Abstract
The vitamin D receptor (VDR) is a nuclear receptor expressed in a number of different cells of the immune system. This study was performed to determine the effect of VDR deficiency on immune function and inflammation of the gastrointestinal tract in a model of inflammatory bowel disease, namely interleukin-10 (IL-10) knockout mice. IL-10 knockout mice were generated which either could or could not respond to vitamin D (double IL-10/VDR knockout; DKO). The distribution and function of lymphocytes in both the primary and secondary lymphoid organs were compared and determined as a function of the severity of intestinal inflammation. DKO mice had normal thymic development and peripheral T-cell numbers at 3 weeks of age, but a week after intestinal disease was detected the thymus was dysplastic with a reduction in cellularity. The atrophy was coupled with increased apoptosis. The spleen weight of DKO mice increased as a result of the accumulation of red blood cells; however, there was a 50% reduction in the numbers of T and B cells. Conversely, the mesenteric lymph nodes were enlarged and contained increased numbers of lymphocytes. The T cells from DKO mice were of a memory phenotype and were hyporesponsive to T-cell receptor stimulation. Colitis in the DKO mice was associated with local and high expression of IL-2, interferon-γ, IL-1β, tumour necrosis factor-α and IL-12. The primary and secondary lymphoid organs in DKO mice are profoundly altered as a consequence of the fulminating inflammation in the gastrointestinal tract. VDR expression is required for the T cells and other immune cells to control inflammation in the IL-10 KO mice.
Keywords: inflammatory bowel disease, mice, T cells, vitamin D receptors
Introduction
Crohn's disease is a chronic intestinal inflammatory disorder of unknown aetiology. The pathogenesis of this disease is dictated by environmental factors that lead to an inappropriate and exaggerated mucosal immune response to gastrointestinal antigens in genetically susceptible individuals.1 Mouse models of colitis offer new opportunities for identifying inflammatory bowel disease (IBD) genes or pathways that may lead to the development of new therapies for this disease. Mice carrying targeted mutations in a variety of mouse genes, such as interleukin-2 knockout (IL-2 KO), T-cell receptor-β KO and IL-10 KO mice,2 spontaneously develop IBD symptoms resembling certain aspects of human ulcerative colitis or Crohn's disease.
A growing body of evidence suggests that vitamin D is an environmental factor that influences the course and severity of IBD.3 Low vitamin D status has been reported in patients with IBD4 and, in IL-10 KO mice, vitamin D deficiency accelerates the development of experimental IBD.5 In vivo treatment of IL-10 KO mice with active vitamin D [1,25(OH)2D3] blocked the progression of disease and prevented their death. In addition, the vitamin D receptor (VDR) gene maps to a region on chromosome 12 that has been linked to IBD by genome screening techniques, and polymorphisms in the VDR gene are associated with susceptibility to IBD.6 There are data in human and animal models of IBD suggesting that vitamin D status and VDR signal transduction are important in determining IBD susceptibility and severity.
The discovery of the VDR in cells of the immune system and the presence of the 1α-hydroxylase in dendritic cells and macrophages7 suggest that locally produced 1,25(OH)2D3 has regulatory autocrine and paracrine properties at the site of inflammation. Synthesis of active vitamin D requires the presence of 1α-hydroxylase, which catalyses the conversion of 25(OH)D3 to 1,25(OH)2D3. The pleiotropic actions of 1,25(OH)2D3 are mediated by its binding to the VDR, which acts as a transcription factor to modulate the expression of specific genes in a tissue-specific manner. The VDR is constitutively expressed in a variety of immune cells.8 Resting T cells (both CD4+ and CD8+) express low levels of VDR, which are up-regulated following activation. Although a membrane form of the VDR has been described, its role has not been demonstrated in the regulation of the immune system.9,10
The role of vitamin D in immunity is complex. In vitro, 1,25(OH)2D3 inhibits the proliferation of activated T cells by blocking cell-cycle progression from G1a to G1b11 and suppresses the production of IL-2, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ).12 Monocytes and dendritic cells are also targets of VDR ligands. Dendritic cell maturation is arrested in the presence of 1,25(OH)2D3, while in macrophages antigen-presenting capacity, phagocytic activity, and production of granulocyte–macrophage colony-stimulating factor, IL-1, TNF-α and IL-12 are inhibited.13 The in vitro effects of 1,25(OH)2D3 suggest that vitamin D may preferentially suppress T helper type 1 (Th1) -driven immune responses.
