Abstract
Interleukin (IL)-12 (p70) is a heterodimeric cytokine composed of p40 and p35, that plays a major role in the protective immune response to Mycobacterium tuberculosis. To define the role of p40 in lungs during pulmonary M. tuberculosis infection we generated transgenic (Tg) mice overexpressing p40 under control of the surfactant protein C promoter. Tg mice expressed the transgene in their lungs, yet demonstrated elevated pulmonary p40 protein levels. After infection, Tg mice displayed higher pulmonary p40 and p70 levels than wild type mice. Interferon-γ concentrations were similar in uninfected and infected Tg and wild type mice, arguing against agonistic effects of p40. Tg mice demonstrated reduced recruitment of macrophages, lymphocytes and neutrophils to the lungs early after infection. This was accompanied by reduced pulmonary tumour necrosis factor-α, macrophage inflammatory protein (MIP)-2 and MIP-1 α levels. This suggests that elevated p40 concentrations inhibited the chemotactic effects of p70 on leucocytes. Furthermore, Tg mice displayed slightly higher pulmonary mycobacterial outgrowth late in the infection than wild type mice. Taken together, we demonstrate that constitutive overexpression of p40 in lungs negatively influences IL-12-mediated leucocyte migration and protection against lung tuberculosis. This suggests a novel antagonistic role for p40 homodimers in regulating the chemotactic bioactivity of IL-12 after pulmonary mycobacterial infection.
Keywords: Mycobacterium tuberculosis, IL-12, chemotaxis, murine, leucocytes
Introduction
Tuberculosis, caused by Mycobacterium tuberculosis, is one of the main threats to mankind with one-third of the world's population being infected.1 Cell-mediated immunity (CMI) is known to be critical in host defence against M. tuberculosis infection and is tightly regulated by a balance between type 1 and type 2 cytokines.2 Interleukin-12 (IL-12) is a key regulatory cytokine produced by antigen-presenting cells, such as macrophages (Mϕs) and dendritic cells, which is decisive in the regulation of the T helper 1 (Th1)/Th2 balance.3 IL-12 plays a pivotal role in promoting Th1 while suppressing Th2 responses, and hence promotes CMI against intracellular pathogens like M. tuberculosis.4 IL-12 furthermore increases the cytolytic activity of CD8+ cells and natural killer (NK) cells, which is critical for an effective control of mycobacterial infection.4 Several in vivo studies, using IL-12 neutralizing antibodies or IL-12-deficient animals, showed an important role for IL-12 in the development of functional Th1 responses and protection against intracellular pathogens.5,6 Accordingly, it was demonstrated that IL-12 receptor (IL-12R)-deficient individuals demonstrated an increased susceptibility to intracellular pathogens.7,8
IL-12 is a heterodimeric protein (p70) composed of two disulphide-linked subunits: p35 and p40. For the generation of bioactive IL-12p70, both subunits are required although their expression is independently regulated.9 Secreted IL-12p70 binds to and signals through the high affinity IL-12R, which consists of a constitutively expressed IL-12Rβ1 chain and an inducible low affinity IL-12Rβ2 chain. The p40 subunit is responsible for IL-12R binding10 and is mainly produced as monomer and homodimer ((p40)2) in excess of the p70 heterodimer.11 Although p40 monomer is neither antagonistic nor agonistic with respect to IL-12 bioactivity, the homodimeric form seems to inhibit IL-12 function in vitro by competing with heterodimer for the high affinity IL-12R on responder T lymphocytes and NK cells. 12–14 Because secretion of IL-12p70 is associated with excess production of the p40 subunit, it has been suggested that (p40)2 plays a critical role as a natural antagonist of IL-12. However, (p40)2 has also been reported to stimulate, rather than to inhibit, Th1 development in vitro, resulting in an increase in interferon-γ (IFN-γ) production15. An agonistic role for (p40)2 has also been suggested by three studies revealing phenotypic differences between IL-12p35−/− and IL-12p40−/− mice in alloantigen Th1 responses following heart transplantation16 in defence of infection with Cryptoccoccus neoformans17 and M. bovis.18 Because p40 has been shown to have both agonistic and antagonistic effects on bioactive IL-12, we wanted to obtain more insight in the role of p40 during pulmonary mycobacterial infection in vivo. Therefore, we generated transgenic mice that overexpress p40 under control of the lung specific surfactant protein C promoter19 (SP-C), and studied host defence mechanisms in p40 Tg mice during pulmonary tuberculosis.
Materials and methods
Animals
FVB/N mice were obtained from Harlan Nederland (Horst, the Netherlands). Foster mothers were on the BDF background (C57BL6/DBA F1 hybrid) and were bred in our central animal facility. For the experiments with M. tuberculosis, female mice between the ages of 7 and 9 weeks were used. Each experimental group consisted of eight mice per group. In all experiments, sex and age matched controls were used. All experiments were approved by the Institutional Animal Care and Use Committee of the Academic Medical Center (University of Amsterdam, the Netherlands).
Generation of lung specific IL-12p40 transgenic mice
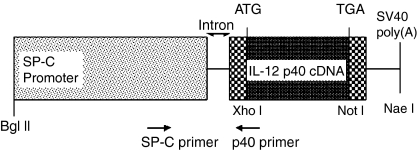
All DNA manipulations were done by standard methods. A 3·7 kb fragment consisting of the SP-C promoter, kindly donated by Dr Stephan W. Glasser, was excised from the pUC18SP-C plasmid.19 This promotor confers expression in bronchiolar and type II epithelial cells in the adult mouse lung.19,20 The SP-C promoter was inserted by blund-end ligation in the expression vector pCI-cytomegalovirus (CMV) (Promega, Leiden, the Netherlands) thereby replacing the CMV promoter. The pCI/SP-C/p40 construct was obtained by introducing mouse IL-12-p40 cDNA in to the XhoI/NotI site of the pCI vector containing the SP-C promoter. The SP-C-p40 construct was released by digestion with BglII and NaeI and purified by agarose gel electrophorese followed by dialysis against AnalaR water. A 3 ng/ml solution of the linear construct was microinjected into pronuclei of fertilized FVB/N eggs. After egg transplantation into pseudo-pregnant foster mothers, transgenic offspring was identified by Southern blot analysis and polymerase chain reaction (PCR) of genomic DNA isolated from tail biopsies.
