Abstract
T-lymphocyte activation triggered by anti-CD3, endogenous or exogenous superantigen, and mitogens was suppressed in a cell-dose-dependent fashion by peritoneal cavity (PerC) leucocytes. Study of lymphocyte-deficient mice and the use of multiparameter fluorescence-activated cell sorter analyses revealed that macrophages were responsible for this form of immune regulation. Interferon-γ was essential to trigger suppression, which, by enzyme inhibition studies, was shown to be the result of tryptophan and arginine catabolism. These results illustrate that macrophages, which are classically defined by their innate effector function as antigen-presenting cells, have the potential to temper adaptive immunity.
Keywords: macrophage, peritoneal, suppression, T cell
Introduction
Immunity relies upon cellular collaboration within organized lymphoid tissue. T lymphocytes require cognate interaction with B cells, macrophages, or dendritic cells, collectively known as antigen-presenting cells (APCs). Macrophages and dendritic cells gather antigen from tissue spaces, process this material, and deliver it to regional lymphoid tissue to present in association with major histocompatibility complex (MHC) molecules to T cells. The differentiation state of the APC is critical for promoting T-cell activation or tolerance. APCs patrol tissue spaces performing non-inflammatory housekeeping duties.1 Primarily as a result of the difficulty of obtaining sufficient numbers of them, most studies of APC function do not work with such ‘resident’ cells. Instead, studies of dendritic cells have focused upon those generated in vitro from cytokine-treated bone marrow cells and macrophage research primarily has relied upon cells drawn to the peritoneum by thioglycollate, peptone, or lipopolysaccharide (LPS) administration.2–4 The methods traditionally employed to isolate these cells commit them to their classic functional role as inducers of T-cell activation.5
APCs with the capacity to temper inflammation and lymphocyte activation have been described. Myeloid suppressor cells (MSCs) have been observed in situations of chronic immune activation, tumour development and autoimmunity.6 In addition to cases of immune perturbation, there are examples of MSCs promoting tissue homeostasis. MSCs at sites of high microbial traffic, such as respiratory and intestinal epithelia, have been shown to curb lymphocyte activation.7–11 These surfaces are also known for their high turnover rate and generation of cell corpses which are removed by shedding and phagocytosis. Other sites of significant apoptotic corpse burden, such as thymus, bone marrow and brain, have resident myeloid cells that serve in this vital housekeeping function.12–15 The steady-state or ‘default mode’ of APCs in these sites is to remove this material avoiding inflammation in the process.1,15
There is much to be learned regarding MSC heterogeneity, anatomic distribution and mechanism of action. Our earlier studies of peritoneal cavity (PerC) lymphocyte biology revealed suppression of superantigen-induced T-cell activation by MSCs.16,17 In this report, we show that PerC macrophages suppress all forms of T-cell activation by catabolism of tryptophan and arginine. These observations are discussed relative to the role of macrophages in the maintenance of immune homeostasis.
Materials and methods
Mice
Two- to four-month-old male and female C.B-17.scid (SCID; severe combined immune-defective), DBA/2J, BALB.xid (XID), C.B-17, and BALB/c mice, bred and maintained at Rider University, were studied. C3H/HeJ, C3H/HeSnJ and the mutant mouse strains NU/JFoxn1, B6.129S4-Cd80tm1ShrCd86tm1Shr/J, B6.129S2-Igh-6tm1Cgn/J, B6.129P2-Il-10tm1Cgn/J, B6.129S7-Ifngr1tm1Agt/J, and their age- and sex-matched wild-type controls, were obtained from The Jackson Laboratory, Bar Harbor, ME. All mice were handled in accordance with National Institutes of Health, Animal Welfare Act, and Rider University IACUC guidelines.
