Abstract
The incidence of allergy is steadily increasing, but the molecular mechanisms involved in the allergic immune response are still not fully understood. In particular, further investigations focusing on dendritic cells, which are central in orchestrating the immune response, are needed. The objective of this study was to investigate the ability of myeloid leukaemia-derived cell lines, such as KG-1, THP-1 and MUTZ-3, to serve as in vitro models for dendritic cells. The ability of these cell lines to mature into functional dendritic cells, expressing costimulatory molecules, was assessed by functional and transcriptional profiling and compared with that of monocyte-derived dendritic cells, which are now used as a standard source of dendritic cells. High-density microarray analysis was utilized to study the transcriptional activity and kinetics of activation of the differentiated MUTZ-3 cell line, in response to a cocktail of inflammatory cytokines. The data obtained clearly demonstrate that MUTZ-3 cells have the ability to induce antigen-independent proliferation in CD4+ CD45RA+ T cells, whereas KG-1 and THP-1 only induced a marginal response. Furthermore, MUTZ-3 displayed the phenotypic and transcriptional profiles of immature dendritic cells, after differentiation with granulocyte–macrophage colony-stimulating factor and interleukin-4. Upon activation with inflammatory cytokines, MUTZ-3 matured phenotypically and exhibited a gene induction similar to that of monocyte-derived dendritic cells. This delineation of the cellular and transcriptional activity of MUTZ-3, in response to maturational stimuli, demonstrates the significance of this cell line as a model for functional studies of inflammatory responses.
Keywords: dendritic cells, myeloid cell line, high-density microarray, inflammation, allergy
Introduction
Dendritic cells (DCs) play a fundamental role in the pathogenesis of allergic disease through their effects on the induction and control of effector T-cell responses.1 Located in allergen-exposed tissue, DCs rapidly produce cytokines and other mediators after allergen encounter, which result in the recruitment of other immune cells and the establishment of allergic inflammation.2,3 DCs are important regulators in, for example, allergic asthma, as the number of local DCs increases in allergic airway inflammation, administration of antigen-pulsed DCs induces disease and, conversely, removal of airway DCs from sensitized mice eliminates features of the disease, for example reducing generation of Th2 effector cells.1 The ability of DCs to orchestrate allergic immune responses makes them unique targets for functional studies in allergy, and also potential candidates for prevention and treatment of allergic diseases.
Because of the limited availability of in vivo DCs, efficient protocols for in vitro differentiation of human DCs have been developed, using either bone marrow or blood precursors.4,5 However, to facilitate functional in vitro studies and even to predict allergenicity, the development of cell lines exhibiting the characteristics of human DCs is crucial. A DC cell line should be able to display diverse phenotypes, reflecting both the immature in vivo antigen-sampling DC and the mature, antigen-presenting DC-equivalent in lymphoid tissue.
Various human myeloid leukaemia-derived cell lines have been considered as potential precursor cells for the development of DC lines, with phenotypic and functional characteristics of in vivo DCs.6–9 After induction of differentiation by, for example, phorbol 12-myristate 13-acetate (PMA) and calcium ionophore, these cell lines acquire some DC phenotypic properties, such as morphological characteristics, expression of costimulatory molecules and MHC class II molecules, and the ability to pinocytose macromolecules and to activate resting T cells. THP-1, KG-1 and MUTZ-3 are myeloid cell lines that have also been shown to be sensitive to cytokine-induced differentiation in experiments using, for example, granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4 and tumour necrosis factor (TNF)-α,10–12 which in contrast to treatment with pleiotropic PMA and ionomycin offer the possibility of more fine-tuned and physiologically relevant studies of DC responses. Masterson et al. recently suggested that MUTZ-3 could act as an unlimited source of CD34+ DC progenitors, and demonstrated that MUTZ-3 acquired a cytokine-induced phenotype similar to that of Langerhans-like DCs.11 They also showed that antigen-loaded MUTZ-3 had the ability to induce specific T-cell proliferation. In this report, we assessed the ability of KG-1, THP-1 and MUTZ-3 to differentiate into DCs displaying either immature or mature phenotypes and functions. In addition to phenotypic and functional studies, our main focus was on performing extensive transcriptional analysis of MUTZ-3-derived DCs (MUTZ-3-DCs) and monocyte-derived DCs (MoDC), using high-density microarray analysis.13,14 We studied the kinetics of transcription in these two in vitro DC models in response to inflammatory stimulation.
We found that MUTZ-3 acquired the most DC-like phenotype after stimulation, compared with KG-1 and THP-1, and that MUTZ-3 was superior in activating resting T cells. Furthermore, transcriptional analysis revealed that MUTZ-3-DCs had a similar expression profile to MoDCs, both in the immature and the mature forms. Our data demonstrate that MUTZ-3-DCs are similar to in vivo DCs in many respects, which suggests that this cell line can profitably be used as a model of DCs when studying immune regulation.
Materials and methods
Cell culture
The human myeloid leukaemia-derived cell line MUTZ-3 (DSMZ, Braunschweig, Germany) was maintained in α-MEM (Invitrogen, Paisley, UK), supplemented with 20%[volume/volume (v/v)] fetal bovine serum (Hyclone Laboratories, Logan, UT) and 40 ng/ml rhGM-CSF (Leukomax; Novartis, Basel, Switzerland). The myelomonocytic cell line KG-1, obtained from the American Type Culture Collection (ATCC) (Manassas, VA), was cultured in Iscove's modified Dulbecco's medium (IMDM) (Invitrogen) supplemented with 20% (v/v) fetal bovine serum. The myeloid leukaemia-derived cell line THP-1 (ATCC) and MoDCs were cultured in RPMI 1640, supplemented with 10% (v/v) fetal bovine serum, 2 mM L-glutamine and 1% (v/v) non-essential amino acids (Sigma-Aldrich, St Louis, MO); this medium is referred to below as R10 complete medium. In addition, 50 µg/ml gentamicin was added to cultures of the primary MoDCs.
Reagents and cytokines
RhIL-4, rhTNF-α and rhIL-1β were purchased from R&D systems (Minneapolis, MN). Prostaglandin E2 (PGE2) was obtained from Sigma-Aldrich. Monocyte-conditioned medium (MCM) was generated by culturing monocytes in R10 complete medium for 24 hr at a cell density of 2 × 106 cells/ml, after which the supernatants were harvested. Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich.
Fluorescence-activated cell sorter (FACS) analysis
The FACS analysis was performed on a FACScan (Becton Dickinson, San Jose, CA), using FLOWJO software (Star, San Carlos, CA) for data analysis. Phosphate-buffered saline (PBS) (without calcium and magnesium) containing 1% bovine serum albumin (BSA) (Sigma-Aldrich) was used in all cell labelling and washing steps. Cells were stained for 10 min at 4°, washed and resuspended in PBS/BSA. The following labelled antibodies were used for FACS analysis: HLA-DR-PE, CD80-PE, CD4-PE (Becton Dickinson Pharmingen, San Jose, CA), CD1a-FITC, CD14-PE, CD86-FITC, CD3-PeCy5 and CD45RA-FITC (DakoCytomation, Glostrup, Denmark). Chrome Pure Mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was used in all experiments to block non-specific binding.
