Abstract
Annexin-1 (ANXA1) is a glucocorticoid-regulated protein that modulates the effects of bacterial lipopolysaccharide (LPS) on macrophages. Exogenous administration of peptides derived from the N-terminus of ANXA1 reduces LPS-stimulated inducible nitric oxide synthase (iNOS) expression, but the effects of altering the endogenous expression of this protein are unclear. We transfected RAW264.7 murine macrophage-like cell lines to over-express constitutively ANXA1 and investigated whether this protein modulates the induction of iNOS, cyclooxygenase-2 (COX-2) and tumour necrosis factor-α (TNF-α) in response to LPS. In contrast to exogenous administration of N-terminal peptides, endogenous over-expression of ANXA1 results in up-regulation of LPS-induced iNOS protein expression and activity. However, levels of iNOS mRNA are unchanged. ANXA1 has no effect on COX-2 or TNF-α production in response to LPS. In experiments to investigate the mechanisms underlying these phenomena we observed that activation of signalling proteins classically associated with iNOS transcription was unaffected. Over-expression of ANXA1 constitutively activates extracellular signal regulated kinase (ERK)-1 and ERK-2, components of a signalling pathway not previously recognized as regulating LPS-induced iNOS expression. Inhibition of ERK activity, by the inhibitor U0126, reduced LPS-induced iNOS expression in our cell lines. Over-expression of ANXA1 also modified LPS-induced phosphorylation of the ERK-regulated translational regulation factor eukaryotic initiation factor 4E. Our data suggest that ANXA1 may modify iNOS levels by post-transcriptional mechanisms. Thus differential effects on iNOS expression in macrophages are seen when comparing acute administration of ANXA1 peptides versus the chronic endogenous over-expression of ANXA1.
Keywords: annexin-1, inflammation, lipocortin-1, lipopolysaccharide, nitric oxide
Introduction
Annexin-1 (ANXA1) is a 37 000 molecular weight calcium-and phospholipid-binding protein with two separate functional domains, the N-terminus of up to 35 amino acids, and the rest of the protein (the core domain1,2). ANXA1 is regulated by corticosteroids and mediates some of the beneficial actions of glucocorticoids, such as inhibition of cellular proliferation, anti-inflammatory effects, the regulation of cell differentiation and membrane trafficking.3,4 The N-terminal domain is critical for the anti-inflammatory effects of ANXA1, including reduced oedema,5 decreased polymorphonuclear cell migration,6,7 antipyretic effects,8,9 and an antiendotoxic action.9 N-terminal peptides of ANXA1 bind to members of the N-formyl peptide (FRP) receptor family to modify the inflammatory responses of leukocytes.10,11 The downstream molecular mechanisms by which ANXA1 modulates these cellular responses are unclear although this protein modulates the activity of extracellular signal regulated kinase (ERK), a mitogen-activated protein kinase (MAPK).12,13
Lipopolysaccharide (LPS) activates Toll-like Receptor-4 (TLR-4), in a complex with CD14, LPS-binding protein and MD-214 to activate a number of intracellular signalling proteins, including the MAPK p38, ERK1, ERK2 and c-jun N-terminal kinase (JNK).15–18 This culminates in the activation of several transcription factors, including nuclear factor-κB (NF-κB).19 Each of these signalling proteins plays a key role in the expression of pro-inflammatory mediators,20,21 the genes encoding many of which have NF-κB recognition sequences in their promoters. These include inducible nitric oxide synthase (iNOS),22 cyclooxygenase-2 (COX-2),23 interleukin-1 (IL-1),24 IL-625 and tumour necrosis factor-α (TNF-α),26 which together contribute to the pathophysiology of inflammation. Glucocorticoids inhibit the activation of JNK and NF-κB and prevent the expression of proteins such as iNOS, COX-2 and TNF-α.27
ANXA1 modulates the LPS-induced expression and activity of iNOS in macrophages.9,28 Exogenous administration of peptides derived from the sequence of ANXA1 results in the inhibition of LPS-induced iNOS expression in J774 murine macrophage-like cells.9 Anti-sera raised against ANXA1 partially inhibit the glucocorticoid-induced inhibition of LPS-induced iNOS expression both in vivo and in vitro.9,28 These studies used transient, exogenous, administration of ANXA1 peptides or anti-ANXA1 antisera before incubation with LPS rather than investigating changes in endogenous levels or sustained expression of ANXA1.
ANXA1 alters the activity of enzymes involved in prostaglandin and leukotriene production, specifically by inhibiting the activation of cytosolic phospholipase A2 (cPLA2) which may also explain some of its anti-inflammatory effects.2,9,28 Inhibition of cPLA2 limits the substrate availability of arachidonic acid for COX-2 to generate pro-inflammatory prostaglandins such as prostaglandin E2 (PGE2). The effects of ANXA1 on the expression of COX-2 induced by LPS are controversial. For example, in rodent or macrophage models of endotoxaemia ANXA1 had no effect on either COX-2 expression or activity, but in experiments using cells from the central nervous system ANXA1 inhibited LPS-induced COX-2 expression.9,29 This cell-type-specific differential regulation of COX-2 expression is further highlighted in mice lacking ANXA1, where COX-2 is up-regulated only in the lung and thymus.30
A few in vivo and in vitro studies suggest that ANXA1, either after induction by dexamethasone or by administration of exogenous peptidomimetics, inhibits TNF-α production by an unknown mechanism.9,31–34 In contrast, ANXA1 is not involved in the modulation of TNF-α production stimulated by mediators such as endothelin.35 All of these studies either used dexamethasone or the exogenous application of ANXA1 peptidomimetics to up-regulate ANXA1 expression acutely. There are scant data on whether increases in endogenous expression of ANXA1 will modulate LPS-induced iNOS, COX-2 or TNF-α production. Similarly, little is known about the effects of sustained expression of ANXA1. Given that the binding of ANXA1 to the FRP receptors results in receptor desensitization,11 it is likely that the consequences of chronic exposure to this protein may differ from those of transient exogenous administration.
