Abstract
Neutrophils are a normal constituent of the female reproductive tract and their numbers increase in the late secretory phase of the menstrual cycle prior to menses. Several cytokines are produced in female reproductive tract tissue. In particular granulocyte–macrophage colony-stimulating factor (GM-CSF), a potent activator of neutrophils, is secreted in high concentrations by female reproductive tract epithelia. We previously observed that GM-CSF synergizes strongly with interleukin-8 (IL-8) in enhancing chemotaxis of neutrophils. Thus we investigated whether pretreatment of neutrophils with GM-CSF would prime subsequent chemotaxis to IL-8 in the absence of GM-CSF. Surprisingly, a 3-hr pulse of GM-CSF severely diminished chemotaxis to IL-8, whereas N-formyl-methyl-leucyl-phenylalanine (fMLP)-mediated chemotaxis was retained. Conversely, when cells were incubated without GM-CSF they retained IL-8-mediated migration but lost fMLP chemotaxis. These changes in chemotaxis did not correlate with expression of CXCR1, CXCR2 or formyl peptide receptor. However, IL-8-mediated phosphorylation of p44/42 mitogen-activated protein kinase was greatly reduced in neutrophils that no longer migrated to IL-8, and was diminished in cells that no longer migrated to fMLP. Oestradiol, which is reported by some to exert an anti-inflammatory effect on neutrophils, did not change the effects of GM-CSF. These data suggest that neutrophil function may be altered by cytokines such as GM-CSF through modulation of signalling and independently of surface receptor expression.
Keywords: chemotaxis, granulocyte–macrophage colony-stimulating factor, interleukin-8, N-formyl-methionyl-leucyl-phenylalanine, mitogen-activated protein kinase, signalling
Introduction
Chemokines, a class of cytokines that induce cellular migration, are crucial players in initiating both innate immunity and the adaptive immune response. Neutrophils, the key cellular mediators of early, non-specific defence against microbes, are recruited to sites of injury and potential infection by a chain response initiated by sentinel neurones and tissue mast cells.1 A series of very early inflammatory events induces activation of tissue and endothelial cells and culminates in production of chemokines such as interleukin-8 (IL-8) that induce migration of neutrophils to the affected site where they inactivate pathogens by phagocytosis or release of microbicides.2,3 Interestingly, neutrophils are also recruited into the tissues of the female reproductive tract during the late secretory phase of the menstrual cycle, prior to menses.4 Although the mechanism driving this influx is incompletely understood it is notable that IL-8 production increases in endometrial tissue at this time.5
Another cytokine with potent effects on neutrophil function is granulocyte–macrophage colony-stimulating factor (GM-CSF), originally described as a myeloid cell growth factor.6 GM-CSF is also referred to as one of the inflammatory cytokines and as such has been found to augment many of the innate protective functions of neutrophils.7,8 This cytokine also prevents neutrophil apoptosis, allowing neutrophils to persist at sites of inflammation.9 GM-CSF is produced by the tissues of the female reproductive tract10 and its synthesis is now known to be hormonally regulated, at least in rodents.11 Female reproductive tract tissues that produce IL-8 are also reported to produce GM-CSF. Indeed, we showed in an earlier report that the potent chemoattractant properties of female reproductive tract epithelial cell secretions were a result of synergy between IL-8 and GM-CSF produced by epithelial cells.12
While cultured primary epithelial cells make IL-8 and GM-CSF in quantities that promote enhanced neutrophil chemotaxis, within the tissue environment other cell types also synthesize chemokines and cytokines and the exact locations and timing of peak IL-8 and GM-CSF synthesis with respect to the menstrual cycle may be very complex. There is evidence that greater amounts of IL-8 are produced by epithelial cells than by the underlying stromal cells.13 Additionally, both these cell types make GM-CSF.11 The chemokine and cytokine environment that exists in the female reproductive tract and the means through which it drives neutrophil functional activation have yet to be elucidated.
In this report we set out to analyse the mechanism whereby GM-CSF enhances neutrophil IL-8-mediated chemotaxis. Surprisingly we found that preactivation of neutrophils with GM-CSF did not augment their response to IL-8. Rather, we observed an inhibition. However this did not appear to extend to chemotaxis mediated by another agent, N-formyl-methionyl-leucyl-phenylalanine (fMLP), even though the receptors for IL-8 and fMLP are both in the G-protein-coupled receptor family.14
Materials and methods
Neutrophil separation and culture
Neutrophils were isolated as described previously12 from venous blood taken from healthy laboratory volunteers. Donors signed an informed consent form approved by the Protection of Human Subjects Committee. All manipulations were performed in a sterile, laminar flow tissue culture hood with sterile reagents. Briefly, red cells were sedimented by the addition of 1 ml Hetasep (Stem Cell Technologies Inc., Vancouver, Canada) per 5 ml blood. The upper, leucocyte-rich fraction was layered onto a discontinuous density gradient of Histopaque 1·077 g/ml (Sigma, St Louis, MO) over Optiprep 1·095 g/ml (Axis Shield, Oslo, Norway) and centrifuged at 500 g for 25 min at room temperature. The neutrophil fraction was recovered from the interface between the Histopaque and Optiprep layers and washed three times in Liebovitz's L-15 medium (Gibco, Grand Island, NY) at room temperature. Purity of the neutrophil preparation was routinely 95% or greater. All separation and culture reagents contained less than 0·01 ng/ml lipopolysaccharide.