Vitamin D and VDR-mediated regulation of the immune system has been well described in vitro; however, there has been little research to assess the effects of the VDR signalling pathway during intestinal inflammation in vivo. We have recently reported that lymphocytes from VDR KO mice are of an inflammatory phenotype, produce more IFN-γ upon stimulation and have a stronger response in a mixed lymphocyte reaction compared to wild-type (WT) cells.14 Moreover, CD4+ CD45RBhigh T cells from VDR KO mice transfer more severe colitis than CD4+ CD45RBhigh T cells from WT mice when injected into leukopenic hosts (recombinase-activated gene KO mice). In addition, we have shown that the VDR/IL-10 double KO (DKO) mouse develops a fulminating form of colitis, leading to premature death at 3–5 weeks of age.14
Here we characterize the immune changes that contribute to the severe IBD found in the DKO mice. We show that the VDR is highly expressed in the thymus and colon of WT and IL-10 KO mice and that VDR deficiency alters lymphocyte homing and function in both the primary and secondary lymphoid organs during intestinal inflammation. As the DKO mice develop colitis symptoms, the thymus undergoes rapid atrophy, which corresponds to the relative absence of the double-positive CD4+ CD8+ (DP) T-cell subtype. T cells in the periphery are fewer in number, non-responsive to stimulation and of a memory phenotype. Activated lymphocytes are found in the colon where they overproduce a number of inflammatory proteins including IFN-γ, TNF-α and IL-12. The fulminating colitis in the DKO mice is accompanied by alterations in all of the lymphoid compartments.
Materials and methods
Animals
All of the mice were from a C57BL/6 background and housed in conventional animal facilities. IL-10 KO (The Jackson Laboratory, Bar Harbor, ME), VDR KO, WT mice (gift from M. Demay, Harvard University, Cambridge, MA) and DKO mice (previously described14) were bred and maintained at The Pennsylvania State University. All VDR KO mice (DKO and single VDR KO) mice were fed diets high in calcium that were sufficient to maintain serum at a normal level (8·5–9 mg/dl) and at the same level as found in WT and IL-10 KO mice. All procedures were reviewed and approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Histopathology
The thymus, spleen, small intestine and colon were removed from the mice, fixed in formalin and sent to the Penn State University Animal Diagnostic Laboratories (University Park, PA) for haematoxylin & eosin (H&E) staining. The inflammation and epithelial injury of the colon were scored blindly on a scale from 0 to 4 using previously described criteria.14,15
Reverse transcription-polymerase chain reaction (RT-PCR)
One-centimetre segments of ascending colon were harvested immediately upon euthanasia and snap-frozen in liquid nitrogen. Colonic mRNA was extracted using RNeasy Midi kit (Qiagen, Valencia, CA) and 5 μg total RNA were reverse-transcribed (Promega, Madison, WI). The PCR primer sequences were designed to cross intron/exon borders and were as follows: VDR [129 base pairs (bp)], 5′-TTCATCATGCCAATGCCAATGTCCAC-3′ and 5′-GTTCACCTGCCCCTTCAAT-3′; IL-2 (413 bp), 5′-TTCAAGCTCCACTTCAAGCTCTACAGCGGAAG-3′ and 5′-GACAGAAGGCTATCCATCTCCTCAGAAAGTCC-3′; IFN-γ (365 bp), 5′-TGCATCTTGGCTTTGCAGCTCTTCCTCATGGC-3′ and 5′-TGGACCTGTGGGTTGTTGACCTCAAACTTGGC-3′; IL-12p40 (550 bp), 5′-CTCACATCTGCTGCTCCACAA-3′ and 5′-CTCCTTCATCTTTTCTTTCTT-3′; IL-12p35 (850 bp), 5′-TTGCCCTCCTAAACCACCTCA-3′, 5′-CTTGCTCTTCTGCTAACACAT-3′; IL-1β (563 bp), 5′-ATGGCAACTGTTCCTGAACTCAACT-3′ and 5′-CAGGACAGGTATAGATTCTTTCCTTT-3′; TNF-α (354 bp) 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC-3′ and 5′-GTATGAGATAGCAAATCGGCTGACGGTGTGGG-3′. PCR cycling conditions were as follows: denaturation at 94° for 1 min, annealing at 59° for 1 min, and extension at 72° for 1 min. The number of cycles was adjusted (30–35) based on the titration of cDNA (for each gene) required to ensure that the PCR reaction was run during the exponential phase of the reaction. PCR products were analysed on 1·5% agarose gels and the intensity of the bands was determined using densitometry scanning software (Scion Image for Windows, Frederick, MD) and corrected for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) expression.
Flow cytometry and apoptosis assays
Spleen cells (106) were stained for flow cytometry. The antibodies were phycoerythrin (PE)-conjugated anti-mouse CD4 (L3T4), CD44 (IM7), CD69 (H1.2F3), immunoglobulin M (IgM; AF6-78) (BD Pharmingen, San Diego, CA), F4/80 (BM8) (Bioscience, San Diego, CA), fluoroscein isothiocyanate (FITC) -conjugated anti-mouse CD8 (53-6.7), CD62L (MEL-14), B220 (RA3-6B2), CD25 (7D4) (BD Pharmingen) and CD11b (M1/70) (Bioscience).