Preparation of cell suspensions and cell culture
Lymph node (LN) and spleen (SP) cell suspensions were obtained by gentle disruption of the organ between the frosted ends of sterile glass slides. PerC cells were obtained by flushing the peritoneum with 10 ml warm (37°) Hanks' balanced salt solution supplemented with 3% fetal bovine serum. Red blood cells were depleted from SP cell preparations by hypertonic lysis followed by Ficoll–Hypaque centrifugation. Natural killer (NK) cells were depleted by intraperitoneal injection of rabbit anti-asialo GM1 antibody 24 hr before PerC cell harvest as recommended by the manufacturer (Wako Chemical, Richmond, VA). CD11b+ cells were enriched or depleted by panning on Petri dishes coated with anti-CD11b (BD-Pharmingen, San Diego, CA) as described previously.18 CD11b-enriched cells were greater than 95% F4/80+; CD11b-depleted cells were less than 10% F4/80+. Viable cell counts were determined by Trypan blue exclusion. LN T cells (4 × 106/ml) and various dilutions (0·125 × 106−4·0 × 106/ml) of PerC cells, in RPMI-1640 culture media (Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 0·1 mm non-essential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 2 mm l-glutamine, 20 μm 2-mercaptoethanol, and 10 mm HEPES, were incubated in a humidified atmosphere of 5% CO2 at 37° in 96-well microtitre plates (Costar, Cambridge, MA). For anti-CD3 stimulation microtitre plates were either precoated with 10 μg/ml hamster anti-mouse CD3ε monoclonal antibody (mAb; BD-Pharmingen) in sterile phosphate-buffered saline or soluble anti-CD3ε mAb was added at 2 μg/ml. Where exogenous costimulation was tested anti-CD28 (BD-Pharmingen) was added at 2 μg/ml. Mitogens [phytohaemagglutinin, (PHA) and concanavalin A (Con A)] and soluble superantigen [staphylococcal enterotoxin B (SEB), Sigma Chemical, St Louis, MO] were added at 2 and 5 μg/ml. For the minor lymphocyte stimulatory (Mls) mixed lymphocyte reaction, DBA/2J SP cells were added to all wells at 4 × 106/ml. Anti-interferon-γ (IFN-γ) mAb (XMG1.2, eBioscience, San Diego, CA) at 10 μg/ml or the indoleamine 2,3-dioxygenase (IDO) and inducible nitric oxide synthase (iNOS) inhibitors 1-methyl tryptophan (1-MT; Sigma Aldrich) and NG-monomethyl-l-arginine (l-NMMA; CalBiochem, San Diego, CA) were added at culture initiation. Optimal concentrations were determined in titration experiments with 1-MT at 1·0 mm and l-NMMA at 0·5 mm. After 44 hr (anti-CD3, SEB, PHA, or Con A cultures) or 68 hr (Mls mixed lymphocyte reaction), 1 μCi [3H]thymidine (Amersham, Boston, MA) was added to each well. The plates were frozen 4 hr after labelling, then thawed for harvesting onto filter paper mats using a semi-automated cell harvester (Skatron Instruments, Richmond, VA). Radioactivity was measured by scintillation spectrometry. For each experiment four or five wells were established for each test group.
Cytokine enzyme-linked immunosorbent assay
Amounts of interleukin-2 (IL-2), IL-4, IL-10 and IFN-γ present in the supernatants of the 44-hr cultures were determined by sandwich enzyme-linked immunosorbent assay. Capture and detection reagents and all buffers were employed following instructions provided by the supplier (BD-Pharmingen).
Immunofluorescence staining and flow cytometric analyses
PerC cell suspensions were stained for myeloid composition using titred amounts of fluorescein isothiocyanate-labelled rat anti-mouse F4/80, cychrome-labelled rat anti-mouse CD11b, and one of the following phycoerythrin-labelled rat anti-mouse mAbs: CD11c, CD14, CD23, CD40, CD49b, anti-class II MHC (clone M5/114.15.2), CD80, CD86, B7-H1 (PD-L1), B7-DC (PD-L2), B7RP-1 (ICOS-L), 4-1BBL, and Gr-1 (Ly-6c) (all from eBioscience). Fluorescein isothiocyanate-labelled CD68 (Serotec, New York, NY), with phycoerythrin-labelled F4/80 (eBioscience) and Cy-5-labelled CD11b served as another staining combination. Isotype- and fluorochrome-matched, non-specific mAb controls were employed to establish gates. The percentage of myeloid cells coexpressing sets of these markers was determined via multiparameter flow cytometric analyses on a FACSCalibur™ flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) by forward scatter/side scatter gating using cellquest software.
Statistical analyses
The T-cell proliferative response is presented as the average counts per min (c.p.m.) ± SEM. Data sets were compared using Student's t-test.
Results
PerC cells inhibit all forms of T-cell proliferation
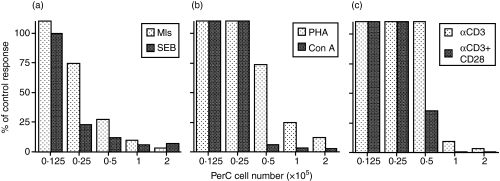
Our previous studies comparing Mls antigen presentation by SP and PerC cells revealed that PerC cells actively inhibit the T-cell response to this endogenous superantigen (Fig. 1a).16,17 This observation was not Mls-specific as the response to the soluble superantigen SEB was also inhibited by PerC cells (Fig. 1a). A cell-dose-dependent inhibition of the T-cell response to the mitogens Con A and PHA was also observed (Fig. 1b). T-cell-receptor-mediated activation, induced by either soluble or insoluble (plate-bound) anti-CD3 was also blocked by PerC cell coculture and was not rescued by costimulation with anti-CD28 (Fig. 1c). For all forms of T-cell stimulation, the highest concentrations of PerC cells suppressed the T-cell response while the lower concentrations revealed APC function by permitting T-cell activation. These data illustrate that resident PerC cells, depending upon cell density, can serve as either suppressor cells or APC.
Figure 1.
PerC cells inhibit T-cell activation in vitro. The lymph node T-cell response to (a)superantigen – Mls, SEB; (b)mitogen – Con A, PHA; and (c)anti-CD3 stimulation was assessed when cocultured with graded numbers of PerC cells. Data are presented as the per cent of control (no PerC cells added) response. The results are representative of more than 10 separate experiments for each T-cell stimulator.