Cells were resuspended at 2 × 106 cells/ml in a 1 : 1 mixture of L-15 and Medium 199 (Gibco) supplemented with 10% charcoal-stripped fetal bovine serum (HyClone, Logan, UT) and 50 μg/ml gentamicin (Gibco) (complete medium.) They were then transferred to flat-bottom culture wells. Parallel cultures received either recombinant GM-CSF (Peprotech, Rocky Hill, NJ) at a final concentration of 4 ng/ml or were left untreated. After 3 hr at 37° in a 5% CO2 incubator the cells were washed once and either added to the chemotaxis assay or incubated in complete medium for a further 3 hr before assessment of chemotaxis. Viability was greater than 95% at the end of the culture period.
Chemotaxis assay
A modification of the under-agarose chemotaxis assay as described by Nelson et al. was employed.15 As previously described,12 a sterile, lipopolysaccharide-free, glass bottle containing a 2·4% suspension of UltraPure agarose (Gibco) in sterile Hanks' buffered salt solution was heated in a bath of boiling water to bring the agarose into solution. The agarose was cooled to 65° and an equal volume of complete L-15 medium at 37° was added and mixed immediately. The final agarose concentration was 1·2%. Agarose (3 ml) was dispensed into Falcon 3001 Petri dishes and allowed to solidify. A sterile, stainless-steel punch guided by a template was used to cut circular wells in the agarose and the gel plugs were removed with a sterile needle. The template produced three peripheral wells spaced equally around a central well. The central well was filled with 23 μl chemoattractant and the peripheral wells were filled with 23 μl of the neutrophil suspension. All dilutions were made in complete L-15 medium. The dishes were incubated at 37° in a humid, 5% CO2-gassed incubator for 16 hr. The contents of the dishes were then fixed for 1 hr at room temperature by addition of 1 ml 37% formaldehyde (Fisher Scientific, Pittsburgh, PA.) The agarose layers were removed, the dishes were rinsed in distilled water and the cells were stained with Coomassie Brilliant Blue stain (Sigma) for 30 min. The dishes were then rinsed with distilled water and air-dried.
Quantification of chemotaxis
Chemotaxis was quantified as follows. The area between each peripheral well (neutrophil well) and the central well (chemoattractant well), including the leading edge of the neutrophil well, was photographed in black-and-white using a Nikon Coolpix 5700 digital camera attached to an inverted microscope. The equivalent area on the side of the peripheral well facing away from the central well was also photographed to record non-directional migration. Images were transferred to a Macintosh G3 computer and converted to bitmaps. Neutrophils in a 1·7 × 2·4-mm ‘target region’ at a distance of 1·7 mm ahead of the edge of the peripheral well were enumerated using the NIH ImageJ Particle Analyzer program. In almost all cases, non-directional migration of neutrophils did not extend as far as 1·7 mm and therefore was excluded by counting only cells that reached the ‘target region’. Results are expressed as the mean and standard deviation of triplicate neutrophil wells.
Western blot
Neutrophils were washed and resuspended at 5 × 106 cells/ml in serum-free L-15 medium supplemented with 1 mm phenylmethylsulphonyl fluoride (Sigma) and the cells were incubated at room temperature for 15 min. They were then spun down, resuspended at 1·5 × 107cells/ml and dispensed into microfuge tubes in aliquots of 3 × 106 cells. The cells were stimulated at 37° with 100 ng/ml IL-8 (Peprotech) or 10−8 m fMLP (Sigma) for the time periods indicated. Controls were held at 37° for the duration of the time–course but received no stimulus. The tubes were then transferred to ice and 1 ml of cold phosphate-buffered saline (PBS) containing 500 μm sodium orthovanadate (Sigma) was immediately added. The cells were spun down and the pellets were lysed in Tris-buffered saline (pH 7·4) containing sodium deoxycholate (0·25%), nonidet P-40 (1%), sodium dodecyl sulphate (0·1%), sodium orthovanadate (10 mm), sodium fluoride (0·02 m), β-glycerolphosphate (0·04 m) and Protease Inhibitor Cocktail III (1 : 60) (Calbiochem, La Jolla, CA.) After 30 min on ice the lysates were centrifuged and the supernatants were frozen for later analysis.
Lysates (106 cell equivalents) were analysed by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and electrotransferred onto nitrocellulose membranes. The blots were incubated with an anti-phospho p44/42 mitogen-activated protein kinase (MAPK) antibody (Cell Signaling Technology, Beverly MA) at 4° for 18 hr, washed and incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin G for 1 hr at ambient temperature (Upstate, Charlottesville, VA).12 Phosphorylated p44/42 MAPK was detected using enhanced chemiluminescence and autoradiography. The membranes were also probed by the above method using an antibody to myeloperoxidase (Upstate) to assess gel loading.
