Abstract
Specific immune suppression and induction of tolerance are essential processes in the regulation and circumvention of immune defence. The balance between allergen-specific type 1 regulatory (Tr1) cells and T helper (Th) 2 cells appears to be decisive in the development of allergy. Tr1 cells consistently represent the dominant subset specific for common environmental allergens in healthy individuals. In contrast, there is a high frequency of allergen-specific interleukin-4 (IL-4)-secreting T cells in allergic individuals. Allergen-specific immunotherapy can induce specific Tr1 cells that abolish allergen-induced proliferation of Th1 and Th2 cells, as well as their cytokine production. Tr1 cells utilize multiple suppressor mechanisms, such as IL-10 and transforming growth factor-β (TGF-β) as secreted cytokines and various surface molecules, such as cytotoxic T-lymphocyte antigen 4 and programmed death-1. IL-10 only inhibits T cells stimulated by low numbers of triggered T-cell receptors, which depend on CD28 costimulation. IL-10 inhibits CD28 tyrosine phosphorylation, preventing the binding of phosphatidylinositol 3-kinase p85 and consequently inhibiting the CD28 signalling pathway. In addition, IL-10 and TGF-β secreted by Tr1 cells skew the antibody production from immunoglobulin E (IgE) towards the non-inflammatory isotypes IgG4 and IgA, respectively. Induction of antigen-specific Tr1 cells can thus re-direct an inappropriate immune response against allergens or auto-antigens using a broad range of suppressor mechanisms.
Keywords: allergen-specific immunotherapy, interleukin-10, suppression, T-cell tolerance, transforming growth factor-β, T regulatory cells
Introduction
The interaction of environmental and genetic factors can lead to the development of atopic disorders in some individuals, but not others, following allergen exposure. The generation of allergen-specific CD4+ T helper (Th) cells provides the initial event responsible for the development of allergic diseases. The current view is that interleukin-4 (IL-4) -influenced naive T cells differentiate into Th2 cells when activated by antigen-presenting cells (APC).1 These effector Th2 cells produce IL-4, IL-5 and IL-13, which mediate several regulatory and effector functions. These functions include allergen-specific immunoglobulin E (IgE) production by B cells, eosinophil development and recruitment, mucus production and smooth muscle contraction, as well as tissue homing of Th2 cells (Fig. 1).1–3 Th1 cells, on the other hand, may contribute to chronicity and the effector phase in allergic disease (Fig. 2).4–6 For example, keratinocyte apoptosis is induced by Th1 cells and mediated by interferon-γ (IFN-γ) and Fas, forming an essential pathogenetic event in eczematous dermatitis.4 IFN-γ up-regulates Fas, intercellular adhesion molecule type 1 and human leucocyte antigen (HLA)-DR rendering keratinocytes susceptible to apoptosis.7 In addition, IFN-γ up-regulates several chemokines in epithelial cells such as IFN-γ-inducible protein 10 (IP-10), monokine-induced by IFN-γ (mig) and IFN-γ-inducible α-chemoattractant (iTac), which further attract Th0/Th1 cells towards epidermis by binding to CXCR3 in atopic dermatitis.8 In asthma, tumour necrosis factor-α (TNF-α) and IFN-γ are potent in inducing bronchial epithelial apoptosis leading to epithelial shedding.4
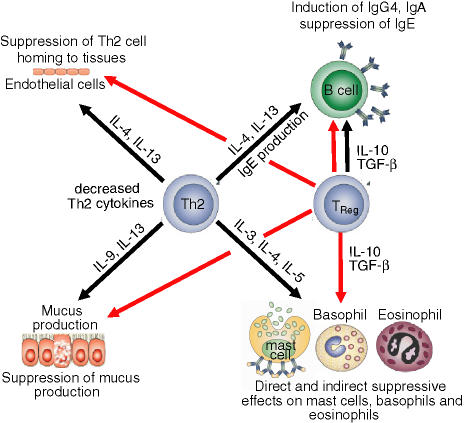
Figure 1.
Suppression of Th2 cell-mediated features of allergic inflammation by TReg cells. TReg cells utilize multiple suppressor factors to regulate undesired activity of effector Th2 cells. IL-10 and TGF-β suppress IgE production and induce the non-inflammatory immunoglobulin isotypes IgG4 and IgA, respectively. Furthermore, these two cytokines directly suppress allergic inflammation induced by effector cells such as mast cells, basophils and eosinophils. In addition, Th2 cells are suppressed by TReg cells and can therefore no longer provide cytokines such as IL-3, IL-4, IL-5, IL-9 and IL-13. These cytokines are required for the differentiation, survival and activity of mast cells, basophils, eosinophils and mucus-producing cells, as well as for the tissue homing of Th2 cells (red line indicates suppression, black line indicates stimulation).
Figure 2.
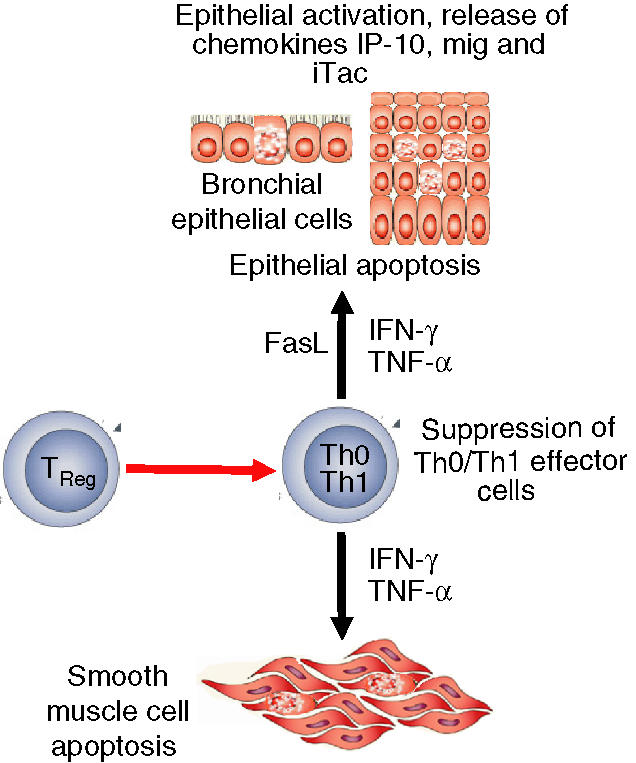
Suppression of Th1 cell-mediated features of allergic inflammation by TReg cells. The Th1 cytokine IFN-γ in combination with TNF-α and/or FasL, induces apoptosis of smooth muscle cells, keratinocytes and bronchial epithelial cells as essential tissue injury events in atopic dermatitis and asthma. TReg cells suppress the stimulation of Th0/Th1 cells, leading to the abrogation of tissue injury mechanisms (red line indicates suppression, black line indicates stimulation).
