Abstract
Relatively little is known about the effector mechanisms whereby the human immune system controls Mycobacterium tuberculosis infection. In this study we elaborate on the immune response and mechanisms of persistence of mycobacteria in lesions by analysing, using immunohistochemistry, the expression of cytokines [tumour necrosis factor-α (TNF-α), interleukin-10 (IL-10), transforming growth factor-β (TGF-β) and interferon-γ (IFN-γ)], apoptotic cells and apoptosis-related proteins [Bcl2, Bax, Fas ligand (FasL) and Fas] in the human tuberculous lymphadenitis lesions. The expression of apoptosis-related proteins has been shown to be exploited by mycobacteria to evade the immune response and persist in the host. Foreign body (FB) granulomas were used as controls. In tuberculosis (TB) granulomas, epithelioid cells and multinucleated giant cells expressed cytokines differently. In epithelioid cells, the numbers of TNF-α-, IL-10- and TGF-β-stained cells were higher than IFN-γ-stained cells (P < 0·01). TGF-β and FasL were strongly expressed in the necrotic centres as compared with other cytokines. More giant cells expressed IL-10 and TGF-β than expressed TNF-α and IFN-γ (P < 0·01). Staining of consecutive sections revealed that some giant cells expressed IL-10 but not TNF-α. Apoptotic TB giant cells correlated positively with the expression of TNF-α, IFN-γ and TGF-β, but not with the expression of IL-10. The percentage of giant cells expressing Bax was lower than those expressing Fas, unlike the epithelioid cells, suggesting that TB giant cells are less susceptible to apoptosis. Compared with FB giant cells, there were fewer TB giant cells showing TNF-α, IFN-γ, FasL, Fas expression or undergoing apoptosis (P < 0·05). Taken together, these observations show that the cellular microenvironment of TB granulomas down-regulates microbicidal functions, favouring bacillary survival and persistence. TGF-β and FasL may be responsible for tissue destruction. The giant cells, being less susceptible to apoptosis, may remain a continuous source of pro-inflammatory cytokines, causing immune pathology.
Keywords: apoptosis, cytokines, granulomas, lymphadenitis, tuberculosis
Introduction
Primarily considered a pulmonary disease, tuberculosis (TB) has the potential to affect almost any organ system, with lymph nodes being the most common form of extrapulmonary TB.1 Human immunodeficiency virus (HIV) infection and other immune-compromising conditions have resulted in an increase in the incidence of tuberculous lymphadenitis.2–4 The mechanisms by which mycobacteria persist in the lesions and lead to chronicity of disease are not well known. Following processing and presentation of bacillary proteins by infected macrophages, the immune system reacts with a cell-mediated immune response.5,6 Subsequently, epithelioid cell granulomas are generated. The formation of the granulomatous immune response is considered to be a correlate of protective immunity to TB. Most TB patients, in parallel with the induction of a cell-mediated immune response, develop an inappropriate necrotising pattern of response to Mycobacterium tuberculosis, which is not a correlate of optimal protective immunity.7 There is a need to understand the differences between protective immunity and the necrotising response, and the factors that determine which response pattern is present, in order to develop better prophylactic and therapeutic vaccines.
Studies have shown that tumour necrosis factor-α (TNF-α), together with interferon-γ (IFN-γ), enhances the bactericidal activity of monocytes and macrophages.8 Both of these cytokines are also responsible for granuloma formation.9,10 Interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), released from the regulatory T cell, have been shown to suppress inflammation in models of allergy, inflammatory bowel disease and autoimmunity.11 These regulatory T cells have received little attention in the context of TB. IL-10 and TNF-α are shown to play opposing roles during mycobacterial infections.12 TNF-α has been shown to be associated with the activation of macrophages.13,14 Conversely, IL-10 inhibits macrophage functions,15 resulting in the enhanced intracellular growth of bacteria.16 IL-10 has also been shown to cause inactivation of TNF-α by inducing the release of soluble TNF receptor.17 TGF-β suppress the anti-mycobacterial immune responses of humans and experimental animals. It plays an important role in down-regulating the production of TNF-α18 and also causes deactivation of macrophages.19–21 These observations suggest that the inappropriate secretion of IL-10 and TGF-β seems to be a possible mechanism of failure of the immune response in TB.