Analysis of surface markers by immunofluorescence
Neutrophils (1 × 106) were dispensed into microtitre wells and incubated with agitation at 4° for 1 hr with 1 μg of fluorochrome-labelled antibodies to CXCR1, CXCR2, class I major histocompatibility complex (MHC), CD45 or isotype controls (BD Biosciences Pharmingen, San Diego, CA) in the presence of 4 mg/ml non-specific human immunoglobulin G to block antibody binding to Fc receptors. The cells were then washed in PBS containing 0·5% bovine serum albumin and 0·02% sodium azide and fixed in 2% paraformaldehyde in PBS. Antibody binding was assessed by flow cytometry. For each sample 10 000 events in the granulocyte gate were recorded. The mean fluorescence of samples stained with isotype control antibodies was subtracted from the mean fluorescence of samples stained with specific antibodies. Results are given as mean ± standard deviation of the corrected mean fluorescence of samples from three separate donors.
Results
We have shown previously that IL-8 and GM-CSF exhibit synergy in mediating neutrophil chemotaxis over a range of concentrations including those used in this report.12 This was initially observed using mixtures of recombinant cytokines. More significantly, we also observed that the potent chemoattractant activity of secretions of epithelial cells from the female reproductive tract resulted from synergy between IL-8 and GM-CSF produced by these cells. This indicates that IL-8–GM-CSF synergy is likely to occur in vivo.
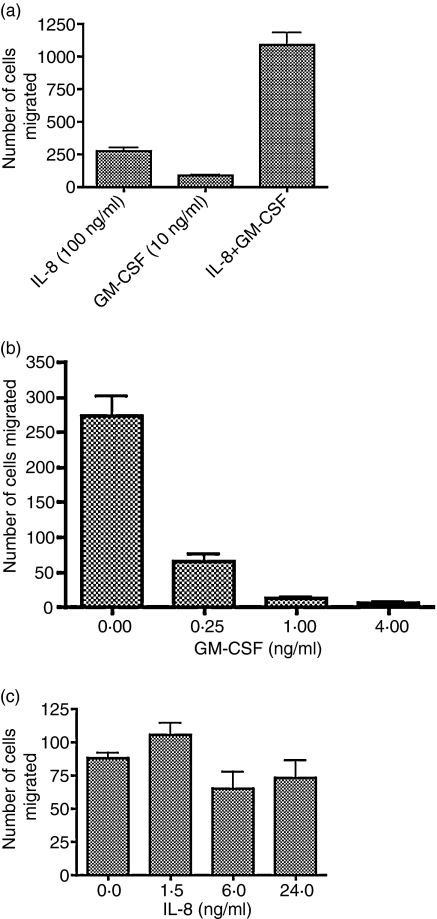
In the present study we set out to address the mechanism of this synergy. Our hypothesis was that GM-CSF, a potent neutrophil activator, enhances the neutrophil chemotactic response to IL-8. Figure 1(a) shows the result of a typical experiment in which neutrophil chemotaxis towards a mixture of IL-8 and GM-CSF (100 ng/ml and 10 ng/ml, respectively) was compared to chemotaxis to either cytokine alone, and confirms our previous observation of a strong synergy between these two compounds in mediating chemotaxis. The same neutrophil preparation was also preincubated with medium or GM-CSF (10 ng/ml) and the subsequent chemotaxis towards IL-8 (100 ng/ml) is shown in Fig. 1(b). Contrary to our expectations, GM-CSF pretreatment did not result in activated cells with greater chemotactic ability. Rather, GM-CSF treatment depressed IL-8-mediated chemotaxis. Figure 1(b) shows the reverse treatment, i.e. the effect of IL-8 pretreatment (100 ng/ml) of neutrophils from the same preparation on chemotaxis to GM-CSF (10 ng/ml). Exposure to IL-8 had no significant effect on GM-CSF-mediated chemotaxis.
Figure 1.
Synergistic effect on neutrophil chemotaxis between IL-8 and GM-CSF cannot be replicated by pretreatment with GM-CSF or IL-8. Neutrophils were prepared as described in the Materials and methods. (a) Neutrophil chemotaxis mediated by IL-8 (100 ng/ml) GM-CSF (10 ng/ml) or a mixture of IL-8 and GM-CSF to the same final concentrations, was assessed as described in the Materials and methods.(a) Neutrophil chemotaxis mediated by IL-8 (100 ng/ml)GM-CSF (10 ng/ml) or a mixture of IL-8 and GM-CSF to the same final concentrations, was assessed as described in the Materials and methods. (b) Neutrophils from the same preparation were preincubated for 3 hr with medium or GM-CSF, then washed and assessed for IL-8-mediated chemotaxis. (c) Neutrophils from the same preparation were preincubated with medium or IL-8, then washed and assessed for GM-CSF-mediated chemotaxis.