DNA isolation and Southern blot analysis
One cm of the tails from FVB/N mice were harvested and 500 µl lysing buffer (100 mm Tris buffer, pH 8·5; 5 mm ethylenediaminetetraacetic acid (EDTA); 0,2% sodium dodecyl sulphate (SDS); 200 mm NaCl; 100 µg/µl proteinase K) was added and incubated overnight at 55° in a shaking disc. DNA extraction was performed by means of phenol and chloroform extraction and isopropanol precipitation. Thereafter, DNA was resuspended in sterile water and stored at −20° until further use. Ten µg DNA was digested by BamHI overnight. For Southern blot analysis DNA was separated on a 0·8% agarose gel. The gel was soaked for 10 min in 0·2 m HCl, thereafter denatured in 0·2 m NaOH/1·5 m NaCl for 45 min and 2 × 20 min neutralized in 0·5 m TRIS/HCl pH 7·4 containing 1·5 m NaCl. Thereafter, DNA was transferred to a Nytran Nylon membrane using the turboblot system (Schleicher & Schuell, Dassel, Germany) and the DNA was UV-crosslinked. The filters were prehybridized at 65° in 5× sodium saline citrate, 5× Denhardt's, 0·5% SDS containing 100 µg/ml herring sperm DNA. Thereafter, the filters were hybridized with IL-12 p40 cDNA labelled with deoxycytidine 5′-[α-32P] triphosphate ([α-32P]dCTP, 3000 Ci/mmol) as described under ‘Northern blot analysis’ and the signal was detected using a Molecular Dynamics phosphor imager (Amersham Biosciences, Sunnyvale, CA).
PCR analysis
PCR was performed with 100 pg genomic DNA in a reaction mixture of 1·0 mm deoxynucleotide triphosphates (dNTPs), 10 mg/ml bovine serum albumin, 2% dimethyl sulphoxide, 10× Pol buffer (0·67 m Tris-HCl, pH 8·8; 67 mm MgCl2; 0,1 mβ-mercaptoethanol; 67 µm EDTA; 0·166 m (NH4)2SO4), 0·5 µm of the SP-C-primer (5′-GGACACATATAAGACCC-TGG-3′), 0·5 µm of the p40 primer (5′-TTTGGTGCTTCACACTTCAGG-3′) and 5 U/µl DNA polymerase (AmpliTaq, Perkin Elmer, Norwalk, CT). Reactions for the p40 transgene were subjected to 30 cycles on a thermocycler (Perkin Elmer Applied Biosystems, Norwalk, CT) consisting of 95° for 1 min, 58° for 1 min and 72° for 2 min, respectively. All PCR mixtures were subjected to denaturation at 95° for 5 min before the first cycle, and to final extension at 72° for 10 min after the last cycle. The amplified products were size fractioned by electrophoresis on a 1·0% agarose gel, followed by ethidium bromide staining for UV-assisted visualization.
Northern blot analysis
RNA was isolated using TRIZOL (Gibco BRL life technologies, Gaithersburg, MD) according to the manufacturer's manual. Samples of 20 µg of total RNA were seperated on a 0·8% agarose gel containing 6·6% formaldehyde and transferred onto Nytran nylon membrane using the turbo-blot system (Schleicher & Schuell). The RNA was fixed to the membrane by 3 min treatment in a microwave oven at full setting (650 W). The filters were prehybridized for 1 hr at 42° in 50% (v/v) formamide, 5× sodium chloride sodium phosphate EDTA buffer (SSPE), 5× Denhardt's, 0·5% (w/v) SDS and 100 µg/ml denaturated herring sperm DNA. Thereafter, filters were hybridized at 42° overnight with mouse IL-12 p40 cDNA, labelled with [α-32P]dCTP according to the random primed labelling techniques using Amersham Rediprime labelling kit. The signal was detected by Molecular Dynamics phosphor Imager (Amersham Bioscience). The ribosomal DNA was stained on filters with methylene blue in order to assess equal RNA loading and transfer.21
Preparation of lung tissue for enzyme-linked immunosorbent assay (ELISA)
Mice were anaesthetized by FFM (fentanyl citrate 0079 mg/ml, fluanisone 2·5 mg/ml, midazolam 1·25 mg/ml in H2O; of this mixture 7·0 ml/kg intraperitonally). Lungs, were harvested and immediately homogenized in nine volumes of 1× lysis buffer (0·5% Triton-X-100, 150 mm NaCl, 15 mm Tris, 1 mm CaCl2 and 1 mm MgCl2.H2O; pH 7·40) at 4° using a tissue homogenizer (Biospec Products, Bartlesville, OK). The homogenates were first centrifuged at 2100 g for 10 min and then at 22 000 g for 10 min to remove cell debris, after which the supernatants were stored at −20°. The following ELISAs were used according to the instructions of the manufacturer: IL-12 p40, IL-12 p70 (Pharmingen, San Diego, CA) and IFN-γ, TNF-α IL-4, MIP-2, MIP-1α (R & D Systems, Minneapolis, MN).
Western Blot analysis
Lung homogenates as prepared for the ELISA from uninfected p40 Tg and wild type (Wt) mice was diluted 1 : 10 in lysis buffer and diluted 1 : 2 in 25 µl of 2 × sample buffer (125 mm Tris/HCl, pH 6·8; 4% SDS; 20% glycerol, bromphenol blue; non-reducing condition). For reducing condition 5%β-mercaptoethanol was added to the sample buffer. Samples mixed with SDS-sample buffer were boiled for 5 min at 95° followed by brief centrifugation. Thirty µl of the samples were loaded onto 10% SDS–polyacrylamide gel electrophorsis (PAGE) and subsequently transferred to a polyvinylidenefluoride membrane. The blots were blocked with 5% bovine serum albumin in TBST (Tris buffered saline with 0·1% Tween-20) for 1 hr at room temperature and washed three times with TBST and incubated with primary antibody (purified rat anti-mouse IL-12 (p40/p70), C15.6, Cat #18491D, Pharmingen) overnight in a 1 : 1000 dilution in TBST supplemented with 5% bovine serum albumin. After three washes with TBST buffer the membrane was incubated with peroxidase-conjugated rabbit anti-rat immunoglobulin G antibodies (P0450, DAKO, Glostrup, Denmark) in a 1 : 1000 dilution for 1 hr at room temperature. The bands were visualized using Lumilight® plus ECL substrate from Roche and a chemiluminescence detector with a cooled CCD camera (GeneGnome) from Syngene.
