Abstract
The chemokine receptor CCR7 is crucial for migration of mature dendritic cells (DC) directed toward secondary lymphoid organs; however, there is little knowledge about the function of the homeostatic chemokine receptor CXCR4 in DC and its contribution to directional migration of DC during inflammation. By comparing the impact of chemokine receptor engagement on mature DC we found that the CCR7 ligand CCL19 holds a stronger chemotactic potency than the CXCR4 ligand CXCL12. Moreover, CCL19 elicited rapid, steep and long-lasting mobilization of intracellular calcium in individual cells and induced intense phosphorylation of extracellular signal-regulated kinase 1/2 and protein kinase B, while the intracellular signals elicited by CXCL12 were in part distinct and significantly weaker. Analysis of chemokine receptor expression revealed that although CCR7 and CXCR4 were expressed by a similar percentage of DC, the mean fluorescence intensity of CCR7 was up to six times higher, suggesting a higher receptor density. Based on these correlations we propose that the type of chemokine signal in conjunction with the expression and functional activity of the respective chemokine receptor is also determining the migration rate and potency of a chemotactic response in mature DC. In conclusion, our data support the fundamental role of CCR7 for rapidly guiding DC toward secondary lymphoid organs at an extra- and intracellular molecular level and on the contrary render CXCR4 a weaker contributor to directional migration of DC during inflammation.
Keywords: chemokines, chemokine receptor, dendritic cell, migration, signalling
Introduction
Chemokines constitute a family of structurally related small proteins that are crucial for directional trafficking and positioning of leukocytes. The chemokine family can be divided into two major subfamilies depending on the position of the two N-terminal cysteine residues, CXC chemokines which have one amino acid in between the cysteines and CC chemokines which do not. Their function on leukocytes is mediated by interaction with hepta-helical transmembrane G protein-coupled receptors, which is followed by intracellular calcium mobilization and activation of signalling pathways promoting actin polymerization and directional migration. Apart from regulation of cell traffic during an inflammatory response, chemokines also play an important role in haematopoietic development, angiogenesis, metastasis and constitutive cell traffic.1–4
The function of dendritic cells (DC), a system of professional antigen-presenting cells,5,6 is essentially connected to their ability to migrate along a gradient of chemotactic factors and chemokines. DC precursors are recruited from the blood periphery into the tissues where they are surrounded by a distinct cytokine milieu that promotes differentiation into DC with an immature phenotype.7–9 These are attracted to sites of infection and inflammation in response to chemotactic factors and chemokines.10,11 Upon exposure to inflammatory signals, DC rapidly switch their chemokine receptor repertoire with down-regulation of inflammatory chemokine receptors, which enables the maturing DC to leave the site of inflammation. Simultaneously, DC up-regulate the chemokine receptors CCR7 and CXCR4 and gain responsiveness to the CCR7 ligands secondary lymphoid organ chemokine (SLC, also CCL21) and macrophage inflammatory protein-3β (MIP-3β, also CCL19). During emigration from the periphery, CCL21 is responsible for guiding the maturing DC into the lymphatic vessels, while CCL19, in collaboration with CCL21, drives further migration to the T-cell zones of lymphoid tissues.12–21 Furthermore, evidence was recently provided that CCR8 also plays a specific role in the migration of monocyte-derived DC directed toward lymph nodes.22
Because of the impaired migration of activated DC from CCR7-deficient mice to draining lymph nodes23 and the accurate anatomical localization of the ligands of CCR7, up-regulation of CCR7 is regarded as the essential step for the migration of mature DC directed toward secondary lymphoid organs.23–27 Hence, the contribution of stromal cell-derived factor-1 (SDF-1, also CXCL12) and its receptor CXCR4 to the directional migration of mature DC has been barely investigated and is still far from being well defined. To date, the CXCL12–CXCR4 axis has been commonly associated with basal cell traffic and developmental processes, as CXCR4 is expressed by many cell types and its ligand CXCL12 is known to be expressed in various tissues, even in secondary lymphoid organs.28–34 Of note, functional CXCR4 is readily present on the surface of immature DC and is further up-regulated upon maturation. In contrast, CCR7 is hardly detectable on immature DC, though it is vigorously up-regulated under inflammatory conditions.11–13,17,35,36
To obtain insights into the selective contribution of CCR7 and CXCR4 to directional migration of DC during inflammation we compared the surface expression, intracellular signalling pathways and in vitro chemotactic responses mediated through these chemokine receptors. We found a strong correlation between different functional levels of CCR7 and CXCR4 in mature DC, which in conclusion highlights the pivotal role of CCR7 for rapidly guiding mature DC to secondary lymphoid tissues at an extra- and intracellular molecular level. On the other hand, our data suggest that the CXCL12–CXCR4 axis might be much less important for this process when compared to CCR7 at a quantitative level. Regarding these correlations we also propose that the outcome of chemokine-induced migration is regulated by differential surface densities of chemokine receptors which influence the rate and potency of a chemotactic response in mature DC.
