Abstract
CD45 has been recognized as an important player in regulating signalling in lymphocytes. However, compared with tyrosine kinases, phosphatases are still poorly understood in terms of the details of their specificity and regulation. Here, the recent progress in understanding the biology of the first recognized receptor tyrosine phosphatase, CD45, is reviewed.
Keywords: CD45, isoforms, phosphatase, polymorphism, protein structure
Introduction
Despite more than 30 years of research and thousands of publications, key aspects of CD45 biology are still poorly understood. What are CD45's significant physiological substrates, what is the function of different isoforms and how is its activity regulated? There have been many reviews of CD45 written over the years, so this article will focus mainly on new results and on the light that recent advances shed on extant problems. Inevitably the brief format means that much work will be overlooked. For more detailed reviews of the CD45 opus you are referred to earlier articles by Hermiston et al.1 and Alexander.2
CD45 gene and protein structure
One of the most significant advances in the study of CD45 has been the long-awaited publication of a three-dimensional (3D) structure of the cytoplasmic domains.3 Before considering the implications of this study, however, we should first review the state of knowledge. The CD45 gene in mammals has 35 well-characterized exons (not including the recent ‘extra’ one in humans; see below), two of which act as alternative transcription start sites (e1a, e1b); exons 4, 5, 6 and 7 are involved in alternative mRNA splicing (detailed below). CD45 is a type 1 transmembrane glycoprotein, containing a single transmembrane domain. Its cytoplasmic portion consists of tandem protein tyrosine phosphatase (PTPase) domains and a C-terminal tail of ≈ 80 amino acids. Only the membrane proximal PTPase domain is enzymatically active, but both domains are required for function in cells. The extracellular portion of the molecule consists of five structural regions. The N-terminal region does not appear to form a domain structure, rather it is quite extended and bears O-linked glycan chains. It is this region of the molecule that provides all the protein isoform variation. Sequence homology and expression studies strongly suggest that all mammalian CD45 molecules possess four folded domains in their extracellular regions, all of similar size. The three membrane-proximal domains are members of the type III fibronectin (fnIII) domain family, and these three domains are conserved in all species, including hagfish, the most primitive organism in which CD45 has been definitively identified to date.4,5 (A putative CD45 orthologue has been identified in lampreys but there are uncertainties about the sequence; if it is CD45 it has no more than two globular extracellular domains.) Between the extended O-glycan tract and the fnIII domains, there is a cysteine-rich region. In mammals this appears to form a globular domain with a high β-sheet content and lacking alpha helices.6 This domain contains five conserved cysteines in all mammalian CD45 molecules, which form two intradomain disulphide bonds and one interdomain disulphide bond with the adjacent fnIII domain.6 This novel cysteine-rich domain is less well conserved in non-mammalian species, with a considerable variation in length – chicken being the smallest at about half the size of mammals – and a lack of conservation of cysteines (for example, chicken has one, shark three and hagfish five).4,5 Thus, within all species, although the actual amino acid identity is low, the general structure of the extracellular domain is conserved, the main variation being in the length of the O-glycosylated region and the cysteine-rich domain, where differences are seen between major evolutionary groups.
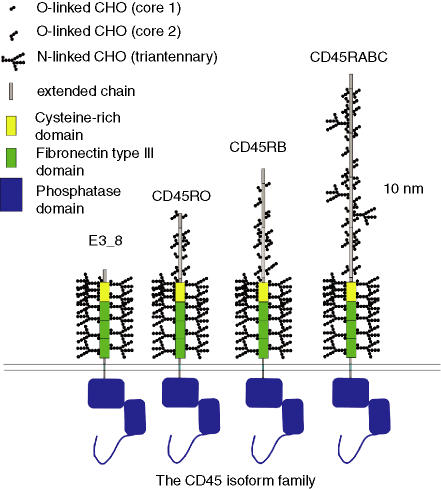
Figure 1 shows a cartoon model of four members of the CD45 isoform family in mammals, drawn to scale as far as possible. The model is based on the available evidence, which consists of electron microscopy (EM) measurements of low-angle rotary shadowed molecules, together with protein biochemistry and expression studies; most of these studies originated from Barclay's group in Oxford.6–8 These data have been combined with sequence analysis using the program NetOGlyc 3·19 and studies of the carbohydrate chains of CD45 by Furukawa and colleagues.10,11 Regrettably, no crystallographic or nuclear magnetic resonance (NMR) data have been obtained for any part of this extracellular region. The EM data depict CD45 as a relatively rigid rod; however, we should expect the actual glycans, and possibly also the O-glycan region (especially its less densely glycosylated segments) to be mobile in solution.
Figure 1.
Cartoon structure of four isoforms of CD45 in mammals. The protein domains are based on electron microscopy (EM) data,7,8 expression studies6 and crystallography.3 The assignment of glycans is not straightforward. No comprehensive data exist to indicate which asparagines, serines and threonines actually carry glycan moieties. In constructing the model therefore, use was made of the available data and of prediction algorithms (NetOGlyc 3.1; CBS9). Barclay et al.103 found that all, bar one, of the asparagines of rat CD45RO was glycosylated. They also determined that the O-glycosylation was confined to the N-terminal region and identified two threonines that appeared to be O-glycosylated; one threonine and two serines were found to be non-glycosylated. NetOGlyc 3.1 made the correct prediction in four of of five cases. Note that the predicted sites are not identical for shared exons as local sequence affects transferase recognition.9 There are also some data on the actual structure of the glycan moieties. Sato et al.11 investigated the structure of N-glycans from human CD45 (derived from peripheral blood mononuclear cells) and Furukawa et al.10 studied the O-linked sugars. The N-glycans were diverse but the major species were tetra-, tri- and bi-antennary complex moieties. Approximately 80% were sialated and about 70% were fucosylated. A fucosylated, disialated triantennary moiety is used for the model. Interestingly, Furukawa et al. only detected α2→6 linked sialic acid residues.10 Somewhat in contrast, the O-linked sugars tended towards the smaller core-1 moieties; the model shows both core 1 and core 2 (almost identical to disialated core 1 in size).
For convenience, standard nomenclature have been adopted, whereby the protein products are given the names with letters specifying the alternative exons and the mRNA is described according to the alternatively spliced exons present; thus, CD45RABC is produced by an mRNA, termed 345678. I have adopted the convention of numbering the amino acid residues from the mature N terminus of the longest ‘standard’ form (known as CD45RABC).
Nam and colleagues have recently published the solution of the 3D structure of the cytoplasmic domain of human CD45 for both native and catalytically inactive (C828S) proteins, as well as two structures of the catalytically inactive version bound to different phosphopeptides.3 One of the phosphopeptides was a fragment of the putatively physiologically relevant substrate, CD3zeta. It was found to be necessary to truncate the CD45 fragment at the C terminus to obtain sufficiently good crystals, so that no information about the position of the final 76 amino acids was obtained. The structural information is extremely interesting. The two CD45 phosphatase domains adopt very similar structures and their cores closely resemble the structure of other PTPase domains, which consists of a highly twisted nine-stranded β-sheet flanked by six alpha helices, four on one side, two on the other. The two domains also adopt the same spatial relationship to one another seen in the structure of the dual PTP domains of the leucocyte common antigen-related phosphatase (LAR), previously published by the same group.12 The structure of CD45 is displayed in cartoon form in Fig. 2. The two domains are bound closely together. Some CD45-specific features are notable. Domain 2 has two unique insertions relative to LAR and other PTPases (including CD45d1); the acidic loop is partly disordered in the crystals (and not visible in Fig. 2) but clearly lies between the two domains as does the smaller basic loop.