Experimental infection
Pulmonary tuberculosis was induced intranasally (i.n.) as described previously.22,23 Briefly, a virulent laboratory strain of M. tuberculosis H37Rv was grown in liquid Dubos medium containing 0·01% Tween-80 for 4 days. A replicate culture was incubated at 37°, harvested at mid-log phase and stored in aliquots at −70°. For each experiment, a vial was thawed and washed twice with sterile 0·9% NaCl. Mice were anaesthetized by inhalation with isoflurane (Abbott Laboratories Ltd, Queenborough, UK) and i.n. infected with 1 × 104 live M. tuberculosis bacilli in 50 µl sterile saline, as determined by viable counts on 7H11 Middlebrook agar plates. Bacterial counts recovered from lungs one day postinfection (p.i.) were shown previously to be similar to the number of bacteria in the inoculum.22
Enumeration of bacteria
Groups of eight mice per time point were killed 2 or 5 weeks p.i., and lungs, and liver were removed aseptically. Organs were homogenized with a tissue homogenizer (Biospec Products, Bartlesville, OK) in 5 volumes of sterile 0·9% NaCl, and 10-fold serial dilutions were plated on Middlebrook 7H11 agar plates to determine bacterial loads. Colonies were counted after 21-day incubation at 37°.
Histology
Lungs were removed 2 or 5 weeks after inoculation with M. tuberculosis and fixed in 4% paraformaldehyde in phosphate-buffered saline for 24 hr. After embedding in paraffin, 4-µm-thick sections were stained with haematoxylin–eosin for histology analysis.
Lung cell differentiation
Pulmonary cell suspensions were obtained by crushing lungs through a 40 µm cell strainer (Becton Dickinson, Franklin Lakes, NJ) as described previously.22 Erythrocytes were lysed with ice-cold isotonic NH4Cl solution (155 mm NH4Cl, 10 mm KHCO3, 100 mm EDTA, pH 7·4), the remaining cells were washed twice with RPMI-1640 (Bio Whittaker, Verviers, Belgium), and counted by using a haemocytometer. The number of Mϕs, polymorphonuclear cells (PMNs) and lymphocytes were calculated from these totals, using cytospin preparations stained with modified Giemsa stain (Diff-Quick, Baxter, McGraw Perk, IL).
Splenocyte stimulation
Single cell suspensions were obtained by crushing spleens through a 40 µm cell strainer. Erythrocytes were lysed, and the remaining cells were washed and counted by using a haemocytometer. Splenocytes were suspended in medium (RPMI-1640, 10% fetal calf serum (FCS), 1% antibiotic–antimycotic (GibcoBRL, Life Technologies, Rockville, MD)), seeded in 96-well round bottom culture plates at a cell density of 1 × 106 splenocytes in triplicate, and stimulated with 20 µg/ml tuberculin-purified protein derivative (PPD; Statens Seruminstitut, Copenhagen, Denmark). Supernatants were harvested after a 48-hr incubation at 37° in 5% CO2, and cytokine levels were analysed by ELISA.
Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). Comparisons were done with Mann–Whitney U-tests. Values of P ≤ 0·05 were considered statistically significant.
Results
Establishment of SP-C/p40 transgenic mice
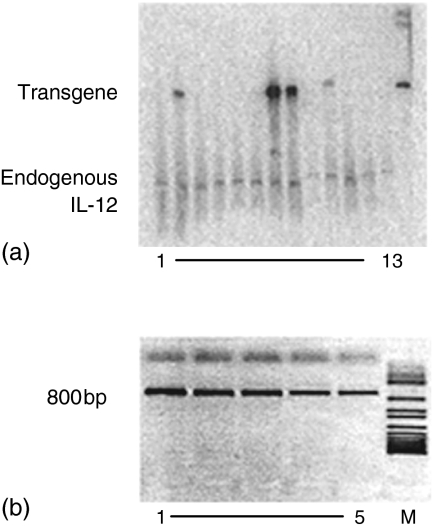
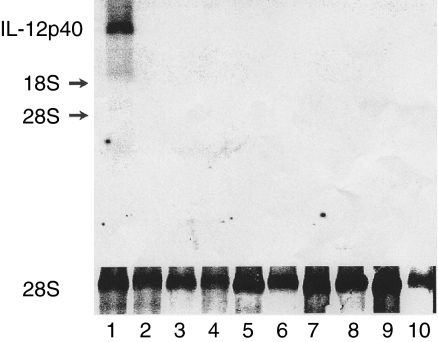
The SP-C/p40 construct shown in Fig. 1 was microinjected into fertilized eggs to generate transgenic mice in which p40 expression was targeted to the lungs. Transgenicity was confirmed by performing Southern blotting, using a specific probe for murine p40, and by PCR using a primer set specific for the SP-C promoter and murine p40 as indicated in Fig. 1. A representative Southern blot and PCR analysis are shown in Fig. 2(a and b), respectively. Twelve mice carrying the p40 transgene were generated. One of the mice with the highest expression, containing about 11 copies of the transgene compared to endogenous p40, was mated with a FVB/N mouse and transmitted the transgene to the offspring. After five generations homozygotic Tg mice were identified and these were mated to generate homozygotic offspring. Upon gross necroscopy, no apparent abnormality was detected in organs of the p40 Tg mice, including lung. p40 Tg mice were apparently normal size and weight, and both sexes were fully fertile. To examine tissue specificity of the p40 transgene expression, total RNA was prepared from various tissues of p40 Tg animals when they were 7 weeks of age and Northern blot analysis was performed. As can be seen in Fig. 3, p40 mRNA was detected in the lung of Tg animals. No p40 mRNA was detected in the lungs of control mice, nor in other tissues from control or p40 Tg mice. Hence, transgenic p40 was exclusively produced in the lungs of p40 Tg animals.
