Abstract
Macropinocytosis is the actin-dependent formation of large vesicles, which allow the internalization of large quantities of fluid-phase solute. In the majority of cells examined, an exogenous stimulus is required to induce the initiation of this endocytic pathway. However, dendritic cells are thought to constitutively macropinocytose large quantities of exogenous solute as part of their sentinel function. In this review we discuss the evidence that dendritic cells macropinocytose exogenous solute and subsequently present antigenic peptides derived from internalized material to T cells. In addition, we put these data into the context of immune surveillance in vivo.
Keywords: dendritic cells, antigen presentation, antigen processing, phagocytosis
Introduction
Following infection with a pathogen at a peripheral site, T-cell responses (both CD4+ and CD8+) can be triggered in the lymph node draining the site of challenge. T cells then produce cytokines that aid the clearance of the pathogen and can also act directly by killing infected cells. To respond effectively to antigen expressed in a peripheral site, a system must be in place to efficiently transport antigen to secondary lymphoid organs where the T-cell response is initiated. The transport of antigen is accomplished via two mechanisms. First, antigen present in high concentrations at a peripheral site can drain through lymphatic vessels directly to lymph nodes and spleen. Antigen concentrates in the subcapsular sinus that drains afferent lymphatic vessels and is then distributed to the T-cell areas of lymph nodes via reticular fibres that form a distribution system known as the conduit network.1 An alternative mechanism involves internalization of antigen at the site of infection by peripheral sentinel dendritic cells (DC). The DC then transport antigen to draining lymph nodes, where antigen presentation to naive T cells can occur. In this review we will examine the role of the fluid-phase endocytic pathway, macropinocytosis, in the internalization of antigen that is subsequently presented to naive T cells and stimulates their response to infection.
Much recent work has focused on the expression of pattern recognition receptors (PRR) by DC. These receptors bind to, or otherwise mediate recognition of, structures that are conserved amongst a wide variety of pathogens. The conserved structures are known as pathogen-associated molecular patterns (PAMPs) and primarily consist of viral, bacterial and protozoal polysaccharides or glycoproteins.2 PRR can be roughly divided into two groups, one that mediates activation of DC and other cell types and is primarily composed of the Toll-like receptor (TLR) family, and another comprised primarily of C-type lectins that mediate internalization of PAMPs and subsequent degradation. However, despite a surprisingly wide variety of pathogen-encoded structures that can be recognized by PRR, these receptors lack the genetic diversity required to recognize the huge number of foreign antigens recognized by the T- or B-cell receptors. Thus, secondary mechanisms must be in place to allow the internalization of pathogenic material by DC and other cells. One such mechanism is the internalization of large amounts of exogenous soluble material via the macropinocytic pathway.
Endocytic pathways
A large number of distinct endocytic pathways exist to allow the internalization of nutrients and growth factors by virtually all nucleated cells, including DC. The majority of endocytic processes involve binding of exogenous ligand to membrane-bound receptors such as the transferrin receptor, B-cell receptor, Fc receptors or scavenger receptors. The pathway utilized for internalization of the ligand after receptor binding often depends upon the nature of the antigen and/or the nature of the receptor. Soluble ligand is most often internalized after clustering of receptors in clathrin-coated pits. The pits then become invaginated and pinch off to form clathrin-coated vesicles 85–110 nm in diameter that are trafficked to early endosomal compartments. Depending upon the receptor/ligand combination the ligand may be released in an early endosomal compartment (e.g. transferrin) or in a later endosomal compartment with a much lower pH (e.g. low-density lipoprotein). Along with ligands bound to their receptors, small quantities of fluid-phase solute are also internalized in clathrin-coated vesicles, a process known as micropinocytosis. Endocytic processes are tightly regulated, but the extent to which clathrin-mediated endocytosis contributes to fluid-phase uptake varies significantly between cell types.
In contrast to the internalization of receptor ligands, particulate ligands are internalized via phagocytosis, a process that can occur via a number of distinct phenotypic mechanisms.3 For example, Fc receptor-mediated phagocytosis involves the extension of a large pseudopod-like membrane extension around an antibody-coated particulate. In contrast, particulates coated with complement are internalized in the absence of membrane protrusions. Both Fc receptor-mediated and complement receptor-mediated phagocytosis involve the internalization of minimal amounts of exogenous solute, as receptors bind tightly to the surface of the particle. In marked contrast to the ‘tight zippering’ around particles that occurs during conventional phagocytosis, bacteria can also be internalized by a process known as ‘spacious phagocytosis’. This can occur in macrophages4,5 or in epithelial cells6 after exposure to Salmonella typhimurium and is mediated by proteins injected via the type III secretion machinery of the bacterium. It appears that internalization into these spacious phagosomes is advantageous for the bacteria, as avirulent strains are not internalized via this mechanism. These spacious phagosomes closely resemble macropinosomes, and may reflect pathogen subversion of a natural process to gain a selective advantage, a topic that will be touched upon later in this review.