Figure 2.
The three-dimensional (3D) structure of the CD45 phosphatase domains. The 3D structure of the CD45 phosphatase domains is shown in cartoon form. The figure was generated from the PDB file, 1YGU, as determined by Nam et al.,3 using the software Protein Explorer.104 The membrane proximal domain (D1) is shown in light blue, the membrane distal domain (D2) in green and the peptide backbone of the substrate peptide is shown in red. The position of the active site is marked by displaying the phosphotyrosine (PY) of the substrate in spacefill. Similarly, the position of the putative ‘wedge’ residue, E624, is marked by displaying this amino acid in spacefill.
If we compare the CD45 structure with the separate structures for domain 1 and domain 2 of receptor protein tyrosine phosphatase-α (RPTPα),13,14 the principal difference is in the N-terminal part of D2. In RPTPa, the first 29 residues of the fragment used were disordered, whereas in CD45 they form the same α1′helix–loop–α2′helix seen in domain 1 of both molecules. In the RPTPα D1 crystal, this unit contacted the opposite partner, D1, in the crystal dimer and was described as an inhibitory ‘wedge’ as it occluded the substrate-binding pocket. That observation gave rise to a hypothesis about dimerization regulating phosphatase activity,13 considered below in this article. Nam et al. state that such inhibition could not take place between CD45 molecules with the structure found in their crystals.3 In Fig. 2, the residue E624, which forms the point of the wedge, is highlighted, as is the phosphotyrosine occupying the substrate pocket. It is certainly possible that RPTPα possesses a quite different, flexible, link between its two domains; a resolution of the tandem domains of RPTPα might shed further light on this.
Isoform functions
One of the most remarkable features of CD45 biology is the degree to which the differential expression of distinct mRNA and protein isoforms has been a conserved feature of its expression over hundreds of millions of years of evolution. Although the data do not extend to the detailed picture we have in mammals, CD45 orthologues have been clearly identified in chickens, sharks and hagfish, as well as bony fish and all mammalian species investigated.4,5,15–18 There is also clear evidence for the production of alternatively spliced isoforms involving coding units at the N terminus of the molecule in all species studied. The record for complexity is currently held by the Channel Catfish (Ictalurus punctatus), in which 13 exons were found to be involved; note, however, that the total length variation is only about 50% greater than in humans.18
In mice and humans it has been often suggested that three exons (exons 4–6) are involved in alternative splicing. In fact, however, there is very reproducible evidence for the involvement of exon 7 in alternative splicing in both species. The existence of an isoform lacking the four exons (exons 4–7) is consistently observed at the mRNA level (refs 19,20 and our unpublished results). A protein isoform is observed in Western blots of extracts of both thymocytes and spleenocytes from mice, which closely corresponds to the protein expressed in cells transfected with this isoform (which we term E3_8); the minor differences in mobility probably correspond to cell-type specific variation in glycosylation (N. Holmes & H. Gouda, unpublished). This protein isoform, which is clearly smaller than CD45RO, can also be seen in published data (e.g. Figure 3 of ref. 21). Kountikov and colleagues have observed the very close correlation between alternative splicing and predicted sites of O-glycosylation in CD45 from diverse species.18 While there are no data to suggest that the E3_8 isoform has any functional significance, it is notable that this is the isoform which differs most in O-glycoslyation (see Fig. 1), being least as different from CD45RO as CD45RB.
Although theoretically the number of possible isoforms is quite large, only six are observed at reasonable levels: e3_8, 378 (RO), 3578 (RB), 34578 (RAB), 35678 (RBC) and 345678 (RABC). Interestingly, these show cell-type and differentiation-stage specific expression, the pattern of which is quite well conserved in mammals (see ref. 1 for more details). An entirely novel alternatively spliced exon has been recently demonstrated in humans by Screaton and colleagues.22 This exon, termed D, encodes 41 amino acids between the ubiquitous exon 3 and the alternative exon 4 in a 3D4567 transcript. It remains to be determined whether this isoform is expressed as protein and at what level. Whether this or any other additional exon exists in other species is not certain, although we have consistently detected a transcript larger than RABC (34567) in reverse transcription–polymerase chain reaction (RT–PCR) products of mouse splenocytes (N. Holme, unpublished results).
Despite much investigation, it has been difficult to establish robust differences in function for the different isoforms. In T-cell lines, most studies found that CD45RO produced more efficient T-cell receptor (TCR) signalling than higher molecular-weight forms (e.g. CD45RABC and CD45RBC),23,24 but others found contrary results,25 including in transgenic mice.26 In order to avoid the possible confusion of different physiological states among cell lines (including subclones of the same cell line), we and others have generated transgenic mice expressing single isoforms of CD45 on a null (knockout) background.27–29 The general conclusions from these studies are that there is little evidence for clear functional differences between isoforms, but that the level of expression of CD45 is a critical parameter and that different levels of expression are required at different stages of T-cell development. CD45RO, CD45RB and CD45RABC were all competent to rescue thymic development and peripheral T-cell function but, expressed alone, none of these isoforms was able to permit normal B-cell maturation.28,29 By contrast, Virts et al. showed that both T- and B-cell functions could be restored by transgenesis of a CD45 minigene that produced an apparently normal pattern and level of expression.30 As the level of CD45 expressed in the single isoform transgenics was, in all cases, lower than the wild-type level, it remains to be determined whether full function in B cells requires a near wild-type level of expression or a combination of isoforms. Further studies will be required to determine whether more subtle quantitative or qualitative differences are produced (e.g. TCR repertoire shifts). We should bear in mind that the glycosylation of CD45 (as with other molecules) varies according to the lineage and differentiation stage of the cell (reviewed in ref. 31). The pattern shown in Fig. 1 is a simplified form of that found on human peripheral blood lymphocytes,9,10 but important differences in size and charge of the glycans exist; this variation is overlayed on the protein isoform variation and is still present in single isoform transgenics.
Genetic polymorphism and evolution of CD45
There is considerable evidence that CD45 is under selection for polymorphism in a variety of mammalian species. The evidence for polymorphism is clear in mice, rats, cattle, humans and hagfish.4,32–39
In humans, at least 14 sites of variation in the coding region have been found. These are listed in Table 1. This list excludes the three non-functional variants described by Kung (large deletion and a splice site mutation)40 and Tchilian (deletion of amino acids 339 and 340).41 Notably, only four of these 14 variants are synonymous. Furthermore, at least one of the synonymous variants (e4_C77G) has phenotypic consequences through an effect on splicing.35,36,39 It is also likely that the other variant at this position (e4_C77T) may alter splicing decisions. The high ratio of variants with phenotypic consequences, particularly within the extracellular region (9 : 1 or 10 : 0), suggests that this part of the PTPRC (CD45) gene is still under selection for variation in humans. This is unlikely to be explained by ascertainment bias because all but two of the variants (e4_G59A and e4_C77G) were detected by non-phenotypic screens. By contrast, ascertainment may well account for the apparent bias of variation towards the variable exons. The dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/index.html) also contains 111 variants in non-coding regions of the PRPRC (CD45) gene, whose distribution suggests normal levels of basal mutation.