Materials and methods
Culture media and reagents
The medium used for DC generation from leukapheresis products was RPMI-1640 (Bio Whittaker, Walkersville, MD) supplemented with 1% of heat-inactivated autologous plasma, 2 mM L-glutamine (Bio Whittaker) and a penicillin–streptomycin mixture with 100 IU/ml penicillin and 100 μg/ml streptomycin (Gibco, Invitrogen, Karlsruhe, Germany). For generation of DC from buffy coats X-Vivo 15 (Bio Whittaker) supplemented with 1% of heat-inactivated pooled human plasma, 2 mM L-glutamine and penicillin–streptomycin was used. CCL3, CCL19 and CXCL12 were purchased from PeproTech (London, UK).
DC generation from buffy coats and leukapheresis products
Leukapheresis products and buffy coats of healthy donors were prepared according to institutional guidelines. Human monocytes from peripheral blood mononuclear cells were obtained by Ficoll gradient separation using Lymphoprep (Axis-Shield, Oslo, Norway) and by depletion of non-adherent cells after incubation for 1 hr in cell factories (Nunc, Roskilde, Denmark). Remaining cells were cultured with the appropriate medium at a final concentration of 5 × 105 cells/ml. Generation of immature DC from peripheral blood monocytes was achieved by adding 1000 IU/ml of granulocyte–macrophage colony-stimulating factor and 800 IU/ml of interleukin-4 (both from Novartis Pharma, Nuremberg, Germany) every 2 days. Maturation was induced after 6 days of cultivation by stimulation with a cocktail consisting of 10 ng/ml interleukin-1β, 1000 IU/ml interleukin-6 (Strathmann, Hamburg, Germany), 1 μg/ml prostaglandin E2 (Minprostin®; Pharmacia & Upjohn, Erlangen, Germany) and 10 ng/ml tumour necrosis factor-α (Bender, Vienna, Austria). Cells were harvested 36–48 hr after stimulation with the maturation cocktail and used for experiments.
Antibodies and flow cytometry
The following monoclonal antibodies (mAb) were used to study surface expression of chemokine receptors and maturation markers: phycoerythrin (PE)-conjugated mouse anti-human CD86 (BU63) and mouse anti-human CD83 were from Chemikon (Hofheim, Germany). PE-conjugated mouse anti-human CXCR4 (12G5) was from BD Pharmingen (Hamburg, Germany). A rat anti-human CCR7 antibody was kindly provided by Reinhold Förster and Markus Lipp from the Max-Delbrück-Centre for Molecular Medicine (MDC), Berlin, Germany. For visualization, this antibody was stained with a PE-conjugated goat anti-rat immunoglobulin antibody from BD Pharmingen (Hamburg, Germany) as secondary antibody, which was also used as control antibody without previous staining with CCR7. Isotype control antibodies were PE-conjugated murine immunoglobulin G1 (IgG1; BD Pharmingen) and IgG2a (Chemikon). For surface staining, cultured cells were washed, suspended at 3 × 105 in 150 μl of cold phosphate-buffered saline (PBS) containing 0·1% sodium azide and 1% human serum albumin (HSA). Subsequent staining with labelled mAb or appropriate isotype controls was performed for 30 min. Cells were washed twice, and re-suspended in 300 μl of cold PBS containing sodium azide and HSA. Stained cells were analysed for three-colour fluorescence with a FACSscan cell analyser (Becton-Dickinson, Mountain View, CA). Cell debris was eliminated from the analysis using a gate on forward- and side-scatter. Results were processed using CELL-QUEST software (Becton-Dickinson).
Chemotaxis assay
Migration assays were performed by using a 96-transwell chemotaxis chamber (Chemo TX system MBA96, Neuro Probe, Gaithersburg, MD) with polycarbonate filters (5-μm pore size). In brief, 410 μl of either CCL3, CCL19 or CXCL12 diluted in RPMI-1640 at various concentrations up to 200 ng/ml, or as a control RPMI-1640 alone, were placed in the lower wells. Upper wells were loaded with 200 μl cell suspensions of mature DC at a concentration of 5 × 105/ml in RPMI-1640. Each condition was set up in triplicate. The complete chamber was kept at 37° in the incubator for 90 min. After that, cell suspensions in the upper wells were removed and cells that had migrated through the filter to the lower wells were counted completely by a flow cytometer. DC were identified according to size and granularity by using the forward and sideward scatter.