Macropinocytosis
Macropinosomes are heterogeneous in size but are generally much larger than the clathrin-coated vesicles discussed above (up to 5 μm in diameter). They form almost exclusively at sites of membrane ruffling, and there is no evidence to suggest that they concentrate receptors as their clathrin-coated counterparts do. Internalization of solutes via macropinocytosis is much more efficient than via micropinocytosis mediated by clathrin-coated vesicles and other endocytic pathways7 and thus can potentially provide an endocytic pathway to complement immune monitoring via PRR.
Membrane ruffling is intimately linked to the formation of macropinosomes. Different cell types ruffle to different extents, and macropinocytosis has been extensively studied in cells in which ruffling is normally minimal but can be stimulated with growth factors or phorbol esters. Stimulation of MDCK cells with hepatocyte growth factor (scatter factor)8 or A431 epidermal carcinoma cells with epidermal growth factor9 causes formation of circular ruffles that can close off to form macropinosomes. Similarly, stimulation of macrophages with macrophage colony stimulating factor (M-CSF, CSF-1) causes the formation of circular ruffles and macropinosomes.7,10 Not all circular ruffles close over to form macropinosomes, although it is not uncommon for one area of membrane in which ruffling is occurring to generate a number of macropinosomes (C.C.N., unpublished observations). Studies examining the dynamics of actin association with macropinosomes in Dictostelium cells indicate that although actin has a central role in the formation of macropinosomes, it dissociates rapidly (< 1 min) once a vesicle is formed.11
The observation that phorbol esters trigger macropinocytosis in some cell types stimulated investigations into the regulatory signalling events that may be involved in this endocytic pathway. The injection of oncogenic Ras triggers ruffling and an increase in fluid-phase uptake in fibroblasts.12 Injection of activated Rac-1 can induce ruffling without the need for any other stimuli13 and this observation has formed the basis for studies examining the signals responsible for the modulation of actin polymerization in many varied processes, including macropinocytosis. A number of small GTPases have been localized to ruffling membranes and macropinosomes. Rac1 localizes to ruffling membranes in a cholesterol-dependent manner.14 Rab5 has been implicated in the formation of the circular ruffles required for macropinosome formation.15 However, it is another small GTPase, rah/Rab34, that appears to be closely associated with the initial formation of macropinosomes, whereas Rab5 associates at a later stage of vesicle development. Transformation of cells with K-ras or V-src also induces constitutive ruffling and macropinocytosis16,17 and this is dependent upon phosphoinositide 3 (PI3)-kinase activity.18,19 Indeed, inhibition of PI3-kinase blocked both macropinocytosis and Fc receptor-mediated phagocytosis, but not receptor-mediated endocytosis.20 Interestingly, this blockade appears at the level of vesicle formation, as cells treated with the PI3-kinase inhibitors wortmannin or LY294002 did not diminish ruffling.20,21
Wortmannin has been used as an inhibitor of macropinocytosis experimentally22 but its use has the disadvantage that it will also block phagocytosis as well as cellular migration. The efficacy of numerous inhibitors has been examined in an attempt to selectively block macropinocytosis. However, although inhibitors that selectively block fluid-phase uptake but not receptor-mediated uptake have been identified, their action is often cell type-specific. In addition, the similarities between the signalling and mechanical pathways used in phagocytosis and macropinocytosis often mean that inhibitors block both pathways, as is the case with PI3-kinase inhibitors.20,21 Both phagocytosis and macropinocytosis are blocked in the presence of cytochalasins, agents that block actin polymerization.10 Macropinocytosis is acutely sensitive to cytoplasmic pH in some cell types, as treatment with inhibitors of the Na+/H+ exchange pump in the plasma membrane, such as amiloride or amiloride analogues, inhibits fluid-phase uptake.9 However, in many cell types, or at high concentrations, the amilorides also inhibit receptor-mediated endocytosis, so under experimental conditions the inhibitory effects of these agents must be carefully controlled. Most recently, a careful study by Sarkar et al.23 demonstrated that the PKCδ inhibitor rottlerin specifically inhibited fluid-phase but not receptor-mediated endocytosis in DC. However, the concentrations required for this inhibitory effect were well below those necessary for the inhibition of PKCδ, so the mechanism of this inhibitory effect remains undescribed.23 Interestingly, many immunosuppressive compounds also inhibit macropinocytosis in DC24–27 but this may reflect an effect of these compounds upon DC maturation and viability, rather than a direct effect upon endocytic mechanisms.