A further subset of T cells termed T regulatory (TReg) cells, which have an immunosuppressive function and cytokine profile distinct from that of either Th1 or Th2 cells, has been described.9,10 TReg cells are able to inhibit the development of allergic Th2 cell responses (Fig. 1),11,12 as well as effector Th1 cell responses (Fig. 2), and therefore play an important role in allergen-specific immunotherapy (SIT).13,14
As a function of TReg cells, specific immune suppression and induction of peripheral tolerance are essential processes in the regulation and circumvention of immune defence. Analysis of the current literature suggests that anergy, peripheral tolerance and active suppression are events related to different stages of TReg cell-mediated immune deviation. Anergy describes a process by which antigen is presented to T-cell clones without the aid of professional APC and results in the induction of a hyporesponsive state, which affects IL-2 production and proliferation upon re-stimulation.15 Reversible functional limitations characterize anergic T cells, including cell division, cell differentiation and cytokine production.16,17 The induction of T-cell unresponsiveness upon antigen encounter provides the major characteristic of T-cell tolerance. Central immune tolerance results from self-antigen exposure in the thymus before the immune response develops, thereby specifically abrogating the response to that antigen thereafter.17,18
T regulatory cells
T cells capable of suppressing the immune response were first described in the early 1970s.19 However, the mechanisms behind this suppression were not clarified and therefore research within this field was abandoned by the 1980s. Investigation into T-cell-mediated immune suppression strengthened during the 1990s and the concept of TReg cells suppressing immune responses via cell–cell interactions and/or the production of suppressor cytokines is currently well established. However, many aspects behind these mechanisms remain to be elucidated.10,20
Type 1 regulatory cells
Type 1 regulatory (Tr1) cells, also known as inducible TReg cells, are defined by their ability to produce high levels of IL-10 and transforming growth factor-β (TGF-β).10,20 Antigen-specific Tr1 cells arise in vivo, but may also differentiate from naive CD4+ T cells. Reports have shown that by stimulating naive CD4+ T cells in the presence of IL-10, IFN-α or a combination of IL-4 and IL-10, a Tr1 cell subset can be generated in vitro.10,20 It is now clear that during the early course of allergen-SIT, IL-10- and/or TGF-β-producing Tr1 cells in humans are generated in vivo, which implies that Tr1 cells are induced by high and increasing doses of allergens.13,14,21 Allergen-specific Th1 and Th2 responses are down-regulated by these Tr1 cells.12 Therefore, Tr1 cells could be used as a promising target for the development of new therapeutic agents, as well as in cellular therapy for peripheral tolerance modulation in allergy and autoimmunity.22,23
CD4+ CD25+ TReg cells
This distinct subset of TReg cells, also called constitutive TReg cells, accounts for 5–10% of peripheral CD4+ T cells and inhibits the activation of effector T cells in the periphery.24 These cells, along with CD4 and CD25 expression, are also associated with the transcription factor FoxP3.25 The suppressive mechanism of CD4+ CD25+ TReg cells has been shown to function via the inhibition of the IL-2 receptor α-chain in target T cells, which is induced by the combined activity of cytotoxic T-lymphocyte antigen 4 (CTLA-4)26 and membrane-bound TGF-β.27
Various autoimmune diseases such as arthritis, diabetes and X-linked immune dysregulation, polyendocrinopathy and enteropathy syndrome (IPEX) may develop spontaneously when CD4+ CD25+ TReg cells are eliminated, indicating the presence of a population of professional TReg cells.28 IPEX is characterized by dermatitis, enteropathy, type I diabetes, thyroiditis, haemolytic anaemia and thrombocytopenia, and results from mutations of FoxP3.29
There is some evidence in adult humans that constitutive CD4+ CD25+ TReg cells and inducible IL-10- and TGF-β-secreting Tr1 cells represent overlapping populations, because of CD25 expression on CD4+ Tr1 cells. Regulatory CD4+ CD25+ T cells normally inhibit Th2 cytokine expression and proliferation of peripheral blood mononuclear cells (PBMC) from non-atopic donors, in response to allergen. This suppression has been shown to be associated with the control of allergic disease.30
Th3 cells
Similar to Tr1 cells, Th3 cells are inducible upon activation with an appropriate antigen or anti-CD3 antibody and produce high levels of TGF-β, with variable amounts of IL-4 and IL-10.9,31 Neutralizing antibodies have demonstrated TGF-β and IL-10 to be the key molecules because of their abrogation of the disease-protective effects of these cells. Furthermore, Th3 cells have been shown to exert bystander immune suppression in vitro.31
Other TReg cells
It has been proposed that in addition to CD4+ T cells, CD8+ TReg cells may also have a role in oral tolerance.32 Furthermore, CD8+ TReg cells have been described as controlling proliferation of CD4+ T cells in a Qa-1-dependent (HLA-E in humans) manner.33 CD8+ CD28– FOXP3+ T suppressor cells have recently been reported to induce tolerogenic endothelial cells34 by inducing inhibitory receptors and down-regulating costimulatory and adhesion molecules.