The apoptosis-regulating proteins, Fas, Fas ligand (FasL), Bcl-2 (anti-apoptotic protein) and Bax (pro-apoptotic protein), play an important role in the regulation of apoptosis and the immune response.22,23 The expression of these proteins is exploited by mycobacteria to evade the immune response and persist in the host, as shown by studies carried out on the murine models of TB24–26 and in in vitro studies.27 Mycobacterial-infected cells, by virtue of increased Bcl-2 and reduced Bax expression, can evade activation-induced apoptotic cell death. Furthermore, these infected cells can induce apoptosis in any approaching immunocyte by virtue of increased expression of FasL and reduced expression of Fas.24–26 This makes these infected cells an immune sanctuary for mycobacteria. Cytokines involved in the host immune response are shown to influence the process of apoptosis. In different experimental systems it has been shown that TNF-α and IL-10 have opposite roles in the induction of apoptosis.28 TNF-α induces apoptosis,29–33 as signals transduced through TNFR1 (p55) can induce activation of various proteases,29 including activation of a family of caspases that are recognized mediators of apoptosis.30 On the other hand, IL-10 expression has been shown to be associated with increased cell survival by inhibition of apoptosis.34,35 IFN-γ and TGF-β are also involved in the induction of apoptosis.28 In human alveolar macrophages, IL-10 is shown to reduce IFN-γ-induced apoptosis.28 Based on this background, we elaborate on the immune response and mechanisms of persistence of mycobacteria in the lesions by analysing the expression of apoptotic cells and apoptosis-related proteins (Bcl2, Bax, FasL, Fas) and cytokines (such as TNF-α, IL-10, TGF-β and IFN-γ) involved in the regulation of apoptosis and the immune response in the tuberculous lymphadenitis lesions. Foreign body (FB) granulomas were used as controls.
Materials and methods
The study was performed on 48 cases of TB lymph node biopsies. Diagnosis of TB lymphadenitis was based on the clinical criteria and presence of histological features characteristic of TB granulomas. Seventeen cases were obtained from the archives of the Department of Pathology, Haukeland University Hospital, Norway. Thirty-one cases were from patients diagnosed with mycobacterial lymphadenitis in an epidemiological study from rural Tanzania.36,37 The findings from these two groups are pooled together in the Results. The majority of patients presented with swelling in the neck; other symptoms, such as fever, pain and weight loss, were infrequent. The lymph nodes were from the cervical region in 82% of cases and the rest were from axillary, inguinal and mesenteric regions. Among the TB patients, 46% were children (2–14 years) and 54% were adults (18–86 years). Sixty-one per cent were male and 39% were female. For some Tanzanian cases, the mycobacterial culture, HIV and bacille Calmette–Guérin (BCG) vaccination status results were available. Among the 17 cases where culture results were available, only one was positive for M. tuberculosis. HIV results based on serology were available for seven cases, and one case was positive. Information on BCG vaccination status was available for 13 patients and, among these, 11 were BCG vaccinated. Verbal consent was given by the participants of the study. Ethical clearance was obtained from the Medical Research Co-ordinating committee in Tanzania and from the ethical committee in Norway.
Six cases of skin biopsies with FB granulomas obtained from the archives of Haukeland University Hospital were used as controls.
Histological examination and immunohistochemistry
Parallel, 5-µm-thick sections were prepared from each specimen. Two sections were stained, one with haematoxylin and eosin and the other with the Ziehl–Nielsen stain for acid-fast bacilli. Immunostaining was carried out using the kit from DakoCytomation (EnVision + System-HRP; DakoCytomation, Denmark A/S, Glostrup, Denmark). Briefly, after deparaffinization and rehydration, the sections were exposed to microwave antigen retrieval using citrate buffer, pH 6·0, at 750 W for 10 min, and at 350 W for 15 min. The sections were cooled for 20 min at room temperature and then incubated with hydrogen peroxide for 5 min. Primary antibodies (Table 1) were then applied to the sections for 45 min followed by incubation for 40 min with anti-rabbit or anti-mouse dextran polymer conjugated to horseradish peroxidase. Visualization was with 3-amino-9-ethylcarbazol (AEC) containing H2O2 (for cytokines and caspase 3), or diaminobenzidine (DAB) (for apoptosis-related proteins) substrate applied for 10 min. Sections were counterstained with haematoxylin. In negative controls, primary antibody was substituted with antibody diluent only.
Table 1.