Table 1. Coding sequence variants identified in PTPRC (human CD45).
| Base identity | |||||||
|---|---|---|---|---|---|---|---|
| Designation1 | AA no.2 | Codon pos.3 | Major | Minor | Phenotype.4 | Freq.5 | Refs |
| e4_A54G | 27 | 1 | A | G | Thr→Ala, splicing | ND | 38 |
| e4_C59A | 28 | 3 | C | A | His→Gln, splicing | ND | 35 |
| e4_C 77G | 34 | 3 | C | G | Splicing | 0·011 | 34,36 |
| e4_C 77T | 34 | 3 | C | T | Synonymous, splicing? | ND | 37 |
| e5_G69C | 98 | 1 | G | C | Asp→His | ND | 37 |
| e6_T127A | 164 | 2 | T | A | Ile→Asn | ND | 37 |
| e6_A138G | 168 | 1 | A | G | Thr→Ilesplicing | 0·0150·2376 | 36,37 |
| rs12129883 | 261 | 1 | A | C | Ile→Leu | 0·005 | dbSNP7 |
| rs2274367 | 304 | 1 | G | A | Glu→Lys | ND | dbSNP |
| rs6696162 | 398 | 2 | C | T | Thr→Ile | ND | dbSNP |
| rs12136658 | 545 | 3 | T | A | His→Gln | ND | dbSNP |
| rs7540378 | 747 | 3 | C | T | Synonymous | ND | dbSNP |
| rs1058191 | 1253 | 3 | T | C | Synonymous | 0·02 | dbSNP |
| rs2298872 | 1260 | 1 | A | C | Ser→Arg | 0·003 | dbSNP |
ND, insufficient data.
Where a published name exists, this is used prefixed with the exon number, otherwise the dbSNP id no. is used.
Amino acid numbered from the mature N terminus of CD45RABC.
Position of variation within the amino acid codon.
Known phenotypic effects; other effects probably exist.
Allele frequency, where evidence in Caucasians, except for6, varies between populations.
In Japanese.36
Filip & Mundy showed that CD45 was under positive selection during the evolution of catarrhine primates.42 In their study, which compared six exons from the extracellular domain-encoding region (comprising ≈ 60% of the extracellular domain), exons 9 and 14 were found to be particularly variable between Old World primates; this places the variation within the cysteine-rich and the third (membrane proximal) fnIII domains, respectively. This conclusion is reinforced by studies of CD45 evolution in cattle34 and within three species of Owl Monkey;43 in both cases the ratio of non-synonymous to synonymous substitution was high. All of the globular extracellular domains varied significantly. In cattle, the cysteine-rich and fnIIId1 domains were the most variable; among Owl monkeys, variation was greatest in the fnIIId3 domain. Note that the Old World primate study did not examine exons 10–13 encoding the two N-terminal fnIII domains.
The level of heterozygosity at the CD45 locus seems to be somewhat lower in humans than in some other species. Most of the human variants for which population data have been obtained are found at low frequency; observed allelic frequencies are generally only a few per cent or less (see Table 1). Systematic studies of CD45 polymorphism have not been performed in any species but, for example, three alleles were found in a library made from six individual hagfish,5 and at least six alleles (forming four quite distinct families) were found in cattle; each family was present at high frequency (> 50%) in one of the partially inbred populations studied.34 One exception to the generally observed low frequency in humans is e6_A138G, where the G variant is found in almost 40% of Japanese (estimated allele frequency 0·237)37 and at a much lower frequency in Caucasians (≈ 0·015).38 e6_138G is the only human variant for which homozygotes have been observed (they were found at Hardy–Weinberg frequency in Japanese),37 but there is no evidence that homozygotes of other variants are not present at the expected low frequencies, given their presumed allele frequencies and the modest numbers of individuals screened.
Many of the variants within the alternatively spliced, O-glycan-rich, region cause alterations in the splicing pattern. The e4_C77G and e4_C59A variants bias against silencing of expression of mRNAs containing exon 4(A)35,36 as normally occurs in antigen-activated T cells; e4_A54G may do something similar.39 By contrast, e6_A138G appears to promote the expression of CD45RO mRNA, dramatically so in the case of individuals homozygous for the e6_138G variant.37 The effects of other variants on splicing remain to be determined. Boxall et al. have shown that e6_138G-positive individuals have an increased frequency of interferon-γ-expressing T cells.44
What could be the driving force behind the apparent selection for sequence variation within the CD45 extracellular segment, and are there different pressures on splice regulation and protein sequence variation in the four folded domains? Here we are somewhat at sea. No solid evidence for an external ligand for the protein domains of CD45 has been found, despite much effort; this may imply a lack of selection for sequence conservation but it does not explain the apparent positive selection for diversity. Three well-characterized ligands bind to the carbohydrates that CD45 displays: Galectin-1;45 CD22;46 and the cysteine-rich domain of the mannose receptor.47 Although the significance of these interactions is unclear, their conservation is unlikely to constrain protein sequence variation. While important protein–protein interactions within the same membrane probably exist, it is unlikely that these can account for the selection. The suggestion that pathogen-driven selection is responsible34 is at present entirely speculative. No data exist on the phenotypic consequences of variation in animals.
Association of polymorphic variants with disease susceptibility
Some studies point to immune phenotypic consequences of CD45 variation in humans. Jacobsen et al.48 reported that e4_C77G was associated with an increased susceptibility to multiple sclerosis. However, subsequent studies have been unable to confirm this initial finding, and a recent meta-analysis of six studies found that the five later studies could be combined with low heterogeneity and that the initial results of Jacobsen were clearly different from these.38 Together, the five negative studies analysed more than 2000 patients and an equivalent number of controls from European or American populations, and yielded an odds ratio of 1·11 (not significantly different from unity). There remains the possibility that e4_77G (or a gene linked to the G variant) may make a contribution to multiple sclerosis susceptibility in a subfraction of genetically susceptible individuals.49 Other studies have failed to find a significant association of this variant with common variable immunodeficiency, type I diabetes, Graves' disease or systemic lupus erythematosis (SLE);50–53 however, one study has reported evidence for an increased risk of autoimmune hepatitis in e4_77G-positive individuals.54
Beverley and colleagues found a reduction in the frequency of e6_A138G heterozygotes among Japanese cohorts with Graves' disease and also with hepatitis B virus-associated hepatic disease.44 They also found an increased frequency of e4_C77G and e4_A54G in human immunodeficiency virus (HIV)-infected individuals,39,55 although in the latter case the sample size was too small for the association to reach significance.
Substrates of the phosphatase
In vitro data on CD45 activity and function has to be treated with caution. Even evidence in cell lines has proved to be confusing, with many contradictory results. Ideally, primary cells would be used to support any proposed substrates and functional interactions, although for reasons of time and practicality this has not always been possible.