Expression of ANXA1 is increased by glucocorticoids, which can be used in chronic therapy. It is important therefore to determine the effects of sustained expression of this protein. To investigate this we have established a RAW264.7 murine macrophage-like cell model where ANXA1 is continually over-expressed.13 This cellular model allows the effects of increased endogenous ANXA1 expression to be studied directly. In these cells, increased ANXA1 is detectable on the cell surface, the site of the biologically active pool of ANXA1. As expected, cellular proliferation is inhibited, confirming that the endogenously increased levels of this protein are biologically active.13,36 In this cell model, over-expression of ANXA1 causes constitutive activation of ERK, which is inhibited by treatment of the cells with LPS.13
In this study we have investigated the effects of chronic over- or under-expression of ANXA1 on LPS-induced iNOS, COX-2 and TNF-α expression. Over-expression of ANXA1 in cells enhances LPS-induced iNOS protein expression and activity, but not mRNA transcription, whilst COX-2 and TNF-α are unaffected. Transient cotransfection of RAW264.7 cells with ANXA1 and an iNOS reporter construct shows no effect on LPS-induced reporter activity. ANXA1 does not affect LPS-induced activation of several proteins associated with iNOS transcription, but inhibition of ERK activity abolishes the effects of ANXA1. We also show that ANXA1 modifies LPS-induced phosphorylation of the ERK-regulated translational regulation factor eukaryotic initiation factor 4E (eIF4E). We conclude therefore that chronic over-expression of ANXA1 results in post-transcriptional control of iNOS levels via an ERK-dependent mechanism, but that it has no effect on the expression of COX-2 or TNF-α.
Materials and methods
Cell culture
This study used either our RAW264.7 cell lines stably transfected with ANXA1 constructs13 or untransfected RAW264.7 cells. Except where stated, experiments were performed in the clone of our well characterized cell line which maximally over-expresses ANXA1.13 The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum supplemented with 2 mm l-glutamine, 200 U/ml penicillin, 100 μg/ml streptomycin and, in the case of transfected cells, 500 μg/ml of G418. Cell surface ANXA1 expression was confirmed at least every 2 weeks to ensure that protein expression was consistently maintained.13 For the experiments, different cell lines were plated onto six-well or 96-well plates at a plating density of 1 × 106 or 1 × 105 per well, respectively. Experiments were conducted when the cultures were confluent. Viable cell counts, using trypan blue exclusion, were made to normalize experimental results to 106 viable cells. LPS (Escherichia coli serotype 0127; B8; 0–1 μg/ml; Sigma-Aldrich, Poole, UK) was dissolved in distilled water and sonicated before being added to the cells. Murine interferon-γ (IFN-γ; 10 IU/ml; Sigma-Aldrich, Southampton, UK) was dissolved in medium prior to addition to the cells. U0126 (Promega; 35) was dissolved in ethanol and diluted in medium. Cells were pretreated for 15 min with U0126 or the appropriate vehicle control prior to addition of LPS. The effect of U0126 on cell viability was assessed by trypan-blue exclusion (Sigma-Aldrich). Salmonella enterica serovar Typhimurium strain (S. Typhimurium) C537 was used for the bacterial study. S. Typhimurium C5 was grown overnight in LB broth at 37° with agitation, diluted (1 : 10) in fresh LB broth and incubated for a further 2 hr. The bacteria were pelleted by centrifugation, resuspended in phosphate-buffered saline and diluted as required in DMEM. The S. Typhimurium was added to the cells at a multiplicity of infection (MOI) of 1.
Transient transfection of RAW264.7 cells
Cells were plated out at 1 × 105 cells per well in 48-well plate 24 hr before the study. Cells were transiently transfected with the luciferase reporter construct pGL3 or with pGL3 containing the full-length iNOS promoter sequence (0·15 μg38), the renilla reporter construct phRG-TK (Promega; 0·05 μg) and the expression construct pRcRSV or pRcRSV-k-ANXA1 (0·1 μg13). An equal amount of DNA (total 2 μg) was transfected into each well using Effectene-plus (Qiagen, Crawley, UK). After 24 hr, cells were treated with LPS for 6 hr and reporter gene activity was measured using the Dual reporter Stop and Glo kit (Promega) on a Galaxy-Lumistar luminometer (BMG Labtechnologies, Aylesbury, UK). Luciferase data were normalized to the renilla activity of the transfection control phRG-TK.
iNOS expression and activity
Cellular iNOS mRNA and 18S rRNA levels, in the presence and absence of 1 μg/ml LPS, were quantified using SYBR Green in a real-time reverse transcription–polymerase chain reaction (RT-PCR) (Qiagen). Total RNA was prepared from 3 × 106 cells using the RNeasy mini kit (Qiagen), including on-column DNAse treatment, following the manufacturer's instructions. Primers (18S rRNA-forward: CGCCGCTAGAGGTGAAATTCT; 18S rRNA reverse: CATTCTTGGCAAATGCTTTCG; iNOS forward: CGCAGCTGGGCTGTACAA; iNOS reverse: TGATGTTTGCTTCGGACATCA) were designed using the primer express software program (PE Applied Biosystems, Warrington, UK). The real-time RT-PCR was performed using the QantiTect SYBR Green RT-PCR kit (Qiagen) with the Rotor-Gene RG-3000 (Corbett Research, Cambridge, UK) according to the manufacturer's instructions.
To generate standard curves for the specific reactions for iNOS mRNA and the 18S rRNA, total RNA extracted from stimulated RAW264.7 macrophages was serially diluted in sterile RNAse-free water and dilutions were made from 10−1 to 10−7. Each RT-PCR experiment contained three no-template controls, test samples and a 1 : 10 dilution series. Each experiment was performed in triplicate. Regression analyses of the mean values of three replicate RT-PCRs for the 1 : 10 diluted RNA were used to generate standard curves. To control for variation in sampling and RNA preparation, the threshold cycle value (Ct) values for products specific for iNOS for each sample were standardized using the Ct value of the 18S rRNA product for the same sample. To normalize RNA levels between samples within an experiment, the mean Ct value for 18S rRNA-specific product was calculated by pooling values from all samples in that experiment. The slope of the 18S rRNA dilution series' regression line was used to calculate differences in total input RNA. Using the slope of the iNOS dilution series' regression lines, the difference in total input RNA, as represented by the 18S rRNA, was then used to adjust iNOS-specific Ct values.