Antibodies used in the immunohistochemical staining.
| Antibodies | Specificity | Source |
|---|---|---|
| Mouse monoclonal IgG1 | Human IL-10 | ImmunoTools, Germany |
| Mouse monoclonal IgG2a | Human IFN-γ | ImmunoTools, Germany |
| Mouse monoclonal IgG1 | Human TNF-α | ImmunoTools, Germany |
| Mouse monoclonal IgG1 | Human Bcl-2 | Santa Cruz Biotechnology, SDS Biosciences, Sweden |
| Mouse monoclonal IgG2b | Human Baxα and Baxβ | Santa Cruz Biotechnology, SDS Biosciences, Sweden |
| Mouse monoclonal IgG1 | Human Fas | Santa Cruz Biotechnology, SDS Biosciences, Sweden |
| Mouse monoclonal IgG1, 5G51 | Human FasL | Alexis Biochemicals, USA |
| Rabbit polyclonal | Human Caspase 3 | R & D Systems, UK |
| Rabbit polyclonal | Human TGF-β1, TGF-β2 | Santa Cruz Biotechnology, SDS Biosciences, Sweden |
| Rabbit polyclonal | Multiple antigens of M. bovisBCG Copenhagen strain | Dako A/S, Denmark |
BCG, bacille Calmette–Guérin; IFN-γ, interferon-γ; IgG, immunoglobulin G; IL-10, interleukin-10; FasL, Fas ligand; TGF-β, transforming growth factor-β; TNF-α, tumour necrosis factor-α.
Evaluation of immunostaining and statistical analysis
For each section, stained cells in the granulomas, randomly chosen in two fields, were counted using a ×40 ocular fitted with a 10 × 10-mm grid. Counting was carried out separately for the giant cells and epithelioid cells. The results were presented as average number of stained epithelioid cells and percentage of the giant cells in two fields per section. Apoptotic cells were also presented as percentages. Statistical analysis was carried out using SPSS 12·0 for Windows. Non-parametric tests were used for two-group comparisons. The Wilcoxon signed-ranked test was used for matched analysis, and the Mann–Whitney test was used for comparison of two independent groups. The Pearson Correlation test was used to determine the relationship between two variables. A P-value of <0·05 was taken as significant.
Results
Histopathology
Both necrotic and non-necrotic granulomas characteristics of TB were observed in the patients. Seventy-five per cent of the patients were found to have necrotic granulomas, whereas 25% of patients had both necrotic and non-necrotic granulomas. Only one patient was positive for acid-fast bacilli, as determined by Ziehl–Nielsen staining. Mycobacterial antigens were not detected, in any patient, by immunohistochemical staining with anti-BCG immunoglobulin.
IL-10, TNF-α, TGF-β and IFN-γ: TB granulomas
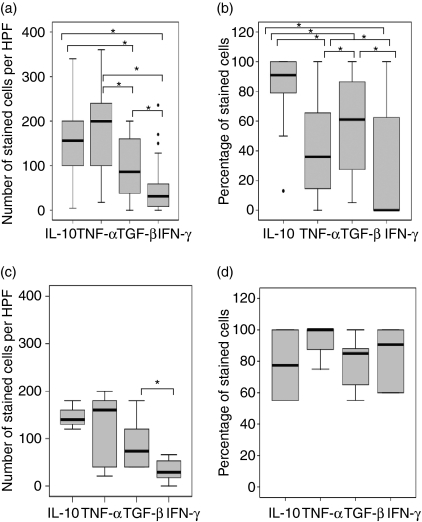
Figure 1(a) shows the percentage of stained epithelioid cells in the granulomas. There was no significant difference in the expression of TNF-α and IL-10. The percentage of cells staining positive for these two cytokines was higher than those staining for TGF-β (P = 0·000, 0·002, respectively, for TNF-α and IL-10) or IFN-γ (P = 0·000). The number of TGF-β-stained cells was higher than IFN-γ-stained cells (P = 0·004), but the intensity of staining for IFN-γ in individual cells was stronger than for TGF-β (Fig. 2). There was no significant difference in the expression of these cytokines between necrotic and non-necrotic granulomas.
Figure 1.
In situ distribution of interleukin-10 (IL-10), tumour necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β) and interferon-γ (IFN-γ) in the tuberculosis (TB) (a,b) and foreign body (FB) (c,d) granulomas analysed by using immunohistochemical staining. The median, 25th and 75th percentiles, and minimum and maximum values are shown. The marks  indicate the extreme values. The Wilcoxon signed-rank test was used for matched analysis and the Mann–Whitney test for two independent group comparisons. Statistical significance with a P-value of <0·05 is marked with an asterisk. (a)Number of stained cells per high power field (HPF) in the epithelioid cells within TB granulomas. There was no significant difference in the expression of TNF-α and IL-10. More cells stained positive for these two cytokines than for TGF-β; however, more cells stained positive for TGF-β than for IFN-γ. (b)Percentage of stained cells in multinucleated giant cells in TB granulomas. A higher percentage of giant cells expressed IL-10 than expressed TNF-α, TGF-β or IFN-γ. A significantly higher percentage of giant cells expressed TGF-β than TNF-α or IFN-γ. A significantly lower percentage of giant cells expressed IFN-γ than TNF-α. (c)Number of stained cells per HPF in the epithelioid cells of FB granulomas. A higher percentage of cells expressed TGF-β than IFN-γ. (d)Percentage of stained cells in multinucleated giant cells in FB granulomas. The expression of cytokines was not significantly different. TB giant cells have significantly lower percentages of TNF-α and IFN-γ as compared with FB giant cells.