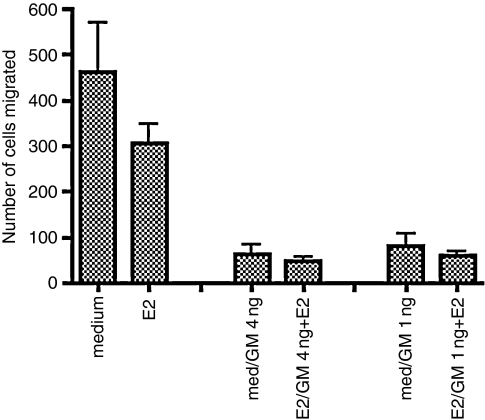
We next examined whether the depression of chemotaxis by GM-CSF was limited to IL-8-mediated migration, or whether this effect extended to other chemoattractants. Figures 2 and 3 show a typical experiment comparing the effect of GM-CSF pretreatment at 4 ng/ml or 1 ng/ml on chemotaxis by neutrophils from the same preparation to IL-8 (100 ng/ml) (Fig. 2) or fMLP (1 × 10−8 m), a formylated peptide representing a class of chemotactic agents derived from bacteria (Fig. 3). In addition, we included oestradiol in some of the pretreatments. Our rationale was that within the tissues of the female reproductive tract, neutrophils would encounter GM-CSF or IL-8 in an oestrogen-enriched environment. Oestradiol has been reported to have an anti-inflammatory effect 16 and it was of interest to determine whether oestradiol might increase the suppressive effect of GM-CSF exposure. Our approach was to culture neutrophils with oestradiol (or medium) for 1 hr, then add GM-CSF (or medium) and culture for a further 3 hr. The cells were then washed once and the chemotaxis assay was performed in the absence of oestradiol and GM-CSF. As shown in Fig. 2, GM-CSF pretreatment dramatically depressed IL-8-mediated chemotaxis and there was no further depression as a result of oestradiol exposure. Oestradiol did not significantly lower chemotaxis in the absence of GM-CSF, although a slight depression was observed.
Figure 2.
Pretreatment of neutrophils with GM-CSF reduces chemotaxis to IL-8 and this is unaffected by oestradiol. Neutrophils were incubated in medium and GM-CSF was added after 1 hr to a final concentration of 4 ng/ml or 1 ng/ml (med/GM 4 ng; med/GM 1 ng) and incubation was continued for 3 hr. Parallel cultures were treated for 1 hr with 10−7 m oestradiol followed by 3 hr with GM-CSF in the continued presence of oestradiol (E2/GM 4 ng + E2; E2/GM 1 ng + E2) Controls were incubated for 4 hr with medium (medium) or 10−7 m oestradiol (E2). The ability of the cells to perform IL-8-mediated chemotaxis was then assessed as described in the Materials and methods using 100 ng/ml IL-8. Data are representative of three independent experiments.
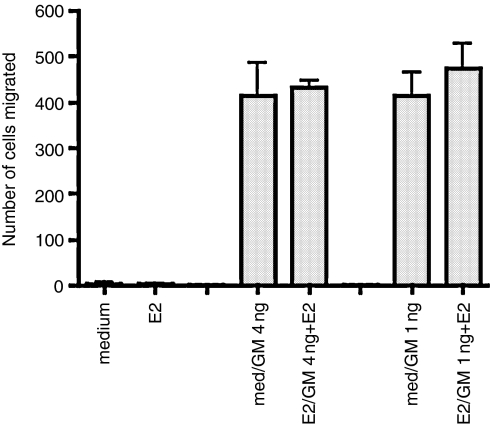
Figure 3.
Pretreatment of neutrophils with GM-CSF preserves fMLP chemotaxis and this is unaffected by oestradiol. Neutrophils from the same preparation used in Fig. 2 were incubated for 1 hr with medium followed by 3 hr with GM-CSF at 4 ng/ml or 1 ng/ml (med/GM 4 ng; med/GM 1 ng) or for 1 hr with 10−7 m oestradiol followed by 3 hr with GM-CSF in the continued presence of oestradiol (E2/GM 4 ng + E2; E2/GM 1 ng + E2) Controls were incubated for 4 hr with medium (medium) or 10−7 m oestradiol (E2). The ability of neutrophils to perform fMLP-mediated chemotaxis was then assessed, as described in the Materials and methods. fMLP was used at 10−8 m. Data are representative of three experiments.
In the same experiment (Fig. 3) we assessed the ability of neutrophils treated as above to migrate up a concentration gradient of fMLP (added at a starting concentration of 10−8 m) We observed that GM-CSF pretreatment did not depress fMLP-mediated chemotaxis, on the contrary, chemotaxis by GM-CSF-treated cells was superior to that of medium-treated cells, which hardly migrated at all. Oestradiol (10−7 m) did not appear to have a suppressive effect on the migration of GM-CSF-treated cells. Since we had observed that freshly isolated cells migrated strongly towards fMLP (unpublished observations) our results suggested that this ability was lost in culture and that GM-CSF maintained fMLP-mediated chemotaxis. As there was no GM-CSF present in the chemotaxis assay our results indicated that the effect of GM-CSF on neutrophils persisted after the cytokine was removed.