Inducible NOS protein expression was measured by Western blot analysis of total cell lysates. Briefly, stimulated and unstimulated cells were lysed in buffer containing 10 mm ethylenediamine tetraacetic acid, 1% Triton-X100, and 1 mm phenylmethylsulphonyl fluoride, 0·05 mm pepstatin A and 0·2 mm leupeptin as protease inhibitors. Western blot analysis for iNOS was performed as described previously28 using rabbit anti-murine iNOS antibody28 at a concentration of 1 : 10 000. Densitometric analysis of the Western blots was performed (Kodak ID gel analysis) and data were expressed as fold changes in optical density. To determine iNOS activity, the supernatant of cultured macrophages was removed 24 hr after addition of LPS and assayed for nitrite accumulation by the Griess reaction as an indication of iNOS activity.39 Briefly, an equal volume of Griess reagent (1% sulphanilamide and 0·1% naphthylethylenediamine dihydrochloride in 5% phosphoric acid) was added to an equal volume of sample and the optical density was read immediately at 540 nm with reference at 620 nm. The values obtained were compared to standards of sodium nitrite dissolved in the culture medium (0–200 μm) and the concentration of nitrite was calculated in μm.
COX-2 expression and activity
COX-2 expression was determined by Western blot analysis as described above, with sheep anti-COX-2 antisera (Santa Cruz, Wembley, UK) used at a 1 : 1000 dilution. Blots were subjected to densitometric analysis. For activity studies, medium was removed from the cells 24 hr after LPS addition and PGE2 release was assessed by radioimmunoassay (RIA). Briefly, anti-PGE2 antisera (Sigma-Aldrich) and 3H-labelled PGE2 (Sigma-Aldrich) were added to each standard or sample. The tubes were incubated for 18–24 hr at 4°. After incubation the bound and unbound fractions were separated by adding dextran-coated charcoal suspension (20 mg/ml charcoal, 4 mg/ml dextran; Sigma-Aldrich) and centrifuged (2060 g, 4°, 15 min). After addition of scintillant (Optiphase HiSafe II) to the supernatant the level of PGE2 was determined by counting for one minute and comparing the values of the samples to the standards.
TNF-α detection assay
TNF-α was detected using a Duoset® ELISA development system (R & D Systems, Abington, UK) according to the manufacturer's instructions. A seven-point standard curve of two-fold dilutions from 15·625 pg/ml to 1000 pg/ml of recombinant mouse TNF-α was used. A volume of 100 μl of the standards and samples of the appropriate dilution were added to a 96-well plate. Supernatants taken at 2 hr were diluted 1 : 4 and those taken at 9 hr were diluted 1 : 10.
IκBα, IκBβ, IRF-1, STAT-1 and eIF4E detection
IκBα and inhibitory κBα (IκBα) activation was determined by measuring their disappearance from the cytoplasm using Western blot analysis. Cells were treated with LPS for up to 6 hr, lysed and subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting.13 The blots were probed with either anti-rabbit IκBα (1 : 1000, Santa Cruz) or IκBβ (1 : 1000, Santa Cruz). For interferon regulatory factor-1 (IRF-1) activation, cells were treated with LPS and/or IFN-γ for 2–6 hr, lysed and subjected to Western blot analysis using anti-rabbit IRF-1 antisera at a concentration of 1 : 1000. signal transducer and activator of transcription-1 (STAT-1) activation was determined by treating cells with LPS and/or IFN-γ for 4 hr prior to lysis. The blots were probed with anti-phospho-STAT-1 antibody (Cell Signaling Technology, Beverly, MA, USA) at a concentration of 1 : 1000. Phosphorylation of eIF4E was determined by Western blot analysis using anti-phospho-eIF4E antibody (Upstate Laboratories, Dundee, UK) at a concentration of 1 : 1000. Even protein loading was verified by stripping and re-probing the blots with anti-actin antibodies (1 : 10 000; Sigma-Aldrich). Native eIF4E and 4E-BP1 were determined by mouse monoclonal anti-eIF4E antibody (1 : 2000; BD Biosciences, Oxford, UK) and rabbit polyclonal anti-4E-BP1 antibody (1 : 2000; Abcam, Cambridge, UK), respectively. The appropriate horseradish peroxidase-conjugated secondary antibodies (Dako Corporation, Ely, UK) were used at 1 : 10 000. Protein concentration in each sample was determined by RC-DC Protein Assay Kit (Bio-Rad Laboratories Ltd, Hemelhempstead, UK) prior to SDS–PAGE.
Data analysis
Data were analysed using instat software (Graphpad Software, San Diego, CA, USA). One-way analysis of variance was used for all statistical analyses. Individual values were compared by Dunnett's test with P < 0·05 considered as significant unless otherwise stated.
Results
Chronic over-expression of ANXA1 enhances LPS-induced iNOS activity and protein expression, but not iNOS mRNA transcription
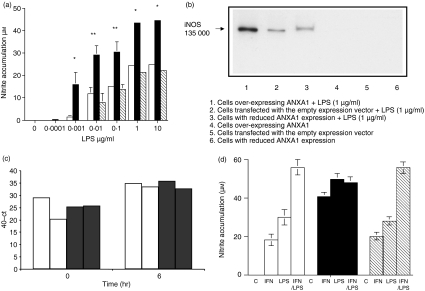
Stimulation of cells over-expressing ANXA113 resulted in a significant enhancement of LPS-induced nitrite production and enhanced iNOS protein expression (Fig. 1a,b). In separate experiments enhanced nitrite levels were also seen with three independent clones of stably transfected RAW264.7 cells over-expressing ANXA1 (NO production was increased by 166% 145% and 125% in comparison to control in each respective clone). In control cells and in cell lines expressing reduced levels of ANXA113 nitrite production and iNOS protein expression levels were similar (Fig. 1a,b). There was no detectable nitrite production or iNOS expression in any of the cells in the absence of LPS stimulation (data not shown). To determine whether increased ANXA1 expression affected iNOS promoter activity, RAW264.7 cells were transiently transfected with ANXA1 and iNOS reporter activity was measured 24 hr later. This had no effect on basal iNOS reporter activity (data not shown; n = 3). In cells treated with LPS (100 ng/ml), transient over-expression of ANXA1 had no detectable effect on iNOS promoter activity. These data suggested that iNOS mRNA levels were not affected by ANXA1. We therefore determined basal and LPS-stimulated iNOS mRNA levels by RT-PCR in control cells and in cells over-expressing ANXA1. As expected, LPS induced iNOS mRNA expression, but there was no difference in this effect between control cells and cells over-expressing ANXA1 (Fig. 1c).