Src family kinases (SFK) are the best-characterized substrates of the CD45 PTPase. The effect of CD45 is complex, however, as the phosphatase dephosphorylates both the C-terminal phosphotyrosine and the kinase-domain phosphotyrosine. In p56lck these are Y505 and Y394, respectively, and both sites have been clearly shown to be dephosphorylated by CD45 in cells, for example primary thymocytes.56,57 The action of the phosphatase on these sites is antagonistic; dephosphorylation of the C-terminal site activates kinase activity, whereas dephosphorylation of the kinase-domain site reduces kinase activity (reviewed in ref. 2). Although it is not yet certain that both sites on all SFK are substrates for CD45, the likelihood is that this is generally the case. It appears that that the overall effect of CD45 on the kinase activity may be different for distinct members of the SFK family and, perhaps, even for the same kinase in different situations. It is clearly established that CD45 is required for TCR signalling and this appears to be caused by the up-regulation of kinase activity in p56lck (also probably p59fyn);56 however, the activity of the total cellular pool of p56lck is not reduced in CD45-deficient cells,58 and CD45 may be required to act dynamically during the very early events of TCR signalling.59 Thus, the dominant effect of CD45 action on Lck is positive, at least during the initial TCR signalling events. This does not rule out a negatively regulating role for CD45 at later time-points during TCR signalling, as has been widely hypothesized, but clear direct evidence for such a role is lacking.
In B lymphocytes, CD45 also appears to potentiate B-cell receptor (BCR) signalling, but the mechanism is more obscure. There is certainly evidence that CD45 acts on Lyn (and presumptively other SFKs) in B cells, mainly from cell lines,60–62 but also recently from primary CD45KO B cells;63 these data suggest that CD45 dephophorylates both the inhibitory and activatory phosphotyrosine in Lyn – whether this leads to an up-regulation of total cellular Lyn activity is controversial. The position of SFKs in early BCR signalling is also in doubt, with some evidence that Lyn is critical for negative signalling,64 but not activation;65 other results suggest that Lyn is needed for BCR activation,66 or that SFKs act only late in signalling, at least in pre-B cells.67 The role of Lyn has recently been reviewed.68 Equally unclear is at what point CD45 regulates BCR signalling. Some data indicate that early antigen-receptor signalling events, like ITAM phosphorylation, are not diminished by the absence of CD45 in J558Lµm369 or primary CD45KO B cells (ref. 70 and K. Byth, C. Louis-dit-Sully and N. Holmes, unpublished), whereas CD45 is required for proximal signalling in DT40.61 Some of the discrepancies may relate to differences in other molecules involved (e.g. in DT40 versus J558Lµm3); 61,69 others may relate to the conditions used to activate the cells. Cyster et al. found significant effects on Ca2+ mobilization, ERK phosphorylation, etc., when CD45-defective B cells were activated by suboptimal antigen concentrations, but not when optimal anti-immunoglobulin M (IgM) was used.71 It is worth noting that B cells do not require CD45 for antigen responses in vivo, providing that CD45+ T-cell help is available if the antigen is T dependent,72 and we have confirmed this finding (S. Howlett and N. Holmes, unpublished data).
A recent report has reaffirmed that natural killer (NK) cells do not require CD45 for cytotoxic function or response to cytokines and showed that CD45KO NK cells fail to produce cytokines or chemokines after stimulation with Ly49D or CD16;73 an earlier report had shown that CD45-deficient NK cells failed to secrete interferon-γ (IFN-γ) in response to anti-NK1.1.74 The phenotype might be accounted for by regulation of SFK. The overall effect of CD45 on SFKs is not always activatory. In CD45-deficient macrophages, the SFKs involved in mediating adhesion (presumptively Hck and Lyn) are more functionally active than in wild-type cells.75
A wide variety of other proteins have been proposed as CD45 substrates: TCRzeta, SKAP55, Jak family kinases and PAG/Cbp.76–80 The physiological significance of these potential targets is not easy to assess. In vitro CD45, like many PTPases, has relatively low specificity and particular caution needs to be exercised when interpreting such results. This is further confounded by the complex regulation of SFK activity by CD45, so that observations of increased tyrosine phosphorylation (in CD45-deficient or mutant cells) of any tyrosine that might be the site of SFK kinase activity, can be explained indirectly, whereas another genuine CD45-substrate might appear to be unaffected owing to a balancing combination of decreased kinase and reduced phosphatase activity. Evidence for a direct enzyme–substrate interaction can sometimes be obtained using inactive ‘substrate-trap’ mutants, and TCRzeta, SKAP55, PAG/Cbp and p56lck have all been ‘trapped’76,78,80
Regulation of phosphatase activity
There are essentially three plausible mechanisms of CD45 PTPase regulation: dimerization; localization; and phosphorylation. It must be emphasized that these mechanisms are completely non-exclusive.
The hypothesis that receptor tyrosine phosphatases might be inhibited by the formation of dimers, proposed by Noel and colleagues,13 has received much attention. While there may be other RPTPs for which dimerization is a major regulatory mechanism (e.g. RPTPa),81,82 the evidence that CD45 enzyme activity is regulated by dimerization is circumstantial. The most persuasive data come from studies of a chimaeric molecule composed of the extracellular domain of the epidermal growth factor (EGF) receptor and the transmembrane and cytoplasmic domains of CD45. This chimaera restores TCR signalling in a CD45-negative T-cell line (H45.01) and such signalling is inhibited by EGF, which presumptively dimerizes the chimaeric receptor phosphatase.59 Crucially, mutation of residue E624, which is the equivalent position in huCD45 of a critical contact in the RPTPa ‘wedge’–catalytic site interface, greatly reduces the ability of EGF to inhibit TCR-induced signalling.59
A knock-in of the mutation E613R in transgenic mice (equivalent to E624R in humans) produces a lymphoproliferative syndrome with accompanying autoantibody production and nephropathy in most homozygous E613R mice and 15–25% of heterozygotes.83 This phenotype depends on B cells and does not require T cells; the mutant B cells are hyper-responsive to antigen.84 Although this phenotype is not directly opposite to that seen in CD45 knockout mice, it is not inconsistent with increased CD45 activity. Nevertheless, CD45 activity remains unknown in E613R B cells. The 3D structure of CD45 does not support the suggestion that the E613R mutation would affect dimerization of the enzymatic domain.3 However, the 3D structure offers no other clues as to how E613R could affect phosphatase activity. As shown in Fig. 2, E613R is on the surface of the protein, ≈ 24 Å from the active site. Studies on the effect of E613R mutation on phosphatase activity are surprisingly lacking.