The fate of material internalized within macropinosomes is cell type-dependent. In macrophages macropinosomes move to the perinuclear region of the cell, shrinking as they move. Some newly formed macropinosomes stain positively for markers of early endosomes such as Transferrin receptor, but they progressively lose these markers and acquire the late endosomal markers rab7 and lysosomal glycoprotein A (lgp-A).28 Macropinosomes readily fuse with each other but eventually they appear to fuse with the pre-existing tubular lysosome network.28 In contrast to the observations made in macrophages, macropinosomes in A431 cells do not appear to interact with other endosomal compartments, but instead eventually release the majority of their contents back into the exogenous milieu.29
The study of macropinocytosis
With the implication of the macropinocytic pathway in immune responses, the study of uptake of antigens by this mechanism has become widespread. However, the majority of studies to date have failed to distinguish between the internalization of antigen via macropinocytic, micropinocytic, or receptor-mediated pathways. An important component of the study of macropinocytosis is visualization of the macropinosomes themselves. Measurement of fluid-phase uptake alone does not distinguish between uptake of antigen by a large number of small vesicles of 100 nm diameter (equivalent to the size of clathrin-coated vesicles) or a small number of large macropinosomes of 1–5 μm in diameter. Thus, microscopic examination of exogenous solute uptake is an essential component of the study of macropinocytosis. In addition, it is essential to account for the dynamic nature of endocytic pathways. Exogenous solute internalized into macropinosomes may be recycled back out of the cell, released into the cytosol, delivered to proteolytically active acidic compartments where fluorescent markers may be lost, or may simply remain within macropinosomes. Examining a single time-point after exposure to an exogenous marker may give misleading or inaccurate results, as trafficking of solute to each of these destinations will produce differing results when conditions, or cell types, are altered.
When studying macropinocytosis, the choice of solute marker is a key determinant of which endocytic pathways are actually being examined. Even experienced laboratories often use fluorescently labelled dextran to measure fluid-phase uptake via macropinocytosis. Immune cell subsets, such as macrophages or DC, constitutively express PRR specifically designed to bind glycoproteins or other carbohydrate moieties that are commonly expressed by pathogens. Thus, the measurement of the internalization of soluble carbohydrate in macrophages or DC almost certainly includes a significant component of receptor-mediated internalization. The contribution of receptor-mediated endocytosis to internalization of solute markers such as dextran can be reduced by preincubation with a competitive inhibitor of binding to PRR, such as mannan.30 However, ligation of PRR such as the mannose or scavenger receptors may trigger intracellular signalling that can potentially alter the rate of macropinocytosis at subsequent times. Thus, the use of solute markers that do not bind to PRR is the best way to study macropinocytosis. Racoosin and Swanson used one such marker, Lucifer Yellow, in their landmark studies of macropinocytosis in macrophages7,10,28 and the use of this marker in a greater proportion of studies would certainly aid interpretation of data examining macropinocytosis in DC.
Macropinocytosis in DC
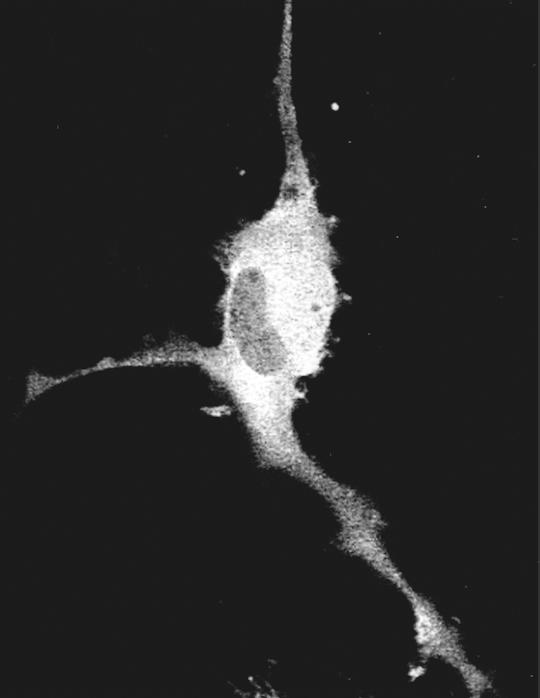
The original observation of macropinocytosis in DC was made by Sallusto and colleagues using DC generated from human blood monocytes by culture in interleukin-4 (IL-4) and granulocyte–monocyte colony-stimulating factor (GM-CSF).30 The authors described a very high rate of fluid-phase uptake of 1100 mm3, or over 40% of the volume of the cell, every hour. The most striking observation was that, in contrast to other cell types, addition of growth factors or phorbol esters to the DC was not necessary to stimulate this uptake. However, addition of stimuli that induced maturation of the DC, such as tumour necrosis factor-α, lipopolysaccharide or IL-1, dramatically reduced fluid-phase uptake 40 hr after addition. High levels of fluid-phase uptake could not be reproduced after withdrawal of the maturation stimuli, indicating an irreversible shut down of macropinocytosis upon maturation. In contrast to the shutdown of macropinocytosis upon exposure to maturation stimuli, receptor-mediated endocytosis did not appear to be affected. Subsequent studies reproduced this observation in DC grown or purified from many species (Fig. 1). It is clear that the mechanisms involved in macropinocytosis in DC differ significantly from those in all other cells examined. For instance, amiloride and amiloride analogues do not specifically inhibit macropinocytosis in DC (C.C.N., unpublished observations and ref. 31). In addition, DC from mice lacking gelsolin, an important regulator of actin polymerization, membrane ruffling, and macropinocytosis in fibroblasts, appear to macropinocytose as efficiently as wild-type DC.32
Figure 1.