To analyse apoptosis freshly isolated thymocytes (106) were resuspended in sterile saline and fixed by the drop-wise addition of 2 ml ice-cold 70% ethanol. The cells were held on ice for 1 hr and then resuspended in 500 μl of propidium iodide (PI)/RNase A solution (BD Pharmingen) and incubated at room temperature for 1 hr in the dark. Annexin V staining alone was performed according to the manufacturer's instructions (Apoptosis Detection Kit, BD Pharmingen). Double staining with Annexin V and PI was carried out using Annexin V staining followed by the incubation of the cells with 50 μg/ml PI. Flow cytometry analysis was performed on an XL-MCL benchtop cytometer (Beckman Coulter, Miami, FL).
Lymphocyte proliferation assay
CD4+ and CD8+ T cells were isolated from the spleen and mesenteric lymph nodes (MLN) using Cell Select Columns (Cedarlane, Hornby, Canada). The purity of the T cells was at least 95%. For stimulation, cells were seeded at 5 × 105 cells/ml on plastic dishes coated with 10 μg/ml anti-CD3 monoclonal antibody. Then, 0·4 μCi [3H]thymidine (ICN, Costa Mesa, CA) was added to each well (96-well plate, Costar, Corning, NY) at 48 hr and the cells were incubated for an additional 24 hr. Radioactive thymidine incorporation was determined by liquid scintillation using a beta plate counter.
Blood analysis
Blood was collected by cardiac puncture in tubes coated with ethylenediaminetetraacetic acid (Becton Dickinson, Franklin Lakes, NJ) and analysed using an ADVIA 120 Hematology System (Bayer Diagnostic, Tarrytown, NY).
Intracellular Ca2+ measurement
Splenocytes were collected and the red blood cells were lysed using hypotonic buffer. Cells were loaded with Fluo-4 (Invitrogen, Eugene, OR; 10 mg/ml) at 37° for 45 min at a concentration of 3–4 mg/ml. The cells were then washed twice and the mean fluorescence intensity vs time was measured. Gating was carried out using the forward and right-angle light scatter after the exclusion of dead cells.
Data analysis
Results are expressed as the mean ± SE. Statistical analysis was performed using the unpaired t-test and analysis of variance (anova; StatView, SAS Institute, Cary, NC). A value of P ≤ 0·05 was considered statistically significant.
Results
VDR distribution in colon, primary lymphoid and secondary lymphoid organs
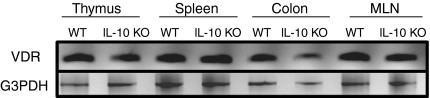
VDR expression was measured by RT-PCR in the thymus, spleen, MLN and colon of IL-10 KO and WT mice. VDR mRNA expression levels were comparable in the thymus, spleen and MLN of IL-10 KO and WT mice (Fig. 1). Within the thymus the VDR was expressed constitutively in DP as well as CD4/CD8 double-negative thymocytes (data not shown). VDR was also highly expressed in the colon, including high VDR expression in colons from young IL-10 KO mice (Fig. 1). The ratios of VDR to G3PDH × 100 were 81 ± 10 in old IL-10 KO mice that developed colonic prolapse and 118 ± 5 in 5-week-old and healthy IL-10 KO mice without symptoms. There was no change in colonic VDR expression in young versus old WT mice (VDR/G3PDH × 100 ratios were ∼ 120 for both). Interestingly, the development of IBD symptoms in IL-10 KO mice was associated with a 31% reduction in VDR expression in the colon.
Figure 1.
Constitutive expression of the VDR in thymus, spleen, MLN and colon of 5-week-old IL-10 KO and WT mice. RNA was isolated, reverse transcribed and then amplified using PCR with primers specific for the VDR and G3PDH. The G3PDH bands indicate that equivalent amounts of mRNA were analysed in each sample. One representative experiment of five is shown.