Because proteins are dynamic molecules in solution, it is not possible to prove or disprove the ‘regulatory dimer’ hypothesis on the basis of crystal structures. Nevertheless, the 3D structures, and the demonstration that the CD45 cytoplasmic region is a stable D1D2 monomer in solution,85 do argue that this configuration is likely to be one of the major forms of CD45 within cells. It is still open to question whether CD45 cytoplasmic domains are capable of forming the type of inhibited D1D1 dimers proposed. There are, however, ample data supporting the proposition that the extracellular domains of CD45 can form dimers,24,83,86 but this process is independent of the intracellular domain.86 The propensity of CD45 to dimerize is greater for CD45RO than for RABC, which can be accounted for, at least in part, by charged carbohydrate repulsion.24,86
There is certainly evidence that the ability of CD45 to access specific substrates depends on its location within the plasma membrane and the proteins with which it is associated. CD45 is predominantly found to be associated with a 32 000 molecular weight membrane protein, CD45-AP (or LPAP). Although CD45-AP knockout mice show little or no phenotypic changes,87–89 certainly nothing as dramatic as in the CD45KO itself,90 there is some evidence that association with CD45AP can modify activity and, indeed, that CD45–CD45AP complexes might alter the ratio of CD45 monomers:dimers.91 CD45 can associate with other proteins, most notably CD4.24,92 Localization within lipid microdomains has also been proposed as a means of controlling CD45's access to specific substrates. The evidence is somewhat contradictory. The consensus is that a proportion, perhaps 10%, of CD45 is associated with detergent-insoluble microdomains (‘rafts’) in resting cells.63,93,94 There is some evidence that CD45 needs to partition into rafts to facilitate TCR signalling.95 However, too much CD45 in rafts inhibits TCR signalling,96,97 although similar inhibition can be obtained by targeting SHP-1.97 The ‘raft’ fraction of CD45 may reduce during lymphocyte activation,93,94 although in B cells any reduction appears transitory.63 It has been suggested that the relative exclusion of CD45 from rafts is needed to sustain TCR signalling;94 other data59,80 argue that CD45 requires ongoing interaction with SFK and PAG/Cbp, which are mainly located in rafts. This dichotomy remains to be resolved. Imaging studies have provided a confusing picture of the location of CD45 within the contact zone of T cells with APC, but they do suggest that the CD45 is involved in the cell–cell contact zone.98,99
There is also evidence for regulation of CD45 by phosphorylation. The CD45-specific acidic loop in PTPd2 is phosphorylated by casein kinase II in vitro,100 and these sites are also phosphorylated in T lymphoblasts and T-cell lines.101 Deletion of the acidic loop, or mutation of four of the serines within it, reduces the ability of CD45 to restore TCR signalling to CD45-deficient Jurkat cells.102
Concluding remarks
Recent progress in CD45 biology includes the solution of the 3D structure of the tandem phosphatase domains, the generation of single-isoform transgenic mice and new insights into the E613R knock-in mouse. Nevertheless, much remains to be clarified. The function of isoforms needs elucidating; studies of human individuals with variant CD45s may present a favourable opportunity to generate testable hypotheses. Given the potential for glycosylation changes to affect the binding of the lectin ligands to CD45, dimerization and other protein–protein interactions, we need to understand the glycobiology of CD45 much better. Rigorous testing of the dimer regulation hypothesis will challenge us; perhaps emerging imaging technology can help. Finally piecing together the complex web of enzyme–substrate interactions represents our most exciting and difficult challenge.
Note added in proof
A recent report105 suggests that transient oxidation could be an additional mechanism for regulating the PTPase activity of CD45. Depending on whether the oxidation is of Cys828 or Cys1144, this mechanism might be independent or dependent on allosteric changes subsequent to oxidation.
Abbreviations
- 3D
three dimensional
- BCR
B-cell receptor
- EGF
epidermal growth factor
- EM
electron microscopy
- fnIII
type 3 fibronectin
- IFN-γ
interferon-γ
- NK
natural killer
- NMR
nuclear magnetic resonance
- PTPase
protein tyrosine phosphatase
- RPTP
receptor protein tyrosine phosphatase
- SFK
Src family kinases
- TCR
T-cell receptor
References
- 1.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 2.Alexander DR. The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Semin Immunol. 2000;12:349–59. doi: 10.1006/smim.2000.0218. [DOI] [PubMed] [Google Scholar]
- 3.Nam H-J, Poy F, Saito H, Frederick CA. Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45. J Exp Med. 2005;201:441–52. doi: 10.1084/jem.20041890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okumara M, Matthews RJ, Robb B, Litman GW, Bork P, Thomas ML. Comparison of CD45 extracellular domain sequences from divergent vertebrate species suggestes the conservation of three fibronectin type III domains. J Immunol. 1996;157:1569–75. [PubMed] [Google Scholar]
- 5.Nagata T, Suzuki T, Ohta Y, Flajnik MF, Kasahara M. The leukocyte common antigen (CD45) of the Pacific Hagfish, Eptatretus stoutii: implications for the primordial function of CD45. Immunogenetics. 2002;54:286–91. doi: 10.1007/s00251-002-0469-1. [DOI] [PubMed] [Google Scholar]
- 6.Symons A, Willis AC, Barclay AN. Domain organisation of the extracellular region of CD45. Protein Eng. 1999;12:885–92. doi: 10.1093/protein/12.10.885. [DOI] [PubMed] [Google Scholar]
- 7.Woollett GR, Williams AF, Shotton DM. Visualisation by low angle shadowing of the leucocyte-common antigen A major cell surface glycoprotein of lymphocytes. EMBO J. 1985;4:2827–30. doi: 10.1002/j.1460-2075.1985.tb04010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCall MN, Shotton DM, Barclay AN. Expression of soluble isoforms of rat CD45 Analysis by electron microscopy and use in epitope mapping of anti-CD45R monoclonal antibodies. Immunology. 1992;76:310–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Julenius K, Mølgaard A, Gupta R, Brunak S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology. 2004;15:153–64. doi: 10.1093/glycob/cwh151. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa K, Funakoshi Y, Autero M, Horejsi V, Kobata A, Gahmberg CG. Structural study of the O-linked sugar chains of human leukocyte tyrosine phosphatase CD45. Eur J Biochem. 1998;251:288–94. doi: 10.1046/j.1432-1327.1998.2510288.x. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Furukawa K, Autero M, Gahmberg CG, Kobata A. Structural study of the sugar chains of human leukocyte common antigen CD45. Biochemistry. 1993;32:12694–704. doi: 10.1021/bi00210a019. [DOI] [PubMed] [Google Scholar]