Figure 1.
Cells over-expressing ANXA1 have increased iNOS activity and protein expression independent of transcription. (a) Cells over-expressing ANXA1 show increased activity of LPS-induced iNOS. Cells transfected with the empty expression vector (open column) or cells over-expressing (closed column) or under-expressing (cross hatched-column) ANXA1 were treated with LPS (1 μg/ml for 24 hr). Cell culture medium was removed, assayed for nitrite utilizing the Griess reaction and normalized to 106 viable cells. The data represent mean ± SEM of at least nine experiments on each cell line. Statistical significance is indicated by *P < 0·05 or **P < 0·01. (b) Cells over-expressing ANXA1 show increased protein expression of LPS-induced iNOS. Cells transfected with the empty expression vector or cells over- or under-expressing ANXA1 were treated with LPS (1 μg/ml for 24 hr). Cells were lysed, the proteins were separated by SDS–PAGE and transferred to PVDF membranes, which were then probed with anti-iNOS antisera. This blot is representative of at least six experiments on each cell line. (c) Cells over-expressing ANXA1 show no increase in LPS-induced iNOS mRNA. Cells transfected with the empty expression vector (open column) or cells over-expressing ANXA1 (closed column) were treated with LPS (1 μg/ml for 6 hr). Cells were lysed and DNA-free RNA was prepared using the RNeasy mini kit (Qiagen). Quantitative RT-PCR was performed using the QantiTect SYBR Green RT-PCR kit (Qiagen). The data show two separate repetitions of experiments in each cell line. (d) Cells over-expressing ANXA1 show increased activity of interferon-γ-induced iNOS. Cells transfected with the empty expression vector (open column) or cells over-expressing (closed column) or under-expressing (hatched column) ANXA1 were treated with LPS (1 μg/ml), murine IFN-γ (10 IU) or both LPS and IFN-γ together for 24 hr. Cell medium was removed, assayed for nitrite using the Griess reaction and normalized to 106 viable cells. The data represents mean ± SEM of at least four experiments on each cell line.
To determine whether the effects of ANXA1 on iNOS expression were stimulus-dependent, we infected cells over-expressing ANXA1 with S. Typhimurium (MOI 1) and found enhanced nitrite production (by 155%) in comparison to control cells whereas LPS (1 μg/ml) enhanced NO production by 168% in comparison with control cells. Similarly, cells over-expressing ANXA1 treated with IFN-γ showed enhanced levels of nitrite production (Fig. 1d) in comparison to control cells treated with IFN-γ. Stimulating control cells or cells expressing reduced levels of ANXA1 with IFN-γ and LPS showed a synergistic increase in NO production, in comparison to stimulation with either IFN-γ or LPS alone, which was similar in both cell lines. In contrast, cells over-expressing ANXA1 did not show a synergistic increase in NO production in response to the combined IFN-γ and LPS stimulation, possibly because the production of NO in response to either LPS or IFN-γ alone in these cells was already maximal.
Increased ANXA1 expression does not affect the production of the inflammatory mediators PGE2, COX-2 or TNF-α
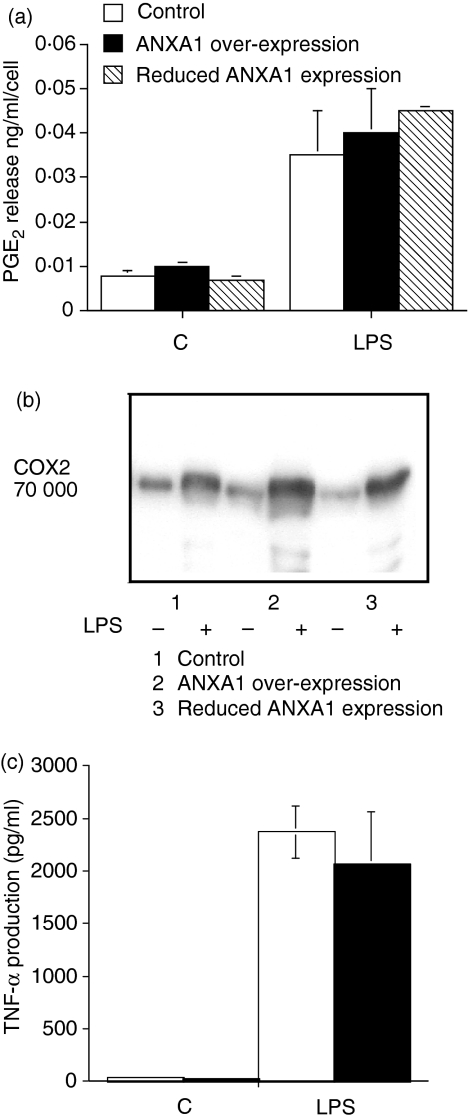
Similar levels of PGE2 were produced in all non-stimulated cell lines. LPS induced a similar increase in PGE2 production and COX-2 expression in each of the different cell lines (Fig. 2a,b). LPS-induced TNF-α production was also unaltered by different levels of ANXA1 expression (Fig. 2c).
Figure 2.