Role of regulatory T cells in peripheral T-cell tolerance in the healthy immune response to allergens and allergen-SIT
Allergen-SIT provides an efficient treatment of insect venom allergy and allergic rhinitis in clinical practice.35,36 Antihistamines, antileukotrienes, β2-adrenergic receptor antagonists and corticosteroids can provide a temporary suppression of mediators and immune cells.37 However, a more long-term solution/treatment can only be provided by allergen-SIT, which specifically restores normal immunity against allergens. Successful allergen-SIT is associated with an increase in allergen-blocking IgG antibodies (particularly IgG4)38 along with the generation of IgE-modulating CD8+ T cells39 and a decrease in the number of mast cells and eosinophils, as well as a decrease in the release of mediators.40 The induction of peripheral T-cell tolerance plays a crucial role in allergen-SIT13,14,22,41 and is initiated by the autocrine action of IL-10 and TGF-β, which are increasingly produced by antigen-specific Tr1 cells.13,14,22 Reactivation of tolerized T cells can result in the distinct production of either Th1 or Th2 cytokine profiles depending on the cytokines present in the tissue microenvironment, and can therefore direct allergen-SIT towards either successful or unsuccessful treatment.41
Tr1 cells have many suppressive mechanisms, including secretion of the suppressive cytokines IL-10 and TGF-β, as well as the surface molecules CTLA-426 and programmed death 1 (PD-1).42,43 IL-10 and TGF-β have been shown to induce T-cell suppression during SIT and in normal immunity to mucosal allergens. Allergen-SIT induced the antigen-specific suppressive activity of CD4+ CD25+ T cells from allergic individuals. This suppression can be partially blocked by the neutralization of secreted or membrane-bound IL-10 and TGF-β.14 It has been shown ex vivo that allergen-specific Th2 cell activation is enhanced when these Tr1 suppressor activities are blocked or when the Th2 cell frequency is enhanced.12
A recent study using IFN-γ-, IL-4- and IL-10- secreting allergen-specific CD4+ T cells (which resemble Th1-, Th2- and Tr1-like cells, respectively) showed that both healthy and allergic individuals exhibit all three subsets but in different proportions. In healthy individuals, Tr1 cells represent the dominant subset for common environmental allergens, whereas a high frequency of allergen-specific IL-4-secreting T cells (Th2-like cells) is found in allergic individuals. Therefore, the frequency of memory effector T cells or TReg cells is decisive in the development of allergy or a healthy immune response.12
In this respect, allergy vaccines that target T cells and induce T-cell tolerance, while bypassing IgE binding, represent a novel opportunity for the prevention and treatment of allergy. For example, immunization of mice with a fusion protein containing linear T-cell epitopes, but not three-dimensional B-cell epitopes of the major bee venom allergens phospholipase A2 and hyaluronidase, has been shown to protect against antibody responses to later encounters with the allergens, therefore suggesting the induction of allergen-specific tolerance.44
IL-10 and TGF-β in immune suppression
Antigen-specific T-cell suppression by IL-10, a known suppressive cytokine of T-cell proliferation and cytokine production, is essential in peripheral tolerance to allergens, autoantigens, transplantation antigens and tumour antigens. The inhibitory effect of IL-10 plays a key role in inducing anergy, and hence has great importance in allergen-SIT (Table 1). IL-10 is a suppressor cytokine of T-cell proliferation in both Th1 and Th2 cells. It was originally thought to be produced by Th2 cells only, however, it is in fact produced particularly by Tr1 cells, but also by Th0, Th1 and Th2 cells as well as B cells, monocytes and keratinocytes.7,45
Table 1.
The mechanisms of action by interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) that aid the deviation of the immune system as observed during allergen-specific immunotherapy
| IL-10 | TGF-β |
|---|---|
| Suppresses allergen-specific IgE | Suppresses allergen-specific IgE |
| Induces allergen-specific IgG4 | Induces allergen-specific IgA |
| Blocks B7/CD2c costimulatory pathway | Suppresses allergen-specific Th1 and Th2 cells |
| Inhibits DC maturation, leading to reduced MHC class II | Down-regulates FcεRI expression on Langerhans cells and costimulatory ligand expression |
| Reduces release of pro-inflammatory cytokines by mast cells | Associated with CTLA-4 expression on T cells |
IgE, immunoglobulin E; DC, dendritic cell; MHC, major histocompatibility complex; Th1, T helper type 1; CTLA-4, cytotoxic T-lymphocyte antigen 4.