PerC cells from all mouse strains inhibit T-cell proliferation
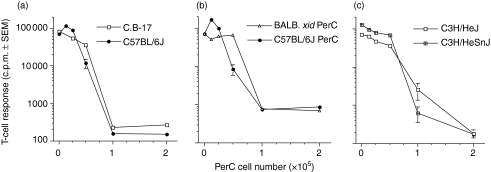
Our initial studies of PerC-cell-mediated suppression focused upon the Mls-expressing DBA/2J strain.16,17 To determine if PerC cell suppression of T-cell activation is a strain-specific phenomenon, other strains of mice were tested. PerC cells from both C57BL/6J [T helper type 1 (Th1)-like] and C.B-17 (BALB/c-derived, Th2-like) mice suppressed T-cell activation (Fig. 2a). PerC cell suppression was MHC-independent because H-2d-restricted T cells were suppressed by allogeneic (H-2b) C57BL/6J PerC cells (Fig. 2b). PerC cells from BALB.xid mice, which have reduced LPS responsiveness, were also suppressive (Fig. 2b). PerC cells from TLR-4 mutant C3H/HeJ mice and their wild-type partner strain (C3H/HeSnJ) also suppressed T-cell activation (Fig. 2c). These results negate a role for LPS preactivation in generating suppressive PerC cells. All mouse strains tested had resident PerC cells capable of suppressing T-cell activation.
Figure 2.
All mouse strains tested have PerC cells that suppress T-cell activation. C.B-17 and C57BL/6J PerC cells were cocultured with autologous T cells (a). BALBxid T cells were cultured with either autologous BALB.xid or allogeneic C57BL/6J PerC cells (b). C3H/HeJ and C3H/HeSnJ T cells were cocultured with autologous PerC cells (c). All cultures employed anti-CD3 for stimulation. Each point represents the average for four wells ± SEM. Data presented are representative of four or more separate experiments.
Myeloid PerC cells suppress T-cell activation
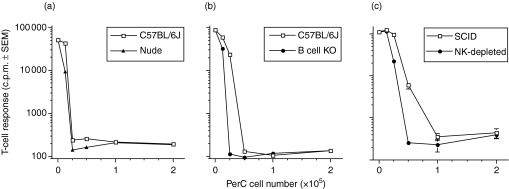
T lymphocytes have been considered the primary cell that regulates lymphocyte activation.19,20 To determine the role of T cells in PerC-cell-mediated immune suppression, nude mice were tested. The results show that T cells are not necessary for PerC cell suppression (Fig. 3a). Via IL-10 production, B cells have been shown to have a role in immune regulation and the peritoneum is enriched for B-1 B cells, a subset that constitutively produces IL-10.21,22 However, PerC cells from B cell knockout mice suppressed T-cell activation (Fig. 3b). Confirmation that B and T lymphocytes were not essential for PerC-cell-mediated suppression was provided by the observation that PerC cells from C.B-17.scid mice suppressed T-cell proliferation (Fig. 3c). Although SCID mice have NK cells, their removal by cytotoxic depletion did not abrogate PerC cell suppression (Fig. 3c). When combined, these results indicate that the cells that mediate suppression are myeloid.
Figure 3.
Non lymphoid PerC cells suppress T-cell proliferation. Graded numbers of PerC cells from C57BL/6J wild-type and C57BL/6J nude mice were cocultured with autologous T cells (a). C57BL/6J wild-type and C57BL/6J B cell knockout PerC cells were cocultured with autologous T cells (b). PerC cells from C.B-17 wild-type, C.B-17.scid, or NK cell-depleted C.B-17.scid mice were cocultured with autologous T cells (c, d). All cultures employed anti-CD3 for stimulation. Each graph point represents the average for five wells ± SEM. Each experiment presented is representative of four or more separate experiments.
Immature macrophage phenotype of suppressor cells
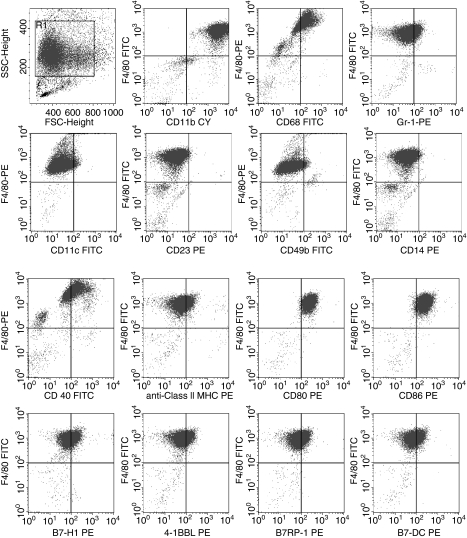
To characterize the suppressor cell, SCID PerC cells were subjected to multiparameter flow cytometric analyses. Forward scatter/side scatter gating focused upon myeloid cells, which because of the scid defect are the predominant (80–90%) leucocyte in the peritoneum (Fig. 4). Virtually all (≥ 94%) of these cells coexpressed CD11b, F4/80, CD80 and CD86. Using F4/80 as a macrophage-specific marker, coexpression of CD68 and CD40 was observed on the majority of these cells (78% and 58%, respectively). A smaller subset of these cells expressed low levels of the costimulatory molecules B7-H1 (PD-L1), B7-DC (PD-L2), B7RP-1 (ICOS-L) and 4-1BBL (28–41%). Low levels of class II MHC and Gr-1 expression were observed on a fraction of these cells (23%, 21%). B-cell (CD23), NK cell (CD49b), and dendritic cell (CD11c) antigens were not expressed. The low level of class II MHC expression and the absence of CD14 indicated that these macrophages were immature. Although the myeloid cell representation was lower (25–40%), similar staining patterns were obtained with PerC cells from BALB.xid, BALB/c, and C57BL/6J mice (not shown). These data illustrate that macrophages with an immature phenotype comprise the majority of myeloid cells residing in the peritoneum.