Generation of MoDCs
Human peripheral blood mononuclear cells were isolated from leucocyte-enriched buffycoats, obtained from Lund University Hospital (Lund, Sweden), by Ficoll-PaqueTM Plus (Amersham Bioscience, Uppsala, Sweden) density gradient centrifugation. CD14+ monocytes were isolated by magnetic antibody cell sorting (MACS), using micro bead-conjugated anti-CD14 antibodies (Abs) (Milenyi Biotec, Bergisch Gladbach, Germany) and magnetic cell separation columns (Miltenyi Biotec), resulting in a purity of > 99% CD14+ cells. To generate DCs, CD14+ monocytes were cultured (5 × 105 cells/ml) for 7 days in R10 complete medium supplemented with rhGM-CSF (150 ng/ml) and rhIL-4 (50 ng/ml). Half the medium was exchanged every 2–3 days. After 7 days of culture, the MoDCs were stimulated with a proinflammatory cocktail containing rhIL-1β (10 ng/ml), TNF-α (10 ng/ml), PGE2 (2 µg/ml) and 25% MCM, or LPS (1 µg/ml). Cell samples of unstimulated and stimulated MoDCs were analysed by FACScan and collected for microarray analysis at 8 and 48 hr after addition of the proinflammatory cocktail. The cell samples were lysed in TRIzol Reagent (Invitrogen) and stored at − 20° until further RNA isolation.
Generation of myeloid leukaemia cell line-derived DCs
To generate dendritoid cell lines, the human myeloid leukaemia cell lines MUTZ-3 (1 × 105 cells/ml), THP-1 (5 × 105 cells/ml) and KG-1 (5 × 105 cells/ml) were differentiated for 7 days in the presence of rhGM-CSF (150 ng/ml) and rhIL-4 (50 ng/ml) into immature DCs. Medium was exchanged every 2–3 days. The three differentiated cell lines (denoted MUTZ-3-DCs, THP1-DCs and KG1-DCs) and MoDCs were further stimulated with either LPS or a cocktail of proinflammatory cytokines, consisting of IL-1β, TNF-α, PGE2 and MCM. Subsequently, the immature and mature MUTZ-3-DCs and MoDCs were collected for microarray analysis, as described above.
T-cell proliferation
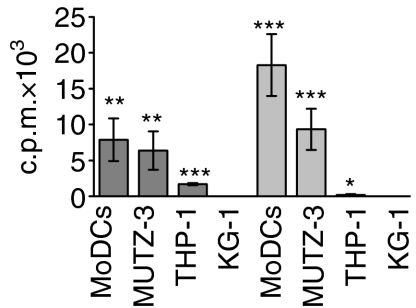
The ability of the matured DC cell lines and MoDCs to activate naïve T-cells was measured in a mixed lymphocyte reaction (MLR). Peripheral blood mononuclear cells were isolated from leucocyte-enriched buffycoats, obtained from Lund University Hospital, by Ficoll-PaqueTM Plus density gradient centrifugation. Isolation of naïve CD4+ T cells was performed by negative selection, using a CD4 isolation kit (MACS) and magnetic cell separation columns. The purified CD4+ cells were subsequently incubated with anti-CD45RO, antiglycophorin A and anti-CD8 (DakoCytomation) mAbs for 15 min at 4°. Thereafter, microbead-conjugated goat anti-mouse antibodies (Miltenyi Biotec) were added (15 min at 4°) to deplete contaminating cells, resulting in 96% purity CD4+ CD45RA+ T cells. Stimulated and unstimulated MoDCs, MUTZ-3-DCs, THP-1-DCs or KG-1-DCs were added to CD4+ CD45RA+ T cells. The detected proliferation was derived from T cells, as the cell lines were irradiated at 5000 rad before the MLR. Triplicates of stimulator cells (1·25 × 104) and naïve T cells (5 × 104) were co-cultured in 96-well plates in 200 µl of R10 complete medium. The cells were cultured for 4 days and, during the last 16 hr of the culture period, 0·5 µCi of 3H-thymidine (Amersham Biosciences, Buckinghamshire, UK) was added to each well. The cells were then harvested and incorporated radioactivity was determined, using a 1450 MicroBetaTM Liquid Scintillation Counter (Wallac, Turku, Finland). Student's t-test was performed to determine whether or not the T-cell proliferation induced by mature DCs was significantly different, as compared with that os immature DCs (Fig. 1).
Figure 1.
Proliferation of CD4+ CD45RA+ T cells after stimulation of monocyte-derived dendritic cells (MoDCs) and differentiated MUTZ-3, THP-1 and KG-1 cell lines. The differentiated cell lines and MoDCs were cultured for 48 hr in the presence of lipopolysaccharide (LPS) (dark grey bars) or a proinflammatory cocktail (light grey bars) prior to co-culture with CD4+ CD45RA+ T cells for 4 days. The results shown represent net proliferation, i.e. proliferation induced by unstimulated cells is subtracted. The values shown are the average of four replicate cultures ± standard deviation (*P < 0·05; **P < 0·005; ***P < 0·0005). c.p.m., counts per minute.
Antigen uptake by MoDCs and MUTZ-3-DCs
The endocytic functionality of MoDCs and MUTZ-3-DCs was evaluated by measuring the levels of cellular uptake of fluorescein isothiocyanate (FITC)-dextran (Sigma-Aldrich), as analysed by flow cytometry. Briefly, immature and mature MoDCs and MUTZ-3-DCs were incubated with 0·5 mg/ml FITC-dextran for 30 min at 37°. As a control, the cells were also incubated with FITC-dextran at 0°. The cells were then washed in ice-cold PBS, containing 1% BSA, and the uptake of antigen was quantified by flow cytometry.
Preparation of cRNA and gene chip hybridization
RNA was isolated from cytokine cocktail-stimulated (8 and 48 hr) and unstimulated control MoDCs and MUTZ-3-DCs. Monocytes from two donors were used to generate MoDCs and the experiment was repeated twice for MUTZ-3 cells. Preparation of labelled cRNA, fragmentation, hybridization and scanning of the human U133A arrays were performed according to the manufacturer's protocol (Affymetrix Inc., Santa Clara, CA). Briefly, cDNA was generated from total RNA (5 µg) using SuperScript II (Invitrogen) and a T7-Oligo(dT) promoter primer (Affymetrix Inc.). Subsequently, cDNA was converted to cRNA by an in vitro transcription reaction (IVT) (ENZO, Farmingdale, NY) with biotin-labelled ribonucleotides and T7 RNA polymerase. Labelled cRNA was purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) and denatured at 94° before hybridization. The samples were hybridized to the human genome U133A arrays at 45° for 16 hr by rotation (60 r.p.m.) in an oven. The arrays were then washed, stained with Steptavidin-PE (Molecular Probes, Eugene, OR), washed again and scanned with a GeneArrayTM Scanner (Affymetrix Inc.).
Microarray data analysis
The fluorescence intensities were analysed using Microarray Suite (MAS) Software 5·0 (Affymetrix Inc.), and scaled based on average intensity on the chip to a target value of 100. Further data normalization and analysis were performed with genespringTM 6·0 software (Silicon Genetics, Redwood City, CA). First, the chip files were normalized to the 50th percentile of the measurements taken from the chips. Secondly, a gene-to-gene normalization was performed to the median of each gene. From the normalized samples, we determined which genes were up- or down-regulated by the cocktail, after 8 and 48 hr of stimulation, by performing a comparative analysis based on ± 2-fold change. The kinetic study was performed by quality threshold (QT) clustering and 10 genes from stereotypical clusters were selected and displayed.
