulted in an increased propensity of DC derived from stem cells of those mice to induce cell-mediated immune responses,28 suggesting long-lived influence acting by a paracrine effect at the level of stem cell precursors. IL-12 also reduced the capacity of DC to produce IL-4.8 Given the effects of many antigens in promoting the production of IL-12 or IL-4 in DC small differences in the exposure of the mice to environmental antigens may contribute to the variability in cytokine production by bone-marrow-derived DC in different experiments.
Additional factors may be influencing the outcome of IL-4 treatment on DC with regard to their knock-on effect on lymphocytes. The ratio of the stimulator DC to responding lymphocyte numbers may influence the balance between Th1 and Th2 profiles directed by the DC.23 However, the importance of IL-4 itself in DC in promoting Th2 responses in vivo is illustrated from the use of the DC cell line X5106; small numbers of these cells containing an IL-4 plasmid stimulated Th2 responses with high IgE and Ig2a responses.39 Furthermore, DC exposed to IL-4 during their differentiation from bone marrow can be relatively poor stimulators of allogeneic cytotoxic T cells even after treatment with interferon-γ.40 The IL-4-treated DC also selectively enhanced the differentiation of IL-4-producing Th2 cells41 and led to the proposal that development of DC in the presence of IL-12 would be preferable for induction of Th1-type responses in T cells. Our earlier results6 and those presented here support this contention and provide direct evidence that the effect of the IL-4 on DC and on the subsequent polarization of T cells may initially be by the promotion of IL-4 production in those DC.
The presence of IL-4 receptors on DC and their stem cells20 provided circumstantial evidence that IL-4 would have a direct effect on developing DC. Exposure of bone marrow stem cells to IL-4 has been routinely used to produce ‘immature’ DC and to remove macrophages from the cultures.42 We confirm the presence of STAT-6 in DC.43,44 The evidence for activation of the STAT-6 signalling pathway by IL-4 suggests that the IL-4 does have a direct effect on DC. Activation is probably via IL-4Rα because STAT-6 activation has only been identified through this receptor,45 although activation by pathways independent of STAT-6 could also be operating. DC are the early producers of IL-12 and are involved in the polarization of stimulated T-cell responses towards the Th1 pathway.1 However, because IL-4 has not been thought of as a DC product, it was previously believed that the main producers of IL-4 during the development of Th2 responses were the T cells, although a source for the early IL-4-producing cells has been sought.46 The observations that DC are capable of producing IL-4 in response to exposure to immunosuppressive retrovirus or to fungal hyphae gave the first indications that DC could also be providing the initial polarizing cytokine for the development of Th2 responses. The combination of the presence of IL-4 receptors on DC and the capacity to produce IL-4 strongly suggested autocrine or paracrine pathways for IL-4 activity.
Many studies fail to identify IL-4 production in DC and the reasons for this failure appear to be that mRNA levels are low in these cells unless they are stimulated and that the IL-4 is rarely detected as secreted protein in the medium. It may be that IL-4 is secreted but also taken up as rapidly because DC do express IL-4 receptors during maturation. Binding of the IL-4 to DC followed by loss of surface binding provides evidence that IL-4 is rapidly bound and then internalized by DC, as has been described for T cells.33–35 IL-10 and IL-12 can be identified in the supernatants of the developing DC on days 3–10 in culture but not at later culture times. By contrast, the IL-4, when detected, was present at later times and in occasional experiments using DC derived from mouse spleen (not shown). The loss of IL-12 in the continued presence of IL-4 production may underlie the switch from stimulation of Th1 cells to stimulation of Th2 cells that occurs with maturing DC.22 The supernatant material might suggest that DC initially produce Th1 promoting cytokines and later switch to Th2 cytokine production when they have been exposed to IL-4 during development. Our studies provide some support for this view although the intracellular identification of IL-4 at day 8 of culture suggests that production of this protein can occur earlier but is not present in the supernatants. This fact may lead to the belief that DC developing in the presence of IL-4 produce, preferentially, IL-12 whereas the reality is that they may additionally be switching towards production of Th2 cytokines in response to their early exposure to IL-4. The influence of the early cytokine exposure may thus be influential in determining the ‘default’ cytokine profiles in DC that may then be modulated by later antigenic exposure. The idea that DC derived in the presence of IL-4 are a good source of stimulation for Th1 responses may be true but only in the short term: a bias towards Th2 may be the – possibly unwanted – longer term outcome.