- 12.Nam H-J, Poy F, Kreuger NX, Saito H, Frederick CA. Crystal structure of the tandem phosphatase domains of RPTP LAR. Cell. 1999;97:521–30. doi: 10.1016/s0092-8674(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 13.Bilwes AM, den Hertog J, Hunter T, Noel JP. Structural basis for inhibition of receptor protein-tyrosine phosphatase-alpha by dimerization. Nature. 1996;382:555–9. doi: 10.1038/382555a0. [DOI] [PubMed] [Google Scholar]
- 14.Sonnenburg ED, Bilwes A, Hunter T, Noel JP. The structure of the membrane distal phosphatase domain of RPTPα reveals interdomain flexibility and an SH2 domain interaction region. Biochemistry. 2003;42:7904–14. doi: 10.1021/bi0340503. [DOI] [PubMed] [Google Scholar]
- 15.Fang KS, Barker K, Sudol M, Hanafusa H. A transmembrane protein-tyrosine phosphatase contains spectrin-like repeats in its extracellular domain. J Biol Chem. 1994;269:14056–63. [PubMed] [Google Scholar]
- 16.Fujiki K, Shin DH, Nakao M, Yano T. Molecular cloning of carp (Cyprinus carpio) leukocyte cell-derived chemotaxin 2, glia maturation factor beta, CD45 and lysozyme C by use of suppression subtractive hybridization. Fish Shellfish Immunol. 2000;10:643–50. doi: 10.1006/fsim.2000.0294. [DOI] [PubMed] [Google Scholar]
- 17.Diaz del Pozo E, Beverley PCL, Timon M. Genomic structure and sequence of the leukocyte common antigen (CD45) from the pufferfish Fugu rubripes and comparison with its mammalian homologue. Immunogenetics. 2000;51:838–46. doi: 10.1007/s002510000214. [DOI] [PubMed] [Google Scholar]
- 18.Kountikov E, Wilson M, Miller N, Clem W, Bengten E. Organisation and expression of thirteen alternatively spliced exons in catfish CD45 homologs. Dev Comparative Immunol. 2004;28:1023–35. doi: 10.1016/j.dci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Chang H-L, Lefrancois L, Zaroukian H, Esselman WJ. Developmental expression of CD45 alternate exons in murine T cells Evidence of additional alternate exon use. J Immunol. 1991;147:1687–93. [PubMed] [Google Scholar]
- 20.Virts E, Barritt D, Raschke WC. Expression of CD45 isoforms lacking exons 7, 8 and 10. Mol Immunol. 1998;35:167–76. doi: 10.1016/s0161-5890(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 21.McNeill L, Cassady RL, Sarkardei S, Cooper JC, Morgan G, Alexander DR. CD45 isoforms in T cell signalling and development. Immunol Lett. 2004;92:125–34. doi: 10.1016/j.imlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Brackenridge S, Screaton GR. A novel CD45 transcript involving the alternative splicing of a new exon is widely distributed in lymphoid tissues. Hum Immunol. 2004;65:1539–45. doi: 10.1016/j.humimm.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 23.Novak TJ, Farber D, Leitenberg D, Hong SC, Johnson P, Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994;1:109–19. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 24.Dornan S, Sebestyen Z, Gamble J, et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002;277:1912–8. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 25.McKenney DW, Onodera H, Gorman L, Mimura T, Rothstein DM. Distinct isoforms of the CD45 protein-tyrosine phosphatase differentially regulate interleukin 2 secretion and activation signal pathways involving Vav in T cells. J Biol Chem. 1995;270:24949–54. doi: 10.1074/jbc.270.42.24949. [DOI] [PubMed] [Google Scholar]
- 26.Chui D, Ong CJ, Johnson P, Teh HS, Marth JD. Specific CD45 isoforms differentially regulate T cell receptor signaling. EMBO J. 1994;13:798–807. doi: 10.1002/j.1460-2075.1994.tb06322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozieradzki I, Kundig T, Kishihara K, et al. T cell development in mice expressing splice variants of the protein tyrosine phosphatase CD45. J Immunol. 1997;158:3130–9. [PubMed] [Google Scholar]
- 28.Ogilvy S, Louis-Dit-Sully C, Cooper J, Cassady RL, Alexander DR, Holmes N. Either of the CD45RB and CD45RO isoforms are effective in restoring T cell, but not B cell, development and function in CD45-null mice. J Immunol. 2003;171:1792–800. doi: 10.4049/jimmunol.171.4.1792. [DOI] [PubMed] [Google Scholar]
- 29.Tchilian EZ, Dawes R, Hyland L, et al. Altered CD45 isoform expression affects lymphocyte function in CD45 Tg mice. Int Immunol. 2004;16:1323–32. doi: 10.1093/intimm/dxh135. [DOI] [PubMed] [Google Scholar]
- 30.Virts EL, Diago O, Raschke WC. A CD45 minigene restores regulated isoform expression and immune function in CD45 deficient mice: Therapeutic implications for human CD45-null Severe Combined Immunodeficiency. Blood. 2003;101:849–55. doi: 10.1182/blood-2002-07-1969. [DOI] [PubMed] [Google Scholar]
- 31.Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;2:903–10. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 32.Zebedee SL, Barritt DS, Raschke WC. Comparison of mouse Ly5a and Ly5b leucocyte common antigen alleles. Dev Immunol. 1991;1:243–54. doi: 10.1155/1991/52686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Symons A, Barclay AN. Molecular basis of antigenic differences between the RT7 alleles of rat CD45. Immunogenetics. 2000;51:747–50. doi: 10.1007/s002510000204. [DOI] [PubMed] [Google Scholar]
- 34.Ballingall KT, Waibochi L, Holmes EC, Woelk CH, MacHugh ND, Lutje V, McKeever DJ. The CD45 locus in cattle: allelic polymorphism and evidence for exceptional positive natural selection. Immunogenetics. 2001;52:276–83. doi: 10.1007/s002510000276. [DOI] [PubMed] [Google Scholar]
- 35.Thude H, Hundreiser J, Wonigeit K, Schwinzer R. A point mutation in the CD45 gene associated with defective splicing of exon A. Eur J Immunol. 1995;25:2101–6. doi: 10.1002/eji.1830250745. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen M, Hoffman S, Cepok S, Stei S, Ziegler A, Sommer N, Hemmer B. A novel mutation in PTPRC interferes with splicing and alters the structure of the human CD45 molecule. Immunogenetics. 2002;54:158–63. doi: 10.1007/s00251-002-0455-7. [DOI] [PubMed] [Google Scholar]
- 37.Stanton T, Boxall S, Hirai K, et al. A high-frequency polymorphism in exon 6 of the CD45 tyrosine phosphatase gene (PTPRC) resulting in altered isoform expression. Proc Natl Acad Sci USA. 2003;100:5997–6002. doi: 10.1073/pnas.0931490100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez-Lira M, Liguori M, Magnani C, et al. CD45 and multiple sclerosis: the exon 4 C77G polymorphism (additional studies and meta-analysis) and new markers. J Neuro Immunol. 2003;140:216–21. doi: 10.1016/s0165-5728(03)00208-x. [DOI] [PubMed] [Google Scholar]
- 39.Stanton T, Boxall S, Bennett A, et al. CD45 variant alleles: possibly increased frequency of a novel exon 4 CD45 polymorphism in HIV seropositive Ugandans. Immunogenetics. 2004;56:107–10. doi: 10.1007/s00251-004-0668-z. [DOI] [PubMed] [Google Scholar]
- 40.Kung C, Pingel JT, Heikinheimo M, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–5. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 41.Tchilian EZ, Wallace DL, Wells RS, Flower DR, Morgan G, Beverley PC. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J Immunol. 2001;166:1308–13. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- 42.Filip LC, Mundy NI. Rapid evolution by positive Darwinian selection in the extracellular domain of the abundant lymphocyte protein CD45 in primates. Mol Biol Evol. 2004;21:1504–11. doi: 10.1093/molbev/msh111. [DOI] [PubMed] [Google Scholar]