Results
In an attempt to delineate functional, phenotypic and transcriptional similarities during differentiation between MoDCs and human myeloid leukaemia-derived cell lines, we performed cell proliferation assays, FACS and global genomic analysis. The aim was to generate a continuously growing cell line, with characteristics comparable to those of MoDCs, as this would provide us with an in vitro system for studying DC biology in immune regulation.
The capacity of differentiated and matured myeloid cell lines to stimulate T cells
The three differentiated cell lines MUTZ-3-DCs, THP1-DCs and KG1-DCs, as well as MoDCs, were further stimulated with either LPS or a cocktail of proinflammatory cytokines to enable efficient maturation and thus up-regulation of costimulatory and MHC class II molecules. To assess the stimulatory capacity of the in vitro differentiated cell lines, we co-cultured CD4+ CD45RA+ T cells, in a mixed lymphocyte reaction, with unstimulated or stimulated MUTZ-3-DCs, THP1-DCs, KG1-DCs and MoDCs (Fig. 1). Irradiation of the cell lines prior to co-culture ensured that the detected proliferation was derived from T cells. From this functional discriminator of how well the differentiated cell lines mirrored MoDCs, it was clear that stimulated MUTZ-3-DCs were superior at inducing T-cell proliferation, compared with THP1-DCs and KG-1-DCs. MUTZ-3-DCs, stimulated with either LPS or the proinflammatory cocktail, displayed cytokine responsiveness and a capacity to activate naïve T cells, which was similar to that of stimulated MoDCs. This suggests that stimulated MUTZ-3-DCs and MoDCs, in this case, possess comparable phenotypic profiles.
Phenotypic analysis of differentiated and matured myeloid cell lines
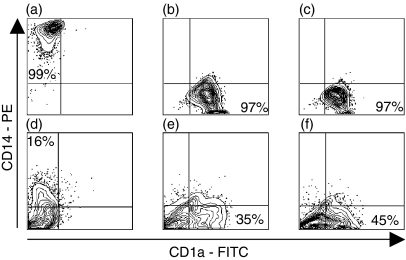
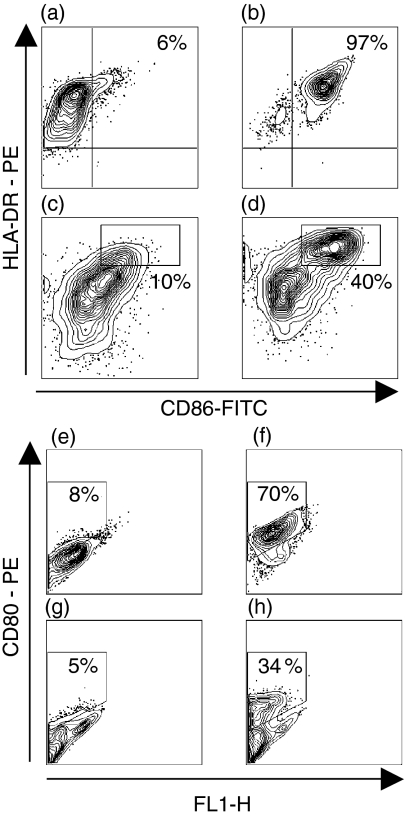
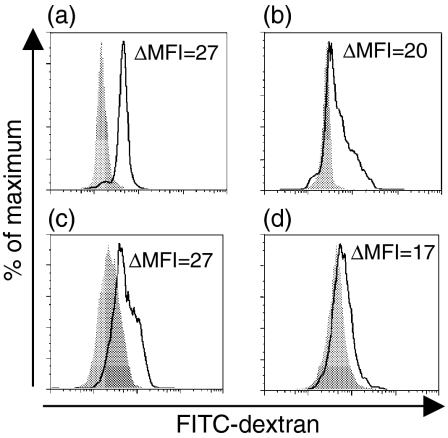
Differentiation of CD14+ monocytes into immature MoDCs for 7 days with GM-CSF and IL-4 typically resulted in CD1a+, CD14–, CD80– and CD86– cells (Figs 2a–c and 3a–e). After stimulation with maturational agents, such as LPS or a proinflammatory cocktail, MoDCs gradually increased their expression of costimulatory molecules CD86 and CD80, as well as MHC class II molecules (Figs 3b–f). THP-1, KG-1 and MUTZ-3 were differentiated in the same way as MoDCs and then stimulated with LPS or proinflammatory cocktail to induce a mature DC phenotype. The cells were then analysed phenotypically and compared to immature and mature MoDCs. Independent of maturation status, only a few KG1-DCs (3–6%) expressed CD1a. Virtually no expression of HLA-DR was detected on THP1-DCs, independently of stimuli, whereas CD86 was up-regulated upon maturation (10–20% positive cells). Unstimulated KG-1-DCs expressed HLA-DR and low amounts of CD86. However, KG-1-DCs were difficult to maturate by stimulation with either LPS or the proinflammatory cocktail, as only a small fraction (0–9%) of the cells up-regulated CD86. The low or absent capacity of THP1-DCs and KG-1-DCs to activate resting T cells is probably a result of cytokine unresponsiveness and suboptimal expression of costimulatory molecules. In contrast, MUTZ-3-DCs displayed a similar expression profile to MoDCs (Fig. 2), as MUTZ-3 lost expression of CD14 during the 7-day culture period and acquired CD1a expression. When stimulated with the proinflammatory cocktail for 48 hr, MUTZ-3-DCs matured into HLA-DR+, CD86+ (40%) and CD80+ (34%) cells, denoted mMUTZ-3-DCs (Fig. 3). A similar expression profile was acquired after LPS stimulation (data not shown). To evaluate the functional efficiency of antigen uptake, both immature and mature MoDCs and MUTZ-3-DCs were incubated with FITC-dextran. As expected, the ability to ingest antigens was higher in cells expressing a more immature phenotype, compared with cells that were allowed to mature. Immature MoDCs and MUTZ-3-DCs showed very similar abilities to ingest the FITC-dextran antigen (Figs 4a–c). In addition, matured MUTZ-3-DCs displayed a similar morphology to mature MoDCs (mMoDCs), with an irregular shape and extended dendrites.
Figure 2.
Changes in CD14 and CD1a expression during differentiation and maturation of monocyte-derived dendritic cells (MoDCs) and MUTZ-3, as assessed by flow cytometry. CD14+ peripheral blood monocytes (a) were differentiated for 7 days into immature MoDCs (b) and subsequently stimulated with a proinflammatory cocktail for 48 hr (c).In a similar fashion, MUTZ-3 cells (d) were allowed to differentiate into an immature DC-like phenotype (e) and were further stimulated with a proinflammatory cocktail for 48 hr (f). Gates were set to exclude debris and dead cells. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Figure 3.
Changes in human leucocyte antigen (HLA)-DR, CD86 and CD80 expression during maturation of monocyte-derived dendritic cells (MoDCs) and MUTZ-3 DCs, as assessed by flow cytometry. Immature MoDCs (a, e) were stimulated with a proinflammatory cocktail for 48 hr (b, f). Similarly, differentiated immature MUTZ-3 DCs (c, g) were allowed to mature for 48 hr after stimulation (d, h). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Figure 4.