indicate the extreme values. The Wilcoxon signed-rank test was used for matched analysis and the Mann–Whitney test for two independent group comparisons. Statistical significance with a P-value of <0·05 is marked with an asterisk. (a)Number of stained cells per high power field (HPF) in the epithelioid cells within TB granulomas. There was no significant difference in the expression of TNF-α and IL-10. More cells stained positive for these two cytokines than for TGF-β; however, more cells stained positive for TGF-β than for IFN-γ. (b)Percentage of stained cells in multinucleated giant cells in TB granulomas. A higher percentage of giant cells expressed IL-10 than expressed TNF-α, TGF-β or IFN-γ. A significantly higher percentage of giant cells expressed TGF-β than TNF-α or IFN-γ. A significantly lower percentage of giant cells expressed IFN-γ than TNF-α. (c)Number of stained cells per HPF in the epithelioid cells of FB granulomas. A higher percentage of cells expressed TGF-β than IFN-γ. (d)Percentage of stained cells in multinucleated giant cells in FB granulomas. The expression of cytokines was not significantly different. TB giant cells have significantly lower percentages of TNF-α and IFN-γ as compared with FB giant cells.
Figure 2.
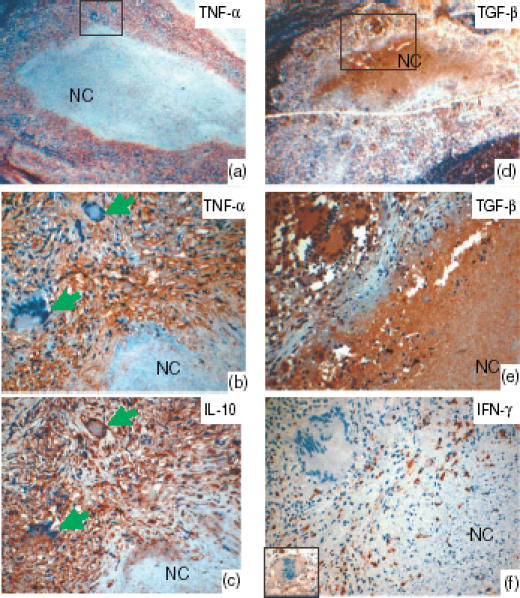
Lymph node tissue showing the staining pattern of tumour necrosis factor-α (TNF-α)-, interleukin-10 (IL-10)-, transforming growth factor-β (TGF-β)- and interferon-γ (IFN-γ)-positive cells in the tuberculosis granulomas, as detected by immunohistochemical staining with 3-amino-9-ethylcarbazol (AEC). Panels (a) and (d) show the low-power image, and the marked areas are shown with high power view in panels (b) and (e), respectively. All cytokines studied were expressed by the epithelioid cells. Some giant cells expressed IL-10, but not TNF-α, as seen in the consecutive sections (b and c) stained with IL-10 and TNF-α (arrows). IFN-γ (f) was expressed weakly (see inset) or was absent in the giant cells, as shown. TGF-β was strongly expressed in the necrotic centres (NC) as compared to IL-10 and TNF-α. IFN-γ was not detected in the necrotic centres. During early necrosis, where cells undergoing necrosis could be seen, the expression of TGF-β was strong, as seen in the periphery of the necrotic centres. In late necrosis there was more homogenous distribution, as seen in the centre of the necrotic areas. TNF-α and IL-10 showed a patchy distribution in the necrotic centres.
The expression of cytokines in the giant cells was assessed separately, as shown in Fig. 1(b). Unlike the epithelioid cells in the granulomas, more giant cells expressed IL-10 than expressed TNF-α (P = 0·000). Some giant cells expressed IL-10 but not TNF-α, as shown in the consecutive sections in Fig. 2. The percentage of IL-10-positive giant cells was also higher than the percentage of TGF-β- (P = 0·01) or IFN-γ- (P = 0·001) positive giant cells. A significantly higher percentage of giant cells expressing TGF-β was observed than of giant cells expressing TNF-α (P = 0·01) or IFN-γ (P = 0·001). Fewer giant cells were observed to express IFN-γ than TNF-α (P = 0·04). The intensity of staining for IFN-γ in the giant cells was weak (Fig. 2). In summary, more giant cells expressed IL-10 and TGF-β than expressed TNF-α and IFN-γ.