Our results showed that a 3-hr exposure to GM-CSF altered the function of neutrophils, maintaining their ability to migrate to fMLP but diminishing the ability to migrate to IL-8. One reason for this observation might be the modulation of expression of receptors for IL-8 and fMLP on neutrophils. We therefore examined the effect of a 3-hr treatment with GM-CSF (4 ng/ml) on neutrophil chemokine receptor expression. In addition, we examined receptor expression after a 3-hr incubation with GM-CSF followed by a further 3-hr incubation in medium. The rationale for this further incubation was that we wished to study neutrophils that most closely resembled those in the agarose wells at the start of chemotaxis. After the addition of cells and chemokines to the agarose plates the diffusion of IL-8 or fMLP to form a concentration gradient for chemotaxis takes a few hr.17 Thus we felt that an additional 3-hr incubation after the removal of GM-CSF would create conditions comparable to those that neutrophils experience in the chemotaxis assay.
The results in Table 1 show that there were no significant differences in expression of CXCR1 and CXCR2 between control neutrophils and any of the GM-CSF-treated samples. While culture for 3 hr with GM-CSF produced a slight increase in formyl peptide receptor expression, a difference between GM-CSF-treated cells and controls was not observed in cells that received an additional 3-hr incubation in medium. All stainings used 1 μg of antibody per 106 cells. From these observations it seemed unlikely that the differences in chemotaxis could be explained on the basis of differences in receptor expression.
Table 1.
Culture with GM-CSF does not alter the expression of chemotactic receptors
| 3 hr medium | 3 hr GM-CSF | 6 hr medium | 3 hr medium+ 3 hr GM-CSF | |
|---|---|---|---|---|
| CXCR | 367 ± 28 | 344 ± 63 | 384 ± 85 | 434 ± 111 |
| CXCR2 | 69 ± 26 | 52 ± 47 | 90 ± 38 | 44 ± 37 |
| FPR | 17 ± 3 | 30 ± 19 | 28 ± 13 | 30 ± 14 |
| MHC I | 114 ± 45 | 136 ± 61 | 117 ± 46 | 111 ± 47 |
| CD45 | 438 ± 147 | 324 ± 188 | 390 ± 174 | 387 ± 221 |
Neutrophils of three donors were treated for 3 hr with medium or GM-CSF (4 ng/ml) and then stained for surface expression of chemotactic receptors CXCR1, CXCR2 and formyl peptide receptor (FPR), and of Class I MHC and CD45 as controls. Separate aliquots of cells were cultured in medium for 6 hr, or in GM-CSF (4 ng/ml) for 3 hr followed by medium for 3 hr, before staining. Results are expressed as mean of MFI of cells from the three donors followed by standard error. Staining used 1 μg of antibody per 106 cells and all values are corrected for isotype control staining.
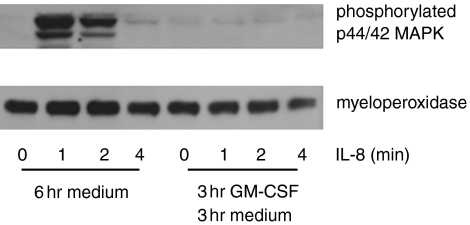
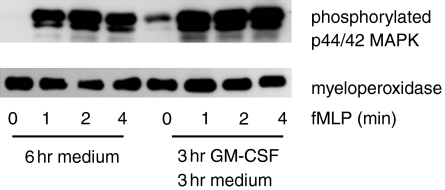
Stimulation of neutrophils through chemokine receptors initiates a signalling cascade involving the phosphorylation of many intracellular proteins that mediate the cellular response to chemokines.18 The phosphorylation of MAPK on threonine and tyrosine appears central to the response to chemokines and cytokines. In particular p44/42 MAPK are phosphorylated in response to both IL-8 and fMLP in neutrophils.19 Since we were unable to account for differences in the chemotaxis of cultured neutrophils by differences in chemokine receptor expression, we sought to determine whether events after receptor ligation could explain our observations. Therefore we examined the phosphorylation of p44/42 MAPK in neutrophils stimulated with IL-8 (100 ng/ml) after a 3-hr incubation with GM-CSF (4 ng/ml) followed by 3 hr in medium. Our rationale was that at least 3 hr elapse between the addition of the IL-8 and neutrophils to their respective wells and the start of neutrophil migration. Thus, after GM-CSF treatment, the neutrophils in the agarose well are exposed to medium alone until IL-8 reaches them by diffusion. We therefore sought to replicate this condition in the phosphorylation studies by giving the GM-CSF-treated neutrophils a 3-hr incubation in the same medium used for the chemotaxis assay, before stimulating them with IL-8 (Fig. 4).
Figure 4.
Phosphorylation of p44/42MAPK in response to IL-8 is reduced by a 3-hr GM-CSF pulse followed by 3 hr in culture. Neutrophils were cultured in GM-CSF (4 ng/ml) or medium for 3 hr followed by culture in medium for a further 3 hr before staining (6 hr medium; 3 hr GM-CSF 3 hr medium.) Following culture the cells were stimulated with IL-8 (100 ng/ml) for 0, 1, 2 and 4 min and assessed for phosphorylation of p44/42 MAPK by Western blot as described in the Materials and methods. The same nitrocellulose membranes were subsequently probed by Western blot for myeloperoxidase. Data are representative of three experiments.