Figure 1.
SP-C/p40 DNA construct. The construct contains a 3·7 kb fragment from the SP-C promoter, a 1 kb murine IL-12p40 cDNA fragment, an intron and SV 40 polyadenylation sequences. Restriction sites and primers used in the PCR are indicated.
Figure 2.
A representative Southern blot (a) and PCR analysis (b) of the screening of transgenic mice.(a) Southern blot analysis of the genomic DNA isolated from mouse tails of the first generation mice after injection of the construct. On this blot four mice carry the transgene. (b) PCR analysis was performed on the fifth generation of transgenic mice. A PCR product of 800 bp because of the appearance of the p40 transgene in combination with the SP-C promoter was detected in all five mice tested. M = marker.
Figure 3.
p40 mRNA expression in the lung of SP-C/p40 Tg mice. Organs were obtained from 7 weeks old Tg and Wt mice. Cellular RNA (20 µg) was analysed by Northern blotting as described in the Methods section. Lane 1: lung Tg, 2: liver Tg, 3: heart Tg, 4: intestine Tg, 5: kidney Tg, 6: lung Wt, 7: liver Wt, 8: heart Wt, 9: intestine Wt, 10: kidney Wt.
High lung p40 levels in p40 Tg mice
To examine p40 levels in the lungs of Tg animals and to establish whether p40 was circulating, lung homogenates were assayed for p40 in a sandwich ELISA when mice were 7 weeks of age. High concentrations of lung p40, 18·0 ± 1·1 ng/ml were found in p40 Tg animals compared to control levels (0·55 ng/ml). Furthermore, we tried to detect lung IL-12 p70 in an ELISA in order to see if high levels of IL-12p40 resulted in an increase of bioactive IL-12 p70. The concentration of p70 in p40 Tg animals was around the detection limit of the ELISA (62·5 pg/ml) in lung homogenates and comparable with that in control mice, indicating that this enhanced p70 production did not occur in p40 Tg mice. Because it was shown that p40 homodimer can induce IFN-γ production by T cells15 we wanted to investigate lung IFN-γ levels. No difference between lung levels in Tg animals compared to controls was observed (data not shown).
Generation of p40 homodimer in p40 Tg mice
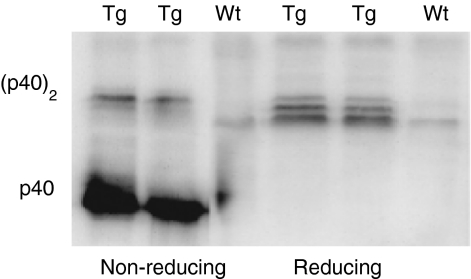
Although p40 monomer is neither antagonistic nor agonistic with respect to IL-12 bioactivity, the homodimeric form strongly inhibits IL-12 function in vitro. To determine whether monomeric or homodimeric forms of p40 could be detected, lung homogenates from p40 Tg and Wt mice were characterized by SDS–PAGE and Western blot analysis. Under reducing conditions, p40 was recognized as a triple band, representing p40 monomeric forms with variable glycosylation with apparent molecular masses of around 40 000 MW (Fig. 4).13 Under non-reducing conditions, p40 was detected as a homodimer with a molecular mass of approximately 80 000 MW (Fig. 4). In the non-Tg animals both the homodimer and monomer were hardly detectable by Western blot analysis (Fig. 4).
Figure 4.
Identification of monomeric and homodimeric p40 on a Western blot under reduced and nonreduced conditions in lungs of p40 Tg and Wt mice. Lung p40 monomeric and homodimeric levels were both elevated in p40 Tg mice as compared to Wt mice.
Increased production of p40 and p70 in p40 Tg mice during tuberculosis
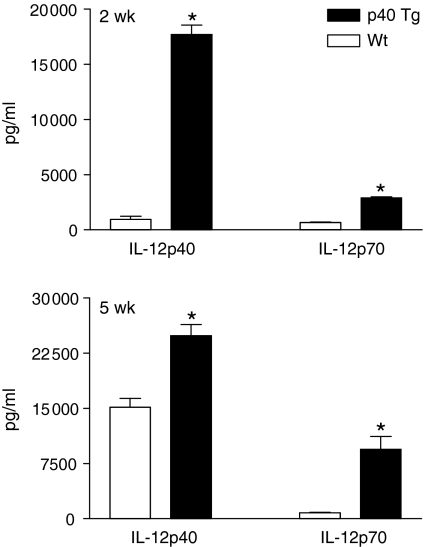
IL-12 has been demonstrated to play an important role in the development of protective immunity against intracellular pathogens. In order to examine the effect of p40 on p40 and p70 protein overexpression levels during mycobacterial infection, we i.n. inoculated p40 Tg and Wt mice with a virulent strain of M. tuberculosis and killed them after 2 and 5 weeks. As expected, very high concentrations of IL-12p40 were measured in lungs of p40 Tg mice at both time points following infection. Lungs of p40 Tg mice contained 18-fold and 1·6-fold more IL-12p40 than Wt mice at, respectively, 2 (P = 0·001) and 5 weeks p.i. (P = 0·003, Fig. 5). In addition, the level of IL-12p70 was 4·4 (2 week p.i., P = 0·001) and 11·8 (5 week p.i., P = 0·002) times elevated in the lung of p40 Tg mice compared to Wt mice (Fig. 5).
Figure 5.
Pulmonary p40 and p70 levels are significantly increased in p40 Tg mice compared to Wt mice 2 and 5 weeks after infection with M. tuberculosis. Data are presented as the mean and SEM for 8 mice per group. *P < 0·05.