TGF-β was strongly expressed in the necrotic centres as compared with IL-10 and TNF-α, while IFN-γ was not detected in the necrotic centres (Fig. 2). In early necrosis, where cells undergoing necrosis could be seen, the expression of TGF-β was strong. In late necrosis there was a more homogenous and stronger distribution of TGF-β in the necrotic centres, whereas TNF-α and IL-10 showed a patchy distribution. The epithelioid cells in the granulomas expressed TGF-β at a lower intensity than the necrotic centres.
We also investigated the relationship between the cytokines. IL-10 correlated positively with TNF-α (r = 0·3, P = 0·04) in the epithelioid cells, but not in the giant cells. In both epithelioid cells and giant cells, TGF-β correlated positively with TNF-α (epithelioid cells: r = 0·7, P = 0·000; giant cells: r = 0·4, P = 0·01) and IFN-γ (epithelioid cells: r = 0·3, P = 0·007; giant cells: r = 0·6, P = 0·001). TNF-α also correlated positively with IFN-γ (epithelioid cells: r = 0·3, P = 0·03; giant cells: r = 0·7, P = 0·000).
Comparison between TB and FB granulomas
Figure 1(c,d) shows the distribution of IL-10, TNF-α, TGF-β and IFN-γ in the FB granulomas. There was no significant difference in the number of cells expressing these cytokines, except that more epithelioid cells expressed TGF-β than expressed IFN-γ. No significant correlation was found between the cytokines in the epithelioid cells. The giant cells expressing TGF-β correlated positively with IFN-γ-expressing cells.
There was no difference in cytokine distribution between the TB and FB epithelioid cells. However, TB giant cells had significantly lower percentages of TNF-α- and IFN-γ-positive cells compared with FB giant cells.
FasL, Fas, Bcl-2, Bax and apoptosis: TB granulomas
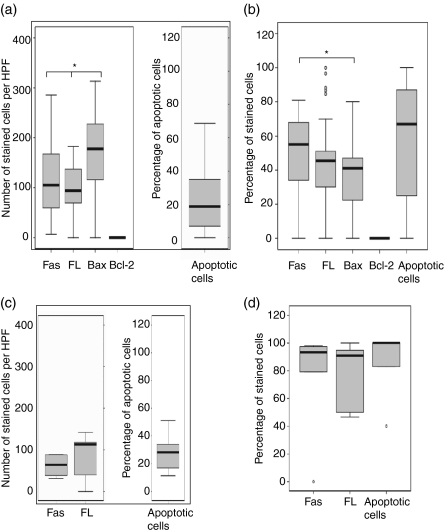
Figure 3(a) shows the number of epithelioid cells that were positive for Fas, FasL, Bax and Bcl-2, and the percentage of apoptotic cells detected by caspase 3. A higher proportion of cells expressed Bax than expressed either Fas (P = 0·01) or FasL (P = 0·006). Cells expressing Bcl-2 were either scarce or undetectable in the granulomas. However, the surrounding lymphoid tissue expressed Bcl-2 (Fig. 4). There was a significant, positive correlation between FasL- and Fas-expressing cells (r = 0·4, P = 0·008). Unlike the epithelioid cells, the percentage of giant cells expressing Bax was lower than those expressing Fas (P = 0·05), as shown in Fig. 3(b), indicating a reduced susceptibility of giant cells to apoptosis. Giant cells expressing Bax correlated positively with giant cells expressing Fas (r = 0·4, P = 0·03) and FasL (r = 0·3, P = 0·04). In 54% of the cases, the necrotic centres expressed high levels of FasL, but the expression of Fas was absent or weak.
Figure 3.
In situ distribution of Fas ligand (FasL), Fas, Bcl-2, Bax and apoptosis in the tuberculosis (TB) (a,b) granulomas, and FasL, Fas, and apoptosis in foreign body (FB) (c,d) granulomas, analysed by using immunohistochemical staining. The median, 25th and 75th percentiles, and minimum and maximum values are shown. The marks indicate the extreme values. The Wilcoxon signed-rank test was used for matched analysis and the Mann–Whitney test for two independent group comparisons. Statistical significance with a P-value of <0·05 is marked with an asterisk. (a) Number of stained cells per high power field (HPF) in the epithelioid cells within TB granulomas. Bax-expressing cells were more numerous than either Fas (P = 0·01) or FasL-expressing cells. Bcl-2-expressing cells were very few or could not be detected in the granulomas. (b) Percentage of stained cells in multinucleated giant cells in TB granulomas. The percentage of giant cells expressing Bax was lower than those expressing Fas (P = 0·05). (c) Number of stained cells per HPF in the epithelioid cells of FB granulomas. There was no significant difference in the expression of cytokines. (d) Percentage of stained cells in multinucleated giant cells in FB granulomas. There was no significant difference in the expression of cytokines. Among TB giant cells there were significantly lower percentages of FasL and Fas and apoptotic cells as compared with FB giant cells.