Over-expression of inflammatory cytokines in the colons of DKO mice during severe IBD
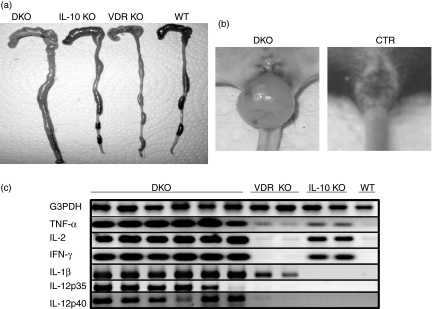
DKO mice developed severe IBD that involved the whole intestinal tract and necropsies of the mice showed the most dramatic changes in the gross anatomy and inflammation of the colon (Fig. 2a), with the majority of the DKO mice developing rectal prolapse (80%, Fig. 2b). Cytokine expression in the colon was measured by RT-PCR using mice that were age-matched (5 weeks old) to the DKO mice. Consistent with their non-inflammatory status, WT mice did not express any cytokines in the colon (Fig. 2c). In contrast, VDR KO mice expressed IL-1β in the colon as well as faint TNF-α bands (Fig. 2c). Old VDR KO mice (9 months) had 10-fold higher IL-1β expression and 20-fold more TNF-α in the colon (data not shown). IL-10 KO mice with minor symptoms of IBD expressed TNF-α, IL-2 and IFN-γ but not IL-1β in the colon (Fig. 2c). The DKO mice expressed two- to three-fold higher levels of IL-1β, IL-2, IFN-γ and TNF-α mRNA in their colons compared with the colons of single KO control mice (Fig. 2c). Interestingly, only the DKO mice expressed IL-12p35 and p40 in the colon (Fig. 2c). Th2 genes (IL-4 and IL-5) were undetectable in mRNA from the colons of WT, VDR KO, IL-10 KO and DKO mice.
Figure 2.
Fulminant colitis and cytokine expression in the colons of DKO mice. (a) The caecum and colon of DKO, IL-10 KO, VDR KO and WT mice. The DKO colon is enlarged compared to the colons of age-matched single KO or WT mice. (b) Rectal prolapse is common in DKO mice at 5 weeks of age. Age-matched IL-10 KO, VDR KO and WT (CTR-control) mice did not develop colonic prolapse. (c) Cytokine expression in the colon of DKO mice. RT-PCR amplification of G3PDH, TNF-α, IL-2, IFN-γ, IL-1β, IL-12 p35 and IL-12 p40 mRNA extracted from colonic tissue of mice at 5 weeks of age. The PCR products were run on a 1·5% agarose gel and stained with ethidium bromide. Since the G3PDH bands are of approximately equal intensity, changes in the intensities of other bands indicate differences in expression of those genes in those samples.
Accelerated thymic involution in mice with severe IBD
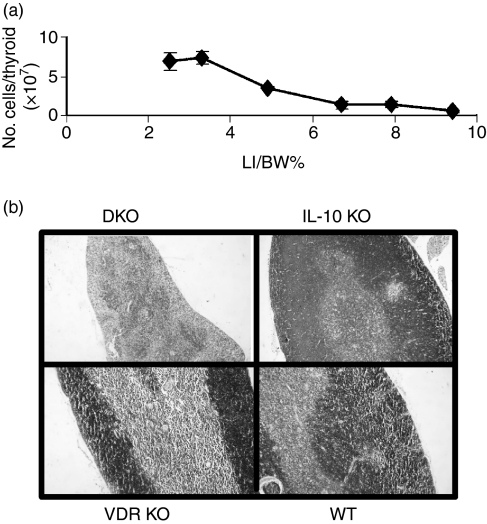
Thymic size and cellularity was normal in young DKO mice (3–4 weeks old), which had not developed IBD [low large intestine (LI)/body weight (BW)% ratio]. We have shown previously that the LI/BW ratios are an objective measure of the severity of IBD in IL-10 KO mice.15 The DKO thymus weight and cellularity declined rapidly as the mice developed IBD (increase in LI/BW percentage; Fig. 3a), whereas the size and cellularity of the thymus in single KO and WT mice increased over the ensuing weeks (data not shown). The total thymocyte number in the thymus of DKO mice at the peak of IBD disease was less than 10–20% of that of the single KO mice (Fig. 3a).
Figure 3.
Thymic involution parallels colitis development in DKO mice. (a) Cell number in the thymus of DKO mice as a function of the ratio of the LI/BW%. (b) H&E staining of the thymus.
Histological staining revealed pronounced structural changes in the thymus of DKO mice at 5 weeks of age (Fig. 3b). DKO mice with progressive disease had thymuses with no clear corticomedullary demarcation, scattered cortical cells and an accumulation of thymocytes in the medulla (Fig. 3b). The thymuses of VDR KO and IL-10 KO mice at 5 weeks of age were no different from those of WT mice (Fig. 3b).