Histopathology of lung tissue
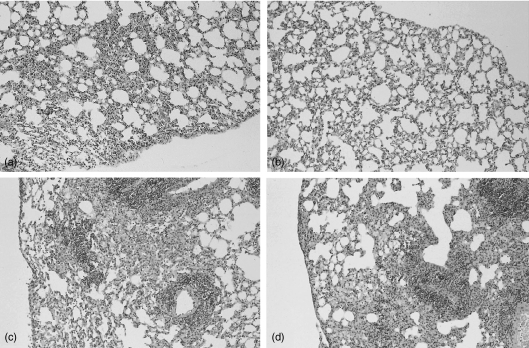
To examine tissue responses, histological assessment of lungs from mice killed at 2 and 5 weeks after mycobacterial infection was conducted. Two weeks after M. tuberculosis inoculation lungs of Wt mice exhibited prominent lymphocytic interstitial inflammation with formation of small granulomas and slight pleuritis (Fig. 6a). In contrast, lungs of p40 Tg mice presented only slight interstitial inflammation (Fig. 6b). After 5 weeks the inflammatory infiltrates in lungs of all mice became more diffuse and intense with prominent lymphocytic perivascular inflammation and foamy macrophages in the alveoli. The degree and the cellular composition of the inflammatory infiltrates were comparable in Wt (Fig. 6c) and p40 Tg (Fig. 6d) mice.
Figure 6.
Representative histologic sections of lungs of Wt (a and c) and p40 Tg mice (b and d) 2 (a and b) and 5 (c and d) weeks after inoculation with M. tuberculosis, demonstrating less inflammatory infiltrate in p40 Tg mice 2 week p.i. as compared to Wt mice. Haematoxylin and eosin staining, original magnification ×33.
Impaired leucocyte recruitment to lungs of p40 Tg mice
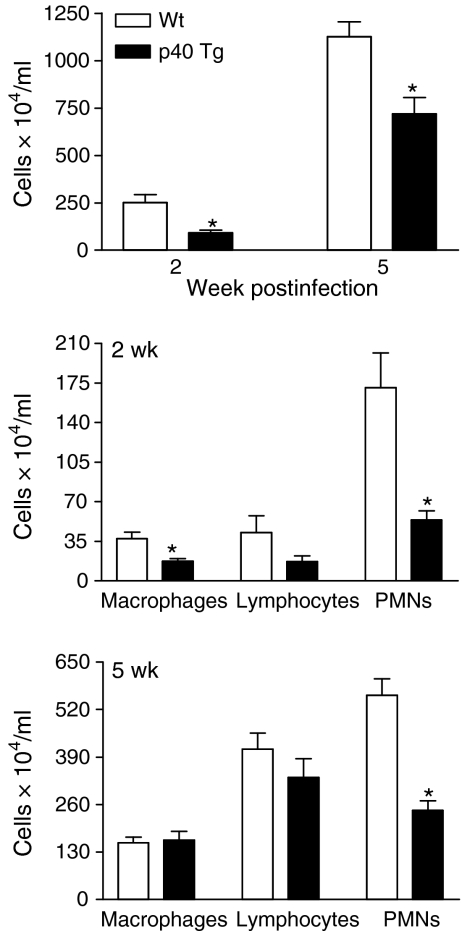
To analyse leucocyte influx in a more quantitative way, pulmonary cells were isolated, counted and differentiated by performing a modified Giemsa staining on cytospin preparations. An increase in total cell numbers was observed in each group of mice throughout the course of the experiment (Fig. 7). However, p40 Tg mice had 3- (2 weeks, P = 0·003) and 1·6- (5 weeks, P = 0·009) fold less leucocytes isolated from their lungs compared with Wt controls (Fig. 7). Analysis of lung cell populations revealed that two times less Mϕs (P = 0·01), three times less PMNs (P = 0·005) and 2·5 times less lymphocytes (P = 0·06) were present in p40 Tg mice compared to Wt mice 2 weeks p.i. At 5 weeks p.i., the absolute number of Mϕs was similar in both groups. Although, 23% less lymphocytes were present at this time point in lungs from p40 Tg mice, this was not significantly different from Wt mice. The absolute number of PMNs were 2·3 times lower in the p40 Tg mice as compared with Wt mice 5 weeks p.i. (P < 0·001).
Figure 7.
Analysis of leucocyte populations in lungs of Wt and p40 Tg mice infected i.n. for 2 or 5 weeks with M. tuberculosis. The number of leucocytes in lungs of p40 Tg mice was significantly lower than of Wt mice at both time points. Leucocyte differentiation revealed that p40 Tg mice had significantly less Mϕs (2 weeks), lymphocytes (2 weeks), and PMNs (2 and 5 weeks) in their lungs compared to Wt mice. Data are presented as the mean and SEM for eight mice per group. *P < 0·05.
Reduced chemokine levels in p40 Tg mice early in the infection
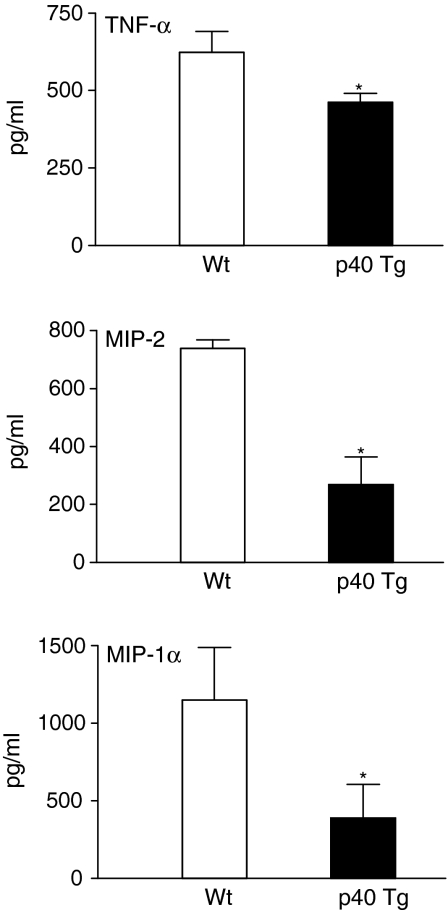
Because the inflammatory infiltrate was less profound in p40 Tg mice early in the infection, we measured pulmonary TNF-α, a potent agonist of chemokine expression, and C-X-C chemokine macrophage inflammatory protein (MIP)-2 and C-C chemokine MIP-1α. MIP-2 is predominantly chemotactic toward PMNs24 whereas MIP-1α preferentially augments monocyte/macrophage and lymphocyte recruitment.25 Interestingly, p40 Tg mice had, respectively, 1·4, 2·7, and three times less TNF-α (P = 0·047), MIP-2 (P = 0·006), and MIP-1α (P = 0·017) in their lungs than Wt mice 2 week p.i. (Fig. 8). After 5 weeks TNF-α and MIP-2 levels were similar in both group of mice, whereas MIP-1α was two times lower in p40 Tg mice compared to Wt mice (P = 0·02) (data not shown).