In mice, IL-10 administration before allergen treatment induced antigen-specific T-cell unresponsiveness and demonstrated the pivotal role of IL-10 in the establishment of peripheral T-cell tolerance.46 Moreover, the inhibition of graft-versus-host disease by IL-10 and the allograft rejection in severe combined immunodeficiency patients who have undergone human leucocyte antigen-mismatched, bone marrow transplants provide further evidence for a key role of this cytokine in the induction and maintenance of an anergic state.47 Similarly, inappropriate stimulation of tumour-reactive human T cells was shown to result from increased endogenous IL-10 production by these cells,48 indicating a role for IL-10 in tumour-specific anergy. Recently, IL-10-derived regulatory CD4+ T cells producing IL-10, but not IL-2 and IL-4, which suppressed the antigen-specific T-cell response in vitro and prevented antigen-induced murine colitis, were identified in both humans and mice.10 During allergen-SIT, IL-10 levels increased significantly by day 7, and reached a maximum by day 28. At this time peripheral tolerance was fully established. The proliferative and cytokine responses could be reconstituted by ex vivo neutralization of endogenous IL-10, indicating that IL-10 is actively involved in the development of anergy in specific T cells.22 Furthermore, antigen- and peptide-induced proliferative responses and Th1 and Th2 cytokine production decreased in both bee venom-SIT and phospholipase A-peptide immunotherapy (PLA-PIT), whereas IL-10 production simultaneously increased and reached maximal levels after 4 weeks, when specific anergy was fully established. The cellular origin of IL-10 was demonstrated by intracytoplasmic IL-10 staining in PBMC and by coexpression of cellular surface markers.13 Intracellular IL-10 significantly increased after 7 days of allergen-SIT in the antigen-specific T-cell population and activated CD4+ T lymphocytes. After 4 weeks of allergen-SIT intracytoplasmic IL-10 was also increased in monocytes and B cells, suggesting an autocrine action of T-cell-secreted IL-10 as a pivotal step in the induction phase of T-cell anergy and its maintenance by IL-10-producing APC and non-specific bystander T cells.13 Interestingly, the same features of peripheral tolerance were found in the T cells of healthy beekeepers who had previously been stung by high numbers of bees. These naturally anergized individuals show increased numbers of IL-10-producing CD4+ CD25+ T cells and monocytes similar to allergic patients after bee venom-SIT. Neutralization of endogenous IL-10 in PBMC cultures from these individuals fully reconstituted the proliferative T-cell response and cytokine production. Recently, a study using the mouse model of experimental allergic encephalomyelitis indicated a possible connection between non-allergen-specific CD4+ CD25+ TReg and antigen-specific IL-10-secreting Tr1 cells. Here, it was found that adoptively transferred CD4+ CD25+ TReg cells induced a high expression of IL-10 by autoantigen-specific T cells, and that the neutralization of IL-10 led to the abrogation of the suppressive effect of these cells.49
Studies with bronchoalveolar lavage fluid from asthmatic patients have revealed lower IL-10 levels than in healthy controls, and the T cells from children suffering from asthma have been shown to produce less IL-10 mRNA than T cells from healthy children.50,51 Together these findings indicate an association between increased IL-10 production and decreased allergic reactions. As TReg cells are a major source of IL-10, it has been speculated that TReg cells secreting IL-10 are involved in the suppression of allergic Th2 cell responses in humans, which is supported by several human allergen-SIT studies.13,23,52 In contrast, some studies demonstrated that increased IL-10 levels are not associated with less allergic disease.53 IL-10 may also promote airway hyperresponsiveness54 and even eosinophilia55 in allergy models.
The TGF-β superfamily consists of more than 35 members with TGF-β1 being the proteotypic member. TGF-β is an important pleiotropic cytokine with potent immunoregulatory properties and is essential for the maintenance of immunological self-tolerance in the CD4+ T-cell compartment.56 TGF-β1-deficient mice develop the phenotype, as a result of activated CD4+ T cells, of a rapidly wasting syndrome leading to death by the age of 3 or 4 weeks.57,58 Although controversial, TGF-β has been implicated in the conversion of naive CD4+ CD25– T cells into CD4+ CD25+ T cells by the induction of FoxP3.59 TGF-β signalling has been demonstrated to be required for in vivo expansion and immunosuppressive capacity of CD4+ CD25+ T cells.60 However, studies with TGF-β-deficient mice have shown that CD4+ CD25+ T cells can develop up to 2 weeks and autocrine TGF-β production was not essential for these cells to exhibit suppressive activity in vivo.61
It is thought that during lymphocyte maturation TGF-β can contribute to both apoptosis of self-reactive clones and the maintenance of tolerance, thereby preventing the development of autoimmunity. In vivo, TGF-β-secreting Th3 cells were found to suppress encephalitis induction with myelin basic protein. Th3-derived TGF-β is thought to suppress this disease by inhibiting autoimmune responses within the target organs.62
In contrast to its known T-cell suppressive activity, some reports imply a role for TGF-β in the pathogenesis of asthma, particularly in the remodelling of injured lung tissue in humans.63 The increased allergic inflammation observed after blocking of CTLA-4 is clearly associated with decreased TGF-β levels in the bronchoalveolar lavage fluid of these animals.64 Furthermore, inhibition of experimental tracheal eosinophilia was also the result of the induction of CD4+ T cells secreting TGF-β.65
The TGF-β superfamily can act on virtually all mammalian cell types by engaging an intracellular cascade of Smad family proteins through ligand-induced activation of TGF-β receptor kinases. Activated Smad complexes accumulate in the nucleus to participate in the transcriptional activation of target genes, some of which stimulate tumorigenesis, while others suppress it. TGF-β receptors are also able to activate Smad-independent signalling mechanisms, including mitogen-activated protein kinases and phosphatidylinositol 3-kinase (PI3-K).66 However, the exact suppressive mechanisms behind TGF-β activation of Smad pathways remain to be elucidated.
TGF-β was thought to play a role in CTLA-4-mediated inhibition of T-cell activation because of the similarities observed in phenotypes of mice deficient for either CTLA-4 or TGF-β1.58,67 However, studies using TGF-β-neutralizing antibodies did not reverse CTLA-4-mediated inhibition and the proliferation of wild-type, TGF-β1-deficient and Smad3-deficient T cells was equally inhibited by CTLA-4 ligation.68 The effect of TGF-β1 on T-cell proliferation can be modified by CD28 costimulation. In the absence of CD28 costimulation TGF-β1 can potently suppress T-cell receptor (TCR)-stimulated proliferation of naive T cells, whereas in the presence of CD28 costimulation, TGF-β1 inhibits apoptosis and enhances TCR-stimulated proliferation. Interestingly, TGF-β inhibited IL-2 production in both cases, thereby suggesting a correlation in downstream signalling between CD28 and TGF-β.69 Studies on the IL-12 signal transduction pathway in T cells revealed that TGF-β blocks IL-12-induced tyrosine phosphorylation and activation of both Jak2 and Tyk2 kinases. This inhibition was associated with a decrease in tyrosine phosphorylation of both STAT3 and STAT4 protein, resulting in decreased T-cell proliferation and IFN-γ production, with an increase in apoptotic cell death.70 Enforcement of STAT4 expression partly prevented inhibition of IFN-γ production by TGF-β during priming, but did not prevent inhibition of Th1 development, indicating the use of distinct mechanisms by TGF-β.71
Antibody isotype regulation by IL-10 and TGF-β