Increased ANXA1 expression does not affect the inflammatory mediators PGE2, COX-2 and TNF-α. (a) ANXA1 has no effect on LPS-induced PGE2 release. Cells transfected with the empty expression vector or cells over- or under-expressing (R1A) ANXA1 were treated with LPS (10 μg/ml for 24 hr). Cell medium was removed, assayed for PGE2 production by radioimmunoassay and normalized to 106 viable cells. The data represents mean ± SEM of four experiments on each cell line. (b) Over-expression of ANXA1 has no effect on LPS-induced COX-2 expression. Cells transfected with the empty expression vector or cells over- or under-expressing ANXA1 were treated with LPS (1 μg/ml for 24 hr). The proteins were separated by SDS–PAGE and after transfer to PVDF membranes the blots were probed with anti-COX-2 antisera. This blot is representative of at least four experiments on each cell line. (c) ANXA1 has no effect on LPS-induced TNF-α release. Cells transfected with the empty expression vector (open column) or cells over-expressing (closed column) ANXA1 were treated with LPS (1 μg/ml for 24 hr). Cell medium was removed, assayed for TNF-α production by ELISA and normalized to 106 viable cells. The data represent mean ± SEM of four experiments on each cell line.
The enhanced production of iNOS by LPS in cells over-expressing ANXA1 is ERK-dependent and not the result of altered NF-κB, STAT-1 or IRF-1 activation
We have previously shown that cells over-expressing ANXA1 do not show altered LPS-induced translocation of NF-κB to the nucleus.13 Unsurprisingly cells over-expressing ANXA1 showed similar changes in IκBα and IκBβ disappearance to control cells and cells with reduced levels of ANXA1 (data not shown). IRF-1 and STAT-1 are linked to LPS-induced expression of iNOS22,40,41 but cells over-expressing ANXA1 showed no constitutive activity of these proteins and similar levels of LPS-induced STAT-1 and IRF-1 (data not shown) activation to control cells.
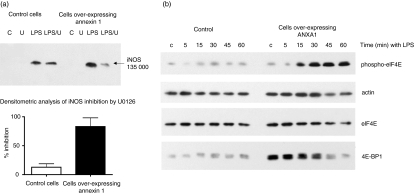
Pre-treatment of cells with U1026 (an inhibitor of ERK activation by MAPKK) markedly reduced the enhanced LPS-induced iNOS protein expression in cells over-expressing ANXA1 (Fig. 3a) without affecting cellular viability. In contrast U1026 did not significantly affect the LPS-induced iNOS production in the control cell line.
Figure 3.
Cells over-expressing ANXA1 have increased iNOS activity and protein expression by an ERK-dependent post-transcriptional mechanism. (a)Inhibition of ERK by U0126 inhibits the enhanced LPS-induced expression of iNOS in cells over-expressing ANXA1. Control or ANXA1 over-expressing cells were pretreated for 1 hr with U0126 (10 μm) or 0·1% ethanol (vehicle control). LPS (1 μg/ml) was added to the cells and, after 24 hr, the expression of iNOS protein was measured by Western blot analysis. The figure shows a representative blot and densitometric analysis from four different experiments. (b) Cells over-expressing ANXA1 have altered levels of phospho-eIF4E expression. Control or ANXA1 over-expressing cells were treated with LPS (1 μg/ml) for 0–60 min and the levels of phospho- and native eIF4E and 4E-BP1 present in the cells lysates were measured by Western blot analysis. Protein loading was confirmed by re-probing the blot for actin expression. A representative blot is shown from four different experiments.
To determine if ERK activity was regulating translational mechanisms, and thus iNOS expression, we measured the levels of phosphorylated eIF4E in control cells and in cells over-expressing ANXA1. In control cells basal levels of phosphorylated eIF4E were seen and LPS stimulation had little effect on these levels (Fig. 3b). In contrast, cells over-expressing ANXA1 had lower basal phospho-eIF4E and an increase in phosphorylation of this protein could be seen after 15–60 min of stimulation with LPS (Fig. 3b). Similar changes in phospho-eIF4E were seen in other cell lines over-expressing ANXA1 (unpublished data). There was no significant difference in the levels of native eIF4E in control cells (169760 ± 110500 optical density units) and in cells over-expressing ANXA1 (266 751 ± 30 400 optical density units). However, basal levels of an eIF4E inhibitory protein, 4E-BP1, in over-expressing cells were significantly higher (334 532 ± 300 optical density units) than in control cells (14 544 ± 1500 optical density units). When stimulated with LPS, the native form of 4E-BP1 decreases, presumably because it becomes hyperphosphorylated.42
Discussion
Here we show that chronic over-expression of ANXA1 resulted in increased protein expression and activity of iNOS in cells stimulated by LPS. Cells with reduced levels of ANXA1 behaved indistinguishably from controls in terms of LPS-induced NO production. This contrasts with results from studies that used glucocortiocoid-induced up-regulation of ANXA1 or exogenous addition of ANXA1 peptidomimetics to cells, in which iNOS protein expression and activity decreased upon LPS stimulation.9,13,28 Our results were unexpected, but consistent in LPS-treated RAW264.7 cell lines over-expressing ANXA1. RAW264.7 cells infected with S. Typhimurium produce NO by an LPS-dependent mechanism.37 ANXA1 over-expressing cells when infected with these bacteria produced more NO compared to control cells with normal ANXA1 levels, confirming the interaction of ANXA1 with the LPS-induced signalling pathway. Induction of iNOS transcription by LPS in control cells and in cells over-expressing ANXA1 is similar, suggesting that ANXA1 increases iNOS protein by a post-transcriptional mechanism. The data from the transient transfection experiments support this idea because ANXA1 failed to alter LPS-induced activity of an iNOS reporter construct. The control of iNOS expression is complex and involves activation of transcription factors such as NF-κB and IRF-1,22,40 activation of STAT-141 and inhibitory regulation, for example, through the complexing of STAT-3 to the NF-κB isoforms p65 and p50.43 None of these transcriptional control mechanisms were affected in our cell lines, confirming our observation that LPS-induced NF-κB activation is unaffected in macrophage-like cells constitutively over-expressing ANXA1.13 Regulation of iNOS by ANXA1 therefore, whether transiently or chronically expressed, is likely to be post-transcriptional utilizing either a translational control mechanism or changes in the stability of the iNOS protein, although our experiments do not rule out the possibility that ANXA1 may prolong the transcriptional activation of the iNOS gene. Furthermore, the induction of iNOS by IFN-γ alone, not in combination with LPS, was enhanced by ANXA1. Induction of iNOS by IFN-γ-dependent, LPS-independent pathways was resistant to the effects of the glucocorticoid analogue dexamethasone.44 However, other studies have shown that dexamethasone inhibits iNOS expression in response to LPS and/or IFN-γ.45 This suggests that the effects of ANXA1, unlike steroids, may not be stimulus-dependent within macrophage-like cells. Dexamethasone suppresses iNOS induction by both transcriptional and translational mechanisms46 and if ANXA1 only inhibits one of these mechanisms it may explain why the dexamethasone modulation of iNOS is only partially ANXA1-dependent.