When neutrophils received a 3-hr incubation in medium following GM-CSF, the GM-CSF-treated population no longer phosphorylated p44/42 MAPK in response to IL-8, whereas cells that were not exposed to GM-CSF retained this function. Thus, the reduced migration after GM-CSF treatment appeared to correlate with a loss of IL-8-mediated signalling.
Similar experiments were performed to compare p44/42 MAPK phosphorylation in response to fMLP (10−8 m) after a 3-hr culture with medium following GM-CSF treatment. As shown in Fig. 5, fMLP-stimulated p44/42 MAPK phosphorylation was somewhat greater in neutrophils that were cultured for 3 hr with GM-CSF (4 ng/ml) followed by 3 hr in medium when compared to cells that received identical culture in the absence of GM-CSF. Thus, neutrophils that received GM-CSF migrated robustly in response to fMLP (Fig. 3) and also exhibited somewhat enhanced p44/42 MAPK phosphorylation. However, while medium-treated cells lost the ability to migrate in response to fMLP (Fig. 3) this was not reflected by a complete loss of p44/42 MAPK phosphorylation (Fig. 5).
Figure 5.
Phosphorylation of p44/42MAPK in response to fMLP is enhanced by a 3-hr GM-CSF pulse followed by 3 hr in culture. Neutrophils were cultured in GM-CSF (4 ng/ml) or medium followed by culture in medium for a further 3 hr before staining (6 hr medium; 3 hr GM-CSF 3 hr medium.) Following culture all samples were stimulated with fMLP (10−8 m) for 0, 1, 2 and 4 min and assessed for phosphorylation of p44/42 MAPK by Western blot as described in the Materials and methods. The same nitrocellulose membranes were subsequently probed by Western blot for myeloperoxidase. Data are representative of three experiments.
In all signalling experiments, probing with an antibody to myeloperoxidase showed that the differences in density of the bands corresponding to phosphorylated p44/42 MAPK could not be accounted for by differences in loading of the gel lanes.
Discussion
We present data demonstrating that a 3-hr pulse with GM-CSF altered the functional capacity of neutrophils. The chemotactic response to IL-8 was lost, whereas the fMLP response was maintained following GM-CSF treatment. Neutrophils that were cultured for the same length of time without GM-CSF exhibited the opposite phenotype, characterized by a loss of fMLP-mediated migration and maintenance of IL-8 chemotaxis. Interestingly, these differences appeared to originate at the signalling level and were not linked to expression levels of receptors for IL-8 or fMLP. These observations suggested that as neutrophils aged they could turn into functionally different subpopulations depending on the stimuli they encountered.
We have made the unexpected observation that neutrophil chemotaxis to IL-8 was depressed by prior exposure to GM-CSF. This contrasts with the marked enhancement of IL-8-mediated chemotaxis in the simultaneous presence of GM-CSF,12 which is in agreement with the generally accepted view that GM-CSF augments or primes many neutrophil functions.20 One difference between these contrasting observations was the temporal relationship between GM-CSF-priming/activation and stimulation with the chemotactic agent IL-8. When neutrophils encountered IL-8 and GM-CSF simultaneously, function was stimulated, whereas when there was a delay between GM-CSF treatment and encountering IL-8, function was depressed. However, it was not clear why pretreatment with GM-CSF would result in inhibition of only IL-8-mediated chemotaxis and not fMLP-mediated chemotaxis. One effect of GM-CSF is to increase expression of some adherence molecules on neutrophils, in particular CD11b/CD18, a β2-integrin.21 It is reported that certain adherence molecules can promote CXCR1 phosphorylation.22 This cross-phosphorylation of chemokine receptors has been cited as a means of desensitizing them and it is generally accompanied by receptor internalization and loss from the cell surface.23 However, the down-regulation of chemotaxis we observed was not through reduction of surface IL-8 receptors but appeared to be through a loss of signalling in response to IL-8. Interestingly, binding of a ligand of integrin β1 to neutrophils has been shown to inhibit IL-8-mediated MAPK activation.24 However, such inhibition has not been reported for integrin β2, so we can only speculate as to whether there may be integrin involvement in the GM-CSF depression of IL-8 chemotaxis.