Figure 4.
FACS analyses of SCID PerC cells: 87·2% of total PerC cells resided in the myeloid gate, 94·4% of which coexpressed CD11b and F4/80; 98·2% and 97·2% of these cells were F4/80+ CD80+ and F4/80+ CD86+. F4/80 was also coexpressed with the following antigens: CD68 (78·0%), CD40 (58·6%), B7-H1 (41·4%), B7-RP1 (39·3%), B7-DC (36·3%), 4-1BBL (28·3%), class II MHC (23·4%), and Gr-1 (21·0%). Negligible levels of CD11c (4·2%), CD49b (3·5%), CD23 (0·7%), and CD14 (1·0%) were expressed by SCID PerC cells. These data are representative of more than 12 analyses of SCID mice.
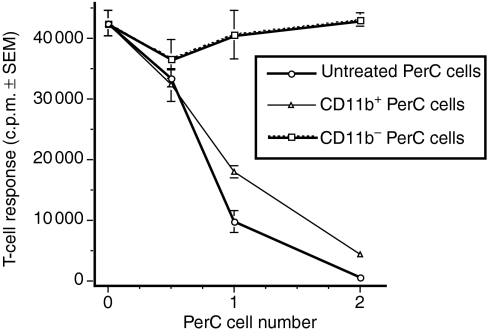
To confirm that the suppressor cell belonged within the cell pool defined by flow cytometry, PerC cells were subjected to selection by panning on anti-CD11b-coated plates and were tested for their ability to inhibit T-cell activation. Only CD11b+ cells suppressed T-cell activation (Fig. 5). In summary, cells with an immature macrophage phenotype are responsible for the suppression mediated by PerC cells.
Figure 5.
CD11b+ PerC cells suppress T-cell activation. BALBxid PerC cells were panned on anti-CD11b-coated Petri dishes as described in the Materials and methods section. Graded numbers of adherent (CD11b+) and non-adherent (CD11b−) cells were tested for suppression of the autologous T-cell proliferative response to anti-CD3. Each graph point represents the average for five wells ± SEM. The data presented are representative of four separate enrichment experiments.
No role for traditional costimulatory molecules in PerC MSC function
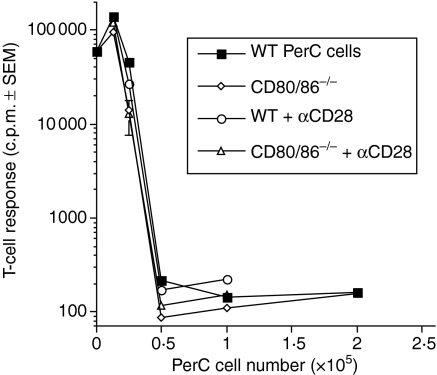
In addition to T-cell receptor engagement, T-cell activation requires costimulation whereby CD28 interacts with CD80/86 on the APC.23 Negative signalling through CD80/86 has also been described.24 To determine the role that costimulation might have in PerC macrophage suppression, PerC cells from CD80/86−/− mice were tested for their ability to suppress T-cell activation. PerC macrophages lacking CD80/86 still suppressed T-cell activation (Fig. 6). Confirmation that signalling through CD28 cannot recover T-cell activation was provided by this experiment (Figs 1 and 6). These data illustrate that CD28–CD80/86 interaction is not needed for PerC macrophage-mediated suppression.
Figure 6.
No role for costimulatory molecules in PerC MSC function. Titered numbers of PerC cells from C57BL/6J CD80/86−/− and wild-type control mice were tested for their ability to suppress the T-cell response to anti-CD3. Anti-CD28 was added to determine if exogenous costimulation could rescue the proliferative response. Each graph point represents the average for five wells ± SEM. The data presented are representative of four separate experiments.
Role of cytokine production in PerC MSC function
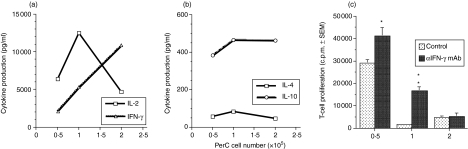
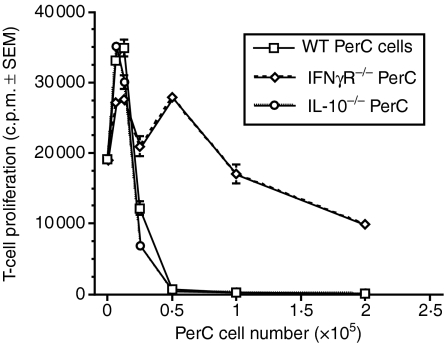
To attempt to define the mechanism whereby PerC macrophages suppress T-cell activation, cytokine production was assessed. Significant levels of the Th1 cytokines IL-2 and IFN-γ were produced in suppressed cultures (Fig. 7a). In contrast, production of the Th2 cytokines IL-4 and IL-10 was more modest (Fig. 7b). The addition of anti-IFN-γ mAb at the initiation of culture released T-cell suppression at the intermediate (0·5 × 105 and 1·0 × 105) PerC cell concentrations. The increased production of IFN-γ with increasing PerC macrophage number and the established role of IL-10 in the regulation of T-cell activation invited further investigation of these cytokines. PerC macrophages from IL-10 knockout mice were as effective as wild-type PerC macrophages in suppressing T-cell activation (Fig. 8). In contrast, PerC macrophages from IFN-γ receptor knockout (IFNγR−/−) mice were less effective as suppressors (Fig. 8). These results show that IFN-γ is necessary for PerC macrophages to regulate T-cell activation.