Time-lapse video-microscopy of macropinosome formation in murine bone-marrow-derived DC. The formation and fate of two macropinosomes formed at the leading edge of a DC cultured in the absence of growth factors for 24 hr prior to examination. At 0 seconds a macropinsome forms (black arrow) and subsequently (40–280 seconds) traffics towards the nucleus. At 40 seconds a large circular ruffle forms at the leading edge of the DC (white arrow), closes off to form a macropinosome (100 seconds), fuses with other large vesicles (180 seconds) and finally fuses with the initial macropinosome.
The observation that macropinocytosis appeared to occur constitutively via novel mechanisms in DC stimulated investigations of the mechanisms behind this phenomenon. As small GTP-binding proteins have an integral role in membrane ruffling, these proteins formed the focus of the investigations. Injection of dominant negative forms of the GTPase Rac blocked macropinocytosis in immature DC33,34 but injection of activated Rac failed to reconstitute macropinocytosis in mature cells. Two studies, however, differed significantly in the data produced addressing the role of Cdc42. In bone-marrow-derived DC grown in media supplemented with the supernatant from cells producing GM-CSF, dominant negative Cdc42 blocked macropinocytosis and injection of activated Cdc42 could restore high rates of pinocytic uptake. In marked contrast, DC derived from splenocytes cultured in GM-CSF and transforming growth factor-β did not shut down macropinocytosis following microinjection of dominant negative Cdc42. The latter cells do not undergo spontaneous maturation, as bone marrow cultures tend to do,35 perhaps implicating the means by which DC maturation is triggered in the signalling pathways regulating macropinocytosis. Despite appearing to produce contradictory results, the two studies do show that Cdc42–Rac interactions are unlikely to be a simple switch by which macropinocytosis in DC is modulated.
The phenomenally high rate of fluid-phase uptake in cultured DC is likely to be a very energy intensive process that utilizes a significant portion of cellular resources, including plasma membrane and actin. In addition, macropinocytosing DC are loaded with large quantities of exogenous solute that must be disposed of to avoid a deleterious effect upon cellular viability. To handle internalization of large volumes of solute, DC express the aquaporins AQP3 and AQP7.36 These molecules are water channels commonly expressed in cells that have a high capacity for water transport, such as the epithelial cells of the kidney.37 Immature DC express these aquaporins, but down-regulate their expression concomitantly with maturation and the down-regulation of constitutive macropinocytosis. Blockade of the aquaporins reduced fluid-phase, but not receptor-mediated, endocytosis.36 Thus, DC are highly specialized to internalize large volumes of solute.
Although large quantities of membrane are required for macropinocytosis, the surface to volume ratio of the large vesicles formed reduces the quantity of membrane used when compared to internalization of solute in much smaller, clathrin-coated, vesicles. Phagocytosis has been proposed to require membrane donation from the endoplasmic reticulum (ER), and proteins normally found in the ER can be isolated from purified phagosomes.38 However, phagosome shrinkage after internalization is limited by the rate of degradation of the phagocytosed particle. In contrast, macropinosomes shrink rapidly after formation (Fig. 1), potentially allowing the rapid return of internalized membrane to the cell surface. Nevertheless, the role of ER-derived membrane in macropinocytosis remains an interesting area of investigation at this time.
As described above, macropinocytosis involves the use of large quantities of actin during ruffling and the formation of large vesicles. DC are highly motile cells in culture and express high levels of actin. Actin is one of the most abundant proteins in any cell, and so is unlikely to be limiting during any normal processes. However, studies from West et al. indicated that actin may be recruited from structures called podosomes to the cell membrane. This occurs during a burst of macropinocytic activity that is triggered in DC by encounter with TLR ligands.22 Fluid phase uptake was increased almost five-fold within 20–40 min of exposure to TLR ligands but returned to normal levels within 2 hr.22 The increased uptake of solute correlated with the rapid disappearance of podosomes, structures that are normally associated with cell migration and tissue invasion.39 The podosomes subsequently reappeared as the rate of macropinocytic uptake declined. As it was not possible to track actin from podosomes to the ruffling edges of the plasma membrane, there are two possible explanations for actin remodelling upon TLR stimulation. The first is that TLR stimulation causes transient dissociation of podosomes as part of the programming of DC maturation, perhaps as a trigger for enhanced motility or increased migration of DC away from peripheral tissues. The second is that actin is at least partially limiting during the massive up-regulation of fluid-phase uptake. The suggestion that actin relocalization may be required during TLR-mediated up-regulation of macropinocytosis, however, raises the intriguing possibility that the very high rates of fluid-phase uptake exhibited by DC in vitro may be unsustainable for significant periods of time in vivo.