Figure 4.
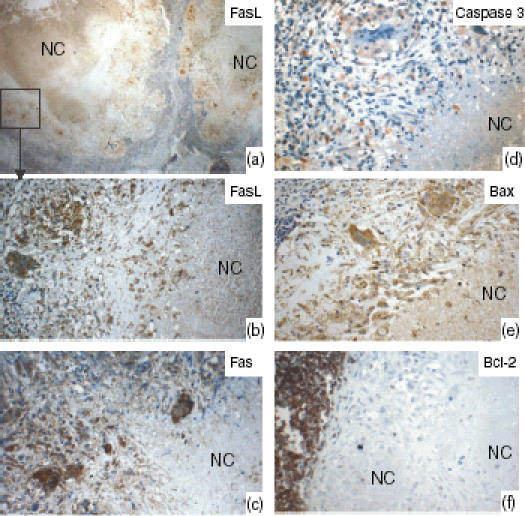
Lymph nodes tissue showing the staining pattern of Fas ligand (FasL), Fas, and caspase 3 for detecting apoptotic cells, Bax, and Bcl-2 in the tuberculosis granulomas, as detected by immunohistochemical staining. (a) Low power view; the marked area is shown at high power in (b). The epithelioid cells and the giant cells expressed FasL, Fas and Bax. In 54% of cases, the necrotic centres (NC) expressed high levels of FasL, but Fas expression was absent or weak. Bcl-2-expressing cells were very few or could not be detected in the granulomas, whereas the surrounding lymphoid tissue expressed Bcl-2 strongly.
The correlation of cytokines with apoptotic cells and apoptosis-regulating proteins was also studied. Apoptotic giant cells correlated positively with the giant cells positive for TNF-α (r = 0·3, P = 0·04), IFN-γ (r = 0·6, P = 0·001) and TGF-β (r = 0·8, P = 0·000), but not with IL-10-positive cells, whereas in the epithelioid cells there was no such correlation. Giant cells expressing FasL correlated positively with those expressing IFN-γ (r = 0·7, P = 0·000) and TGF-β (r = 0·6, P = 0·000).
Comparison between TB and FB granulomas
Figure 3(c,d) shows the number of Fas- and FasL-positive cells and the percentage of apoptotic cells in the FB granulomas. There was no difference or correlation in the expression of these apoptosis-related proteins. There was no difference in the distribution of Fas, FasL or apoptotic cells between TB and FB epithelioid cells. However, fewer giant cells in TB granulomas expressed FasL, Fas and caspase 3 (apoptotic cells) as compared with FB giant cells.
Difference between Norwegian and Tanzanian populations
The number of giant cells positive for TNF-α, IFN-γ and FasL in granulomas in Norwegian patients was significantly higher than in Tanzanian patients. The numbers of IL-10-, TNF-α-, TGF-β- and IFN-γ-positive epithelioid cells were also higher in Norwegian patients. These differences could not be explained based on the information available. Differences in the processing of biopsy specimens, or variation in the mycobacterial species involved in the aetiology, may provide some explanation. Further studies are being carried out in our laboratory to clarify this.
Discussion
Relatively little is known about the effector mechanisms whereby the human immune system controls M. tuberculosis infection and the influence of specific cytokines in this process. One of the hallmarks of an effective immune response against M. tuberculosis is the formation of granulomas containing multinucleated giant cells. Although felt to play an important role in the control of M. tuberculosis infection,38 the function of giant cells and epithelioid cells in the tuberculous granulomas are not completely understood. This study has shown that giant cells are highly active and express cytokines involved in inflammation and apoptosis. We have shown that in the TB granulomas, more giant cells expressed IL-10 and TGF-β and fewer expressed TNF-α and IFN-γ. Significantly lower percentages of TB giant cells expressed TNF-α and IFN-γ as compared with FB giant cells. Staining of consecutive sections from TB granulomas revealed that some giant cells expressed IL-10, but not TNF-α (Fig. 2). Furthermore, apoptotic giant cells expressing caspase 3 correlated positively with the expression of TNF-α, IFN-γ and TGF-β, but not with IL-10. This is in agreement with previous studies, which have shown that TNF-α,29–33 IFN-γ28 and TGF-β39 are involved in the induction of apoptosis, whereas IL-10 expression has been associated with the inhibition of apoptosis.34,35 In TB giant cells there were significantly lower percentages of FasL, Fas and apoptotic cells as compared with FB giant cells. In TB granulomas the percentage of giant cells expressing Bax was lower than those expressing Fas, unlike the epithelioid cells. Bax is known to promote cell susceptibility to apoptosis,40 implying that the TB giant cells are less susceptible to apoptosis as compared to epithelioid cells and FB giant cells. Taken together, these observations show that increased expression of IL-10 and TGF-β, as compared to TNF-α and IFN-γ, by the giant cells in TB granulomas may down-regulate microbicidal functions, thus favouring the survival and persistence of bacilli in the cellular microenvironment of granulomas. These giant cells are not able to rid themselves of bacilli and, being less susceptible to apoptosis, may remain a continuous source of pro-inflammatory cytokines, causing immune pathology.