DKO thymocytes are hypersensitive to apoptosis
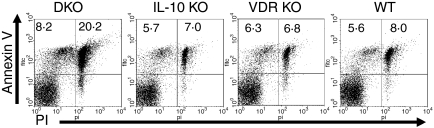
DP cells are the major cell type that undergo apoptosis during normal thymic development16 and are extremely sensitive to systemic mediators of apoptosis.17 Because the DP population is severely reduced during the progression of the disease, we investigated whether increased apoptosis was the mechanism responsible (Fig. 4). Ten per cent of DKO thymocytes were PI-positive compared with only 1% in single KO mice; therefore, more apoptotic events had occurred in the DKO thymus. In addition, evidence of early apoptosis was found in the DKO thymocytes which showed higher levels of Annexin V staining, 25%, compared to 14% in control thymocytes. Double staining with Annexin V and PI was used to discriminate between early stage (annexin V+ PI−) and late-stage (annexin V+ PI+) apoptotic cells. A similar percentage of cells was in the early stage of apoptosis in all groups (Fig. 4). However, the DKO thymuses had significantly more thymocytes in the late-stage of apoptosis (20%) compared with VDR KO, IL-10 KO and WT mice (7%, Fig. 4).
Figure 4.
Increased thymocyte susceptibility to apoptosis. Thymocytes were stained with annexin V and PI. The percentage of cells that were in early apoptosis (annexin V+ PI−) or late apoptosis (annexin V+ PI+) were determined. The results are representative of experiments with six mice per group. DKO values for late apoptosis were significantly different from single KO and WT values. P < 0·05.
Alterations in the peripheral lymphoid compartments
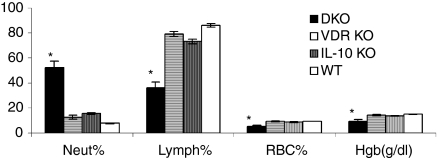
Severe IBD symptoms (bleeding, weight loss and rectal prolapse, Fig. 2b) in DKO mice were associated with four-fold increases in blood neutrophil counts and two-fold decreases in lymphocyte cell counts (P > 0·05, Fig. 5). In addition, the decreased number of erythrocytes and lower haemoglobin concentrations in the DKO mice (Fig. 5) suggested that the DKO mice were anaemic.
Figure 5.
Anaemia and alterations in neutrophil and lymphocyte subpopulations in the peripheral blood of DKO mice. Blood was analysed using the Advia system and the percentages of neutrophils (Neut), lymphocytes (Lymph), red blood cells (RBC) and haemoglobin (Hgb) in the blood of each strain were determined. *Data are expressed as mean % ± SE of six mice per group. DKO values were significantly different than those from VDR KO, IL-10 KO and WT mice. P < 0·05.
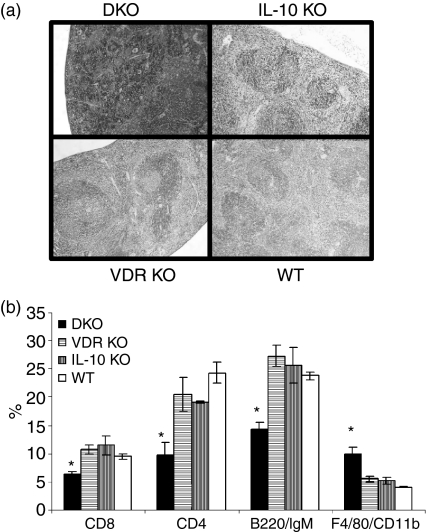
The spleens from DKO mice were two-fold larger than single KO and WT mice. Histological sections of the spleen from DKO mice (Fig. 6a) revealed an almost complete absence of the white pulp and an expanded red pulp that had a congestive aspect because of the accumulation of red blood cells. The spleens from both single KO and control mice were normal showing the obvious presence of germinal centres and good demarcation of the red and white pulp (Fig. 6a). The overall cellularity of the spleen from DKO mice was reduced, with a decrease in the percentage of CD4+, CD8+ and B cells to approximately half the levels found in VDR KO, IL-10 KO or WT mice (Fig. 6b). Conversely, the F4/80/CD11b macrophages were higher in the spleen of DKO mice than in other strains of mice (Fig. 6b).
Figure 6.
The spleen of DKO mice is larger and contains more macrophages but fewer T and B cells than the spleens of single KO or WT controls. (a) H&E staining of the whole spleen sections. (b) Single cell suspensions of spleens from DKO, IL-10 KO, VDR KO and WT mice were analysed by flow cytometry for the expression of CD4, CD8, B220/IgM, and F4/80 CD11b. Reduced CD4, CD8 and, B220/IgM proportions and increased F4/80 CD11b proportions in splenocytes from DKO mice. *DKO values were significantly different than the corresponding VDR KO, IL-10 KO and WT values. P < 0·05 n = 6 to n = 12.
The MLN from DKO mice were three-fold to four-fold enlarged and had increased cell numbers relative to other strains of mice (data not shown). The percentages of CD4+ and CD8+ T cells in the MLN were the same in the DKO, VDR KO, IL-10 KO and WT mice, but the absolute number of CD4+ and CD8+ T cells were three-fold higher in MLN from DKO mice.