The observation that up-regulation of ANXA1 enhances LPS-induced iNOS expression and activity contrasts with previously published data. Up-regulation of ANXA1 by dexamethasone or administration of exogenous ANXA1 inhibits the induction of iNOS both in vitro and in vivo.9,28 Our study is fundamentally different as we increased endogenous expression of ANXA1 and our cell lines over-express ANXA1 in a sustained fashion, thus this protein is being chronically, rather than acutely, expressed. This may explain the differential effect of ANXA1 on the LPS-induced stimulation of iNOS. In leukocytes, exogenous administration of ANXA1 peptidomimetics activates G-protein-coupled receptors, such as the lipoxin A4 and FPR receptors, to inhibit inflammatory effects.10,11 ANXA1 N-terminal peptides desensitize FPR receptors. Therefore, we speculate that transient administration of exogenous ANXA1 activates a G-protein-coupled receptor to inhibit LPS-induced iNOS production in macrophages. In our cell models the receptors may be down-regulated because of the continued expression of ANXA1 revealing alternative cellular effects of this protein.
The differential responses seen in this study may reflect the fact that biologically active ANXA1 is endogenously over-expressed13 rather than exogenously applied or induced by the action of steroids. Glucocorticoids have a limited effect on ANXA1 gene expression, but do increase its translocation to the cell surface.47,48 In addition, the phosphorylation status of ANXA1, which plays a critical role in modulating the function of this protein, may be profoundly affected by glucocorticoids.49 Dexamethasone causes serine phosphorylation of ANXA1 in the rat anterior pituitary gland.50 Truncated forms of ANXA1 and ANXA1 peptidomimetics, are highly susceptible to N-terminal cleavage that is likely to effect their biological activity.49 In our cell lines over-expressing ANXA1 we did not detect the presence of truncated forms of this protein although we did detect a pool of tyrosine-phosphorylated ANXA1 using anti-phosphotyrosine antisera.13 In conclusion, the chronic endogenous over-expression of ANXA1, which we have shown to enhance iNOS activation, may have effects upon the cell that are different from those seen in response to acute exogenous administration of ANXA1 where iNOS activation is inhibited.13,28
Cells over-expressing ANXA1 have constitutively active ERK that is ablated following LPS treatment.13 The protein eIF4E is an important translational regulation factor in most cell systems. Phosphorylation of eIF4E by the ERK-regulated mitogen-activated protein kinase-interacting kinases (MNKs) correlates with increased translational activity. However, after activation of cells with either TNF-α or IL-1β51,52 phosphorylation of eIF4E did not correlate with enhanced translational activity. Activation of MNKs by the ERK pathway results in negative control of translation.24 Given the effects of ANXA1 on ERK activity and the links between ERK and translational regulation we decided to investigate whether there was any possible link between ANXA1, ERK and eIF4E activity. To determine if ANXA1 modulates LPS-induced iNOS expression through an ERK-dependent pathway we inhibited the activation ERK with the inhibitor U0126. This inhibitor reduced the induction of iNOS in response to LPS in cells over-expressing ANXA1 whereas in the control cells it had no significant effect. The effect of U0126 on LPS-induced NO production is controversial as one paper suggests it has no effect53 whereas a recent study suggests that this inhibitor enhances NO production.54 Our work suggests that U0126 does not affect LPS-induced iNOS production in control cells but does in ANXA1 over-expressing cells. Overall, these data suggest that ERK may well modulate NO production in RAW cells. From these data, and our data on iNOS mRNA expression and reporter activity, it is tempting to speculate that the constitutively active ERK in cells over-expressing ANXA1 alters eIF4E activity to modify protein translation within the cell.24 Our cell lines over-expressing ANXA1 showed enhanced levels of 4E-BP1, an inhibitory protein which prevents the formation of the translation–initiation complex.55 The levels of 4E-BP1 are reduced following cell stimulation with LPS, presumably as a result of phosphorylation of this protein. Consistent with this, the level of eIF4E phosphorylation was markedly increased in the cells over-expressing ANXA1. Similar changes in 4E-BP1 or phospho-eIF4E were not observed in the control cells. ERK activates the kinases MNK1 and MNK2 to phosphorylate eIF4E and this pathway has been suggested to regulate acute-phase inflammatory protein production.56 This may explain the enhanced level of iNOS activity in the LPS-stimulated ANXA1 over-expressing cells.
Altered levels of phospho-eIF4E and 4E-BP1 in our ANXA1-over-expressing cell line in comparison to control cells suggest that there is a potential link between ANXA1 and altered translational activity. The expression of cyclin D1, a key protein involved in cell cycle regulation and subject to regulation by eIF4E, is markedly reduced in cells over-expressing ANXA136 therefore providing further evidence for a link between ANXA1 and translational regulation. Cells over-expressing ANXA1 show enhanced levels of 4E-BP1, which is likely to suppress translational activity within the cell and our data therefore suggest a further mechanism by which ANXA1 induces growth arrest. We cannot rule out, however, a mechanism involving inhibition of the down-regulation of iNOS protein expression as an alternative explanation for our observations. Little is known at present about mechanisms leading to down-regulation of iNOS, and we have not yet pursued this. LPS-induced activation of TNF-α and COX-2 was also unaffected by ANXA1 in our cell lines. This was expected because our cells show no changes in LPS-induced NF-κB, JNK or p38 MAPK activation.13 The failure to activate inflammatory mediators other than iNOS suggests that chronic over-expression of ANXA1 specifically regulates iNOS rather than a general inflammatory response.