Video-microscopic measurement of intracellular calcium elevation
For the microscopic analysis of intracellular calcium increase a T.I.L.L.-Photonics digital video imaging system, consisting of a CCD camera and a monochromator connected to an inverted fluorescence microscope (Olympus, IX-71), was used. Mature, monocyte-derived DC (106 cells) were loaded with the calcium sensitive dye FURA2-AM (Sigma, Deisenhofen, Germany) at a concentration of 1 μM for 20 min. Then, 300 μl of a suspension of FURA2-AM-labelled DC at a concentration of 2 × 106/ml were placed on poly-L-lysine-coated slides and cells were left untreated in the heated chamber for 3–5 min to allow for adhesion to the surface of the slides. Adherent cells were superfused with RPMI-1640 using a peristaltic pump (Ismatec, Wertheim, Germany) which was connected to the heated incubation chamber. Measurement was started with a constant flow rate of 500 μl/min for 20 cycles (1 cycle = 1 image/second). Mature DC were stimulated with the indicated chemokines at various concentrations ranging from 50 to 500 ng/ml for another 100 cycles by adding the respective chemokine to the RPMI-1640 until the desired concentration was reached, then switching the pump to RPMI-1640 alone to rinse out any remaining chemokines until the measurement was finished at 400 cycles. The collected single frames were combined to produce a moving film using TILLVISION software from Photonics. The ratio of the FURA2-AM emission wavelengths of 355 nm and 380 nm was calculated and the calcium signalling curve was calculated for the total measuring time, representing the calcium response of each single DC.
Calculation of calcium signalling parameters
To determine the relative calcium concentrations (relC) and increase elicited by chemokine receptor triggering, expressed as Δ area, the total area below the signalling curve was subtracted from the baseline area. The baseline was defined as the mean value of the first 100 cycles. The formula for this being: Δ area = total area − baseline area = (x1 + x2 + xn + ċ + x400) − {[(x1 + x2 + xn + ċ + x100)/100] × 400}. To compare the characteristics of the individual calcium fluxes, cells that did not show a response over the mean baseline were excluded from analysis. The Δ peaks of each calcium curve were calculated by subtracting the peak value from the baseline value, which was defined as the value just before the rise of the calcium curve (Δ peak = total peak − baseline peak). The Δ time, defined as the time needed for increasing calcium concentrations from the baseline value to the peak value, was obtained by subtracting the peak time-point from the baseline time-point: Δ time = t(peak) − t(baseline). The velocity v of the relative calcium increase to the peak value was calculated as follows: v = Δ peak/Δ time and is expressed as relative increase of calcium concentration per second (relC/second).
Phosphorylation assay and Western blot
Mature monocyte-derived DC were harvested and brought up to a concentration of 1 × 106 cells/ml in RPMI-1640. DC were kept in the incubator at 37° for another 120 min to allow for down-regulation of intracellular signalling pathways. Then DC were stimulated with various chemokines at concentrations from 50 to 100 ng/ml for 3 min. Stimulation was terminated by subsequent addition of ice-cold PBS and immediate centrifugation at 32 914 g Supernatants were removed and pellets were immediately frozen in liquid nitrogen and kept at − 80°. The following antibodies and secondary reagents were used for Western blotting: monoclonal mouse β-actin 1 : 5000 (Sigma, Deisenhofen, Germany), polyclonal rabbit ERK1/2 and monoclonal P-ERK1/2 (Thr202/Tyr204) 1 : 1000 (Cell Signaling, Beverly, MA), goat anti-rabbit HRP 1 : 1000 to 1 : 5000 (Dianova, Hamburg, Germany), rabbit anti-mouse IgG HRP 1 : 1000 to 1 : 5000 (Dianova), mouse monoclonal P-PKB (Ser473) 1 : 1000 (Cell Signaling), rabbit polyclonal PKB 1 : 1000 (Cell Signaling). Samples were treated with lysis buffer (40 mM Tris–base, pH 10·5, 1% Triton X-100, phenylmethylsulphonyl urea 1 mM) and the total amount of protein was assessed according to Bradford. Samples were then resolved in sodium dodecyl sulphate (SDS)-loading buffer (100 mM Tris–HCl, pH 6·8, 2% SDS, 10 mM dithiothreitol) and boiled at 95° for 10 min. Then, 100 μg protein per probe was separated on an SDS–polyacrylamide gel. After blotting on polyvinylidene fluoride (PVDF) membrane (Millipore, Schwalbach, Germany), proteins were detected by the appropriate antibodies (usually diluted in PBS containing 3% milk). Chemoluminescence was measured with Supersignal (Pierce, Rockford, Il, USA) on Biomax ML films (Kodak). The luminosity of the samples was assessed by densitometric scanning and is expressed in arbitrary units (AU).
Statistical analysis
One-way analysis of variance (anova), combined with the multiple comparison test according to Bonferroni and Student's t-test, was used to detect statistical significant differences. P < 0·05 was regarded as statistically significant.