Antigen presentation
One of the major functions of DC is to degrade foreign antigens to peptides that then form complexes with major histocompatibility complex (MHC) molecules and are then presented to T cells. Ablation of CD11c+ cells prevents the induction of a CD8+ T-cell response to some pathogens.40 Thus, the delivery of macropinocytosed antigen to compartments where processing of antigen and subsequent binding of peptides to MHC molecules could occur, is an important step in the initiation of T-cell responses to soluble antigens (Fig. 3). A portion of antigen internalized by macropinocytosis in human monocyte-derived DC was delivered to compartments rich in MHC class II.30 In these studies it was difficult to distinguish between antigen uptake via macropinocytosis or receptor-mediated endocytosis, and targeting of antigen to PRR did enhance MHC class II-restricted presentation. However, it is likely that antigen delivered via macropinosomes is delivered to the MHC class II processing pathway. Antigen presentation by DC is prolonged compared to presentation by macrophages. The persistence of antigen within DC has been correlated to reduced levels of lysosomal proteases and proteolysis in these cells41 but some antigen may be retained in neutral macropinosomes in which proteolysis of antigen does not occur for prolonged periods.42
Figure 3.
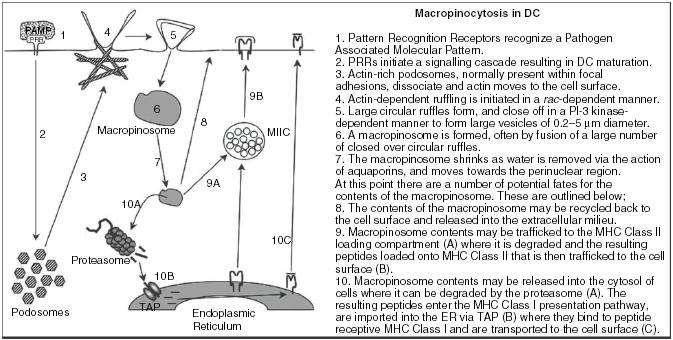
Schematic of macropinocytosis in DC.
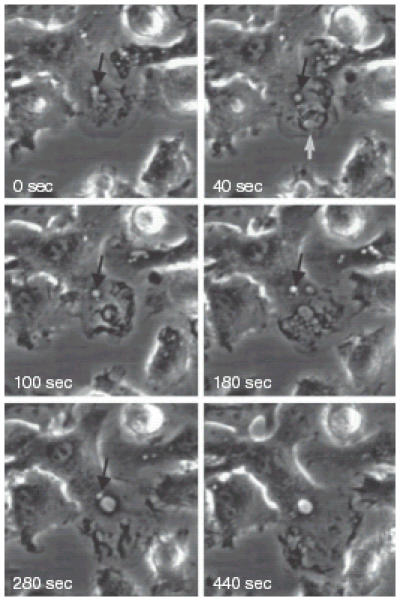
Antigen endocytosed within macropinosomes is also available for processing and presentation by the MHC class I pathway. This is accomplished via release of antigen from internal vesicles to the cytosol of the cell. The release of macropinocytosed antigen into the cytosol was first described in A431 cells and macrophages upon stimulation of macropinocytosis with phorbol esters or growth factors43 but occurs constitutively in bone-marrow-derived mouse DC in vitro(Fig. 2).31 Although the concentrations of antigen utilized in these studies were high (> 0·5 mg/ml) the exposure times were very short (> 20 min), potentially mimicking the release of large quantities of antigen from virus-infected cells. Transient up-regulation of macropinocytosis upon ligation of TLR increases presentation of a soluble exogenous antigen on MHC class I, although the antigen in this case, immune complexes, was probably internalized by receptor-mediated endocytosis in addition to macropinocytosis.22
Figure 2.
Release of exogenous solute into the cytosol of a murine bone-marrow-derived DC. DC were cultured at 37°C in the presence of 1 mg/ml fluorescein isothiocyanate (FITC)-dextran (40 000 MW), then washed and chased in the presence of Trypan Blue that will quench FITC fluorescence if it is accessible from outside the cell. FITC fluorescence is not quenched, and so is in an intact living cell.