- 43.Montoya GE, Vernot JP, Patarroyo ME. Comparative analysis of CD45 proteins in primate context: owl monkeys vs humans. Tissue Antigens. 2004;64:165–72. doi: 10.1111/j.0001-2815.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 44.Boxall S, Stanton T, Hirai K. Disease association and altered immune function in CD45 138G variant carriers. Hum Mol Genet. 2004;13:2377–84. doi: 10.1093/hmg/ddh276. [DOI] [PubMed] [Google Scholar]
- 45.Symons A, Cooper DN, Barclay AN. Characterization of the interaction between galectin-1 and lymphocyte glycoproteins CD45 and Thy-1. Glycobiology. 2000;10:559–63. doi: 10.1093/glycob/10.6.559. [DOI] [PubMed] [Google Scholar]
- 46.Van der Merwe PA, Crocker PR, Vinson M, Barclay AN, Schauer R, Kelm S. Localization of the putative sialic acid-binding site on the immunoglobulin superfamily cell-surface molecule CD22. J Biol Chem. 1996;271:9273–80. [PubMed] [Google Scholar]
- 47.Martínez-Pomares L, Crocker PR, Da Silva R, Holmes N, Colominas C, Rudd P, Dwek R, Gordon S. Cell-specific glycoforms of Sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the Mannose Receptor. J Biol Chem. 1999;274:35211–8. doi: 10.1074/jbc.274.49.35211. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen M, Schweer D, Ziegler A, et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nat Genet. 2000;26:495–9. doi: 10.1038/82659. [DOI] [PubMed] [Google Scholar]
- 49.Vyskina T, Leist TP, Shugart YY, Kalman B. CD45 (PTPRC) as a candidate gene in multiple sclerosis. Mult Scler. 2004;10:614–7. doi: 10.1191/1352458504ms1115oa. [DOI] [PubMed] [Google Scholar]
- 50.Vorechovsky I, Kralovicova J, Tchilian E, et al. Does 77C-G in PTPRC modify autoimmune disorders linked to the major histocompatibility locus? Nat Genet. 2001;29:22–3. doi: 10.1038/ng723. [DOI] [PubMed] [Google Scholar]
- 51.Wood JP, Bieda K, Segni M, Herwig J, Krause M, Usadel KH, Badenhoop K. CD45 exon 4 point mutation does not confer susceptibility to type 1 diabetes mellitus or Graves' disease. Eur J Immunogenet. 2002;29:73–4. doi: 10.1046/j.1365-2370.2002.00262.x. [DOI] [PubMed] [Google Scholar]
- 52.Thude H, Rosenhahn S, Hunger-Dathe W, Muller UA, Barz D. A transmembrane protein-tyrosine phosphatase receptor type C (CD45) exon A point mutation (77 C to G) is not associated with the development of type 1 diabetes mellitus in a German population. Eur J Immunogenet. 2004;31:245–7. doi: 10.1111/j.1365-2370.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 53.Johanneson B, Lima G, von Salome J, Alarcon-Segovia D, Alarcon-Riquelme ME. A major susceptibility locus for systemic lupus erythematosus maps to chromosome 1q31. Am J Hum Genet. 2002;71:1060–71. doi: 10.1086/344289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogel A, Strassburg CP, Manns MP. 77 C/G mutation in the tyrosine phosphatase CD45 gene and autoimmmune hepatitis: evidence for a genetic link. Genes Immun. 2003;4:79–81. doi: 10.1038/sj.gene.6363918. [DOI] [PubMed] [Google Scholar]
- 55.Tchilian EZ, Wallace DL, Dawes R, Imami N, Burton C, Gotch F, Beverley PC. A point mutation in CD45 may be associated with an increased risk of HIV-1 infection. AIDS. 2001;15:1892–4. doi: 10.1097/00002030-200109280-00024. [DOI] [PubMed] [Google Scholar]
- 56.Stone JD, Conroy LA, Byth KF, Hederer RA, Howlett S, Takemoto Y, Holmes N, Alexander DR. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-zeta and ZAP-70. J Immunol. 1997;158:5773–82. [PubMed] [Google Scholar]
- 57.Zhao R, Yang FT, Alexander DR. An oncogenic tyrosine kinase inhibits DNA repair and DNA-damage-induced Bcl-xL deamidation in T cell transformation. Cancer Cell. 2004;5:37–49. doi: 10.1016/s1535-6108(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 58.D'Oro U, Ashwell JD. The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol. 1999;162:1879–83. [PubMed] [Google Scholar]
- 59.Majeti R, Bilwes AM, Noel JP, Hunter T, Weiss A. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 60.Katagiri T, Ogimoto M, Hasegawa K, Mizuno K, Yakura H. Selective regulation of Lyn tyrosine kinase by CD45 in immature B cells. J Biol Chem. 1995;270:27987–90. doi: 10.1074/jbc.270.47.27987. [DOI] [PubMed] [Google Scholar]
- 61.Yanagi S, Sugawara H, Kurosaki M, Sabe H, Yamamura H, Kurosaki T. CD45 modulates phosphorylation of both autophosphorylation and negative regulatory tyrosines of Lyn in B cells. J Biol Chem. 1996;271:30487–92. doi: 10.1074/jbc.271.48.30487. [DOI] [PubMed] [Google Scholar]
- 62.Katagiri T, Ogimoto M, Hasegawa K, et al. CD45 negatively regulates lyn activity by dephosphorylating both positive and negative regulatory tyrosine residues in immature B cells. J Immunol. 1999;163:1321–6. [PubMed] [Google Scholar]
- 63.Shrivastava P, Katagiri T, Ogimoto M, Mizuno K, Yakura H. Dynamic regulation of Src-family kinases by CD45 in B cells. Blood. 2004;103:1425–32. doi: 10.1182/blood-2003-03-0716. [DOI] [PubMed] [Google Scholar]
- 64.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits. Lyn, CD22 and SHP-1 are limiting elements of a biochemical pathway regulating BCR signalling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 65.Chan VWF, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 66.Meade J, Fernandez C, Turner M. The tyrosine kinase Lyn is required for B cell development beyond the T1 stage in the spleen: rescue by overexpression of Bcl-2. Eur J Immunol. 2002;31:1029–34. doi: 10.1002/1521-4141(200204)32:4<1029::AID-IMMU1029>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 67.Saijo K, Schmedt C, Su IH, et al. Essential role of Src-family protein tyrosine kinases in NF-kappa B activation during B cell development. Nat Immunol. 2003;4:274–9. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 68.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase. accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Pao LI, Cambier JC. Syk, but not Lyn, recruitment to B cell antigen receptor and activation following stimulation of CD45– B cells. J Immunol. 1997;158:2663–9. [PubMed] [Google Scholar]
- 70.Benatar T, Carsetti R, Furlonger C, Kamalia N, Mak TW, Paige CJ. Immunoglobulin-mediated signal transduction in B cells from CD45-deficient mice. J Exp Med. 1996;183:329–34. doi: 10.1084/jem.183.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, Goodnow CC. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–8. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 72.Kong Y-Y, Kishihara K, Sumichika H, Nakamura T, Kaneko M, Nomoto K. Differential requirements of CD45 for lymphocyte development and function. Eur J Immunol. 1995;25:3431–6. doi: 10.1002/eji.1830251234. [DOI] [PubMed] [Google Scholar]
- 73.Huntington ND, Xu Y, Nutt SL, Tarlinton DM. A requirement for CD45 distinguishes Ly49D-mediated cytokine and chemokine production from killing in primary natural killer cells. J Exp Med. 2005;201:1421–33. doi: 10.1084/jem.20042294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin SM, Mehta IK, Yokayama WM, Thomas ML, Lorenz RG. Development of intestinal intraepithelial lymphocytes, NK cells, and NK 1.1+ T cells in CD45-deficient mice. J Immunol. 2001;166:6066–73. doi: 10.4049/jimmunol.166.10.6066. [DOI] [PubMed] [Google Scholar]