The DKO-derived CD4+ T cells were large as measured by forward light scatter, but showed normal expression of the activation markers CD25 and CD69 (data not shown). The percentage of CD4+ CD25+ T cells were not different in the spleen, thymus or MLN of DKO, VDR KO, IL-10 KO and WT mice. The DKO CD4+ T cells had a memory phenotype characterized by high expression of CD44, and low expression of CD62L (data not shown). Conversely, the CD4+ T cells from the single KO and WT mice were of a naive phenotype and had low expression of CD44 and high expression of CD62 ligand.
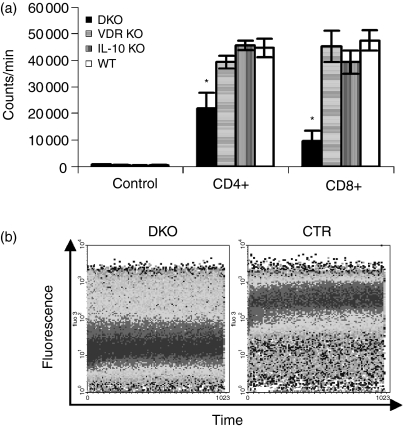
To determine if the cells were functional, the CD4+ and CD8+ T cells from DKO mice were stimulated with anti-CD3 antibodies. Interestingly, both the CD4+ and CD8+ DKO T cells showed reduced proliferative capacity compared with cells from single KO and WT mice (Fig. 7a). In addition, intracellular free calcium concentrations [Ca2+], which are essential for T-cell proliferation, differentiation and apoptosis, were determined. Ca2+ concentrations were significantly lower in unstimulated DKO lymphocytes (mean fluorescence intensity 3·3; Fig. 7b) compared to lymphocytes from single KO and WT mice (mean fluorescence intensity from 7·9 to 8·3, Fig. 7b).
Figure 7.
Altered proliferation of DKO T cells. (a) Purified CD4+ and CD8+ were isolated from DKO, VDR KO, IL-10 KO and WT mice and stimulated for 72 hr with plate-bound CD3 antibodies. The CD4+ and CD8+ T cells from DKO mice were relatively unresponsive to CD3 stimulation compared to the same cells from VDR KO, IL-10 KO and WT mice. Bars represent means ± SE (n = 5 or n = 6). *P < 0·05. (b) Single cell suspensions of splenocytes from DKO mice were compared to single KO and WT mice for the resting levels of intracellular Ca2+. DKO splenocytes had lower levels of intracellular Ca2+ (dark band at lower fluoresence intensity) compared to VDR KO, IL-10 KO, and WT splenocytes (control, CTR).
Discussion
VDR/IL-10 DKO mice spontaneously develop a fulminating form of IBD involving all areas of the small intestine and colon. Because the inflammatory process occurs throughout the small intestine and colon, the disease in DKO mice is most similar to Crohn's disease in humans. Additionally, their thymuses undergo rapid atrophy, while thymocytes and lymphocytes in peripheral organs undergo phenotypic and functional changes. The net result of the immune system alterations in the DKO mice is a severe form of IBD and over-expression of numerous inflammatory cytokines in the colon. In contrast, the disease in IL-10 KO mice with functional VDRs is less pronounced (milder, affected less mice, and took many months to develop), suggesting that VDR deficiency exacerbates IBD severity. The rapid kinetics of the immune alterations in DKO mice argues that VDR signalling is an additional factor controlling disease susceptibility in IL-10 KO mice. It is noted that neither the VDR KO nor IL-10 KO mice developed such an aggressive form of IBD. Therefore, this accelerated and increased severity of disease was a cumulative effect of both VDR and IL-10 deficiencies.
As expected, WT mice did not express inflammatory cytokines in the colon. Age-matched IL-10 KO mice developed minor colitis, and expressed TNF-α, IFN-γ and IL-2 in the colon, but not IL-1β, or IL-12. VDR KO mice with macroscopically and microscopically normal gastrointestinal tracts had high IL-1β expression in the colon and detectable TNF-α expression. Interestingly, older VDR KO mice express more IL-1β and TNF-α in the colon than younger VDR KO mice. Surprisingly, only DKO mice showed up-regulation of IL-12 p35 and p40 in the colon, suggesting that their immune response was strongly Th1 predisposed.