Results
Chemokine-induced migration of mature DC toward CCL19 and CXCL12
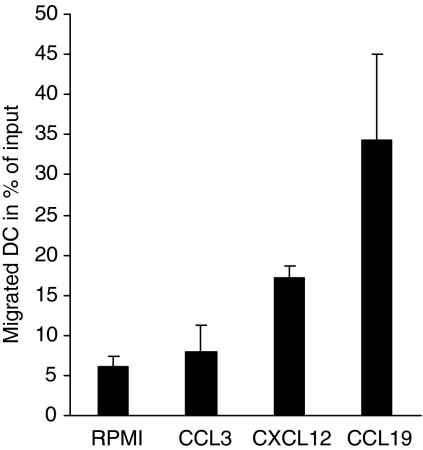
The migratory functionality and chemotactic responsiveness of mature DC to chemokines was examined in a 96-transwell chemotaxis chamber. As recently described, mature DC migrate in response to the CCR7 ligand CCL19 and the CXCR4 ligand CXCL12, whereas migration toward inflammatory chemokines such as CCL3 is abolished as a result of a chemokine receptor switch during the maturation process.12,13,17,26,35 RPMI-1640 containing the probed chemokines at equal and optimal concentrations of 200 ng/ml was loaded into the lower chamber and mature DC were incubated in the upper chamber for 90 min to allow transmigration to occur through the filter. Entire populations that migrated to the lower wells were counted by flow cytometry. The application of lower concentrations resulted in a lower overall migratory response, but there was still a comparable discrepancy between CCL19 and CXCL12 (up to 23% for CCL19 and up to 6% for CXCL12 with 100 ng/ml, data not shown). Figure 1 shows that CCL19 induced a more potent chemotactic response in mature DC compared to CXCL12, assessed by the number of migrated DC within a defined time frame, while little or no migration was observed in response to CCL3.
Figure 1.
Differential chemotactic responses toward CCL19 and CXCL12. The chemotactic responsiveness of mature DC toward the CCR7 ligand CCL19 and the CXCR4 ligand CXCL12 was tested by performing migration assays using 96-well chemotaxis chambers with 5-μm pore polycarbonate filters. Mature monocyte-derived DC (105 cells) suspended in medium were loaded into the upper well and RPMI-1640 containing the probed chemokines at a concentration of 200 ng/ml was placed in the lower wells, while each condition was set up in triplicate. DC that had migrated through the filter to the lower wells were collected after 90-min incubation and counted completely by flow cytometry. One representative experiment of three independent experiments with similar results is shown. Data represent the mean + SD of triplicates.
Chemokine receptor triggered calcium signals of individual DC
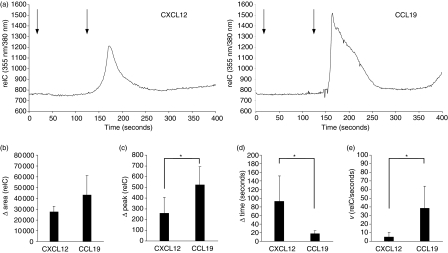
The observation described above led us to go into further detail with our investigations regarding possible functional differences of CCR7 and CXCR4 in mature DC. Chemokine receptor stimulation is followed by mobilization of intracellular free calcium.37,38 To address the question whether there are different signal intensities and patterns upon stimulation of CCR7 and CXCR4 in mature DC, we established a video-microscopic method for single cell calcium release assays to analyse the response of a single DC over time to different stimuli. Mature DC were loaded with the calcium-sensitive dye FURA2-AM and cell suspensions were placed on poly-L-lysine-coated cover slides for 5 min to facilitate adhesion while the cells were maintained in a heated incubation chamber. A constant flow of RPMI-1640 was generated by a peristaltic pump connected to the incubation chamber. Before starting the measurement non-adherent cells were removed from the visual field by applying the flow at a rate of 500 μl/min for 1 min. Stimulation with the indicated chemokines was performed by adding the respective chemokine to the RPMI-1640 to reach the desired concentrations. Bright-field and fluorescence images for the emission wavelengths of 355 nm and 380 nm were shot every second (one cycle) over a total time of 400 seconds. The collected single frames were combined into a moving image and the ratio of FURA2-AM emission wavelengths of 355 nm and 380 nm was calculated. Figure 2(a) shows a typical calcium response of an individual DC after stimulation with CCL19 and CXCL12 at a concentration of 50 ng/ml. Stimulated at equal chemokine concentrations, CCL19 elicited strong and steep needle-shaped intracellular calcium curves, whereas the calcium response to CXCL12 was wave-shaped, shallow and broad. Further increase of the chemokine concentration up to 200 ng/ml did not basically alter the shape and general appearance of the respective calcium signals, but was followed by a more delayed return to the baseline levels and augmented the percentage of responding DC (see also supplemental data for original traces). Stimulation with the control chemokine CCL3 did not show a significant rise of calcium levels (see also supplemental Fig. 3), indicating specificity for the respective receptor–ligand interactions. The kinetics of the intracellular calcium levels were determined by subtracting the total area below the signal curve from the baseline area (Δ area). A moderately stronger capacity of CCL19 to increase and maintain intracellular calcium levels could be confirmed by comparison of the Δ areas below the signalling curve (Fig. 2b). Stimulation with CCL19 also led to significantly higher Δ peaks, which were calculated for each individual calcium curve by subtracting the total peak value from the baseline value, defined as the value just before the rise of the calcium signal (Fig. 2c). Furthermore, we compared the time needed to reach the peak value and the velocity (v) of calcium increase, defined as the quotient of the Δ peak and Δ time and expressed as relC/second. Intracellular calcium levels reached the peak value in a shorter period of time when stimulated with CCL19 compared to CXCL12, and in addition the velocity of calcium increase was higher, indicating a faster release of intracellular free calcium (Figs 2d,e). Taken together, we can show that the type of calcium response elicited by stimulation of CCR7 was in part remarkably different from that elicited by stimulation of CXCR4 with regard to shape, relative calcium levels, peak values, peak times and velocity of calcium increase.