Release of antigen into the cytosol of cells could allow access of antigen to the classical pathway of MHC class I processing and presentation, a potential mechanism to explain the in vivo observation of cross priming of exogenous antigen on MHC class I.44–46 However, the major substrates for MHC class I are thought to be short-lived defective ribosomal products (DRiPs)47–49 in contrast to the long-lived intact proteins that are delivered to the cytosol via macropinocytosis. Thus, the conventional MHC class I processing pathway may not efficiently present long-lived cytosolic proteins. The observation that phagosomes recruit components from ER-membranes38 led to the hypothesis that phagosomes are competent mediators of MHC class I-restricted presentation in macrophages50 or DC.51 This observation was extended to the presentation of soluble exogenous ovalbumin, presumably after internalization via macropinocytosis by human primary DC or a human DC cell line.52 These publications described a pathway in which antigen is released from either phagosomes or macropinosomes, degraded by proteasomes present on the cytosolic side of the vesicle membrane, and the peptides produced are pumped back into the ER-macropinosome/phagosome compartment by the Transporter of Antigen Processing (TAP) transporter complex. Although some parts of this pathway remain to be fully explained, presentation via this mechanism could explain the discrepancy between the sources of peptides from DRiPs versus exogenous proteins. A specialized mechanism such as presentation by a specialized ER-phagosome compartment could readily explain the 100-fold difference in efficiency of MHC class I-restricted presentation between antigen expressed by an endosomal pathogen53 and the much less efficient presentation of cytosolic antigen expressed by a recombinant virus.54 However, recent evidence has suggested that ER components are not recruited to phagosomes,55 bringing the role of ER-macropinosome/phagosome fusion into question. Rationalizing the observations from all of the groups involved in these investigations in identical cell lines, and in primary DC, is a prerequisite before this theory is abandoned. In addition, further examination of the role of the ER in macropinocytosis and cross-presentation of exogenous antigens on MHC class I is required. Nonetheless, the observation that A431 cells are unable to present exogenous antigen on MHC class I (ref. 43 and C.C.N. unpublished observations) strongly indicates a specialization of macrophages and DC to allow cross-presentation of exogenous antigens from macropinosomal or phagosomal compartments.
Potential role in vivo
The wealth of data produced from DC generated in vitro led to the hypothesis that DC constitutively macropinocytose antigen in the periphery, then concurrently mature and migrate to secondary lymphoid organs where they present the endocytosed antigen in complex with both MHC class I and II. Although widely accepted, this hypothesis has yet to be tested fully in vivo, and significant data exist to indicate that this is a somewhat simplified view of antigen uptake and presentation by DC. The obvious flaw in the in vitro systems used to examine cell biological processes is that DC grown from human monocytes, mouse bone marrow, or purified from animal tissues, are unlikely to phenotypically resemble peripheral DC. Any manipulation of DC in vitro is known to stimulate at least partial DC maturation56 and so it is likely that the cells examined in these studies are at least partially activated. Studies from West et al. demonstrate that exposure of DC to PAMPs transiently increases the rate of fluid-phase uptake prior to shut down of this endocytic pathway.22 Thus, in light of the fact that macropinocytosis is probably a very energy-intensive process, it is possible that uptake by peripheral DC is minimal until a maturation stimulus is encountered.
An additional indication that the model of peripheral uptake by DC followed by maturation and migration is only one of the possible mechanisms involved in delivery and presentation of antigen to primary T cells is the observation that a large proportion of DC resident in secondary lymphoid organs are phenotypically immature.57–59 These immature DC may induce peripheral tolerance in T cells to which they present antigen.60 Lymph node-resident DC are able to internalize injected antigen61 and present it to CD4+ T cells. Therefore, DC subsets in the lymph node may be specialized to sample the contents of reticular conduits, allowing enhanced presentation of antigen draining from the periphery.62 The role of macropinocytosis in the internalization of antigen by lymph node-resident DC in vivo remains to be established because the studies outlined above have all used antigen that could be internalized after binding to PRR. Indeed, the involvement of endocytic pathways in a large number of essential processes in vivo have prevented the construction of genetically manipulated mice in which the importance and regulation of these processes can be studied. Although advances in technology offer the tantalizing prospect that we may be able to study these processes in animal models in the future, we currently have to extrapolate our knowledge of endocytic processes from yeast and/or tissue culture models to in vivo situations.
Pathogen manipulation of macropinocytosis
Although scientists have been unable to gain insight into the in vivo roles of macropinocytosis, many other organisms can give us strong indicators of how macropinocytosis functions relative to their survival, persistence and replication. Eukaryotic or viral pathogens can either evolve to take advantage of existing processes during infection or to express modulatory molecules that can subtly alter the host's immune response to the pathogen. As outlined above, Salmonella typhimurium can induce ruffling and phagocytosis in epithelial cells6 or macrophages.4,5 This process is advantageous for the bacterium, as ablation of the gene responsible for the induction of spacious phagocytosis significantly reduces virulence. A number of other intracellular bacteria, including Mycobacterium tuberculosis,63 are also known to exploit this pathway during entry into cells.