Figure 8.
p40 Tg mice had decreased expression of TNFα, MIP-2, and MIP-1α in their lungs 2 weeks after infection as compared with Wt mice. Data are presented as the mean and SEM for eight mice per group. *P < 0·05.
Th1 dominant cytokine profile in lungs from p40 Tg mice late in the infection
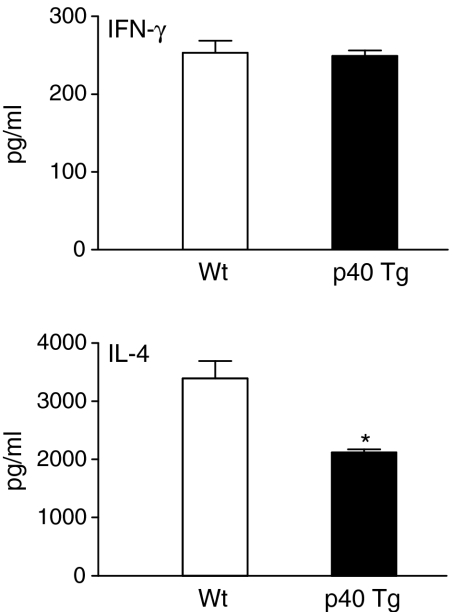
Because the Th1/Th2 balance is critical to antimycobacterial host defence, we examined the pulmonary concentration of type 1 cytokine IFN-γ, and type 2 cytokine IL-4 2 and 5 weeks p.i. IFN-γ, and IL-4 concentrations were similar in Wt and p40 Tg mice 2 weeks p.i. (data not shown). In contrast, later in the infection (5 weeks p.i) IL-4 was 60% lower in lungs of p40 Tg mice compared to Wt mice (P = 0·002, Fig. 9). IFN-γ levels were yet again not different in both groups of mice at this time point (Fig. 9).
Figure 9.
p40 Tg mice have an increased Th1/Th2 balance compared to Wt mice since they have unaltered pulmonary IFN-γ (Th1) levels and reduced IL-4 (Th2) levels when compared to Wt mice 5 weeks p.i. Data are presented as the mean and SEM for eight mice per group. *P < 0·05.
Increased antigen-specific IFN-γ response in p40 Tg mice following M. tuberculosis infection
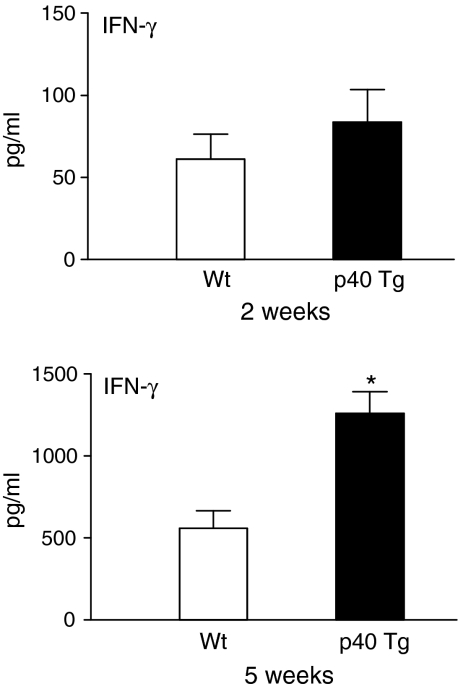
To more directly examine CMI responses in p40 Tg mice and their relationship with susceptibility to M. tuberculosis infection, we analysed the antigen-specific IFN-γ production of splenocytes from p40 Tg and Wt mice following M. tuberculosis infection in response to the recall-antigen PPD. We found that 2 week p.i. IFN-γ levels were similar in both groups of mice. In comparison, later in the infection splenocytes from p40 Tg mice released 2·3 times more IFN-γ upon PPD stimulation than Wt mice (P = 0·005, Fig. 10). These results suggest that increased p70 levels in lungs from p40 Tg mice enhanced the ability of splenocytes to display a antigen-specific IFN-γ response.
Figure 10.
Splenocytes from p40 Tg mice mount an increased antigen-specific IFN-γ response to mycobacterial antigen compared to Wt mice 5 weeks p.i. Splenocytes were harvested 2 and 5 weeks after i.n. inoculation with M. tuberculosis, and stimulated for 48 hr. Data are presented as the mean and SEM for eight mice per group. *P < 0·05.