In conclusion, over expression of ANXA1 in RAW264.7 macrophage-like cells does not affect LPS-induced COX-2 and TNF-α expression, but enhances NO production and iNOS expression by a post-transcriptional mechanism. The enhancement of LPS-induced iNOS expression in cells over-expressing ANXA1 is dependent on ERK activation. Cells over-expressing ANXA1 show elevated levels of the translational regulator 4E-BP1 and decreased levels of phosphorylated eIF4E. One of the mechanisms by which ANXA1 may modulate inflammation is by affecting inflammatory protein translation within the cell.
Acknowledgments
This work was supported by a Wellcome Trust Advanced Fellowship and project grant awarded to C.E.B. We would like to thank Matthew Royle for help with the infection study.
Abbreviations
- ANXA1
annexin-1
- COX-2
cyclooxygenase-2
- cPLA2
cytosolic phospholipase A2
- eIF4E
eukaryotic initiation factor 4E
- 4E-BP1
eIF4E-binding protein
- iNOS
inducible nitric oxide synthase
- IRF-1
interferon regulatory factor-1
- LPS
lipopolysaccharide
- MNKs
mitogen-activated protein kinase-interacting kinases
- MOI
multiplicity of infection
- PGE2
prostaglandin E2
- TLR-4
Toll-like receptor 4
References
- 1.Raynal P, Pollard HB. Annexins. the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 2.Flower RJ, Rothwell NJ. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol Sci. 1994;15:71–6. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 3.Diakonova M, Gerke V, Ernst J, Liautard JP, van der Vusse G, Griffiths G. Localization of five annexins in J774 macrophages and on isolated phagosomes. J Cell Sci. 1997;110:1199–213. doi: 10.1242/jcs.110.10.1199. [DOI] [PubMed] [Google Scholar]
- 4.Traverso V, Morris JF, Flower RJ, Buckingham J. Lipocortin 1 (annexin 1) in patches associated with the membrane of a lung adenocarcinoma cell line and in the cell cytoplasm. J Cell Sci. 1998;111:1405–18. doi: 10.1242/jcs.111.10.1405. [DOI] [PubMed] [Google Scholar]
- 5.Cirino G, Peers SH, Flower RJ, Browning JL, Pepinsky RB. Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci USA. 1989;86:3428–32. doi: 10.1073/pnas.86.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–62. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 7.Perretti M, Flower RJ. Cytokines, glucocorticoids and lipocortins in the control of neutrophil migration. Pharmacol Res. 1994;30:53–9. doi: 10.1016/1043-6618(94)80087-1. [DOI] [PubMed] [Google Scholar]
- 8.Davidson J, Flower RJ, Milton AS, Peers SH, Rotondo D. Antipyretic actions of human recombinant lipocortin-1. Br J Pharmacol. 1991;102:7–9. doi: 10.1111/j.1476-5381.1991.tb12122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CC, Croxtall JD, Perretti M, Bryant CE, Thiemermann C, Flower RJ, Vane JR. Lipocortin 1 mediates the inhibition by dexamethasone of the induction by endotoxin of nitric oxide synthase in the rat. Proc Natl Acad Sci USA. 1995;92:3473–7. doi: 10.1073/pnas.92.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perretti M. The annexin 1 receptor(s): is the plot unravelling? Trends Pharmacol Sci. 2003;24:574–9. doi: 10.1016/j.tips.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Ernst S, Lange C, Wilbers A, Goebeler V, Gerke V, Rescher U. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J Immunol. 2004;172:7669–76. doi: 10.4049/jimmunol.172.12.7669. [DOI] [PubMed] [Google Scholar]
- 12.Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289–98. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alldridge LC, Harris HJ, Plevin R, Hannon R, Bryant CE. The annexin protein lipocortin 1 regulates the MAPK/ERK pathway. J Biol Chem. 1999;274(53):37620–8. doi: 10.1074/jbc.274.53.37620. [DOI] [PubMed] [Google Scholar]
- 14.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 15.Geng Y, Zhang B, Lotz M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J Immunol. 1993;151:6692–700. [PubMed] [Google Scholar]
- 16.Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1:93–103. [PMC free article] [PubMed] [Google Scholar]
- 17.Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–8. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265(5173):808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 19.Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouliot M, Baillargeon J, Lee JC, Cleland LG, James MJ. Inhibition of prostaglandin endoperoxide synthase-2 expression in stimulated human monocytes by inhibitors of p38 mitogen-activated protein kinase. J Immunol. 1997;158:4930–7. [PubMed] [Google Scholar]
- 21.Rose DM, Winston BW, Chan ED, Riches DW, Gerwins P, Johnson GL, Henson PM. Fc gamma receptor cross-linking activates p42, p38 and JNK/SAPK mitogen-activated protein kinases in murine macrophages: role for p42MAPK in Fc gamma receptor-stimulated TNF-alpha synthesis. J Immunol. 1997;158:3433–8. [PubMed] [Google Scholar]
- 22.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 23.Nakao S, Ogata Y, Shimizu-Sasaki E, Yamazaki M, Furuyama S, Sugiya H. Activation of NFkappaB is necessary for IL-1beta-induced cyclooxygenase-2 (COX-2) expression in human gingival fibroblasts. Mol Cell Biochem. 2000;209:113–18. doi: 10.1023/a:1007155525020. [DOI] [PubMed] [Google Scholar]
- 24.Hiscott J, Marois J, Garoufalis J, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–40. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–34. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adcock IM, Caramori G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 2001;79:376–84. doi: 10.1046/j.1440-1711.2001.01025.x. [DOI] [PubMed] [Google Scholar]
- 28.Bryant CE, Perretti M, Flower RJ. Suppression by dexamethasone of inducible nitric oxide synthase protein expression in vivo: a possible role for lipocortin 1. Biochem Pharmacol. 1998;55:279–85. doi: 10.1016/s0006-2952(97)00462-0. [DOI] [PubMed] [Google Scholar]