Figure 7.
Cytokine production in PerC cell cultures. IL-2 and IFN-γ (a)and IL-4 and IL-10 (b)levels were determined in culture supernatants harvested 44 hr after the coculture of BALB.xid PerC cells with autologous T cells. Each point represents the average in pg/ml for five pooled wells. The data presented are representative of two separate experiments. (c) Anti-IFN-γ mAb was added at the start of culture. Each point represents the average of four wells ± SEM. *P < 0·05; **P < 0·005. Data presented are representative of two separate experiments.
Figure 8.
Role of cytokine production in PerC MSC function. Graded numbers of PerC cells from C57BL/6J IFNγR−/−, C57BL/6J IL-10−/−, and wild-type control mice were tested for their ability to suppress the T-cell response to anti-CD3. Each point represents the average of five wells ± SEM. Data presented are representative of four separate experiments.
Arginine and tryptophan catabolism drive PerC macrophage suppression
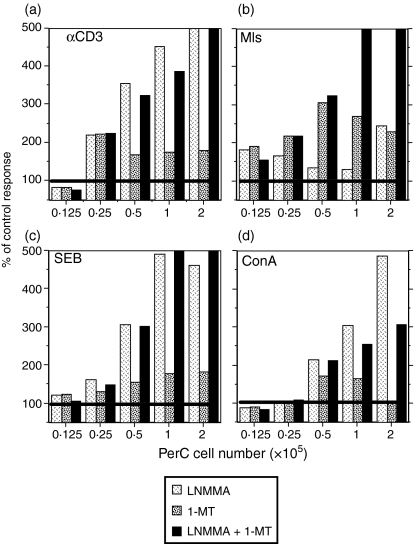
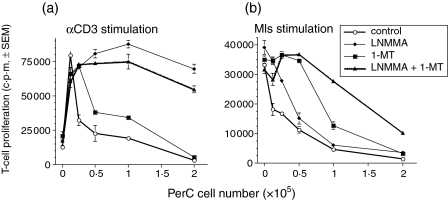
IFN-γ has been shown to induce macrophages to express a pair of amino acid catabolising enzymes that generate products that curb T-cell activation. 25–32 IDO catabolism of tryptophan can be inhibited by the addition of 1-MT and iNOS catabolism of arginine can be inhibited by the addition of l-NMMA. These inhibitors were tested for their ability to block PerC macrophage suppression. For all forms of T-cell activation, suppression was reduced by the addition of either inhibitor (Fig. 9a–d). The degree of reduction differed between groups with the soluble T-cell activators, particularly anti-CD3, exhibiting the greatest recovery of T-cell proliferation at the highest PerC cell concentration. l-NMMA was the better inhibitor of macrophage suppression when T cells were stimulated by soluble stimulators (Figs 9a and 10a). In contrast, 1-MT was better when Mls was the T-cell activator (Figs 9b and 10b). These data illustrate that amino acid catabolism has a primary role in the T-cell suppression mediated by PerC macrophages.
Figure 9.
Arginine and tryptophan catabolism drive PerC macrophage suppression. Titrations of BALB.xid PerC cells were cocultured with autologous T cells in the presence of (a) anti-CD3, (c)SEB, or (d) Con A in the presence of 1-MT, l-NMMA, or both inhibitors. For (b), titrations of DBA/2J PerC cells were cocultured with autologous SP cells and Mls-reactive BALB.xid T cells. Each histogram represents the percentage of the control response for the same PerC cell dose for wells in which no inhibitor was added (n = 4). Data presented are representative of two to four experiments.
Figure 10.
Arginine and tryptophan catabolism drive PerC macrophage suppression. (a) Anti-CD3 and (b) Mls cultures as described for Fig. 9. Each point represents the average of four wells ± SEM. Results are representative of three separate experiments.
Discussion
Macrophages traditionally have been defined by their antigen presentation properties.5 Their activation leads to their service as initiators of acquired immunity. A number of studies, however, have described macrophages that restrict T-cell activation. MSCs have been characterized in disease states and as resident cells that promote homeostasis on epithelial surfaces.6–11 In this report, the suppressive properties of the macrophages that are normal residents of the peritoneal cavity were characterized. These cells had an immature phenotype and were capable of potent suppression of all forms of T-cell activation by catabolism of arginine and tryptophan. These results serve to reinforce the extension of the functional role of macrophages in immunity to include regulation of T-cell activation.