Receptor-mediated endocytosis by immature and mature monocyte-derived dendritic cells (MoDCs) and MUTZ-3 DCs. Fluorescein isothiocyanate (FITC)-dextran uptake at 37° by immature (a) and mature (b) MoDCs compared with the uptake at 0° (grey) was calculated, using the mean fluorescence intensity (MFI). The uptake of FITC-dextran by immature (c) and mature (d) MUTZ-3 DCs demonstrated a similar reduction of antigen uptake compared with MoDCs. ΔMFI is based on the geometric mean value.
Global transcriptional analysis of MoDCs and MUTZ-3-DCs
Similarities in phenotype induced during differentiation and maturation prompted us to assess the mRNA expression profiles of MUTZ-3-DCs and MoDCs in response to a proinflammatory cocktail. The two cell types, MUTZ-3-DCs and MoDCs, were stimulated by a maturational, proinflammatory cocktail for 8 and 48 hr. The expression of DC-specific and characteristic molecules by immature and mature (at 48 hr) MUTZ-3-DCs and MoDCs was compared, using high-density oligonucleotide arrays (with > 22·500 transcripts) (Table 1). Most DC markers, such as 4-1BBL, CCR7, CD13, CD40, CD44, CD80, CD83, CD86, DC-LAMP, DEC205, OX40L, IL-12B (p40) and human leucocyte antigen (HLA) classes I and II, were similarly expressed in MoDCs and MUTZ-3-DCs after stimulation. However, some transcripts coding for DC markers, such as CD1a, CD11c and CD34, showed different expression profiles in mMUTZ-3-DCs as compared with mMoDCs.
Table 1. Dendritic cell markers expressed by monocyte-derived dendritic cells (MoDCs) and MUTZ-3 dendritic cells (MUTZ-3-DCs) in their immature (0 hr) and mature (48 hr) states.
| MoDCs | MUTZ-3-DCs | ||||
|---|---|---|---|---|---|
| GenBank no. | Description | 0 hr | 48 hr | 0 hr | 48 hr |
| NM_001561 | 4-1BB | – | ++ | + | ++ |
| NM_003811 | 4-1BB-L | +– | ++ | + | ++ |
| NM_006401 | APRIL | +++ | +++ | +++ | ++ |
| NM_004925 | AQP3 | ++ | – | ++ | – |
| NM_001295 | CCR1 | ++ | – | + | + |
| NM_000647 | CCR2 | – | – | ++ | + |
| NM_000579 | CCR5 | ++ | – | +– | – |
| NM_001838 | CCR7 | + | +++ | +– | +++ |
| NM_000632 | CD11b | ++ | – | ++ | ++ |
| M81695 | CD11c | ++ | – | – | +– |
| NM_001150 | CD13 | ++ | ++ | ++ | +++ |
| M28825 | CD1a | +++ | +– | ++ | +++ |
| NM_006110 | CD2 | ++ | ++ | ++ | ++ |
| NM_002002 | CD23 | ++ | + | ++ | + |
| NM_000417 | CD25 | – | ++ | – | – |
| NM_021642 | CD32 | ++ | – | ++ | ++ |
| NM_001772 | CD33 | ++ | – | ++ | ++ |
| M81104 | CD34 | – | – | ++ | + |
| NM_001250 | CD40 | ++ | ++ | ++ | ++ |
| BC004372 | CD44 | ++ | ++ | +++ | +++ |
| NM_001251 | CD68 | + | – | + | +– |
| NM_005191 | CD80 | ++ | ++ | ++ | ++ |
| NM_004233 | CD83 | ++ | +++ | ++ | +++ |
| BG236280 | CD86 | ++ | ++ | ++ | ++ |
| AF348491 | CXCR4 | +– | +++ | ++ | ++ |
| NM_014398 | DC-LAMP | ++ | +++ | +– | +++ |
| AF290886 | DC-SIGN | +++ | – | ++ | ++ |
| NM_002349 | DEC-205 | ++ | +++ | + | ++ |
| NM_006140 | GM-CSFR | ++ | +++ | ++ | ++ |
| NM_000201 | ICAM-1 | ++ | +++ | ++ | +++ |
| NM_002187 | IL-12Bp40 | + | ++ | + | ++ |
| NM_000877 | IL-1R1 | ++ | +++ | +– | ++ |
| NM_004633 | IL-1R2 | ++ | ++ | ++ | +++ |
| NM_000418 | IL-4RA | ++ | ++ | ++ | ++ |
| NM_003326 | OX40L | – | ++ | – | ++ |
| NM_000958 | PGER | ++ | ++ | ++ | ++ |
| NM_003839 | RANK | – | ++ | – | + |
| NM_003264 | TLR2 | + | – | ++ | ++ |
| NM_003266 | TLR4 | ++ | – | +– | – |
| NM_001065 | TNFR1 | ++ | ++ | ++ | ++ |
| NM_001066 | TNFR2 | ++ | ++ | + | + |
| AA573862 | HLA-A | +++ | +++ | +++ | +++ |
| L42024 | HLA-B | +++ | +++ | +++ | +++ |
| U655851 | HLA-DR | +++ | +++ | +++ | +++ |
GM-CSFR, granulocyte–macrophage colony-stimulating factor; ICAM, intercellular adhesion molecule; IL, interleukin; TLR, Toll-like receptor; TNF, tumour necrosis factor; HLA, human leucocyte antigen.
Fluorescence signal intensity values are indicated as follows: not expressed (–), fluorescence signal intensity value <100 (+), 100–1000 (++) and >(+++). Genes expressed in one array out of two (+−).
Global genome analyses of stimulated MoDCs and MUTZ-3-DCs were initially performed to monitor overall transcriptional changes, which were quantitatively similar in responding MoDCs and MUTZ-3-DCs (data not shown). However, to produce a more comprehensive picture of cytokine-induced gene regulation in MUTZ-3-DCs and MoDCs, a kinetic analysis was performed. Hundreds of genes that were more than 2-fold up- or down-regulated after 8 and 48 hr of stimulation were identified. These could be grouped into representative functionally associated gene families (Tables 2 and 3). Of note, similar transcripts encoding for cell adhesion molecules, cytokine ligands and receptors involved in inflammation and immune response were induced in response to the proinflammatory cocktail in both MoDCs and MUTZ-3-DCs. Some cell adhesion molecules such as activated leucocyte cell adhesion molecule (ALCAM), intercellular adhesion molecule 1 (ICAM-1), CD58 and CD207 were up-regulated after 8 hr in both MoDCs and MUTZ-3-DCs, whereas others, such as CD151 and C-type lectin, were down-regulated after 8 and 48 hr. After 48 hr, the expression levels were in most cases sustained in MUTZ-3-DCs, whereas MoDCs showed an initial decrease in expression. Chemokines involved in directing cell migration [ thymus- and activation-regulated chemokine (TARC), monocyte derived chemokine (MDC), macrophage inflammatory protein (MIP)-1α, MIP-1β, RANTES, growth-related oncogene (Gro)-α, Gro-β] and chemokine receptors (CCR7, CXCR4) were up-regulated in both cell types, whereas CCR1 was down-regulated. FcγRIIa was down-regulated, while interleukins (IL-1α/β, IL-6, IL-8, IL-12B and IL-23A), interleukin receptors (IL-1R, IL-10R, IL-18R, IL-21R and IL-7R) and prostaglandin E receptor 4 (PTGER4) were up-regulated in both mMUTZ-3-DCs and mMoDCs. Furthermore, TNF stimulating factor 7 (CD70) and several TNF receptors (CD40, OX40 and 4-1BB) were up-regulated. CD80 and CD83 were both up-regulated > 2-fold, whereas CD86 was up-regulated 1·7-fold in MUTZ-3 and 5·7-fold in MoDCs after 48 hr. In summary, the transcriptional kinetics of functionally associated genes involved in immune regulation was similar in mMUTZ-3-DCs and mMoDCs, and only minor discrepancies could be detected.