Although peripheral tolerance has been demonstrated in specific T cells, the ability of B cells to produce specific IgE antibodies is not eliminated during allergen-SIT.41 In fact, the serum levels of both specific IgE and IgG4 antibodies increase during the early phase of treatment. However, the increase in antigen-specific IgG4 is more striking and therefore the ratio of specific IgE to IgG4 decreases between 10-fold and 100-fold. A similar change in specific isotype ratio has been observed in SIT of various allergies.22,72 Moreover, IL-10 produced and progressively secreted during allergen-SIT, appears to counter-regulate the synthesis of antigen-specific IgE and IgG4 antibodies.13 IL-10 potently suppresses both total and allergen-specific IgE, whereas it simultaneously increases IgG4 production.13,73 Therefore, IL-10 not only generates T-cell tolerance, it also regulates specific isotype formation and skews the specific IgE response towards an IgG4-dominated phenotype. High levels of specific IgA and IgG4, low amounts of IgG1 and trace amounts of IgE antibodies in serum were demonstrated in the healthy immune response to Der p 1.14 Specific IgE levels did not significantly change after 70 days of house dust mite-SIT treatment of allergic patients; however, a significant increase in specific IgA, IgG1 and IgG4 was observed.14 The increase of specific IgA and IgG4 in serum coincides with increased TGF-β and IL-10, respectively. This may account for the role of IgA and TGF-β as well as IgG4 and IL-10 in mucosal immune responses to allergens in healthy individuals.13,74
In addition, IL-10 has been shown to reduce pro-inflammatory cytokine release from mast cells,75 down-regulate eosinophil function and activity, and suppress IL-5 production from resting human Th0 and Th2 cells.76
IL-10-mediated suppression of T-cell costimulation
Specific activation of T cells requires stimulation through the TCR and a costimulatory signal, generated by the engagement of multiple cell surface receptors with their ligands.77 A major costimulatory signal is delivered to T cells by the interaction of CD28 with CD80/86, which is essential for antigen-stimulated T-cell proliferation and cytokine production.78,79 TCR stimulation without costimulation or by blocking the costimulation in vivo and in vitro, induces tolerance or anergy in T cells.15,80
The molecular mechanisms of T-cell suppression by IL-10 have been investigated in antigen-specific PBMC cultures, purified CD45RO+ T cells and T-cell clones. IL-10 inhibited the proliferative T-cell response in PBMC to various antigens and to the superantigen staphylococcal enterotoxin B.81 However, IL-10 did not affect the proliferative responses of T cells that were stimulated by anti-CD3. In contrast, IL-10 significantly inhibited the anti-CD28-stimulated proliferation. The analysis of TCR numbers on T cells demonstrated the essential requirement for costimulation in T-cell activation and its relation to the number of triggered TCRs.81 IL-10 inhibited the T-cell proliferation within a certain range of triggered TCRs that T cells require for costimulation. T cells which were stimulated by different concentrations of anti-CD3, and a constant amount of anti-CD28, showed that low numbers of triggered TCRs required CD28 costimulation. Thus, IL-10 suppressed only those T cells that had low numbers of TCRs triggered and which required CD28 for proliferation.
Stimulation of CD28 by monoclonal antibodies induces tyrosine phosphorylation. Ligation of IL-10 receptor (IL-10R) at the time of monoclonal antibody stimulation inhibits tyrosine phosphorylation of CD28.81,82 The inhibitory effect of IL-10 appeared to be specific for the costimulation pathways, as IL-10 did not affect ZAP-70 tyrosine phosphorylation stimulated by CD3 cross-linking.81 As a consecutive event for signal transduction, PI3-K binds to phosphorylated costimulatory molecules by its p85 subunit. This association with the PI3-K p85 molecule was inhibited by IL-10. PI3-K is a heterodimer that comprises an 85 000 MW regulatory subunit and a 110 000 MW catalytic subunit possessing both protein serine-kinase and lipid-kinase activity.83 The p85 subunit contains a p110 binding site as well as two src-homology-2 (SH2) domains. Binding of PI3-K occurs by direct interaction between SH2 domain motifs of p85 PI3-K and a (p)YXXM motif (where Y denotes tyrosine, X denotes any amino acid and M denotes methionine) in the cytoplasmic part of costimulatory molecules.84 This binding requires tyrosine phosphorylation of the tyrosine residues within the pYxxM motif (Y191MNM) in CD28.84 IL-10 exerts its biological functions through the activation of Jak1 and Tyk2, the members of the receptor-associated Janus tyrosine kinase family and Stat1 and Stat3 and in certain cells Stat5.85 Previous studies demonstrated that IL-10 does not only inhibit T cells, it is also a potent inhibitor of activated monocytes and macrophages.86 Since monocytes and macrophages do not express CD28, the inhibitory impact of IL-10 is likely to occur through other mechanisms in non-T cells. In monocytes, IL-10 was shown to induce expression of the suppressor of the cytokine-signalling-3 (SOCS3) gene that may play a role in the inhibition of the IFN-induced tyrosine phosphorylation of Stat1.87
IL-10-secreting B cells have recently been proposed to prevent the development of arthritis.88 In this study, adoptive transfer of B cells generated with anti-CD40 antibody in combination with antigen prevented the development of arthritis upon immunization with bovine collagen in an IL-10-dependent manner. It is generally accepted that immature dendritic cells (DC), which cannot appropriately activate T cells, may induce tolerance.89 In normal immunity, DC receive sufficient signals from the surroundings of the antigen, T cells and other tissue cells, innate immune response stimulating (i.e. Toll-like receptor triggering) substances and via costimulatory ligands and cytokines, and therefore should not have any restriction in maturation. However, the full maturation of DC can be inhibited by IL-10, which induces a state of anergy in alloantigen-specific CD4+ T cells.90 Furthermore, there are indications that mature DC can induce peripheral T-cell tolerance. Pulmonary DC from mice transiently produce IL-10 when exposed to respiratory antigen.91 These phenotypically mature pulmonary DC, which were B7hi, stimulate the development of CD4+ Tr1-like cells. Adoptive transfer of IL-10+/+, but not IL-10–/–, pulmonary DC of mice exposed to respiratory antigen induced antigen-specific unresponsiveness in recipient mice. This study shows that under certain circumstances, phenotypically mature DCReg may exist. It has been clearly demonstrated that natural killer cells, epithelial cells, macrophages and glial cells, amongst others, express suppressor cytokines such as IL-10 and TGF-β. Although they have not been classified as professional regulatory cells, they may also have an involvement in the generation and suppression of an immune response.92–96
CTLA-4, PD-1 and GITR as active suppressor mechanisms of T regulatory cells
CTLA-4, a negative regulator of T-cell function has been reported to associate with the TCR complex ζ-chain in T cells.97 Studies on CD4+ CD25+ TReg cells have shown that they do not proliferate upon normal TCR-mediated stimulation and suppress the proliferation of other T cells. TCR stimulation is required for these cells to exert suppression of other T cells, but this suppression is not confined to T cells specific for the same antigen. CD4+ CD25+ T cells are the only lymphocyte subpopulation in both mice and humans that express CTLA-4 constitutively and this expression apparently correlates with the suppressor function of CTLA-4.98 The addition of anti-CTLA-4 antibody or its Fab (fragment of antigen binding) in cocultures of CD4+ CD25+ and CD4+ CD25– T cells reverses suppression.98 Similarly, the treatment of mice, which are recipients of CD4+ CD45RBlow T cells, with these agents abrogated the suppression of inflammatory bowel disease.26 These studies indicate that signals that result from the engagement of CTLA-4 by its ligands, CD80 or CD86, are required for the induction of suppressor activity. Under some circumstances, the engagement of CTLA-4 on the CD4+ CD25+ T cells by antibody or by CD80/CD86 may lead to inhibition of the TCR-derived signals that are required for the induction of suppressor activity.