The use of mutant mice lacking mature lymphocyte subpopulations and the manipulation of leucocyte subsets suggested that myeloid cells were the suppressor cell in the PerC. Based on coexpression of F4/80, CD68 and high levels of CD11b (Mac-1), and the relative absence of Gr-1, a marker lost as monocytes mature, these cells are macrophages.15,33 Further phenotypic characterization indicated that these cells, immediately ex vivo, have an immature phenotype. In contrast to thioglycollate- or LPS-elicited cells resident PerC macrophages express low levels of class II MHC and no CD14.2,34 Further support for a ‘naive’ macrophage phenotype for the PerC suppressor cell came from the observation that BALB.xid and C3H/HeJ mice, known for their low LPS responsiveness, had resident PerC cells capable of suppression. Most MSCs described in the literature are Gr-1+ and exhibit properties characteristic of neutrophils.6,33 Although heterogeneity in phenotype remains a barrier to the characterization of myelomonocytic cells there is little argument that the extravascular leucocytes that are resident on the epithelial surfaces and in the body cavities of normal, disease-free animals, are macrophages.4,15
An indication of the functional role of resident PerC macrophages can be revealed by assessment of their expression of costimulatory molecules. Modest levels of all costimulatory molecules tested were observed and macrophages that expressed both CD80 and CD86 were the most abundant. Regulation of T-cell activation via maintenance of T regulatory cells has been proposed as one role for the constitutive expression of CD80/86 in the absence of an active immune response.24 However, neither T cells or CD80/86 expression were necessary for suppression. Expressed by a significant percentage (25–45%) of resident PerC macrophages, B7-H1 (PD-L1), B7-DC (PD-L2), B7RP-1 (ICOS-L), and 4-1BBL have roles in costimulating or regulating IFN-γ production.29,35–38 As reported for CD40, which was also expressed by most PerC macrophages, B7-H1 is necessary for iNOS expression in macrophages.29,39 That ex vivo macrophages express these molecules indicates that these cells might be providing a regulatory function, particularly considering their modest expression of class II MHC. Activated macrophages express much greater levels of costimulatory and class II MHC molecules.2,5,15,23 T-cell coculture would certainly modify the expression of some of these molecules, thus ex vitro analysis will be necessary to reveal those potentially required for suppression. This will be particularly revealing employing T-cell activators that differ in terms of their reliance upon costimulation.
The outcome of macrophage interaction with T lymphocytes is influenced by cytokines. Th1 cytokines, particularly IFN-γ, are known to generate pro-inflammatory macrophages whereas Th2 cytokines, such as IL-4 or IL-10, induce the differentiation of anti-inflammatory macrophages.40 The activation history of macrophages would certainly have an impact on their interaction with naive T cells and, based on this premise, anti-inflammatory cytokines would be expected to be predominant in suppressed cultures. However, previous studies did not reveal a significant role for transforming growth factor-β or IL-10 in macrophage-mediated suppression.17,29 In this report, IFN-γ production had the best correlation with macrophage number and degree of suppression and blocking antibody experiments suggested a role for this cytokine. Where production of IL-10 was not essential for suppression, expression of the IFNγR by macrophages was necessary to curb T-cell activation. These data indicated that IFN-γ was essential for inducing the suppressive mechanism in the macrophage. Although all of the gene knockout experiments were performed with C57BL/6-derived (Th1) mice these observations were not strain-specific as suppression was also observed with BALB/c-derived (Th2) strains. Suppression appeared to be slightly greater, i.e., titrated to lower PerC cell numbers, with C57BL/6 mice, an observation consistent with the M-1/M-2 (Th1/Th2) hypothesis of macrophage biology.41
Although several mechanisms for macrophage-mediated suppression of T-cell activation were possible, the essential role of IFN-γ in these studies focused attention upon amino acid catabolism by IDO and iNOS, enzymes whose increased production by macrophages is triggered by this cytokine.27–30 For all forms of T-cell activation, suppression was reduced by the addition of inhibitors of either enzyme. The release from suppression was greatest with soluble activators, all of which induce IFN-γ production.42,43 As noted in a previous study,29 suppression of T cells activated with anti-CD3 was best released by the use of l-NMMA as the inhibitor. However, for T cells activated by Mls, 1-MT was more effective at blocking suppression. These distinctions probably reflect differences in the signals for activating these enzymes, the complexities of which are being intensively studied.6,44–46 The functional overlap of these enzymes is consistent with many redundant control mechanisms evident in adaptive immunity.47 It is important to emphasize the caveat that these are strong activating signals and that it is unlikely that suppression in vivo would require the control of such a large portion of the T-cell pool. However, as evidenced with the fetal allograft of pregnancy, local depletion of critical amino acids is clearly an effective means to control T cells in vivo.44
The results described in this report are consistent with a growing body of work that has revealed an immune regulatory strategy of macrophage catabolism of essential amino acids.6,25,30–32,39,48,49 This form of control operates at the initiation of an adaptive immune response and is cell-density-dependent. Macrophages persist at sites where tempering inflammation is vital and cell density has been shown to play a role in tempering immunity in vivo.11,50 In the absence of pro-inflammatory signals, such as tumour necrosis factor-α, IL-1, or IL-6, which can override this form of suppression (not shown), this is beneficial for macrophage housekeeping functions, e.g. removing apoptotic corpses without triggering an inflammatory response.1 Thus, most macrophages are active but naive in an inflammatory sense, requiring additional ‘danger’ signals to terminally differentiate into classic phagocytic/APC effectors.51 This non-inflammatory steady state, although beneficial in healthy individuals, might be a factor in the failure of immune surveillance in certain cancers. MSC that actively suppress the cellular immunity required for the elimination of transformed cells have been described in several forms of cancer.6,52,53 A better understanding of how such suppression is established is essential for advances in cancer immunotherapy. Time and tolerization are key features of tumorigenesis.53 Although tempering cellular immunity may be beneficial in early life perhaps it is harmful with aging, permitting tumour development. Such a life-history ‘trade-off’ would represent an immune system example of antagonistic pleiotropy, an evolution-based hypothesis for aging.54
Acknowledgments
This work was supported by NIH AREA program grants CA77814-01, AG19631-01, and AI060356-01. We are grateful to Faith Archer, Andrew Cacace, Theron Jenifer, and Jessica Reid for excellent maintenance of our mouse colonies.