Induction of macropinocytosis is also a method by which a number of viruses can either enter or enhance their entry into target cells. Although the exact mode of entry of poxviruses is not known, viral particles known as intracellular mature viral particles (IMV) can trigger actin rearrangement and Rac1 activation. IMV particles bind to filopodia that are formed and viral cores are released into the cytosol, although internalization into vesicles may not be required.64 Adenovirus 2 also induces macropinocytosis through particle binding to an αv integrin, leading to activation of PI3-kinase and Rac1.65 However, in the case of adenovirus it is unlikely that the virus enters by this pathway. Rather, induction of macropinocytosis places additional strain upon the endocytic pathway, perhaps by overcoming the solute control mechanisms utilized by most cells. This increased volume of solute probably reduces the integrity of endosomal vesicles and increases the ease with which adenovirus can lyse these compartments and escape into the cytosol.65
In addition to using macropinocytosis to increase infection, viruses may also reduce fluid phase uptake by immune cells, and particularly DC. Poxviruses encode a homologue of proteins normally involved in neural tube growth, called semaphorins.66 This protein, from the A39R gene in vaccinia virus, binds to the receptor plexin C1 on DC. Ligation of plexin C1 transduces signals that induce cytoskeletal rearrangement, blocking cellular migration67 and phagocytosis.68 Receptor-mediated endocytosis of fluorescein isothiocyanate-dextran was not affected by exposure to A39R68 but the method of action of this viral modulator strongly suggests that it will inhibit macropinocytosis. Vaccinia virus encoding A39R caused increased inflammation compared to virus that did not encode the gene.69 Thus, viruses may modulate endocytic pathways as mechanisms by which to gain a selective advantage.
Conclusion
The extent to which internalization of soluble antigen by DC via macropinocytosis contributes to the initiation of a pathogen-targeted immune response remains unclear at present. The logistics of DC in the periphery constitutively internalizing large quantities of solute are probably beyond the realm of possibility. However, it is clear that DC are specialized to internalize enormous quantities of solute upon exposure to a maturation stimuli. This would constitute a back-up mechanism to sample large quantities of antigenic material upon triggering by PRR that do not generally mediate internalization of its ligand, such as TLRs. The soluble antigen internalized is presented efficiently on both MHC class I and class II, allowing generation of a fully functional immune response in all compartments. This model makes a compelling argument that DC macropinocytosis plays a vital role in immune surveillance, but only further investigations in vivo will reveal the significance of this process in induction of a T-cell response.
Acknowledgments
This work was supported by National Institutes of Health Grant AI056094 to C.C.N. We would like to acknowledge Dr Emmy Truckenmiller and members of the Norbury laboratory for carefully reviewing the manuscript.
Abbreviations
- AQP
aquaporin
- DC
dendritic cell
- DRiPs
defective ribosomal products
- ER
endoplasmic reticulum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–monocyte colony-stimulating factor
- IL-4
interleukin-4
- IMV
intracellular mature viral
- M-CSF (CSF-1)
macrophage colony-stimulating factor
- MHC
major histocompatibility complex
- PAMPs
pathogen-associated molecular patterns
- PI3
phosphoinositide 3
- PRR
pattern recognition receptors
- TLR
Toll-like receptor
References
- 1.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–40. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S. Toll-like receptors and innate immunity. Adv Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 3.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 4.Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–8. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpuche-Aranda CM, Berthiaume EP, Mock B, Swanson JA, Miller SI. Spacious phagosome formation within mouse macrophages correlates with Salmonella serotype pathogenicity and host susceptibility. Infect Immun. 1995;63:4456–62. doi: 10.1128/iai.63.11.4456-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galan JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–26. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 7.Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–80. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- 8.Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur J Cell Biol. 1993;61:44–53. [PubMed] [Google Scholar]
- 9.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–9. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989;170:1635–48. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E, Knecht DA. Visualization of actin dynamics during macropinocytosis and exocytosis. Traffic. 2002;3:186–92. doi: 10.1034/j.1600-0854.2002.030304.x. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–8. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 13.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 14.Grimmer S, van Deurs B, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–62. [Google Scholar]
- 15.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–14. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 16.Veithen A, Cupers P, Baudhuin P, Courtoy PJ. v-Src induces constitutive macropinocytosis in rat fibroblasts. J Cell Sci. 1996;109:2005–12. doi: 10.1242/jcs.109.8.2005. [DOI] [PubMed] [Google Scholar]
- 17.Veithen A, Amyere M, Van Der Smissen P, Cupers P, Courtoy PJ. Regulation of macropinocytosis in v-Src-transformed fibroblasts: cyclic AMP selectively promotes regurgitation of macropinosomes. J Cell Sci. 1998;111:2329–35. doi: 10.1242/jcs.111.16.2329. [DOI] [PubMed] [Google Scholar]
- 18.Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–67. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–94. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 20.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–60. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki N, Hatae T, Furukawa A, Swanson JA. Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci. 2003;116:247–57. doi: 10.1242/jcs.00235. [DOI] [PubMed] [Google Scholar]