Abbreviations
- cDNA
copy deoxyribonucleic acid
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- ELISPOT
enzyme-linked immuno-SPOT
- FACS
fluorescence-activated cell sorter
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL
interleukin
- mRNA
messenger riboneucleic acid
- PCR
polymerase chain reaction
- PMA
phorbol 12-myristate 13-acetate
- RNA
ribonucleic acid
- Rα
receptor alpha
- RT
reverse transcriptase
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis;
- STAT
signal transducer and activator of transcription
- Th1
T helper 1
- Th2
T helper 2
- TNF-α
tumour necrosis factor-α
References
- 1.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 2.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 3.Pulendran B, Smith JL, Caspary G, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8alpha+ and CD8alpha– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 6.Kelleher P, Knight SC. IL-12 increases CD80 expression and the stimulatory capacity of bone marrow-derived dendritic cells. Int Immunol. 1998;10:749–55. doi: 10.1093/intimm/10.6.749. [DOI] [PubMed] [Google Scholar]
- 7.Grohmann U, Belladonna ML, Bianchi R, et al. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity. 1998;9:315–23. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 8.Kelleher P, Maroof A, Knight SC. Retrovirally induced switch from production of IL-12 to IL-4 in dendritic cells. Eur J Immunol. 1999;29:2309–18. doi: 10.1002/(SICI)1521-4141(199907)29:07<2309::AID-IMMU2309>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Gorak PM, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–95. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, Knight SC. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur J Immunol. 1999;29:1803–11. doi: 10.1002/(SICI)1521-4141(199906)29:06<1803::AID-IMMU1803>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Reis e Sousa C, Yap G, Oliver S, et al. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–47. doi: 10.1016/s1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- 12.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic IL-12, while Gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–6. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.d'Ostiani CF, Del Sero G, Bacci A, et al. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–74. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 15.Martino A, Sacchi A, Sanarico N, et al. Dendritic cells derived from BCG-infected precursors induce Th2-like immune response. J Leukoc Biol. 2004;76:827–34. doi: 10.1189/jlb.0703313. [DOI] [PubMed] [Google Scholar]
- 16.Monti P, Leone BE, Zerbi A, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol. 2004;172:7341–9. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- 17.Pulendran B. Modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol Res. 2004;29:187–96. doi: 10.1385/IR:29:1-3:187. [DOI] [PubMed] [Google Scholar]
- 18.Didierlaurent A, Ferrero I, Otten LA, et al. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172:6922–30. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 19.Ho LJ, Shaio MF, Chang DM, Liao CL, Lai JH. Infection of human dendritic cells by dengue virus activates and primes T cells towards Th0-like phenotype producing both Th1 and Th2 cytokines. Immunol Invest. 2004;33:423–37. doi: 10.1081/imm-200038680. [DOI] [PubMed] [Google Scholar]
- 20.Fisher GM, Iqball S, Knight SC. Gene expression during differentiation of human dendritic cells from cord blood CD34 stem cells. Cytokine. 1999;11:111–17. doi: 10.1006/cyto.1998.0403. [DOI] [PubMed] [Google Scholar]
- 21.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–18. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 22.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation. impact on priming of Th1, Th2 and nonpolarized T cells. Nature Immunol. 2000;1:311–16. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Demeure CE, Rubio M, Delespesse G, Sarfati M. Human monocyte-derived dendritic cells induce naive T cell differentiation into T helper cell type 2 (Th2) or Th1/Th2 effectors. Role of stimulator/responder ratio. J Exp Med. 2000;192:405–12. doi: 10.1084/jem.192.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalinski P, Smits HH, Schuitemaker JH, et al. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J Immunol. 2000;165:1877–81. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 25.Feili-Hariri M, Falkner DH, Morel PA. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J LeukocBiol. 2005;78:656–64. doi: 10.1189/jlb.1104631. [DOI] [PubMed] [Google Scholar]