The loss of fMLP-mediated chemotaxis over time in the absence of GM-CSF could have several mechanisms. It is possible that factors necessary to maintain this function are lacking in tissue culture, although it is noteworthy that IL-8 chemotaxis, mediated by a receptor with a common signalling pathway to the formyl peptide receptor, was not lost under the same conditions. Neutrophils have a relatively short half-life in the circulation and eventually lose their chemotactic function and undergo apoptosis.25 It is possible that neutrophils lose their response to some chemokines before others once they start down the path to apoptosis. In this regard it is of interest to note that loss of fMLP-mediated chemotaxis was not reversed by oestradiol, a sex steroid hormone, whereas glucocorticoid steroids are reported to reduce neutrophil apoptosis26
Published reports demonstrate the importance of p44/42 MAPK in chemotaxis, by use of MAPK-inhibiting drugs or MAPK mutants.27–29 However, our observations appeared to indicate that the relationship between phosphorylation and chemotaxis in response to fMLP was not as striking as with IL-8. Although there was greater phosphorylation of p44/42 MAPK in GM-CSF-treated cells that retained their ability to migrate in response to fMLP, the medium-treated cells that did not migrate appreciably still phosphorylated p44/42 MAPK after fMLP stimulation. Since, in our modification of the under-agarose assay, the neutrophils need to migrate extensively (over 1 mm in distance) before they are counted it is possible that a modest impairment of the migration machinery would result in a dramatic reduction in numbers of cells scored. It is also likely that neutrophils encounter fMLP in the agarose gel at lower concentrations than that used to stimulate measurable phosphorylation, because of diffusion within the agarose. These factors could combine to accentuate the difference in fMLP-mediated chemotaxis between medium-treated and GM-CSF-treated cells.
The levels of GM-CSF in blood are usually extremely low,30 whereas certain tissues, such as the epithelia of the female reproductive tract, secrete GM-CSF in very large amounts.10 The concentration of GM-CSF used to treat neutrophils in our experiments (4 ng/ml) is well within this range. It is possible that one of the many actions of GM-CSF on neutrophils could be to down-regulate responses when they are no longer necessary to the role of the cell. For instance, once a cell has crossed the female reproductive tract epithelium, where it would encounter high levels of GM-CSF, it would enter the lumen of the female reproductive tract. In this locale the sole function of the neutrophil would be to destroy micro-organisms. A response to IL-8, which might attract the cell back into the tissue, would be counterproductive. On the other hand it would be important to retain the ability to migrate towards bacteria.
Our studies demonstrated that neutrophil chemotaxis was unaffected by oestradiol treatment either alone or in combination with GM-CSF. This appears in contradiction to the findings of Miyagi et al.31 who reported that neutrophil chemotaxis to fMLP was diminished by oestradiol. One explanation for the differences seen is that these authors used pharmacological concentrations of oestradiol. The level of oestradiol we used is considered to be at the high end of the levels found in female reproductive tract tissue32 and is higher than that found in blood. Measurement of blood neutrophil chemotaxis over the course of the menstrual cycle did not reveal any effects of varying levels of oestrogen.33 While oestradiol may not affect chemotaxis directly it is important to note that oestradiol acts through uterine stromal cells to mediate the secretion of cytokines, and probably chemokines, by epithelial cells.34
In summary, these observations suggest that one of the effects of GM-CSF on neutrophils may be to down-regulate functions such as IL-8-mediated chemotaxis under certain conditions while maintaining responsiveness to bacterial products. Here, the down-regulation was not related to loss of surface IL-8 receptors but to a loss of receptor signalling. It appears that in this system GM-CSF may affect neutrophil chemotactic responses by altering the activation of signalling intermediates upstream of MAP kinases.
Acknowledgments
This work was supported by NIH Program Project Grant number 1 PO1A151877 and NIH award AI43837.
References
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Peterson PK, Verhoef J, Sabath LD, Quie PG. Extracellular and bacterial factors influencing staphylococcal phagocytosis and killing by human polymorphonuclear leukocytes. Infect Immun. 1976;14:496–501. doi: 10.1128/iai.14.2.496-501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci. 2001;58:978–89. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update. 2000;6:16–27. doi: 10.1093/humupd/6.1.16. [DOI] [PubMed] [Google Scholar]
- 5.Arici A, Seli E, Senturk LM, Gutierrez LS, Oral E, Taylor HS. Interleukin-8 in the human endometrium. J Clin Endocrinol Metab. 1998;83:1783–7. doi: 10.1210/jcem.83.5.4754. [DOI] [PubMed] [Google Scholar]
- 6.Rapoport AP, Abboud CN, DiPersio JF. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF). receptor biology, signal transduction, and neutrophil activation. Blood Rev. 1992;6:43–57. doi: 10.1016/0268-960x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan GW, Carper HT, Mandell GL. The effect of three human recombinant hematopoietic growth factors (granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and interleukin-3) on phagocyte oxidative activity. Blood. 1993;81:1863–70. [PubMed] [Google Scholar]