- 22.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar K, Kruhlak MJ, Erlandsen SL, Shaw S. Selective inhibition by rottlerin of macropinocytosis in monocyte-derived dendritic cells. Immunology. 2005;116:513–24. doi: 10.1111/j.1365-2567.2005.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 25.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100:1084–7. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 26.Hackstein H, Morelli AE, Larregina AT, et al. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J Immunol. 2001;166:7053–62. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- 27.Woltman AM, Schlagwein N, van der Kooij SW, van Kooten C. The novel cyclophilin-binding drug sanglifehrin A specifically affects antigen uptake receptor expression and endocytic capacity of human dendritic cells. J Immunol. 2004;172:6482–9. doi: 10.4049/jimmunol.172.10.6482. [DOI] [PubMed] [Google Scholar]
- 28.Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–20. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment. downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 32.West MA, Antoniou AN, Prescott AR, Azuma T, Kwiatkowski DJ, Watts C. Membrane ruffling, macropinocytosis and antigen presentation in the absence of gelsolin in murine dendritic cells. Eur J Immunol. 1999;29:3450–5. doi: 10.1002/(SICI)1521-4141(199911)29:11<3450::AID-IMMU3450>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–34. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 34.West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10:839–48. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Norbury CC, Greenwood R, Bennink JR, Yewdell JW, Frelinger JA. Multiple paths for activation of naive CD8+ T cells: CD4-independent help. J Immunol. 2001;167:1283–9. doi: 10.4049/jimmunol.167.3.1283. [DOI] [PubMed] [Google Scholar]
- 36.de Baey A, Lanzavecchia A. The role of aquaporins in dendritic cell macropinocytosis. J Exp Med. 2000;191:743–8. doi: 10.1084/jem.191.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619–48. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- 38.Gagnon E, Duclos S, Rondeau C, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–31. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 39.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–85. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 40.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–4. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 42.Lutz MB, Rovere P, Kleijmeer MJ, et al. Intracellular routes and selective retention of antigens in mildly acidic cathepsin D/lysosome-associated membrane protein-1/MHC class II- positive vesicles in immature dendritic cells. J Immunol. 1997;159:3707–16. [PubMed] [Google Scholar]
- 43.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 44.Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J Immunol. 1976;117:2233–8. [PubMed] [Google Scholar]
- 45.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staerz UD, Karasuyama H, Garner AM. Cytotoxic T lymphocytes against a soluble protein. Nature. 1987;329:449–51. doi: 10.1038/329449a0. [DOI] [PubMed] [Google Scholar]
- 47.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–4. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 48.Reits EA, Vos JC, Gromme M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–8. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 49.Khan S, de Giuli R, Schmidtke G, Bruns M, Buchmeier M, van den Broek M, Groettrup M. Cutting edge. neosynthesis is required for the presentation of a T cell epitope from a long-lived viral protein. J Immunol. 2001;167:4801–4. doi: 10.4049/jimmunol.167.9.4801. [DOI] [PubMed] [Google Scholar]
- 50.Houde M, Bertholet S, Gagnon E, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–6. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 51.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 52.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA. 2003;100:12889–94. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villanueva MS, Fischer P, Feen K, Pamer EG. Efficiency of MHC class I antigen processing: a quantitative analysis. Immunity. 1994;1:479–89. doi: 10.1016/1074-7613(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 54.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–54. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 55.Touret N, Paroutis P, Terebiznik M, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123:157–70. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 57.Randolph GJ. Is maturation required for Langerhans cell migration? J Exp Med. 2002;196:413–16. doi: 10.1084/jem.20021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geissmann F, Dieu-Nosjean MC, Dezutter C, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–30. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson NS, El-Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, Villadangos JA. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–94. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 60.Wilson NS, El-Sukkari D, Villadangos JA. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood. 2004;103:2187–95. doi: 10.1182/blood-2003-08-2729. [DOI] [PubMed] [Google Scholar]
- 61.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present a subcutaneous antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 62.Sixt M, Kanazawa N, Selg M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Perez BE, Mondragon-Flores R, Luna-Herrera J. Internalization of Mycobacterium tuberculosis by macropinocytosis in non-phagocytic cells. Microb Pathol. 2003;35:49–55. doi: 10.1016/s0882-4010(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 64.Locker JK, Kuehn A, Schleich S, Rutter G, Hohenberg H, Wepf R, Griffiths G. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol Biol Cell. 2000;11:2497–511. doi: 10.1091/mbc.11.7.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, Greber UF. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158:1119–31. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Comeau MR, Johnson R, DuBose RF, et al. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity. 1998;8:473–82. doi: 10.1016/s1074-7613(00)80552-x. [DOI] [PubMed] [Google Scholar]
- 67.Walzer T, Galibert L, Comeau MR, De Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol. 2005;174:51–9. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- 68.Walzer T, Galibert L, De Smedt T. Poxvirus semaphorin A39R inhibits phagocytosis by dendritic cells and neutrophils. Eur J Immunol. 2005;35:391–8. doi: 10.1002/eji.200425669. [DOI] [PubMed] [Google Scholar]
- 69.Gardner JD, Tscharke DC, Reading PC, Smith GL. Vaccinia virus semaphorin A39R is a 50–55 kDa secreted glycoprotein that affects the outcome of infection in a murine intradermal model. J Gen Virol. 2001;82:2083–93. doi: 10.1099/0022-1317-82-9-2083. [DOI] [PubMed] [Google Scholar]