Increased susceptibility of p40 Tg mice to pulmonary M. tuberculosis infection
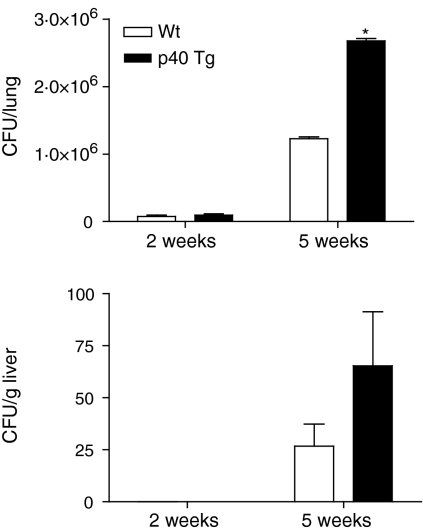
To examine the impact of p40 overexpression on the in vivo growth of M. tuberculosis in the lung, we determined the mycobacterial load 2 and 5 weeks p.i. Early in the infection ∼28% more colony-forming units (CFUs) were found in lungs from p40 Tg mice as compared to Wt mice (Fig. 11, not significantly different). No mycobacteria were disseminated to the liver in either group at this time point. Five weeks after the infection, p40 Tg mice had 118% more M. tuberculosis CFUs than Wt mice (Fig. 11, P = 0·021). In the liver of p40 Tg mice 144% more viable mycobacteria were found, although this difference with Wt mice did not reach statistical significance because of large variation (Fig. 11). Together, these data indicate that p40 Tg mice are more susceptible to M. tuberculosis infection than Wt mice as demonstrated by reduced clearance of M. tuberculosis infection from the lung and liver late in the infection.
Figure 11.
Increased mycobacterial outgrowth in lungs and livers of p40 Tg mice compared to Wt mice 5 weeks after i.n. infection with 1 × 104 M. tuberculosis bacilli. Data are presented as the mean and SEM for eight mice per group. *P < 0·05.
Discussion
In the present study, we generated transgenic mice that overexpress p40 specifically in the lung. We found that p40 Tg animals develop, grow and reproduce normally. We demonstrated that lung p40 levels were profoundly elevated in p40 Tg animals compared to p40 levels in control Wt mice. In contrast, no apparent increase in lung IL12-p70 levels was observed in p40 Tg mice, likely because in non-infected Tg animals not enough p35 is available for dimerization with the p40 subunit. In addition, no increase in IFN-γ production was found in Tg animals, arguing against a direct agonistic effect of p40 overexpression under basal condition.
IL-12 is involved in the chemotactic activity for Mϕs, 26–29 T cells27,30 and PMNs31 by enhancing pro-inflammatory cytokine and chemokine expression.27,29,32 As p40 overexpression led to an early defect in chemokine expression and leucocyte recruitment, these results are most compatible with the inhibition of the chemotactic bioactivity of IL-12 by p40 homodimers that compete with IL-12 for binding to its receptor.13 We showed that p40 homodimers were highly present in lung homogenates of Tg mice. Presumably, this early impaired leucocyte recruitment in p40 Tg mice at least in part was responsible for the relatively enhanced outgrowth of M. tuberculosis in lungs of p40 Tg mice. Indeed, resistance against M. tuberculosis depends primarily on Mϕs and T cells and the interaction between these cells.33 Furthermore, decreased TNF-α levels in lungs from p40 Tg mice early in the infection may play a role in this since TNF-α is important for activating Mϕs to kill intracellular pathogens.34 Together, we suggest that IL-12p40 homodimer acts as an antagonist for IL-12-mediated leucocyte migration to the M. tuberculosis-infected lung by reducing TNF-α, a potent agonist of chemokine expression, and the chemokines MIP-2 and MIP-1α. These data demonstrate a new mechanism by which the bioactivity of IL-12 is regulated during mycobacterial infection. One could hypothesize that p40 homodimers may act as a physiological regulator of IL-12-mediated chemotaxis of inflammatory cells in order to restore pulmonary homeostasis.
Two recent studies revealed a protective role for p40 during mycobacterial infection, as indicated by the finding that p35 knockout (KO) mice were less susceptible for infection with mycobacteria than p40 KO mice.18,30 p40 can also bind to p19 to form IL-23, a novel cytokine that has similar biological activities as IL-12p70.35 While Cooper et al. suggest that the increased resistance could be caused by the formation of IL-23 in p35 KO mice5, Hölscher et al. suggest that the agonistic effect of endogenous and exogenous p40 can accomplish this.18 The present study demonstrates that mice overexpressing p40 in lung have an increased susceptibility for tuberculosis, indicating that p40 by itself does not mediate resistance during mycobacterial infection. Because in the former two studies p70 could not be formed, a possible antagonistic effect of p40 on p70 in vivo could not be studied. By overexpressing p40 in the lung, we introduced an increased competition between p40 and p70 for binding to IL-12R and demonstrated an antagonistic effect of elevated p40 concentrations on previously established IL-12 functions, i.e. chemokine expression and recruitment of leucocytes and resistance against mycobacterial infection.5,27,29 It is unclear at this point whether IL-23 signalling is increased or partly responsible for the impaired inflammatory response and bacterial clearance in p40 Tg mice, although this appears unlikely given the fact that IL-23 gene transfer to lungs of M. tuberculosis infected animals led to an increased bacterial clearance and T-cell influx.36
Remarkably, p40 Tg mice displayed a relative ‘type 1 phenotype’ at 5 weeks p.i., as reflected by a increased IFN-γ/IL-4 ratio in lung tissue and by enhanced antigen-specific IFN-γ release by splenocytes. At this time point profoundly elevated IL-12p70 concentrations were measured in the lungs of p40 Tg mice, suggesting that, unlike the situation in uninfected mice, tuberculosis triggers the production of p35, which in the presence of excess p40 results in the generation of more IL-12p70. It is conceivable that these elevated IL-12p70 concentrations occurring later in the course of the infection overcame the antagonistic effects of p40, especially since at 5 weeks p.i. the difference between p40 Tg and Wt mice with respect to p40 levels was by far less profound as at 2 weeks p.i. In spite of this relative type 1 response in p40 Tg animals 5 weeks p.i., the mycobacterial load was increased in these mice. These data suggest that the early immune response in Tg mice, dominated by elevated p40 concentrations and associated with a reduced recruitment of different leucocyte subsets to the lungs, was more decisive for the number of M. tuberculosis CFUs at 5 weeks p.i.