PD-1 is an immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptor expressed upon T-cell activation. An inhibitory role for PD-1 in immune responses was suggested because of the autoimmune diseases that develop in PD-1-deficient mice.42 The ligands for PD-1 are members of the B7 family, PD-L1 and PD-L2. PD-1 ligation on murine CD4 and CD8 T cells results in the inhibition of proliferation and cytokine production. T cells stimulated with anti-CD3/PD-L1 demonstrate a marked decrease in proliferation and IL-2 production.43 PD-1 : PD-L interactions inhibit IL-2 production even in the presence of costimulation and therefore, following a prolonged activation, the PD-1 : PD-L inhibitory pathway dominates. Exogenous IL-2 is able to overcome PD-L1-mediated inhibition at all times, indicating that the cells maintain IL-2 responsiveness.
Glucocorticoid-induced tumour-necrosis factor receptor family related gene (TNFRSF18, GITR) is expressed by CD4+ CD25+ alloantigen-specific and naturally occurring circulating TReg cells.99,100 Stimulation of CD4+ CD25+ TReg cells through GITR breaks immunological self-tolerance.100 GITR is up-regulated in CD4+ CD25– T cells after T-cell receptor stimulation and it also functions as a survival signal for activated cells.101 In addition, CD103 (αEβ7 integrin) and CD122 (β-chain of IL-2 receptor) are highly expressed on CD4+ CD25+ TReg cells, which correlates with their suppressive activity.102,103
Conclusion
Peripheral T-cell tolerance represents the key mechanism in healthy immune responses to non-infectious, non-self antigens. There is increasing evidence to support TReg cells and immunosuppressive cytokines as key players in mediating successful allergen-SIT and a healthy immune response to allergens. Active suppression involves the utilization of multiple suppressive mechanisms, secreted cytokines (IL-10 and TGF-β) and surface molecules (CTLA-4, PD-1, mIL-10, TGF-βR and IL-10R).104
These mechanisms may have implications in autoimmunity, graft-versus-host disease, tumour cell growth, parasite survival, chronic infections and the development of acquired immune deficiency syndrome. Knowledge of this molecular basis is pivotal in understanding the equilibrated regulation of the immune response and anergy to immunogenic agents and their possible therapeutic applications. Suppression of an immune response may have beneficial effects in the case of hypersensitivity reactions, but it may also be harmful in other cases such as cancer development, chronic infection and tissue remodelling. The over-expression of TReg cells and suppressive cytokines may be responsible for chronicity and tumour tolerance and therefore does not always support a healthy response. Taking this into account, along with the recent advances in knowledge of peripheral tolerance mechanisms, may lead to future developments of safer approaches to treatment of immune-mediated diseases.
Acknowledgments
This work was supported by the Swiss National Science Foundation grants 32–100266 (M.A.), 3100–065436/2 (K.B.) and 32–105865 (C.A.A.).