Abbreviations
- APC
antigen-presenting cell
- Con A
concanavalin A
- c.p.m.
counts per minute
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IL-2
interleukin-2
- iNOS
inducible nitric oxide synthase
- LN
lymph node
- l-NMMA
NG-monomethyl-l-arginine
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- Mls
minor lymphocyte stimulatory
- MSCs
myeloid suppressor cells
- 1-MT
1-methyl tryptophan
- NK
natural killer
- PerC
peritoneal cavity
- PHA
phytohaemagglutinin
- SCID
severe-combined immune-defective
- SEB
staphylococcal enterotoxin B
- SP
spleen
- Th1
T helper type 1
References
- 1.Steinman R, Hawiger D, Nussenzweig M. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Fortier A. Activation of murine macrophages. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current Protocols in Immunology. New York: John Wiley & Sons, Inc.; 1991. pp. 4.1–5. Section 14. [Google Scholar]
- 3.Inaba K, Swiggard W, Steinman R, Romain N, Schuler G. Isolation of dendritic cells. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current Protocols in Immunology. New York: John Wiley & Sons, Inc.; 1991. pp. 7.1–15. Section 3. [Google Scholar]
- 4.Fortier A, Falk L. Isolation of Murine Macrophages. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current Protocols in Immunology. New York: John Wiley & Sons, Inc.; 1991. pp. 1.1–19. Section 14. [Google Scholar]
- 5.Unanue E. Perspective on antigen processing and presentation. Immunol Rev. 2002;185:86–102. doi: 10.1034/j.1600-065x.2002.18510.x. [DOI] [PubMed] [Google Scholar]
- 6.Bronte V, Zanovello P. Regulation of immune responses by 1-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 7.Holt P, Warner L, Mayrhofer G. Macrophages as effectors of T suppression: T-lymphocyte-dependent macrophage-mediated suppression of mitogen-induced blastogenesis in the rat. Cell Immunol. 1981;63:57–70. doi: 10.1016/0008-8749(81)90028-9. [DOI] [PubMed] [Google Scholar]
- 8.De Heer H, Hammad H, Soullie T, Hijdra D, Vos N, Willart M, Hoogsteden H, Lambrecht B. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cailhier J, Partolina M, Vuthoori S, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–42. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 10.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal R, Campbell D, Hwang P, DeKruygg R, Frankel L, Umetsu D. Human alveolar macrophages induce functional inactivation in antigen-specific CD4 T cells. J Allergy Clin Immunol. 2001;107:258–64. doi: 10.1067/mai.2001.112845. [DOI] [PubMed] [Google Scholar]
- 12.Surh C, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–3. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen K, Prasad V, Sidman C, Osmond D. Apoptosis and macrophage-mediated deletion of precursor B cells in the bone marrow of E mu-myc transgenic mice. Blood. 1994;84:2784–94. [PubMed] [Google Scholar]
- 14.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–18. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 15.Taylor P, Martinez-Pomares L, Stacey M, Lin H-H, Brown G, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 16.Riggs J, Howell K, Taylor J, Mahjied T, Prokopenko N, Alvarez J, Coleman C. Mls presentation by peritoneal cavity B cells. Immunobiology. 2004;209:255–64. doi: 10.1016/j.imbio.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Rosini L, Matlack R, Taylor J, Howell K, Yeh K, Pennello A, Riggs J. Nonlymphoid peritoneal cells suppress the T cell response to Mls. Immunobiology. 2004;209:575–84. doi: 10.1016/j.imbio.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Mage M., Xxxx . In: Current Protocols in Immunology. Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. New York: John Wiley & Sons, Inc.; 1991. pp. 5.1–6. Section 3. [Google Scholar]
- 19.Gershon R, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 20.Chatenoud L, Salomon B, Bluestone J. Suppressor T cells – they're back and critical for regulation of autoimmunity! Immunol Rev. 2001;182:149–63. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore K, de Waal Malefyt R, Coffman R, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 22.Hardy R, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 23.Greenwald R, Freeman G, Sharpe A. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 24.Lohr J, Knoechel B, Jiang S, Sharpe A, Abbas A. The inhibitory function of B7 co-stimulators in T cell responses to foreign and self-antigens. Nat Immunol. 2003;4:664–9. doi: 10.1038/ni939. [DOI] [PubMed] [Google Scholar]
- 25.Albina J, Abate J, Henry W. Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. J Immunol. 1991;147:144–8. [PubMed] [Google Scholar]
- 26.Cauley L, Miller E, Yen M, Swain S. Superantigen-induced CD4 T cell tolerance mediated by myeloid cells and IFN-γ. J Immunol. 2000;165:6056–66. doi: 10.4049/jimmunol.165.11.6056. [DOI] [PubMed] [Google Scholar]