- 26.Ebner S, Ratzinger G, Krosbacher B, et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J Immunol. 2001;166:633–41. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- 27.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelleher P, Williams NJ, Knight SC. Interleukin-12 administration in retroviral infection of mice increases the potential to produce functional dendritic cells from bone marrow stem cells. Immunol Lett. 1999;65:51–4. doi: 10.1016/s0165-2478(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 29.Knight SC. Lymphocyte proliferation assays. In: Klaus GGB, editor. Lymphocytes. A Practical Approach. Oxford: IRL Press; 1988. [Google Scholar]
- 30.Colle JH, Falanga PB, Singer M, Hevin B, Milon G. Quantitation of messenger RNA by competitive RT-PCR. A simplified read out assay. J Immunol Meth. 1997;210:175–84. doi: 10.1016/s0022-1759(97)00186-5. [DOI] [PubMed] [Google Scholar]
- 31.Knight SC, Farrant J. Comparing stimulation of lymphocytes in different samples: separate effects of numbers of responding cells and their capacity to respond. J Immunol Meth. 1978;22:63–71. doi: 10.1016/0022-1759(78)90058-3. [DOI] [PubMed] [Google Scholar]
- 32.Rigby RJ, Knight SC, Kamm MA, Stagg AJ. Production of interleukin (IL)-10 and IL-12 by murine colonic dendritic cells in response to microbial stimuli. Clin Exp Immunol. 2005;139:245–56. doi: 10.1111/j.1365-2249.2004.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galizzi JP, Zuber CE, Cabrillat H, Djossou O, Banchereau J. Internalization of human interleukin-4 and transient down-regulation of its receptor in the CD23-inducible Jijoye cells. J Biol Chem. 1989;264:6984–9. [PubMed] [Google Scholar]
- 34.Friedrich K, Kammer W, Erhardt I, Brandlein S, Arnold S, Sebald W. The two subunits of the interleukin-4 receptor mediate independent and distinct patterns of ligand endocytosis. Eur J Biochem. 1999;265:457–65. doi: 10.1046/j.1432-1327.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 35.Ewen C, Baca-Estrada ME. Evaluation of interleukin-4 concentration by ELISA is influenced by the consumption of IL-4 by cultured cells. J Interferon Cytokine Res. 2001;21:39–43. doi: 10.1089/107999001459141. [DOI] [PubMed] [Google Scholar]
- 36.Morita Y, Gupta R, Seidl KM, McDonagh KT, Fox DA. Cytokine production by dendritic cells genetically engineered to express IL-4: induction of Th2 responses and differential regulation of IL-12 and IL-23 synthesis. J Gene Med. 2005;7:869–77. doi: 10.1002/jgm.730. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins SJ, Mountford AP. Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect Immun. 2005;73:395–402. doi: 10.1128/IAI.73.1.395-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J Immunol. 2004;173:2419–27. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi S, Johnston SA, Takashima A. Induction of Th2-directed immune responses by IL-4-transduced dendritic cells in mice. Vaccine. 2000;18:3097–105. doi: 10.1016/s0264-410x(00)00140-7. [DOI] [PubMed] [Google Scholar]
- 40.Sato M, Iwakabe K, Ohta A, et al. Functional heterogeneity among bone marrow-derived dendritic cells conditioned by T(h)1- and T(h)2-biasing cytokines for the generation of allogeneic cytotoxic T lymphocytes. Int Immunol. 2000;12:335–42. doi: 10.1093/intimm/12.3.335. [DOI] [PubMed] [Google Scholar]
- 41.Sato M, Iwakabe K, Kimura S, Nishimura T. Functional skewing of bone marrow-derived dendritic cells by Th1- or Th2-inducing cytokines. Immunol Lett. 1999;67:63–8. doi: 10.1016/s0165-2478(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welte T, Koch F, Schuler G, Lechner J, Doppler W, Heufler C. Granulocyte-macrophage colony-stimulating factor induces a unique set of STAT factors in murine dendritic cells. Eur J Immunol. 1997;27:2737–40. doi: 10.1002/eji.1830271038. [DOI] [PubMed] [Google Scholar]
- 44.Frucht DM, Aringer M, Galon J, et al. Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J Immunol. 2000;164:4659–64. doi: 10.4049/jimmunol.164.9.4659. [DOI] [PubMed] [Google Scholar]
- 45.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 46.Haas H, Falcone FH, Holland MJ, et al. Early interleukin-4: its role in the switch towards a Th2 response and IgE-mediated allergy. Int Arch Allergy Immunol. 1999;119:86–94. doi: 10.1159/000024182. [DOI] [PubMed] [Google Scholar]