Table 2.
Down-regulated transcripts in monocyte-derived dendritic cells (MoDCs) and MUTZ-3 dendritic cells (MUTZ-3-DCs) in response to inflammatory stimulation
| MoDCs | MUTZ-3-DCs | ||||
|---|---|---|---|---|---|
| GenBank no. | Description | 0–8 hr | 0–48 hr | 0–8 hr | 0–48 hr |
| Cell adhesion | |||||
| NM_004357 | CD151 | −2·2 | −2·2 | −1·8 | −2·5 |
| BC005254 | CLECSF2 | −12·9 | −16·0 | −1·9 | −5·4 |
| NM_014880 | DCL-1 | −5·8 | −8·1 | −2·2 | −2·7 |
| NM_000118 | ENG | −3·2 | −1·2 | −1·1 | −2·2 |
| Cell shape | |||||
| NM_001431 | EPB41L2 | −1·5 | −2·6 | 1·1 | −2·2 |
| T62571 | MAP7 | −54·6 | −90·7 | −1·2 | −2·3 |
| Immune/inflammatory response | |||||
| BF213829 | AIF1 | −2·1 | −206·4 | −1·3 | −3·4 |
| AA995910 | ALOX5 | −4·3 | −5·6 | −1·8 | −2·0 |
| NM_000700 | ANXA1 | −2·6 | −4·8 | −1·5 | −2·1 |
| L04636 | C1QBP | −2·0 | −2·4 | −1·4 | −2·1 |
| U62027 | C3AR1 | −26·6 | −26·7 | −1·1 | −9·6 |
| NM_002984 | CCL4 | 3·2 | −7·8 | 2·9 | −2·1 |
| AI421071 | CCR1 | −42·0 | −39·7 | −2·9 | −1·5 |
| NM_000397 | CYBB | −2·5 | −2·9 | 1·2 | −3·6 |
| M31933 | FCGR2B | −10·6 | −202·1 | 1·4 | −6·0 |
| AF198052 | FYB | −14·9 | −8·5 | −3·1 | −2·9 |
| AF208043 | IFI16 | −30·2 | −13·8 | −2·2 | −2·2 |
| AF109683 | LAIR1 | −1·3 | −3·0 | −1·4 | −3·5 |
| AF000425 | LST1 | −2·8 | −7·8 | −1·5 | −2·0 |
| Regulation of transcription | |||||
| NM_004364 | CEBPA | −9·1 | −5·0 | −2·2 | −1·3 |
| AF109161 | CITED2 | −8·1 | −20·3 | −1·1 | −2·5 |
| NM_002131 | HMGA1 | −6·1 | −5·2 | −1·1 | −2·2 |
| N22468 | MEF2C | −12·8 | −15·0 | −1·7 | −8·2 |
| NM_002432 | MNDA | −29·3 | −10·4 | −4·0 | −4·5 |
| AW592266 | MYBL1 | −6·8 | −30·2 | −1·9 | −2·0 |
| U85430 | NFATC3 | −10·0 | −2·8 | −1·8 | −2·2 |
| L13974 | NFE2 | −129·2 | −96·0 | −4·3 | −7·9 |
| Signalling | |||||
| NM_000677 | ADORA3 | −15·8 | −15·7 | −2·3 | −2·2 |
| NM_001666 | ARHGAP4 | −3·2 | −17·9 | −1·4 | −2·3 |
| NM_019555 | ARHGEF3 | −22·5 | −4·8 | −2·0 | −2·1 |
| NM_024701 | ASB13 | −1·9 | −5·5 | −2·5 | −1·5 |
| NM_001772 | CD33 | −86·0 | −242·1 | −1·7 | −2·2 |
| L07555 | CD69 | −1·7 | −8·7 | 1·0 | −2·4 |
| NM_002183 | IL-3RA | 1·3 | −4·8 | 1·8 | −5·7 |
| NM_013416 | NCF4 | −4·7 | −5·7 | −1·4 | −2·9 |
| AW574504 | PECAM1 | −9·3 | −7·2 | −2·3 | −3·1 |
| NM_002953 | RPS6KA1 | −4·0 | 1·6 | −1·8 | −2·2 |
The fold change in expression level compared with the unstimulated control sample is shown and the transcripts are grouped according to their function based on information from the Gene OntologyTM (GO) Consortium (http://geneontology.org/.)
Table 3.
Up-regulated transcripts in monocyte-derived dendritic cells (MoDCs) and MUTZ-3 dendritic cells (MUTZ-3-DCs) in response to inflammatory stimulation.
| MoDCs | MUTZ-3-DCs | ||||
|---|---|---|---|---|---|
| GenBank no. | Description | 0–8 hr | 0–48 hr | 0–8 hr | 0–48 hr |
| Cell adhesion | |||||
| NM_001627 | ALCAM | 6·5 | 2·8 | 2·4 | 3·2 |
| NM_015717 | CD207 | 5·1 | 1·0 | 1·3 | 7·7 |
| AA700015 | CD58 | 6·1 | 2·7 | 5·4 | 5·2 |
| NM_021101 | CLDN1 | 3·8 | −4·0 | 3·5 | 3·6 |
| AA292373 | COL6A1 | 2·9 | 1·4 | 4·0 | 3·6 |
| NM_030781 | COLEC12 | 2·6 | 1·1 | 1·0 | 2·3 |
| NM_000201 | ICAM-1 | 4·3 | 3·1 | 2·5 | 1·5 |
| NM_003622 | PPFIBP1 | 6·3 | 1·1 | 2·2 | −1·3 |
| Cell shape | |||||
| NM_019034 | ARHF | 9·0 | 6·2 | 3·2 | 2·4 |
| NM_002421 | MMP1 | 21·3 | 7·4 | 2·5 | 6·3 |
| NM_002425 | MMP10 | 340·0 | 61·6 | 8·2 | 3·1 |
| NM_004994 | MMP9 | 5·6 | −4·0 | 3·2 | 2·2 |
| Immune/inflammatory response | |||||
| BC006196 | 4-1BB | 50·7 | 47·3 | 4·2 | 1·1 |
| NM_002987 | CCL17, TARC | 4·3 | 4·4 | 4·4 | 6·1 |
| NM_002990 | CCL22, MDC | 16·2 | 15·0 | 10·7 | 12·9 |