Figure 2.
Intracellular calcium signals of individual DC induced by ligation of CXCR4 and CCR7. FURA2-AM-labelled mature DC were placed on poly-L-lysine-coated cover slides to facilitate adhesion and were maintained in a heated incubation chamber throughout the measurement. A constant flow of RPMI-1640 at a rate of 500 μl/min was generated by using a peristaltic pump and measurement was started after removal of non-adherent cells from the vision field. Bright-field and fluorescence pictures for the emission wavelengths of 355 nm and 380 nm were recorded once a second (one cycle). The chemokine of interest was added to the RPMI-1640 after 20 cycles, then the system was switched to pure RPMI-1640 after another 100 cycles to rinse out any remaining chemokine until the measurement was finished after a total of 400 cycles. (a)Typical calcium responses of individual DC stimulated either with CXCL12 or CCL19 at a concentration of 50 ng/ml. First arrow indicates the addition of chemokine, second arrow indicates the switch to RPMI-1640 for washout (delayed response in relation to actual addition of chemokines is the result of the configuration of the video-microscopic system).(b) The Δ area below the signal curve was calculated by subtracting the total area from the baseline area, representing the kinetics of the relative intracellular calcium concentrations (relC) after stimulation with the respective chemokines at a concentration of 50 ng/ml.(c) Comparison of the Δ peaks elicited by stimulation with CCL19 and CXCL12, which were obtained by subtracting the total peak value from baseline peak value, thus representing the maximum rise of calcium concentrations after chemokine receptor triggering.(d) Comparison of the Δ time, which was defined as the time needed to reach the peak value measured from the baseline value.(e) Comparison of the velocity of calcium increase from the baseline value to the peak value (v = Δ peak/Δ time), expressed as relC/second. Data in (b) to (e) are presented as the mean + SD of the indicated numbers of DC (CCL19: n = 5, CXCL12: n= = 4; *P < 0·05). Data are representative for one of three independent experiments with similar results.
Figure 3.
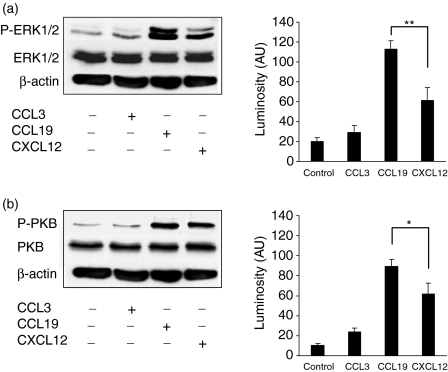
Activation of intracellular signalling pathways following stimulation with CCL19 and CXCL12. Mature DC were stimulated with 50 ng/ml of the indicated chemokines for 3 min. To assess the signalling capacity of the respective chemokine receptors, Western blots against PKB, ERK1/2 and β-actin, and the phosphorylated isoforms of PKB (P-PKB) and ERK1/2 (P-ERK1/2) were performed. (a) Representative Western blot showing phosphorylation of ERK1/2 of unstimulated control and after chemokine receptor stimulation with CCL3, CCL19 and CXCL12 and the respective luminosity (AU) of phosphorylated ERK1/2 presented as mean + SEM of five independent experiments (**P < 0·01).(b) Representative Western blot showing phosphorylation of PKB of unstimulated control and after chemokine receptor stimulation with CCL3, CCL19 and CXCL12 and the respective luminosity (AU) of phosphorylated PKB presented as mean + SEM of four independent experiments (*P < 0·05).