- 75.Roach T, Slater S, Koval M, White L, Cahir McFarland E, Okumura M, Thomas M, Brown E. CD45 regulates Src family member kinase activity associated with macrophage integrin mediated adhesion. Curr Biol. 1997;7:408–17. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 76.Furukawa T, Itoh M, Krueger NX, Streuli M, Saito H. Specific interaction of the CD45-protein tyrosine phosphatase with tyrosine phosphorylated CD3ζ chain. Proc Natl Acad Sci USA. 1994;91:10928–32. doi: 10.1073/pnas.91.23.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kashio N, Watsumoto W, Parker S, Rothstein DM. The second domain of the CD45 protein tyrosine phosphatase is critical for interleukin-2 secretion and substrate recruitment of TCR-ζin vivo. J Biol Chem. 1998;273:33856–63. doi: 10.1074/jbc.273.50.33856. [DOI] [PubMed] [Google Scholar]
- 78.Wu L, Fu J, Shen SH. SKAP55 coupled with CD45 positively regulates T-cell receptor-mediated gene transcription. Mol Cell Biol. 2002;22:2673–86. doi: 10.1128/MCB.22.8.2673-2686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irie-Sasaki J, Sasaki T, Matsumoto W, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–54. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 80.Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adapter. Mol Cell Biol. 2003;23:2017–28. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang G, den Hertog J, Su J, Noel J, Sap J, Hunter T. Dimerization inhibits the activity of receptor-like protein tyrosine phosphatase α. Nature. 1999;401:606–10. doi: 10.1038/44170. [DOI] [PubMed] [Google Scholar]
- 82.Jiang G, den Hertog J, Hunter T. Receptor-like protein tyrosine phosphatase α homodimerizes on the cell surface Mol. Cell Biol. 2000;20:5917–29. doi: 10.1128/mcb.20.16.5917-5929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majeti R, Xu Z, Parslow TG, Olson JL, Daikh DI, Killeen N, Weiss A. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–70. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 84.Hermiston ML, Tan A, Weiss A. Perturbation of B cell homeostasis and function in mice expressing an inactivating point mutation in the inhibitory wedge of CD45. Blood. 2003;102:160. [Google Scholar]
- 85.Felberg J, Johnson P. Characterization of recombinant CD45 cytoplasmic domain proteins. Evidence for intramolecular and intermolecular interactions. J Biol Chem. 1998;273:17839–45. doi: 10.1074/jbc.273.28.17839. [DOI] [PubMed] [Google Scholar]
- 86.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat Immunol. 2002;3:674–1. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 87.Kung C, Okumura M, Seavitt JR, et al. CD45 associated protein is not essential for the regulation of antigen receptor-mediated signal transduction. Eur J Immunol. 1999;29:3951–5. doi: 10.1002/(SICI)1521-4141(199912)29:12<3951::AID-IMMU3951>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 88.Ding I, Bruyns E, Li P, et al. Biochemical and functional analysis of mice deficient in expression of the CD45-associated phosphoprotein LPAP. Eur J Immunol. 1999;29:3956–61. doi: 10.1002/(SICI)1521-4141(199912)29:12<3956::AID-IMMU3956>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 89.Matsuda A, Motoya S, Kimura S, McInnis R, Maizel AL, Takeda A. Disruption of lymphocyte function and signaling in CD45-associated protein-null mice. J Exp Med. 1998;187:1863–70. doi: 10.1084/jem.187.11.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Byth KF, Conroy LA, Howlett S, Smith AJH, May J, Alexander DR, Holmes NJ. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and in B-cell maturation. J Exp Med. 1996;183:1707–18. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takeda A, Matsuda A, Paul RML, Yaseen NR. CD45-associated protein inhibits CD45 dimerization and up-regulates its protein tyrosine phosphatase activity. Blood. 2004;103:3440–7. doi: 10.1182/blood-2003-06-2083. [DOI] [PubMed] [Google Scholar]
- 92.Leitenberg D, Novak T, Farber D, Smith B, Bottomly K. The extracellular domain of CD45 controls association with the CD4 T cell receptor complex and the response to antigen-specific stimulation. J Exp Med. 1996;183:249–59. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent insoluble microdomains in T lymphocytes. J Immunol. 2002;169:5036–42. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 94.Zhang M, Moran M, Round J, Low TA, Patel VP, Tomassian T, Hernandez JD, Miceli MC. CD45 signals outside of lipid rafts to promote ERK activation, synaptic raft clustering, and IL-2 production. J Immunol. 2005;174:1479–90. doi: 10.4049/jimmunol.174.3.1479. [DOI] [PubMed] [Google Scholar]
- 95.Irles C, Symons A, Michel F, Bakker TR, van der Merwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–97. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 96.He X, Woodford-Thomas TA, Johnson KG, Shah DD, Thomas ML. Targeting of CD45 protein tyrosine phosphatase activity to lipid microdomains on the T cell surface inhbits TCR signaling. Eur J Immunol. 2002;32:2578–87. doi: 10.1002/1521-4141(200209)32:9<2578::AID-IMMU2578>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 97.Su MW-C, Yu C-L, Burakoff SJ, Jin Y-L. Targeting Src homology 2 domain-containing tyrosine phosphatase (SHP-1) into lipid rafts inhibits CD3-induced T cell activation. J Immunol. 2001;166:2975–82. doi: 10.4049/jimmunol.166.6.3975. [DOI] [PubMed] [Google Scholar]
- 98.Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci USA. 2000;97:10138–43. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dustin ML. Membrane domains and the immunological synapse: keeping T cells resting and ready. J Clin Invest. 2002;109:155–60. doi: 10.1172/JCI14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Guo W, Liang L, Esselman WJ. Phosphorylation of CD45 by casein kinase 2. Modulation of activity and mutational analysis. J Biol Chem. 1999;274:7454–61. doi: 10.1074/jbc.274.11.7454. [DOI] [PubMed] [Google Scholar]
- 101.Autero M, Saharinen J, Pessa-Morikawa T, et al. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck kinase and activates the phosphatase. Mol Cell Biol. 1994;14:1308–21. doi: 10.1128/mcb.14.2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greer SF, Wang Y, Raman C, Justement LB. CD45 function is regulated by an acidic 19-amino acid insert in domain II that serves as a binding and phospho-acceptor site for casein kinase 2. J Immunol. 2001;166:7208–18. doi: 10.4049/jimmunol.166.12.7208. [DOI] [PubMed] [Google Scholar]
- 103.Barclay AN, Jackson DI, Willis AC, Williams AF. Lymphocyte specific heterogeneity in the rat leucocyte common antigen (T200) is due to differences in polypeptide sequences near the NH2-terminus. EMBO J. 1987;6:1259–64. doi: 10.1002/j.1460-2075.1987.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martz E. Protein Explorer: easy yet powerful macromolecular vizualization. Trends Biochem Sci. 2002;27:107–9. doi: 10.1016/s0968-0004(01)02008-4. (http://www.proteinexplorer.org) [DOI] [PubMed] [Google Scholar]
- 105.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–93. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]