References
- 1.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:142–6. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 2.Ying S, Meng Q, Barata LT, Robinson DS, Durham SR, Kay AB. Associations between IL-13 and IL-4 (mRNA and protein), vascular cell adhesion molecule-1 expression, and the infiltration of eosinophils, macrophages, and T cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J Immunol. 1997;158:5050–7. [PubMed] [Google Scholar]
- 3.Akdis CA, Blaser K, Akdis M. Apoptosis in tissue inflammation and allergic disease. Curr Opin Immunol. 2004;16:717–23. doi: 10.1016/j.coi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Trautmann A, Schmid-Grendelmeier P, Krüger K, et al. T cells and eosinophils cooperate in the induction of bronchial epithelial apoptosis in asthma. J Allergy Clin Immunol. 2002;109:329–37. doi: 10.1067/mai.2002.121460. [DOI] [PubMed] [Google Scholar]
- 5.Trautmann A, Akdis M, Brocker EB, Blaser K, Akdis CA. New insights into the role of T cells in atopic dermatitis and allergic contact dermatitis. Trends Immunol. 2001;22:530–2. doi: 10.1016/s1471-4906(01)02004-x. [DOI] [PubMed] [Google Scholar]
- 6.Yssel H, Groux H. Characterization of T cell subpopulations involved in the pathogenesis of asthma and allergic diseases. Int Arch Allergy Immunol. 2000;121:10–18. doi: 10.1159/000024292. [DOI] [PubMed] [Google Scholar]
- 7.Trautmann A, Altznauer F, Akdis M, Simon HU, Disch R, Brocker EB, Blaser K, Akdis CA. The differential fate of cadherins during T-cell-induced keratinocyte apoptosis leads to spongiosis in eczematous dermatitis. J Invest Dermatol. 2001;117:927–34. doi: 10.1046/j.0022-202x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 8.Klunker S, Trautmann A, Akdis M, Verhagen J, Schmid-Grendelmeier P, Blaser K, Akdis AC. A second step of chemotaxis after transendothelial migration: keratinocytes undergoing apoptosis release IP-10, Mig and iTac for T cell chemotaxis towards epidermis in atopic dermatitis. J Immunol. 2003;171:1078–84. doi: 10.4049/jimmunol.171.2.1078. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 11.Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol. 2000;165:4848–53. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- 12.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of IL-10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis AC. IL-10 and TGF-β cooperate in regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 15.Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983;157:1434–47. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faith A, Akdis CA, Akdis M, Simon H-U, Blaser K. Defective TCR stimulation in anergized type 2 T helper cells correlates with abrogated p56lck and ZAP-70 tyrosine kinase activities. J Immunol. 1997;159:53–60. [PubMed] [Google Scholar]
- 17.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 18.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 19.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 20.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 21.Nasser SM, Ying S, Meng O, Kay AB, Ewan PW. Interleukin-10 levels increase in cutaneous biopsies of patients undergoing wasp venom immunotherapy. Eur J Immunol. 2001;31:3704–13. doi: 10.1002/1521-4141(200112)31:12<3704::aid-immu3704>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Akdis CA, Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. Faseb J. 1999;13:603–9. doi: 10.1096/fasebj.13.6.603. [DOI] [PubMed] [Google Scholar]
- 23.Müller UR, Akdis CA, Fricker M, Akdis M, Bettens F, Blesken T, Blaser K. Successful immunotherapy with T cell epitope peptides of bee venom phospholipase A2 induces specific T cell anergy in bee sting allergic patients. J Allergy Clinimmunol. 1998;101:747–54. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 25.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 26.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+) CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annunziato F, Cosmi L, Liotta F, et al. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med. 2002;196:379–87. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 29.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity–allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 31.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 32.Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–51. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 33.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 34.Manavalan JS, Kim-Schulze S, Scotto L, et al. Alloantigen specific CD8+ CD28− FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. International Immunol. 2004;16:1055–68. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 35.Müller UR, Mosbech H. Position paper: Immunotherapy with hymenoptera venoms. Allergy. 1993;48:36–46. [PubMed] [Google Scholar]
- 36.Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302:265–9. doi: 10.1136/bmj.302.6771.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jutel M, Watanabe T, Klunker S, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–5. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 38.van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- 39.Rocklin RE, Sheffer A, Greineder DK, Melmon KL. Generation of antigen-specific suppressor cells during allergy desensitization. N Engl J Med. 1980;302:1213–19. doi: 10.1056/NEJM198005293022201. [DOI] [PubMed] [Google Scholar]
- 40.Creticos PS, Adkinson NF, Jr, Kagey-Sobotka A, Proud D, Meier HL, Naclerio RM, Lichtenstein LM, Norman PS. Nasal challenge with ragweed pollen in hay fever patients. Effect of immunotherapy. J Clin Invest. 1985;76:2247–53. doi: 10.1172/JCI112233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Muller U, Blaser K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996;98:1676–83. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 43.Carter L, Fouser LA, Jussif J, et al. PD-1: PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–43. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Kussebi F, Karamloo F, Rhyner C, et al. A major allergen gene-fusion protein for potential usage in allergen-specific immunotherapy. J Allergy Clin Immunol. 2005;115:323–9. doi: 10.1016/j.jaci.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 45.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 46.Enk AH, Saloga J, Becker D, Zadeh M, Knop J. Induction of hapten-specific tolerance by interleukin 10 in vivo. J Exp Med. 1994;179:1397–402. doi: 10.1084/jem.179.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacchetta R, Bigler M, Touraine JL, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Becker JC, Czerny C, Brocker EB. Maintenance of clonal anergy by endogenously produced IL-10. Int Immunol. 1994;6:1605–12. doi: 10.1093/intimm/6.10.1605. [DOI] [PubMed] [Google Scholar]
- 49.Mekala DJ, Alli RS, Geiger TL. IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+ CD25+ T lymphocytes. Proc Natl Acad Sci USA. 2005;102:11817–22. doi: 10.1073/pnas.0505445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–96. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 51.Koning H, Neijens HJ, Baert MR, Oranje AP, Savelkoul HF. T cells subsets and cytokines in allergic and non-allergic children. II. Analysis and IL-5 and IL-10 mRNA expression and protein production. Cytokine. 1997;9:427–36. doi: 10.1006/cyto.1996.0185. [DOI] [PubMed] [Google Scholar]
- 52.Pierkes M, Bellinghausen I, Hultsch T, Metz G, Knop J, Saloga J. Decreased release of histamine and sulfidoleukotrienes by human peripheral blood leukocytes after wasp venom immunotherapy is partially due to induction of IL-10 and IFN-gamma production of T cells. J Allergy Clin Immunol. 1999;103:326–32. doi: 10.1016/s0091-6749(99)70509-9. [DOI] [PubMed] [Google Scholar]