- 29.Minghetti L, Nicolini A, Polazzi E, Greco A, Perretti M, Parente L, Levi G. Down-regulation of microglial cyclo-oxygenase-2 and inducible nitric oxide synthase expression by lipocortin 1. Br J Pharmacol. 1999;126:1307–14. doi: 10.1038/sj.bjp.0702423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannon R, Croxtall JD, Getting SJ, et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. Faseb J. 2003;17:253–5. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- 31.de Coupade C, Ajuebor MN, Russo-Marie F, Perretti M, Solito E. Cytokine modulation of liver annexin 1 expression during experimental endotoxemia. Am J Pathol. 2001;159:1435–43. doi: 10.1016/S0002-9440(10)62530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira SH, Cunha FQ, Lorenzetti BB, Michelin MA, Perretti M, Flower RJ, Poole S. Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br J Pharmacol. 1997;121:883–8. doi: 10.1038/sj.bjp.0701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudlow AW, Carey F, Forder R, Rothwell NJ. The role of lipocortin-1 in dexamethasone-induced suppression of PGE2 and TNF alpha release from human peripheral blood mononuclear cells. Br J Pharmacol. 1996;117:1449–56. doi: 10.1111/j.1476-5381.1996.tb15305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Hutchinson P, Morand EF. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum. 1999;42:1538–44. doi: 10.1002/1529-0131(199907)42:7<1538::AID-ANR29>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Ruetten H, Thiemermann C. Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: ET-receptors, signal transduction and inhibition by dexamethasone. J Physiol Pharmacol. 1997;48:675–88. [PubMed] [Google Scholar]
- 36.Alldridge LC, Bryant CE. Annexin 1 regulates cell proliferation by disruption of cell morphology and inhibition of cyclin D1 expression through sustained activation of the ERK1/2 MAPK signal. Exp Cell Res. 2003;290:93–107. doi: 10.1016/s0014-4827(03)00310-0. [DOI] [PubMed] [Google Scholar]
- 37.Royle MC, Totemeyer S, Alldridge LC, Maskell DJ, Bryant CE. Stimulation of Toll-like receptor 4 by lipopolysaccharide during cellular invasion by live Salmonella typhimurium is a critical but not exclusive event leading to macrophage responses. J Immunol. 2003;170:5445–54. doi: 10.4049/jimmunol.170.11.5445. [DOI] [PubMed] [Google Scholar]
- 38.Hume DA, Underhill DM, Sweet MJ, Ozinsky AO, Liew FY, Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 40.Spink JET. Binding of the transcription factor interferon regulatory factor-1 to the inducible nitric-oxide synthase promoter. J Biol Chem. 1997;272:24417–25. doi: 10.1074/jbc.272.39.24417. [DOI] [PubMed] [Google Scholar]
- 41.Meraz MA, White JM, Sheehan KC, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–42. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 42.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–37. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.YuZ, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker G, Pfeilschifter J, Kunz D. Mechanisms of suppression of inducible nitric-oxide synthase (iNOS) expression in interferon (IFN)-gamma-stimulated RAW 264.7 cells by dexamethasone. Evidence for glucocorticoid-induced degradation of iNOS protein by calpain as a key step in post-transcriptional regulation. J Biol Chem. 1997;272:16679–87. doi: 10.1074/jbc.272.26.16679. [DOI] [PubMed] [Google Scholar]
- 45.Korhonen R, Lahti A, Hamalainen M, Kankaanranta H, Moilanen E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol Pharmacol. 2002;62:698–704. doi: 10.1124/mol.62.3.698. [DOI] [PubMed] [Google Scholar]
- 46.Kunz D, Walker G, Eberhardt W, Pfeilschifter J. Molecular mechanisms of dexamethasone inhibition of nitric oxide synthase expression in interleukin 1 beta-stimulated mesangial cells. Evidence for the involvement of transcriptional and posttranscriptional regulation. Proc Natl Acad Sci USA. 1996;93:255–9. doi: 10.1073/pnas.93.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donnelly SR, Moss SE. Functional analysis of the human annexin I and VI gene promoters. Biochem J. 1998;332:681–7. doi: 10.1042/bj3320681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 1998;10:514–21. doi: 10.1006/cyto.1997.0325. [DOI] [PubMed] [Google Scholar]
- 49.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–71. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 50.John C, Cover P, Solito E, Morris J, Christian H, Flower R, Buckingham J. Annexin 1-dependent actions of glucocorticoids in the anterior pituitary gland: roles of the N-terminal domain and protein kinase C. Endocrinology. 2002;143:3060–70. doi: 10.1210/endo.143.8.8965. [DOI] [PubMed] [Google Scholar]
- 51.Fraser CS, Pain VM, Morley SJ. Cellular stress in xenopus kidney cells enhances the phosphorylation of eukaryotic translation initiation factor (eIF) 4E and the association of eIF4F with poly(A)-binding protein. Biochem J. 1999;342(Part 3):519–26. [PMC free article] [PubMed] [Google Scholar]
- 52.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 53.Watters JJ, Sommer JA, Pfeiffer ZA, Prabhu U, Guerra AN, Bertics PJ. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1 beta production. J Biol Chem. 2002;277:9077–87. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- 54.Koide N, Mu MM, Sugiyama T, Hassan F, Islam S, Mori I, Yoshida T, Yokochi T. Inhibition of extracellular signal-regulated kinase 1/2 augments nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophage cells. FEMS Immunol Med Microbiol. 2005;45:213–19. doi: 10.1016/j.femsim.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Haghighat A, Mader S, Pause A, Sonenberg N. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. Embo J. 1995;14:5701–9. doi: 10.1002/j.1460-2075.1995.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–49. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]