Activation of intracellular signalling pathways following ligation of CCR7 and CXCR4
Next we wondered whether the different calcium responses elicited by ligation of CCR7 and CXCR4 with their respective ligands are also reflected at other steps of intracellular signalling. Chemokines transmit their signal through interaction with G-protein-coupled receptors, which usually involves activation of the phosphoinositol-3-kinase-γ (PI3-Kγ) and the mitogen-activated protein kinase (MAPK) pathways39–47. Therefore we tested phosphorylation of protein kinase B (PKB), a major target of the PI3-Kγ, and phosphorylation of extracellular signal-regulated kinase-1 and -2 (ERK1/2) as representative steps of the MAPK pathway. Mature DC were stimulated with the indicated chemokines at a concentration of 50 ng/ml for 3 min. Cells were prepared to perform Western blots of PKB, ERK1/2 and the phosphorylated isoforms of these kinases, while β-actin was used as loading control. As shown in Fig. 3(a,b), CCL19 induced intense phosphorylation of PKB and both ERK1 and ERK2 in mature DC, whereas the signal strength elicited by CXCL12, especially phosphorylation of ERK1, was significantly lower. Stimulation with CCL3, used as control chemokine, did not enhance signal strength beyond baseline phosphorylation activity, indicating specific engagement of CCR7 and CXCR4 with their respective ligands. Comparable results were obtained by stimulation with CCL3, CCL19 and CXCL12 at a concentration of 100 ng/ml (data not shown).
Surface expression and density of CCR7 and CXCR4 on mature DC
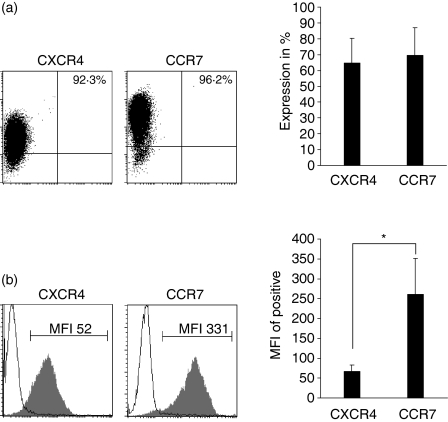
To find a possible explanation for the differential chemotactic responsiveness and intracellular signalling capacity of CCR7 and CXCR4 in mature DC, we determined the surface expression and the relative surface density of these chemokine receptors by flow cytometry. Mature DC (95–99% positive for the maturation markers CD83 and CD86, and negative for the CCL3 receptors CCR1 and CCR5, data not shown) were stained with antibodies to CXCR4 and CCR7, respectively. DC were usually taken from the same culture and at the same time-point as had been used for migration assays, representing maturation and receptor status of mature DC during migration. Figure 4(a) shows that CCR7 and CXCR4 are expressed by a similar percentage of DC. Yet, comparison of the mean fluorescence intensity (MFI), a relative measure of the surface density on a cellular level, revealed that the MFI of CCR7 was up to six times higher than that of CXCR4 (Fig. 4b), strongly suggesting a higher surface density of CCR7 on mature DC. Since our data were obtained by comparison of the binding properties of two antibodies specific to different surface molecules, which might exhibit different affinities for their target or may not recognize all conformational states, this approach might not be sufficient to securely prove that mature DC have a higher surface density of CCR7 compared to CXCR4, although the differences in the MFI are quite impressive. However, our suggestion can be substantially underlined by data from a recent work, where it was shown that at the level of mRNA transcripts CCR7 expression by far exceeds the expression of CXCR4 in mature DC.12 Taken together, the assumed differential surface density of both chemokine receptors was correlated to their functional activity measured by the potency of chemokine-induced directional migration and to the intracellular signalling capacity of the respective chemokine receptors.
Figure 4.
Surface expression and density of CCR7 and CXCR4 on mature DC. DC were stained with antibodies to the chemokine receptors CXCR4 and CCR7, and surface expression and staining intensity was analysed by flow cytometry. (a) Representative dot plot showing the percentage of positively stained DC, which is indicated in the respective histogram and statistical summary of four to six independent measurements presented as the mean + SEM (P > 0·8).(b)Representative histograms of CXCR4 and CCR7 expression of mature DC (filled area) compared to isotype control (black line) showing the mean fluorescence intensity (MFI) and statistical summary of four to six independent measurements presented as mean + SEM (*P < 0·05).