Colitis in IL-10 KO mice is a Th1-mediated disease and Th1 cytokines, especially IFN-γ, IL-12 and TNF-α, have been shown to play an important role in its pathogenesis. TNF-α blockade resulted in clinical improvement of experimental IBD.18,19 The inhibition of IBD symptoms by 1,25(OH)2D3 treatment of IL-10 KO mice has been shown to be partly the result of the inhibition of the TNF-α pathway.20 Administration of neutralizing antibodies specific for IL-12 or IFN-γ prevented colitis in young IL-10 KO mice.21 Like the VDR KO and DKO mice, biopsies from Crohn's disease patients show increased IL-1β expression in both the inflamed and non-inflamed areas.22 In another model of Th1 autoimmune disease (type 1 diabetes), vitamin D deficiency is associated with higher IL-1β expression at the site of inflammation.23 In addition, 1,25(OH)2D3 has been shown to suppress macrophage production of IL-1βin vitro.24 It will be interesting to evaluate the colon of VDR KO mice in aged animals to determine if VDR deficiency alone predisposes them to IBD symptoms. However, 12-week-old VDR KO mice did not have inflammatory cells in the colon (data not shown). The severe IBD and high expression of IL-1β, IL-2, IL-12, IFN-γ and TNF-α in the colons of DKO mice suggest that VDR signalling protected IL-10 KO mice from an early burst of IL-12 and overproduction of IL-1β in the colon, which induces Th1 cells and colitis as early as 3–6 weeks of age.
Approximately 90% of the DKO mice became anaemic at 5 weeks of age with decreased numbers of red blood cells and haemoglobin concentrations, whereas none of the IL-10 KO mice showed haematological abnormalities. The 5-week-old IL-10 KO mice in our colony did show minor microscopic evidence of intestinal pathology; however, they did not develop outward symptoms of disease, or show haematological abnormalities. In some mouse colonies, IL-10 KO mice have been reported to develop anaemia associated with the IBD symptoms, but not before 7–11 weeks of age.25 The abrupt debut of anaemia in 3- to 5-week-old DKO mice must be a consequence of the IBD symptoms, which included intestinal bleeding. In what may be an attempt to bolster the red blood cell number, the spleens from DKO mice increased approximately three-fold in size because of hyperplasia of the red pulp, indicating that extensive extramedullary haematopoiesis occurred to attempt to compensate for the anaemia.
The thymuses of DKO mice with IBD were 10–20% less in both size and cellularity compared to age-matched IL-10 KO, VDR KO, or WT mice. This thymic atrophy might be a consequence of several mechanisms: blockade of thymic development, decreased proliferation, increased apoptosis, or elevation of serum corticosteroids. The number of thymocytes and the thymic mass were normal in young DKO mice but immediately after the onset of disease, acute thymic atrophy was detected. DP cells are the major cell type undergoing apoptosis during normal thymic development26 and are extremely sensitive to systemic mediators of apoptosis such as the corticosteroids.27 Increased numbers of DKO thymocytes were in the early and late stages of apoptosis compared to the single KO and WT thymocytes. However, DKO thymocytes proliferated normally when stimulated with concanavalin A and IL-2 (data not shown). Only 2–3 days separated the onset of macroscopically detectable intestinal symptoms and the drastic reduction of the thymus in DKO mice. The rapidity of the thymic atrophy, which follows the development of a severe form of IBD, argues that corticosteroid production induced by the severe IBD may be the underlying cause of thymic atrophy. It seems likely that during the severe intestinal stress in DKO mice, pro-inflammatory cytokines such as IL-1β and TNF-α stimulated production of corticosteroids, which resulted in the rapid apoptosis of the DP thymocytes. Endotoxic shock (driven by IL-1β and TNF-α) has been shown to up-regulate corticosteroid production and to result in rapid atrophy of the thymus.28 Our results suggest a scenario in which VDR signalling promotes thymocyte survival and during systemic stress protects thymus integrity and function by controlling the level of the systemic inflammatory response.
DKO mice with colitis had large, but lymphopenic, spleens with a 50% reduction in the number of CD4+, CD8+ and B cells. In addition DKO mice had a 50% reduction in lymphocytes in the blood. Conversely, the MLN were enlarged, and had a two- to three-fold higher number of CD4+ and CD8+ T cells. The data suggest that the lymphocytes may have relocated from the spleen and peripheral blood in closer proximity to the site of active inflammation. Both CD4+ and CD8+ T cells from DKO mice had a markedly decreased proliferative response compared with WT and single KO T cells, suggesting that peripheral T cells from DKO mice were anergic. The hyporesponsiveness of DKO T cells might be a result of defective T-cell signalling at the membrane receptor level or downstream of the TCR. Nevertheless, the mitogenic response of lymphocytes is dependent on increases in intracellular free Ca2+, which activates calcineurin, dephosphorylates nuclear factor-AT and induces transcription of IL-2 resulting in proliferation.29 Concentrations of Ca2+ were significantly lower in resting DKO lymphocytes compared to single KO and WT lymphocytes, suggesting that the DKO lymphocytes failed to adequately accumulate intracellular Ca2+. These results suggest that VDR signalling was involved in intracellular calcium regulation and may account for the lymphocyte hyporesponsiveness observed in DKO mice with colitis. Interestingly, serum Ca2+ from DKO mice was not different from the other strains of mice (data not shown). Younger DKO mice, which did not show outward signs of colitis, had normal spleens, MLN and lymphocyte function.