- 8.Kumaratilake LM, Ferrante A, Jaeger T, Rzepczyk C. GM-CSF-induced priming of human neutrophils for enhanced phagocytosis and killing of asexual blood stages of Plasmodium falciparum: synergistic effects of GM-CSF and TNF. Parasite Immunol. 1996;18:115–23. doi: 10.1046/j.1365-3024.1996.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 9.Brach MA, deVos S, Gruss HJ, Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood. 1992;80:2920–4. [PubMed] [Google Scholar]
- 10.Giacomini G, Tabibzadeh SS, Satyaswaroop PG, et al. Epithelial cells are the major source of biologically active granulocyte macrophage colony-stimulating factor in human endometrium. Hum Reprod. 1995;10:3259–63. doi: 10.1093/oxfordjournals.humrep.a135899. [DOI] [PubMed] [Google Scholar]
- 11.Tamura K, Kumasaka K, Kogo H. The expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) and its regulation by ovarian steroids in rat uterine stromal cells. Jpn J Pharmacol. 1999;79:257–62. doi: 10.1254/jjp.79.257. [DOI] [PubMed] [Google Scholar]
- 12.Shen L, Fahey JV, Hussey SB, Asin SN, Wira CR, Fanger MW. Synergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis. Cell Immunol. 2004;230:23–32. doi: 10.1016/j.cellimm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195–204. doi: 10.1016/0303-7207(93)90168-j. [DOI] [PubMed] [Google Scholar]
- 14.Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. J Biol Chem. 1999;274:6027–30. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 15.Nelson RD, Quie PG, Simmons R. Chemotaxis under agarose. a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975;115:1650–66. [PubMed] [Google Scholar]
- 16.Josefsson E, Tarkowski A, Carlsten H. Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell Immunol. 1992;142:67–78. doi: 10.1016/0008-8749(92)90269-u. [DOI] [PubMed] [Google Scholar]
- 17.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–60. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rollet E, Caon AC, Roberge CJ, et al. Tyrosine phosphorylation in activated human neutrophils. Comparison of the effects of different classes of agonists and identification of the signaling pathways involved. J Immunol. 1994;153:353–63. [PubMed] [Google Scholar]
- 19.Torres M, Hall FL, O'Neill K. Stimulation of human neutrophils with formyl-methionyl-leucyl-phenylalanine induces tyrosine phosphorylation and activation of two distinct mitogen-activated protein-kinases. J Immunol. 1993;150:1563–77. [PubMed] [Google Scholar]
- 20.DiPersio JF. Colony-stimulating factors: enhancement of effector cell function. Cancer Surv. 1990;9:81–113. [PubMed] [Google Scholar]
- 21.Yong KL, Linch DC. Differential effects of granulocyte- and granulocyte-macrophage colony-stimulating factors (G- and GM-CSF) on neutrophil adhesion in vitro and in vivo. Eur J Haematol. 1992;49:251–9. doi: 10.1111/j.1600-0609.1992.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 22.Stanton KJ, Frewin MB, Gudewicz PW. Heterologous desensitization of IL-8-mediated chemotaxis in human neutrophils by a cell-binding fragment of fibronectin. J Leukoc Biol. 1999;65:515–22. doi: 10.1002/jlb.65.4.515. [DOI] [PubMed] [Google Scholar]
- 23.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–11. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 24.Xythalis D, Frewin MB, Gudewicz PW. Inhibition of IL-8-mediated MAPK activation in human neutrophils by beta1 integrin ligands. Inflammation. 2002;26:83–8. doi: 10.1023/a:1014836211643. [DOI] [PubMed] [Google Scholar]
- 25.Whyte MK, Meagher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–34. [PubMed] [Google Scholar]
- 26.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–8. [PubMed] [Google Scholar]
- 27.Kuroki M, O'Flaherty JT. Differential effects of a mitogen-activated protein kinase kinase inhibitor on human neutrophil responses to chemotactic factors. Biochem Biophys Res Commun. 1997;232:474–7. doi: 10.1006/bbrc.1997.6296. [DOI] [PubMed] [Google Scholar]
- 28.Hinton DR, He S, Graf K, Yang D, Hsueh WA, Ryan SJ, et al. Mitogen-activated protein kinase activation mediates PDGF-directed migration of RPE cells. Exp Cell Res. 1998;239:11–15. doi: 10.1006/excr.1997.3873. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Liu J, Segall JE. MAP kinase function in amoeboid chemotaxis. J Cell Sci. 1998;111:373–83. doi: 10.1242/jcs.111.3.373. [DOI] [PubMed] [Google Scholar]
- 30.Strandell A, Thorburn J, Wallin A. The presence of cytokines and growth factors in hydrosalpingeal fluid. J Assist Reprod Genet. 2004;21:241–7. doi: 10.1023/B:JARG.0000042009.93520.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyagi M, Aoyama H, Morishita M, Iwamoto Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J Periodontol. 1992;63:28–32. doi: 10.1902/jop.1992.63.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Baird DT, Fraser IS. Blood production and ovarian secretion rates of estradiol-17beta and estrone in women throughout the menstrual cycle. J Clin Endocrinol Metab. 1974;38:1009–17. doi: 10.1210/jcem-38-6-1009. [DOI] [PubMed] [Google Scholar]
- 33.Giuliani A, Mitterhammer H, Burda A, Egger G, Glasner A. Polymorphonuclear leukocyte function during the menstrual cycle and during controlled ovarian hyperstimulation. Fertil Steril. 2004;82:1711–13. doi: 10.1016/j.fertnstert.2004.05.091. [DOI] [PubMed] [Google Scholar]
- 34.Grant-Tschudy KS, Wira CR. Effect of oestradiol on mouse uterine epithelial cell tumour necrosis factor-alpha release is mediated through uterine stromal cells. Immunology. 2005;115:99–107. doi: 10.1111/j.1365-2567.2005.02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]