Discussion
Up-regulation of CCR7 is regarded as a crucial event for the trafficking and entering of activated DC to secondary lymphoid organs throughout an inflammatory response. However, the role of CXCR4 and its contribution to directional migration of DC during inflammation has been scarcely explored, also in part because CXCR4- and CXCL12-deficient mice die within the perinatal period.29,48 To address the question of which differences exist between CCR7 and CXCR4 in mature DC, we compared surface expression and relative density, receptor-mediated activation of intracellular signalling pathways and the chemotactic function of these chemokine receptors. First, we found that the chemotactic responsiveness toward the CCR7 ligand CCL19 is more potent compared to the response to the CXCR4 ligand CXCL12 as assessed by the count of migrated DC within a defined time-frame. This finding could be further linked to a stronger and partly distinct capability of CCL19 to induce mobilization of intracellular calcium and to activate intracellular signalling pathways compared to CXCL12. Finally, we found a close correlation between these parameters and the mean fluorescence intensity of the respective receptors, strongly suggesting a higher surface density of CCR7 on mature DC.
Triggering of chemokine receptors is known to rapidly activate the phospholipase Cβ2 and β3 isoforms which leads to a transient rise of intracellular free calcium via formation of inositol-1,4,5-triphosphate.42 Challenging the requirement of this pathway for chemotaxis, it was recently shown that elevations of intracellular calcium are not necessary for directional migration.42 Nonetheless, calcium signalling is commonly used to test the responsiveness of chemokine receptors to their respective ligands. However, most studies referring to the analysis of chemokine-receptor-triggered calcium responses were applied to total populations, which is a summation of all signal vectors. This provides good information on the general signal strength and changes, but misses information about the characteristics of the elicited signal. Indeed, we can show on a single cell level that the type of calcium signal elicited by stimulation of CCR7 was remarkably different from that elicited by stimulation of CXCR4 with regard to shape, relative increase, peak values, peak times and velocity of calcium increase. Because a rise in intracellular calcium appears to be dispensable for the outcome of a chemotactic response, these findings do not explain the differential chemotactic responsiveness mediated through CCR7 and CXCR4 which we observed, but might implicate quantitative differences at the level of receptor expression.
One of the most established downstream effectors of chemokine receptors is the PI3-Kγ and its target the PKB. Although contradictory studies exist, it is widely accepted that this pathway is very important, if not a prerequisite, for the outcome of a chemotactic response.41–44 By comparing the efficiency of CCR7 and CXCR4 in mediating downstream signals we can show a significantly stronger activation of PKB through CCR7 in mature DC, which could explain the different chemotactic responsiveness elicited by CCL19 and CXCL12. According to the stronger capability of CCL19 to induce activation of PKB, we can also show differences in the capacity of CCR7 and CXCR4 to induce activation of ERK1 and ERK2. However, the role of the MAPK pathways in determining the outcome of a chemotactic response is less clear, although its activation via chemokine receptors is well documented.45–47,49 Several studies showed that the action of ERK mediated by G protein-coupled receptors depends on activation of PI3-Kγ and thus is a direct result of its intrinsic activity.41,50,51 Apart from this, it was demonstrated that this pathway is not essential for directional migration in general.52 The differences in activation of ERK1 and ERK2 that we observed could therefore be a direct consequence of differential PKB activation through CCR7 and CXCR4. Interestingly, the differences were mostly pronounced for ERK1 activation, and were less for ERK2. By providing data that strongly imply that the surface density of CCR7 on mature DC is by far higher than that of CXCR4, it is tempting to propose that the stronger activation of PKB and consecutively of ERK1/2 is a result of the higher receptor density and thus of the amount of functional coupled chemokine receptors.
Taking into account the differences of CXCR4 and CCR7 which we demonstrated at both extra- and intracellular levels, our data might question the importance of CXCR4 for the directional migration of DC under inflammatory conditions. The CXCL12–CXCR4 axis is commonly associated with homeostatic cell traffic, developmental processes and cell survival. CXCR4 is expressed by many cell types and its ligand CXCL12 is known to be found in a large variety of tissues, like bone marrow, liver, lung, skin and secondary lymphoid organs, yet also in inflamed tissues.28–34 A conceivable possibility for its function on mature DC is that CXCR4 is synergising with CCR7 to drive migration forward to the site of T-cell priming, or even that both receptors may operate sequentially, while CXCR4 could be responsible for guiding mature DC to the B-cell areas of secondary lymphoid organs. On the other hand, both chemokines may also act as opponents, whereby CXCL12 expressed in tissues would retain DC at the site of inflammation. Upon activation DC vigorously up-regulate CCR7. However, up-regulation of CXCR4 also occurs during maturation, albeit resulting in a significantly lower receptor density and overall level of function than that of CCR7. Thus, the response to CCL19 could overcome the response to CXCL12 because of higher receptor density by which the overall outcome is migration directed toward secondary lymphoid organs. Apart from interacting with the trafficking properties of DC, CXCR4 could provide important signals for protection against apoptosis during activation and maturation. Conversely, it was recently demonstrated that even in the context of supplying signals for survival, CCR7 appeared to be superior to CXCR4 in fully matured DC.53 In accordance with our results, this finding could also be well explained by differences in the surface density of these two chemokine receptors. As the functionality of both chemokine receptors was only examined in fully mature DC, it remains to be clarified if CXCR4 is responsible for inhibition of apoptosis at the site of inflammation during an early time-point of DC activation, when CCR7 is not yet present on the surface of DC. The precise non-chemotactic function of the CXCL12–CXCR4 axis for recently activated DC is therefore an interesting issue to be explored in future studies.