Together our data in this murine model of experimental colitis define VDR signalling as a potent anti-inflammatory mechanism in vivo. VDR deficiency accelerated the development of experimental IBD in IL-10 KO mice by inducing phenotypic and functional changes in T-cell responsiveness. The combined VDR and IL-10 deficiency resulted in acceleration, 100% incidence and the increased severity of IBD symptoms. Since all of the immune changes (thymus, spleen and MLN) only occur in mice with IBD symptoms it is difficult to determine what effect VDR deficiency has on the immune system to cause the accelerated and fulminant IBD. It is clear that VDR expression is essential for the IL-10 KO mouse to maintain the level of lymphocyte homeostasis required to control the gastrointestinal immune response to commensal bacteria.
Acknowledgments
This work was supported by a Crohn's and Colitis Foundation of America Predoctoral Research Award (to M.F.) and by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grant 1R01 NS38888 (to M.T.C.). We express our thanks to Avery August (Pennsylvania State University, University Park) for his advice, support and critical review of the manuscript.
Abbreviations
- BW
body weight
- DKO
vitamin D receptor/interleukin-10 double knockout
- DP
double-positive CD4+ CD8+
- IBD
inflammatory bowel disease
- IL-10
interleukin-10
- KO
knockout
- LI
large intestine
- MLN
mesenteric lymph nodes
- VDR
vitamin D receptor
- WT
wild-type
References
- 1.Gerd Bouma WS. The immunological and genetic basis of inflammatory bowel disease. Nature Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky D. Lessons from genetic models of inflammatory bowel disease. Acta Gastroenterol Belg. 1997;60:163–5. [PubMed] [Google Scholar]
- 3.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223:230–3. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 4.Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr. 2002;76:1077–81. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 5.Cantorna MT, Humpal-Winter J, DeLuca HF. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D(3) Arch Biochem Biophys. 2000;377:135–8. doi: 10.1006/abbi.2000.1765. [DOI] [PubMed] [Google Scholar]
- 6.Simmons JD, Mulligan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut. 2000;47:211–14. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreutz M, Andreesen R, Krause S, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–7. [PubMed] [Google Scholar]
- 8.Deluca HF, Cantorna MT. Vitamin D. Its role and uses in immunology. Faseb J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 9.Marcinkowska E. A run for a membrane vitamin D receptor. Biol Signals Recept. 2001;10:341–9. doi: 10.1159/000046902. [DOI] [PubMed] [Google Scholar]
- 10.Meehan TF, DeLuca HF. The vitamin D receptor is necessary for 1alpha,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Arch Biochem Biophys. 2002;408:200–4. doi: 10.1016/s0003-9861(02)00580-5. [DOI] [PubMed] [Google Scholar]
- 11.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–5. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49:26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 13.Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004:89–90. 437–41. doi: 10.1016/j.jsbmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 15.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 16.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nature Rev Immunol. 2002;2:309–22. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 17.Ashwell JD, King LB, Vacchio MS. Cross-talk between the T cell antigen receptor and the glucocorticoid receptor regulates thymocyte development. Stem Cells. 1996;14:490–500. doi: 10.1002/stem.140490. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay JO, Hodgson HJ. Review article: the immunoregulatory cytokine interleukin-10 – a therapy for Crohn's disease? Aliment Pharmacol Ther. 2001;15:1709–16. doi: 10.1046/j.1365-2036.2001.01093.x. [DOI] [PubMed] [Google Scholar]
- 19.van Deventer SJ. Immunomodulation of Crohn's Disease. Curr Dir Autoimmun. 2000;2:150–66. doi: 10.1159/000060502. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2004;35:217–24. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 21.Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829–33. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- 22.Ludwiczek O, Vannier E, Borggraefe I, Kaser A, Siegmund B, Dinarello CA, Tilg H. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin Exp Immunol. 2004;138:32–9. doi: 10.1111/j.1365-2249.2004.02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giulietti A, Gysemans, Stoffels K, van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451–62. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 24.Tsoukas CDWD, Escobar SS, Provvedini DM, Dinarello CA, Hustmyer FG, Manolagas SC. Inhibition of interleukin-1 production by 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1989;69:127–33. doi: 10.1210/jcem-69-1-127. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 26.Amsen D, Kruisbeek AM. Thymocyte selection: not by TCR alone. Immunol Rev. 1998;165:209–29. doi: 10.1111/j.1600-065x.1998.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. Glucocorticoid-induced apoptosis in the thymus. Semin Immunol. 1992;4:363–9. [PubMed] [Google Scholar]
- 28.Zuckerman SH, Shellhaas J, Butler LD. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989;19:301–5. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]
- 29.Lewis R. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]