The tissue damage causing caseous necrosis and liquefaction of the necrotic centres is the basic process of tuberculous disease in humans, but the mechanism behind it remains largely unknown. TNF-α has been proposed as a major mediator of necrosis in M. tuberculosis-infected tissue, but this has not been confirmed by previous studies in murine models8,41,42 or in this study where TNF-α expression in the necrotic centres was patchy. TGF-β was, however, strongly expressed in the necrotic centres, but whether TGF-β has any role in causing tissue damage is not known. It has been shown that TGF-β causes up-regulation of matrix metalloproteinase (MMP)-2 and MMP-9.43 MMPs can digest collagens and other matrix proteins, contributing to necrosis.44 MMPs have been shown to be strongly expressed in TB granulomas and are associated with tissue destruction.45 MMPs also cause cleavage of FasL from the cell surface.46 FasL is believed to be involved in tissue damage47 and, in this study, the epithelioid cells and the giant cells surrounding the large necrotic areas expressed high amounts of FasL. In 54% of cases the necrotic centres expressed high levels of FasL, but Fas was absent or weak, implying a role of FasL in tissue damage. Other studies with tumours have also shown that increased production of TGF-β is associated with an increase in FasL, leading to tumour progression.48 It has also been shown that TNF-α and IFN-γ can lead to an increase in FasL expression.49 Taken together, these findings suggest that the M. tuberculosis infection and cytokines produced in the granuloma microenvironment lead to an increase in FasL and TGF-β production, which may be responsible for the tissue destruction and progression of disease. This suggestion is further supported by the finding that levels of soluble FasL, but not Fas, are higher in the serum of these patients as compared with healthy controls.50
The mechanisms that lead to the resolution or progression of granulomas and, consequently, disease, are not understood. Activated immune cells are believed to undergo apoptosis by activation-induced cell death mechanisms, leading to resolution of infection. We have shown that apoptosis occurs in the TB granulomas. The granuloma cells expressed Bax, but not Bcl-2. It is well known that excess Bcl2 blocks cell death following a variety of stimuli and has a death-sparing effect on haematopoietic cells, including macrophages.32 On the other hand, when Bcl2 is absent, the Bcl-associated x protein, Bax, can dimerize with itself. Based on this, one may argue that the lack of Bcl2 and the abundance of Bax in granulomas may render them sensitive to apoptosis. Apoptotic cells were also detectable in the necrotic centres in the granulomas, and this is in agreement with other studies which have shown that caseation is strongly associated with the apoptosis of T cells and macrophages.51 However, the following questions remain: ‘Why do granulomas not resolve despite such high numbers of apoptotic cells?’ and ‘What is the relationship between apoptosis and necrosis within caseous foci?’. The Fas/FasL system has been recognized as a major pathway for induction of apoptosis in cells and tissues,47 and is used by M. tuberculosis to contribute to its virulence.26,27 In murine pulmonary lesions, infection with M. tuberculosis results in an increase in the expression of FasL, but reduced expression of Fas in the cells in which mycobacteria reside, providing mycobacteria with an immune-privileged sanctuary.25 Based on this background we hypothesize on the mechanisms of immune privilege for mycobacterial sanctuary in TB granulomas via the Fas/FasL system. The granuloma microenvironment, by virtue of mycobacterial infection and inappropriate secretion of TGF-β and other cytokines, leads to an increase in expression of FasL in granuloma cells that counterattack cytotoxic T lymphocytes as part of granuloma immune privilege. These findings explain the morphology of granulomas where lymphocytes are found in the periphery in the granulomas and not among the epithelioid cells. This might be caused by apoptosis induced by the FasL-expressing epithelioid cells. This hypothesis is indirectly supported by studies in TB patients where constant apoptosis of T cells has been shown to result in a reduction in T-cell numbers and depressed proliferative responses.52,53 In addition, it has been shown that increased production of TGF-β can result in the down-regulation of FasL in cytotoxic T lymphocytes, inhibiting the cytotoxic potential of these cells.54 As Fas was also expressed on epithelioid cells, autocrine or juxtacrine killing of these cells is expected to occur, leading to resolution of granulomas rather than the expansive granulomas seen. This can be explained by the Fas-mediated resistance to apoptosis of epithelioid cells, as found in tumour cells.55,56 Soluble FasL may be involved in the down-regulation of Fas expression by binding to Fas and inhibiting Fas-mediated apoptosis of the granuloma cells, causing an expansion in the lesions and making these granulomas an immune-privileged site for a small number of intracellular bacilli.