We here report that lung specific overexpression of p40 in mice does not result in a phenotype with evidence of IL-12-like agonistic properties. During pulmonary infection with M. tuberculosis, p40 Tg mice displayed characteristics of an IL-12 antagonistic phenotype, as indicated by a diminished chemokine expression and recruitment of leucocytes to the lung early in the infection and a subsequently slightly increased mycobacterial load. Yet, later during the infection, enhanced expression of p40 also resulted in elevated levels of IL-12p70 and an increased antigen-specific IFN-γ response. Additional research is warranted to further define the role of p40 in the biology of IL-12 and IL-23 during intracellular infections. Together, we suggest that IL-12p40 homodimer acts as an antagonist for IL-12-mediated leucocyte migration to the M. tuberculosis infected lung by reducing the expression of chemokines. This study suggests a novel mechanism by which the bioactivity of IL-12 is regulated during infection with mycobacteria. P40 homodimers may act as a physiological regulator of IL-12-mediated chemotaxis of inflammatory cells in order to restore homeostasis in the lungs after mycobacterial infection.
Acknowledgments
This work was supported by a grant from the Netherlands Organization for Scientific Research to J. C. Leemans. The authors wish to thank Joost Daalhuisen for expert technical assistance, André Geerdink for technical help with the generation of transgenic mice, and Dr Stephan W. Glasser for his kind donation of the SP-C promotor.
Abbreviations
- IL-12
interleukin-12
- IL-12R
interleukin-12 receptor
- Tg
transgenic
- SP-C
surfactant protein C promotor
- TNF-α
tumour necrosis factor-α
- MIP
macrophage inflammatory protein
- IFN-γ
interferon-γ
- CMI
cell-mediated immunity
- Mϕ
macrophage
- PMN
polymorphonuclear cell
- SEM
standard error of the mean
References
- 1.Kaufmann SHE. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines. new players in the regulation of T cell responses. Immunity. 2003;19:641–4. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 4.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 5.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakeham J, Wang J, Magram J, et al. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette–Guerin in IL-12-deficient mice. J Immunol. 1998;160:6101–11. [PubMed] [Google Scholar]
- 7.de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–8. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 8.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 9.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trinchieri G. Interleukin-12. a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 11.D'Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillessen S, Carvajal D, Ling P, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–6. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 13.Ling P, Gately MK, Gubler U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 14.Gately MK, Carvajal DM, Connaughton SE, et al. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann N Y Acad Sci. 1996;795:1–12. doi: 10.1111/j.1749-6632.1996.tb52650.x. [DOI] [PubMed] [Google Scholar]
- 15.Piccotti JR, Chan SY, Li K, Eichwald EJ, Bishop DK. Differential effects of IL-12 receptor blockade with IL-12 p40 homodimer on the induction of CD4+ and CD8+ IFN-gamma-producing cells. J Immunol. 1997;158:643–8. [PubMed] [Google Scholar]
- 16.Piccotti JR, Li K, Chan SY, et al. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–8. [PubMed] [Google Scholar]
- 17.Decken K, Kohler G, Palmer-Lehmann K, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holscher C, Atkinson RA, Arendse B, et al. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–66. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 19.Glasser SW, Korfhagen TR, Wert SE, et al. Genetic element from human surfactant protein SP-C gene confers bronchiolar-alveolar cell specificity in transgenic mice. Am J Physiol. 1991;261:L349–56. doi: 10.1152/ajplung.1991.261.4.L349. [DOI] [PubMed] [Google Scholar]
- 20.Glasser SW, Burhans MS, Eszterhas SK, Bruno MD, Korfhagen TR. Human SP-C gene sequences that confer lung epithelium-specific expression in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L933–45. doi: 10.1152/ajplung.2000.278.5.L933. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson M, Doskow J. Lindsey SRNA blots. staining procedures and optimization of conditions. Nucl Acids Res. 1991;19:679. doi: 10.1093/nar/19.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leemans JC, Juffermans NP, Florquin S, et al. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol. 2001;166:4604–11. doi: 10.4049/jimmunol.166.7.4604. [DOI] [PubMed] [Google Scholar]
- 23.Leemans JC, Florquin S, Heikens M, Pals ST, van der Neut R, van der Poll T. CD44 is a macrophage binding site for Mycobacterium tuberculosis that mediates protective immunity against pulmonary tuberculosis. J Clin Invest. 2003;111:681–9. doi: 10.1172/JCI16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo. involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–602. [PubMed] [Google Scholar]
- 25.Gonzalo JA, Lloyd CM, Wen D, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–67. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha SJ, Lee SB, Kim CM, Shin HS, Sung YC. Rapid recruitment of macrophages in interleukin-12-mediated tumour regression. Immunology. 1998;95:156–63. doi: 10.1046/j.1365-2567.1998.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Geboes K, Heremans H, et al. Role of interleukin-12 in the induction of mucosal inflammation and abrogation of regulatory T cell function in chronic experimental colitis. Eur J Immunol. 2001;31:1550–60. doi: 10.1002/1521-4141(200105)31:5<1550::AID-IMMU1550>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami K, Tohyama M, Xie Q, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immunol. 1996;104:208–14. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearlman E, Lass JH, Bardenstein DS, et al. IL-12 exacerbates helminth-mediated corneal pathology by augmenting inflammatory cell recruitment and chemokine expression. J Immunol. 1997;158:827–33. [PubMed] [Google Scholar]
- 30.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–7. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 31.Papp Z, Middleton DM, Rontved CM, Foldvari M, Gordon JR, Baca-Estrada ME. Transtracheal administration of interleukin-12 induces neutrophil responses in the murine lung. J Interferon Cytokine Res. 2000;20:191–5. doi: 10.1089/107999000312603. [DOI] [PubMed] [Google Scholar]
- 32.Kawakami K, Qureshi MH, Zhang T, et al. Interferon-gamma (IFN-gamma)-dependent protection and synthesis of chemoattractants for mononuclear leucocytes caused by IL-12 in the lungs of mice infected with Cryptococcus neoformans. Clin Exp Immunol. 1999;117:113–22. doi: 10.1046/j.1365-2249.1999.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rook GA, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–84. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch CS, Ellner JJ, Russell DG, Rich EA. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–53. [PubMed] [Google Scholar]
- 35.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 36.Happel KI, Lockhart EA, Mason CM, et al. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect Immun. 2005;73:5782–8. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]