Collectively, our data emphasize the key role of CCR7 for directional migration of mature DC at an extra- and intracellular molecular level and, in contrast, they query the necessity of CXCR4 for this process. Regarding the correlations between surface density, chemotactic responsiveness and signalling capacity of CCR7 and CXCR4, shown here for the first time, we propose that chemokine-induced migration of mature DC is also regulated through the differential expression of chemokine receptors, thus shaping the outcome of the directional movement. In consequence the nature of a chemokine-induced chemotactic response would not only be determined through the direction, by which responding cells migrate along a gradient of chemokines, but also by the cellular density of chemokine receptors and as a result by the summation of single signals which determine the total signalling strength. Although both receptors are known to share the same intracellular signalling pathways, the overall outcome of the chemokine-induced response was marked by diversity. The clue to this diversity appeared to be dependent on the different amount of these chemokine receptors expressed on a single cell, and consequently is likely to be dependent on the summation of signals elicited by each single receptor.
Acknowledgments
We thank Timo Gaber for critically reading the manuscript. This work was supported by the ELAN Fonds of the University of Erlangen, Germany.
References
- 1.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–20. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 7.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sozzani S, Sallusto F, Luini W, et al. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155:3292–5. [PubMed] [Google Scholar]
- 11.Sozzani S, Luini W, Borsatti A, et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997;159:1993–2000. [PubMed] [Google Scholar]
- 12.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Sozzani S, Allavena P, D'Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–6. [PubMed] [Google Scholar]
- 14.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J Leukoc Biol. 1999;66:252–62. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- 16.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–5. [PubMed] [Google Scholar]
- 17.Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol. 1998;161:3096–102. [PubMed] [Google Scholar]
- 18.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 19.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–63. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunn MD, Kyuwa S, Tam C, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu C, Edwards EW, Tacke F, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–41. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 26.Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol. 1999;162:3859–64. [PubMed] [Google Scholar]
- 27.Ohl L, Mohaupt M, Czeloth N, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–88. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 29.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 30.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–71. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 31.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 32.Pablos JL, Amara A, Bouloc A, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–86. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanki T, Hayashida K, El Gabalawy HS, et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165:6590–8. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- 34.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Nat. Medical. Vol. 10. 2004. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1; pp. 858–64. [DOI] [PubMed] [Google Scholar]
- 35.Delgado E, Finkel V, Baggiolini M, Mackay CR, Steinman RM, Granelli-Piperno A. Mature dendritic cells respond to SDF-1, but not to several beta-chemokines. Immunobiology. 1998;198:490–500. doi: 10.1016/s0171-2985(98)80073-9. [DOI] [PubMed] [Google Scholar]
- 36.Lin CL, Suri RM, Rahdon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur J Immunol. 1998;28:4114–22. doi: 10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 38.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 39.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–34. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Kawasaki H, Nagayama H, et al. Signaling events following chemokine receptor ligation in human dendritic cells at different developmental stages. Int Immunol. 2001;13:167–79. doi: 10.1093/intimm/13.2.167. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–9. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 43.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–53. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 44.Thelen M, Uguccioni M, Bosiger J. PI 3-kinase-dependent and independent chemotaxis of human neutrophil leukocytes. Biochem Biophys Res Commun. 1995;217:1255–62. doi: 10.1006/bbrc.1995.2903. [DOI] [PubMed] [Google Scholar]
- 45.Tilton B, Ho L, Oberlin E, et al. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J Exp Med. 2000;192:313–24. doi: 10.1084/jem.192.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones SA, Moser B, Thelen M. A comparison of post-receptor signal transduction events in Jurkat cells transfected with either IL-8R1 or IL-8R2. Chemokine mediated activation of p42/p44 MAP-kinase (ERK-2) FEBS Lett. 1995;364:211–14. doi: 10.1016/0014-5793(95)00397-r. [DOI] [PubMed] [Google Scholar]
- 47.Ganju RK, Brubaker SA, Meyer J, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–75. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 48.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–8. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–7. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 51.Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann MP. Bifurcation of lipid and protein kinase signals of PI3Kgamma to the protein kinases PKB and MAPK. Science. 1998;282:293–6. doi: 10.1126/science.282.5387.293. [DOI] [PubMed] [Google Scholar]
- 52.Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1997;94:3052–7. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez-Sanchez N, Riol-Blanco L, La RG, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104:619–25. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]