- 53.Tillie-Leblond I, Pugin J, Marquette CH, et al. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159:487–94. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 54.Makela MJ, Kanehiro A, Borish L, et al. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci USA. 2000;97:6007–12. doi: 10.1073/pnas.100118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Wang S, Fan Y, Han X. IL-10 deficiency prevents IL-5 overproduction and eosinophilic inflammation in a murine model of asthma-like reaction. Eur J Immunol. 2000;30:382–91. doi: 10.1002/1521-4141(200002)30:2<382::AID-IMMU382>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 56.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 57.Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci USA. 1995;92:12215–19. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 61.Mamura M, Lee W, Sullivan TJ, Felici A, Sowers AL, Allison JP, Letterio JJ. CD28 disruption exacerbates inflammation in Tgf-beta1–/– mice. In vivo suppression by CD4+CD25+ regulatory T cells independent of autocrine TGF-beta1. Blood. 2004;103:4594–601. doi: 10.1182/blood-2003-08-2897. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Inobe J, Kuchroo VK, Baron JL, Janeway CA, Jr, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice. Suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA. 1996;93:388–91. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vignola AM, Chanez P, Chiappara G, et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156:591–9. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 64.Hellings PW, Vandenberghe P, Kasran A, Coorevits L, Overbergh L, Mathieu C, Ceuppens JL. Blockade of CTLA-4 enhances allergic sensitization and eosinophilic airway inflammation in genetically predisposed mice. Eur J Immunol. 2002;32:585–94. doi: 10.1002/1521-4141(200202)32:2<585::AID-IMMU585>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 65.Haneda K, Sano K, Tamura G, Shirota H, Ohkawara Y, Sato T, Habu S, Shirato K. Transforming growth factor-beta secreted from CD4(+) T cells ameliorates antigen-induced eosinophilic inflammation. A novel high-dose tolerance in the trachea. Am J Respir Cell Mol Biol. 1999;21:268–74. doi: 10.1165/ajrcmb.21.2.3576. [DOI] [PubMed] [Google Scholar]
- 66.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 67.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sullivan TJ, Letterio JJ, van Elsas A, et al. Lack of a role for transforming growth factor-beta in cytotoxic T lymphocyte antigen-4-mediated inhibition of T cell activation. Proc Natl Acad Sci USA. 2001;98:2587–92. doi: 10.1073/pnas.051632398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sung JL, Lin JT, Gorham JD. CD28 co-stimulation regulates the effect of transforming growth factor-beta1 on the proliferation of naive CD4+ T cells. Int Immunopharmacol. 2003;3:233–45. doi: 10.1016/S1567-5769(02)00276-X. [DOI] [PubMed] [Google Scholar]
- 70.Bright JJ, Sriram S. TGF-beta inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J Immunol. 1998;161:1772–7. [PubMed] [Google Scholar]
- 71.Lin JT, Martin SL, Xia L, Gorham JD. TGF-beta 1 uses distinct mechanisms to inhibit IFN-gamma expression in CD4+ T cells at priming and at recall. Differential involvement of Stat4 and T-bet. J Immunol. 2005;174:5950–8. doi: 10.4049/jimmunol.174.10.5950. [DOI] [PubMed] [Google Scholar]
- 72.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 73.Punnonen J, De Waal Malefyt R, Van Vlasselaer P, Gauchat J-F, De Vries JE. IL-10 and viral IL-10 prevent IL-4-induced IgE synthesis by inhibiting the accessory cell function of monocytes. J Immunol. 1993;151:1280–9. [PubMed] [Google Scholar]
- 74.Sonoda E, Matsumoto R, Hitoshi Y, et al. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989;170:1415–20. doi: 10.1084/jem.170.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL) -10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996;97:1122–8. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schandene L, Alonso-Vega C, Willems F, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152:4368–74. [PubMed] [Google Scholar]
- 77.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002;2:439–46. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 78.Boussiotis VA, Gribben JG, Freeman GJ, Nadler LM. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr Opin Immunol. 1994;6:797–807. doi: 10.1016/0952-7915(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 79.Wingren AG, Parra E, Varga M, Kalland T, Sjogren HO, Hedlund G, Dohlsten M. T cell activation pathways. B7, LFA-3, and ICAM-1 shape unique T cell profiles. Crit Rev Immunol. 1995;15:235–53. doi: 10.1615/critrevimmunol.v15.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 80.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 81.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. Faseb J. 2000;14:1666–8. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 82.Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30:1683–90. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 83.Ward SG, June CH, Olive D. PI3-kinase: a pivotal pathway in T-cell activation? Immunoltoday. 1996;17:187–97. doi: 10.1016/0167-5699(96)80618-9. [DOI] [PubMed] [Google Scholar]
- 84.Prasad KV, Cai YC, Raab M, Duckworth B, Cantley L, Shoelson SE, Rudd CE. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc Natl Acad Sci USA. 1994;91:2834–8. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–90. [PubMed] [Google Scholar]
- 86.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes. An autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vasquez N, Donnelly RP, Larner AC, Finbloom DS. Interleukin-10 inhibits expression of both interferon α and interferon γ-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–63. [PubMed] [Google Scholar]
- 88.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of athritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reid CD. The biology and clinical applications of dendritic cells. Transfus Med. 1998;8:77–86. doi: 10.1046/j.1365-3148.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 90.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk A. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 91.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 92.Morganti-Kossmann MC, Kossmann T, Brandes ME, Mergenhagen SE, Wahl SM. Autocrine and paracrine regulation of astrocyte function by transforming growth factor-beta. J Neuroimmunol. 1992;39:163–73. doi: 10.1016/0165-5728(92)90185-n. [DOI] [PubMed] [Google Scholar]
- 93.Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol. 2003;170:3806–11. doi: 10.4049/jimmunol.170.7.3806. [DOI] [PubMed] [Google Scholar]
- 94.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–71. [PubMed] [Google Scholar]
- 95.Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol. 2003;50:444–52. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 96.Kitamura M, Suto T, Yokoo T, Shimizu F, Fine LG. Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J Immunol. 1996;156:2964–71. [PubMed] [Google Scholar]
- 97.Lee K-M, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+) CD25(+) immunoregulatory T cells. Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 100.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+) CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 101.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216–21. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lehmann J, Huehn J, de la Rosa M, et al. Expression of the integrin alpha E beta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc Natl Acad Sci USA. 2002;99:13031–6. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levings MK, Sangregorio R, Roncarolo MG. Human CD25(+) CD4(+) T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taylor A, Verhagen J, Akdis CA, Akdis M. T regulatory cells in allergy and health: a question of allergen specificity and balance. Int Arch Allergy Immunol. 2004;135:73–82. doi: 10.1159/000080523. [DOI] [PubMed] [Google Scholar]