| NM_002983 | CCL3, MIP-1a | 2·0 | −2·3 | 2·7 | −1·5 |
| NM_002984 | CCL4, MIP-1b | 3·3 | −8·0 | 2·9 | −2·1 |
| NM_002985 | CCL5, RANTES | 23·4 | 1·1 | 6·7 | 2·2 |
| NM_001838 | CCR7 | 54·2 | 59·6 | 9·9 | 44·6 |
| X60592 | CD40 | 5·1 | 3·1 | 2·4 | 1·5 |
| NM_001252 | CD70 | 9·0 | 10·5 | 4·4 | 4·4 |
| NM_005191 | CD80 | 4·1 | 2·9 | 2·2 | 2·4 |
| NM_004233 | CD83 | 10·0 | 7·3 | 10·4 | 12·6 |
| NM_001511 | CXCL1, Groα | 53·3 | 4·5 | 3·5 | 2·7 |
| M57731 | CXCL2, Groβ | 41·1 | 4·3 | 4·4 | 2·1 |
| AJ224869 | CXCR4 | 10·4 | 5·8 | 1·8 | 3·5 |
| NM_002349 | DEC205 | 6·6 | 11·2 | 1·8 | 10·1 |
| U62824 | HLA-C | 1·8 | 3·0 | 1·8 | 2·5 |
| BE138825 | HLA-F | 2·1 | 2·3 | 1·5 | 2·4 |
| M90686 | HLA-G | 2·0 | 3·1 | 2·5 | 3·8 |
| AF043337 | IL-8 | 56·5 | 10·3 | 2·5 | 1·9 |
| NM_001558 | IL-10RA | 2·0 | 1·4 | 2·6 | 2·7 |
| NM_002187 | IL-12Bp40 | 132·3 | 34·9 | 5·9 | 26·5 |
| NM_003855 | IL-18R1 | 54·5 | 8·5 | 3·0 | 1·3 |
| M15329 | IL-1A | 4·4 | 1·9 | 18·6 | 3·3 |
| M15330 | IL-1B | 21·3 | 3·4 | 6·0 | 3·6 |
| NM_000877 | IL-1R1 | 2·0 | 1·8 | 1·5 | 2·2 |
| BE563442 | IL-1RN | 31·9 | 2·3 | 6·6 | 2·4 |
| AF269133 | IL-21R | -1·3 | 3·0 | 2·3 | 3·4 |
| NM_016584 | IL-23A | 5·0 | 4·3 | 2·8 | 1·3 |
| NM_000600 | IL-6 | 34·3 | 1·9 | 2·3 | 2·8 |
| NM_002185 | IL-7R | 14·9 | 5·4 | 5·8 | 3·2 |
| AJ277151 | OX40 | 97·6 | 23·2 | 5·9 | 1·7 |
| NM_000958 | PTGER4 | 5·3 | 1·0 | 3·9 | 3·5 |
| NM_000963 | PTGS2 | 81·4 | 4·1 | 2·3 | 1·3 |
| Regulation of transcription | |||||
| NM_020183 | ARNTL2 | 7·3 | 8·5 | 3·1 | 2·1 |
| AL021977 | MAFF | 13·8 | 8·9 | 4·6 | 3·7 |
| NM_002908 | REL | 19·6 | 11·5 | 6·2 | 6·0 |
| NM_006509 | RELB | 5·4 | 4·8 | 3·7 | 4·5 |
| Signalling | |||||
| AB003476 | AKAP12 | 28·1 | −1·6 | 5·0 | 1·1 |
| M13436 | INHBA | 129·6 | 12·9 | 10·8 | 3·9 |
| M55643 | NFKB1 | 11·9 | 7·7 | 5·4 | 3·3 |
| NM_007115 | TNFAIP6 | 105·3 | 12·9 | 16·8 | 8·4 |
| M27281 | VEGF | 14·9 | 2·5 | 2·6 | 1·5 |
The fold change in expression level compared with the unstimulated control sample is shown and the transcripts are grouped according to their function based on information from the Gene OntologyTM (GO) Consortium (http://geneontology.org/).ICAM, intercellular adhesion molecule; IL, interleukin; TNF, tumour necrosis factor; HLA, human leucocyte antigen; MIP, macrophage inflammatory protein.
Genes displaying similar expression profiles were finally clustered into groups, as illustrated by a heat map in Fig. 5. Five expression profiles are represented: genes down-regulated after (i) 8 hr or (ii) 48 hr (e.g. AQP3, DAB2, CCR1 and MRC1), genes up-regulated after (iii) 8 hr or (iv) 48 h (e.g. CD80, CD83, DEC-205 and LAMP3) and (v) genes that were initially up-regulated and thereafter down-regulated (e.g. GK, CCL3, IL-8 and MMP3). Taken together, the transcriptional kinetics, in relation to crucial genes involved in orchestrating an allergic response, were similar between MUTZ-3-DCs and MoDCs. However, in some instances it was observed that MUTZ-3-DCs showed slower temporal gene regulation, probably reflecting the fact that MUTZ-3 is a transformed cell line.
Figure 5.
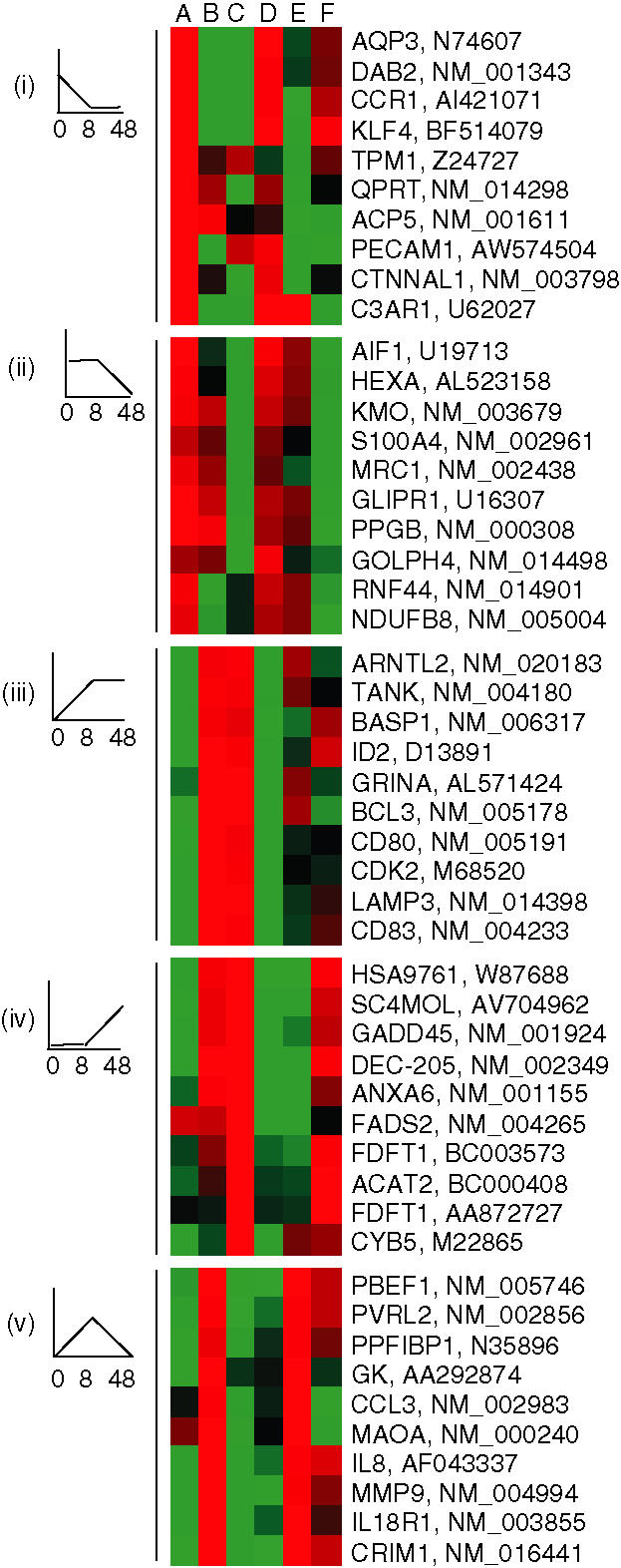
Genes expressed by both monocyte-derived dendritic cells (MoDCs) and MUTZ-3 DCs are clustered into groups of expression profiles. From left to right are A, MoDCs, 0 hr; B, MoDCs, 8 hr; C, MoDCs, 48 hr; D, MUTZ-3 DCs, 0 hr; E, MUTZ-3 DCs, 8 hr; F, MUTZ-3 DCs, 48 hr. A schematic illustration of the expression profile is shown to the left. Red, up-regulated > 2-fold; green, down-regulated > 2-fold; black, intermediate. (i)–(v), as annotated in the text.