- 27.Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn D. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1+ macrophages that suppress naïve CD4+ T cell proliferation via an IFN-γ and nitric oxide-dependent mechanism. J Immunol. 2001;167:4293–302. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- 28.Dupuis M, Ibarra-Sanchez M, Tremblay M, Duplay P. Gr-1+ myeloid cells lacking T cell protein tyrosine phosphatase inhibit lymphocyte proliferation by an IFN-γ and nitric oxide-dependent mechanism. J Immunol. 2003;171:726–32. doi: 10.4049/jimmunol.171.2.726. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki T, Akiba H, Koyanagi A, Azuma M, Yagita H, Okumura K. Blockade of B7–H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-gamma-induced nitric oxide production. J Immunol. 2005;175:1586–92. doi: 10.4049/jimmunol.175.3.1586. [DOI] [PubMed] [Google Scholar]
- 30.Terness P, Bauer T, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolism. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez P, Zea A, DeSalvo J, Culotta K, Zabaleta J, Quiceno D, Ochoa J, Ochoa A. l-Arginine consumption by macrophages modulates the expression of the CD3ζ chain in T lymphocytes. J Immunol. 2003;175:1232–9. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 32.Munn D, Shafizadeh E, Attwood J, Bondarev I, Pashine A, Mellor A. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagasse E, Weissman I. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Meth. 1996;197:139–50. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 34.Akashi S, Saitoh S, Wakabayashi Y, et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–42. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4–1BB and 4–1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–86. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 36.Rutitzky L, Ozkaynak E, Rottman J, Stadecker M. Disruption of the ICOS-B7RP-1 costimulatory pathway leads to enhanced hepatic immunopathology and increased gamma interferon production by CD4 T cells in murine schistosomiasis. Infect Immun. 2003;71:4040–4. doi: 10.1128/IAI.71.7.4040-4044.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwai H, Kozono Y, Hirose S, et al. Amelioration of collagen-induced arthritis by blockade of inducible costimulator-B7 homologous protein costimulation. J Immunol. 2002;169:4332–9. doi: 10.4049/jimmunol.169.8.4332. [DOI] [PubMed] [Google Scholar]
- 38.Tseng S, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian L, Noelle R, Lawrence D. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995;25:306–9. doi: 10.1002/eji.1830250152. [DOI] [PubMed] [Google Scholar]
- 40.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 41.Mills C, Kincaid K, Alt J, Heilman M, Hill A. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 42.Gollob K, Nagelkerken L, Coffman R. Endogenous retroviral superantigen presentation by B cells induces the development of type 1 CD4+ T helper lymphocytes. Eur J Immunol. 1993;23:2565–71. doi: 10.1002/eji.1830231028. [DOI] [PubMed] [Google Scholar]
- 43.Rochford R, Riggs J, Clavo A, Ernst D, Hobbs M. Differential effects of CD28 costimulation upon cytokine production by CD4+ and CD8+ T cells. Immunobiology. 2004;209:513–22. doi: 10.1016/j.imbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Mellor A, Munn D. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 45.Grohmann U, Fallarino F, Bianchi R, Orabona C, Vacca C, Fioretti M, Puccetti P. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J Exp Med. 2003;198:153–60. doi: 10.1084/jem.20030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hucke C, MacKenzie C, Adjogble K, Takikawa O, Daubener W. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect Immun. 2004;72:2723–30. doi: 10.1128/IAI.72.5.2723-2730.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yadav D, Sarvetnick N. Cytokines and autoimmunity: redundancy defines their complex nature. Curr Opin Immunol. 2003;15:697–703. doi: 10.1016/j.coi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson J, Battisto J. In the murine syngeneic mixed lymphocyte reaction, one T cell subset replicates in the presence of B cells or macrophages and replication is inhibited by simultaneous presence of both stimulator cells. Eur J Immunol. 1986;16:508–12. doi: 10.1002/eji.1830160508. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu T, Sano C, Tomioka H. The role of B7 molecules in the cell contact-mediated suppression of T cell mitogenesis in immunosuppressive macrophages induced with mycobacterial infection. Clin Exp Immunol. 2004;35:373–9. doi: 10.1111/j.1365-2249.2004.02403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brys L, Beschin A, Raes G, Ghassabeh G, Noel W, Brandt J, Brombacher F, De Baetselier P. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol. 2005;174:6095–104. doi: 10.4049/jimmunol.174.10.6095. [DOI] [PubMed] [Google Scholar]
- 51.Matzinger P. An innate sense of danger. Ann NY Acad Sci. 2002;961:341–2. doi: 10.1111/j.1749-6632.2002.tb03118.x. [DOI] [PubMed] [Google Scholar]
- 52.Muller A, DuHadaway J, Donover P, Sutanto-Ward E, Prendergast G. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–19. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 53.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 54.Kirkwood T, Austad S. Why do we age? Nature. 2000;408:233–8. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]