Discussion
The fundamental role played by DCs in the pathogenesis of allergy is a consequence of their ability to process and present allergens to effector T cells which eventually results in production of allergen-specific IgE antibodies. Studies of the molecular mechanisms involved in the induction and control of allergic immune responses are hampered by difficulties in isolating and culturing human DCs, which is why these cells have been generated in vitro from monocyte and CD34+ stem cells. A continuous source of DCs would thus be beneficial for many studies, especially for standardization of experiments. Although several human myeloid leukaemia-derived cell lines have the ability to acquire a dendritic phenotype, their functional and transcriptional profiles are either unknown or not very similar to those of human DCs. In the present study, we assessed the functional properties and phenotypic profiles of three myeloid leukaemia-derived cell lines in relation to MoDCs. Furthermore, we performed a transcriptional evaluation of MUTZ-3-DCs, using MoDCs as a reference, with a focus on activation kinetics and differential gene expression in immature and mature cells. PGE2 was included in the stimulation cocktail to reflect in part allergic inflammation, as PGE2-stimulated DCs have been shown to induce Th2 proliferation and suppress IL-12 production.15
The three cell lines MUTZ-3,11 THP-116–18 and KG-112,19 have previously been mentioned as possible model systems for studying DC biology. THP-1 cells have been reported to acquire the ability to induce allogeneic T-cell proliferation after stimulation with PMA,16 which is consistent with our data. However, we could not get KG-1 cells, stimulated with either LPS or a cytokine cocktail, to induce T-cell proliferation, which is in contrast to the findings of Hulette et al., who reported allogeneic T-cell activation by cytokine-responsive KG-1 cells.12 In the present work, MUTZ-3-DCs were shown to induce allogeneic T-cell activation after stimulation with either proinflammatory cytokines or LPS to a similar extent to MoDCs, which was probably attributable to expression of costimulatory molecules such as CD80 and CD86. In comparison, only a low percentage of KG-1-DCs and THP-1-DCs (9 and 20%, respectively) up-regulated CD86 after stimulation with the proinflammatory cocktail. Furthermore, HLA-DR was significantly up-regulated on MoDCs and MUTZ-3-DCs upon stimulation, in contrast to both THP-1-DCs and KG-1-DCs. The main difference between MoDCs and MUTZ-3-DCs was in the kinetics: 48 hr of stimulation did not mature MUTZ-3-DCs to the same extent as MoDCs. However, as the proinflammatory cocktail-driven maturation of MUTZ-3-DCs resulted in a DC-like phenotype and an ability to induce strong T-cell proliferation, these cells were selected for a comparative transcriptional analysis of the immature and mature MUTZ-3-DCs.
Dendritic cells are very effective at taking up antigen, by pinocytosis of soluble compounds, macropinocytosis of high-molecular-weight antigens and phagocytosis of particles.20 DCs also use receptor-mediated uptake to concentrate the engulfed macromolecules in special intracellular compartments, enriched for MHC class II molecules. The uptake of FITC-dextran by MoDCs and MUTZ-3-DCs clearly demonstrates a similar ability with respect to receptor-mediated endocytosis. The fact that MUTZ-3-DCs express MHC class I and II molecules, like MoDCs, suggests that the cell line possesses endosomal pathways important in antigen uptake and presentation. During maturation of DCs, the lysosomal membrane proteins DC-LAMP and CD68 have been shown to co-localize with MHC class II+ vesicles, indicating that these molecules are involved in the endosomal processes that result in presentation of antigen.21 Interestingly, while the expression of DC-LAMP was up-regulated by both MoDCs and MUTZ-3-DCs upon stimulation, the level of CD68 transcripts was instead down-regulated, suggesting that these two lysosomal proteins have different roles in these processes. Functional presentation of antigen by MUTZ-3-DCs via MHC class I, MHC class II and CD1d has previously been demonstrated.11 MUTZ-3-DCs may also be able to present lipid antigens via CD1a,22 as the cell line acquires CD1a expression during differentiation. However, the expression of CD1a was up-regulated in MUTZ-3-DCs during maturation while down-regulated in MoDCs, which may be a result of the slower temporal gene regulation in MUTZ-3-DCs.
After antigen encounter, DCs will migrate to the lymph nodes to activate specific T cells.23 The migration is dependent on the expression of chemokine receptors, which enables the cell to sense chemotactic gradients, and cell adhesion molecules for interaction with endothelial cells. The chemokine receptor CCR7 was found to be up-regulated in both MoDCs and MUTZ-3-DCs. We also found that CCR1 was down-regulated in both MoDCs and MUTZ-3-DCs during the process of maturation. These findings support the notion that MUTZ-3-DCs are capable of regulating chemokine receptors, which allows DCs to be recruited to inflammatory sites and then, after antigen capture, to migrate to T-cell areas in secondary lymphoid organs to interact with antigen or allergen-specific T cells.23 Examples of cell adhesion molecules co-regulated in both MoDCs and MUTZ-3-DCs are ALCAM, CD207, CD58, CLDN1, COL6A1 and ICAM, which again demonstrates the similar behaviour of the two dendritic models. Several of these molecules play important regulatory roles, such as ALCAM, which is expressed on activated T cells and on MoDCs, and might play a role in DC migration.24 The interaction between CD2 on T cells and CD58 on antigen-presenting cells is intimately involved in T-cell-specific antigen recognition.25 ICAM-1 facilitates physical contact with T cells and CD40–CD40L engagement between DCs and T cells, and is intimately involved in activation of the signalling pathways in adaptive immunity.25 The human DC-specific adhesion receptor DC-SIGN regulates primary immune responses by establishing DC–T-cell interactions,26 and LPS and TNF-α are examples of cytokines that regulate the expression of DC-SIGN negatively.16 DC-SIGN was, according to our FACS analysis, expressed on both MoDCs and MUTZ-3-DCs and was down-regulated after stimulation with the cytokine cocktail (data not shown). Upon maturation, transcript levels of DC-SIGN were clearly down-regulated in MoDCs, whereas the levels were unaltered in MUTZ-3-DCs (Table 1). Furthermore, OX40L, a potential costimulatory molecule in T-cell and DC interactions,27 was found to be up-regulated in both MoDCs and MUTZ-3-DCs stimulated with proinflammatory cytokines for 48 hr. Also, CD70, which is possibly involved in CD40-dependent CD8+ T-cell responses,28 was up-regulated in both MoDCs and MUTZ-3-DCs after stimulation. The panel of molecules that are expressed by MUTZ-3-DCs and are involved in interaction with T cells suggests that the cell line acquires the necessary ability for T-cell activation during maturation.
Interestingly, it was recently reported that the exposure of immature DCs to PGE2 resulted in the induction of the inactive p40 subunit of IL-12,29 and this induction was not accompanied by the production of the active p70 heterodimer of IL-12.30 These results are in agreement with the present transcriptional study in that we detected transcript of IL-12p40 in both MoDCs and MUTZ-3-DCs, whereas no transcripts of IL-12p35 could be detected. The ability of MUTZ-3-DCs to respond to PGE2, by suppressing IL-12, demonstrates that the cell line possesses functionality involved in the regulation of Th1 andTh2 polarization.
Human blood DCs can be divided into several distinct phenotypic and functional subpopulations, and both MoDCs and MUTZ-3-DCs are of the myeloid CD1a+ CD2+ CD11c+ CD13+ CD33+ and Lin– lineage, resembling their in vivo myeloid counterpart.31 The low level of surface CD14 on MUTZ-3 suggests that the cell line more closely resembles immature DC precursors than monocytes. Both MUTZ-3-DCs and MoDCs displayed the DC marker CD1a after 7 days in culture. However, after stimulation with the cytokine cocktail, the expression of CD1a, CD11b and CD33 was down-regulated in MoDCs, whereas mMUTZ-3-DCs were still positive for these markers. The differences in gene regulation between MUTZ-3-DCs and MoDCs could be explained by slower gene regulation in MUTZ-3DCs than in MoDCs, which has also been observed for up-regulated genes, as discussed above. The Langerhans cell marker Langerin (CD207) is up-regulated in matured MoDCs and MUTZ-3-DCs.32 The diverse expression of DC markers for blood DCs and LCs in MUTZ-3-DCs indicates that the cell line has the potential to differentiate into different subsets depending on the stimulation.
In summary, a comprehensive picture of matured MUTZ-3-DCs and the biology of their progenitors is evolving, based on functional, phenotypic and genomic data. MUTZ-3-DCs were found to differ significantly from THP-1-DCs and KG-1-DCs and to show a closer resemblance to the most common in vitro generated DCs today, i.e. monocyte-derived DCs. On the basis of the comparative functional and transcriptional profiles of MUTZ-3-DCs, we conclude that this is a continuous cell line that is potentially useful as a model for deciphering the molecular mechanisms of immune regulation in, for example, allergy.
Abbreviations
- DC
dendritic cell
- LPS
lipopolysaccharide
- MCM
monocyte-conditioned media
- MoDC
monocyte-derived dendritic cell
- PGE2
prostaglandin E2.
Acknowledgements
This work was supported by grants from the European Commission FW5/6, the Swedish Fund for Research without Animal Experiment and the National Science Council (VR-M). We would like to thank Ann-Charlotte Ohlsson for excellent technical assistance.
References
- 1.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- 2.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol. 2003;15:620–6. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 3.von Bubnoff D, Geiger E, Bieber T. Antigen-presenting cells in allergy. J Allergy Clin Immunol. 2001;108:329–39. doi: 10.1067/mai.2001.117457. [DOI] [PubMed] [Google Scholar]
- 4.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koski GK, Schwartz GN, Weng DE, Czerniecki BJ, Carter C, Gress RE, Cohen PA. Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J Immunol. 1999;163:82–92. [PubMed] [Google Scholar]
- 7.Koski GK, Schwartz GN, Weng DE, Gress RE, Engels FH, Tsokos M, Czerniecki BJ, Cohen PA. Calcium ionophore-treated myeloid cells acquire many dendritic cell characteristics independent of prior differentiation state, transformation status, or sensitivity to biologic agents. Blood. 1999;94:1359–71. [PubMed] [Google Scholar]
- 8.St Louis DC, Woodcock JB, Fransozo G, et al. Evidence for distinct intracellular signaling pathways in CD34+ progenitor to dendritic cell differentiation from a human cell line model. J Immunol. 1999;162:3237–48. [PubMed] [Google Scholar]
- 9.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170:4178–88. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 10.Reischl IG, Dubois GR, Peiritsch S, Brown KS, Wheat L, Woisetschlager M, Mudde GC. Regulation of Fc epsilonRI expression on human monocytic cells by ligand and IL-4. Clin Exp Allergy. 2000;30:1033–40. doi: 10.1046/j.1365-2222.2000.00859.x. [DOI] [PubMed] [Google Scholar]
- 11.Masterson AJ, Sombroek CC, De Gruijl TD, et al. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood. 2002;100:701–3. doi: 10.1182/blood.v100.2.701. [DOI] [PubMed] [Google Scholar]
- 12.Hulette BC, Rowden G, Ryan CA, Lawson CM, Dawes SM, Ridder GM, Gerberick GF. Cytokine induction of a human acute myelogenous leukemia cell line (KG-1) to a CD1a+ dendritic cell phenotype. Arch Dermatol Res. 2001;293:147–58. doi: 10.1007/s004030000201. [DOI] [PubMed] [Google Scholar]
- 13.Messmer D, Messmer B, Chiorazzi N. The global transcriptional maturation program and stimuli-specific gene expression profiles of human myeloid dendritic cells. Int Immunol. 2003;15:491–503. doi: 10.1093/intimm/dxg052. [DOI] [PubMed] [Google Scholar]
- 14.Lindstedt M, Johansson-Lindbom B, Borrebaeck CA. Global reprogramming of dendritic cells in response to a concerted action of inflammatory mediators. Int Immunol. 2002;14:1203–13. doi: 10.1093/intimm/dxf082. [DOI] [PubMed] [Google Scholar]
- 15.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 16.Puig-Kroger A, Serrano-Gomez D, Caparros E, et al. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem. 2004;279:25680–8. doi: 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida Y, Sakaguchi H, Ito Y, Okuda M, Suzuki H. Evaluation of the skin sensitization potential of chemicals using expression of co-stimulatory molecules, CD54 and CD86, on the naive THP-1 cell line. Toxicol Vitro. 2003;17:221–8. doi: 10.1016/s0887-2333(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 18.Ashikaga T, Hoya M, Itagaki H, Katsumura Y, Aiba S. Evaluation of CD86 expression and MHC class II molecule internalization in THP-1 human monocyte cells as predictive endpoints for contact sensitizers. Toxicol Vitro. 2002;16:711–6. doi: 10.1016/s0887-2333(02)00060-7. [DOI] [PubMed] [Google Scholar]
- 19.Hajas G, Zsiros E, Laszlo T, et al. New phenotypic, functional and electrophysiological characteristics of KG-1 cells. Immunol Lett. 2004;92:97–106. doi: 10.1016/j.imlet.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barois N, de Saint-Vis B, Lebecque S, Geuze HJ, Kleijmeer MJ. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3:894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- 22.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Figdor CG. Molecular characterization of dendritic cells operating at the interface of innate or acquired immunity. Pathol Biol (Paris) 2003;51:61–3. doi: 10.1016/s0369-8114(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 25.Crawford K, Stark A, Kitchens B, et al. CD2 engagement induces dendritic cell activation: implications for immune surveillance and T-cell activation. Blood. 2003;102:1745–52. doi: 10.1182/blood-2002-07-2206. [DOI] [PubMed] [Google Scholar]
- 26.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–85. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen AI, McAdam AJ, Buhlmann JE, et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–98. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 28.Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173:6542–6. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 29.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells. synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 31.Crawford K, Gabuzda D, Pantazopoulos V, Xu J, Clement C, Reinherz E, Alper CA. Circulating CD2+ monocytes are dendritic cells. J Immunol. 1999;163:5920–8. [PubMed] [Google Scholar]
- 32.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]