In summary, our data suggest that epithelioid cells and giant cells in granulomas probably encompass target as well as effector cells, and the inappropriate secretion of IL-10 and TGF-β by these cells may be a possible mechanism of failure of the immune response to resolve the infection. These findings have implications in designing new immune-intervention strategies for TB by modulation of the host immune response.
Acknowledgments
This study was supported by Funds from the University of Bergen, Gades Legat & Connie Gulberg Jansens Legat with University of Bergen, and Skipsreder Tom Wilhelmsens Stiftelse, Norway.
Abbreviations
- BCG
bacille Calmette–Guérin
- FasL
Fas ligand
- FB
foreign body
- HIV
human immunodeficiency virus
- IFN-γ
interferon-γ
- IL-10
interleukin-10
- MMP
matrix metalloproteinase
- TGF-β
transforming growth factor-β
- TNF-α
tumour necrosis factor-α
- TB
tuberculosis
References
- 1.Anon . 1989 Tuberculosis Statistics in the United States. Atlanta, GA: US Department of Health and Human Services, Public Health Services, Centres for Disease Control; 1991. (Report) [Google Scholar]
- 2.Small PM, Schecter GF, Goodman PC, Sande MA, Chaisson RE, Hopewell PC. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1991;324:289–94. doi: 10.1056/NEJM199101313240503. [DOI] [PubMed] [Google Scholar]
- 3.Range N, Ipuge YA, O'Brien RJ, Egwaga SM, Mfinanga SG, Chonde TM, Mukadi YD, Borgdorff MW. Trend in HIV prevalence among tuberculosis patients in Tanzania 1991–98. Int J Tuberc Lung Dis. 2001;5:405–12. [PubMed] [Google Scholar]
- 4.van den Broek J, Mfinanga S, Moshiro C, O'Brien R, Mugomela A, Lefi M. Impact of human immunodeficiency virus infection on the outcome of treatment and survival of tuberculosis patients in Mwanza, Tanzania. Int J Tuberc Lung Dis. 1998;2:547–52. [PubMed] [Google Scholar]
- 5.Dannenberg AM., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–33. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SHE. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 7.Wilson GS, Schwabacher H, Maier I. The effect of the desensitisation of tuberculous guinea-pigs. J Pathol Bacteriol. 1940;50:89–109. [Google Scholar]
- 8.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 9.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 11.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 12.Appelberg R. Opposing effects of interleukin-10 on mouse macrophage functions. Scand J Immunol. 1995;41:539–44. doi: 10.1111/j.1365-3083.1995.tb03605.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flesch IE, Kaufmann SH. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–7. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–21. [PubMed] [Google Scholar]
- 16.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–6. [PubMed] [Google Scholar]
- 17.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol. 1998;161:2636–41. [PubMed] [Google Scholar]
- 18.Chantry D, Turner M, Abney E, Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989;142:4295–300. [PubMed] [Google Scholar]
- 19.Czarniecki CW, Chiu HH, Wong GH, McCabe SM, Palladino MA. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–23. [PubMed] [Google Scholar]
- 20.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- 21.Hernandez-Pando R, Orozco H, Arriaga K, Sampieri A, Larriva-Sahd J, Madrid-Marina V. Analysis of the local kinetics and localization of interleukin-1 alpha, tumour necrosis factor-alpha and transforming growth factor-beta, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oddo M, Renno T, Attinger A, Bakker T, MacDonald HR, Meylan PR. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–54. [PubMed] [Google Scholar]
- 23.Stenger S, Modlin RL. Cytotoxic T cell responses to intracellular pathogens. Curr Opin Immunol. 1998;10:471–7. doi: 10.1016/s0952-7915(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 24.Mogga SJ, Mustafa T, Sviland L, Nilsen R. Increased Bcl-2 and reduced Bax expression in infected macrophages in slowly progressive primary murine Mycobacterium tuberculosis infection. Scand J Immunol. 2002;56:383–91. doi: 10.1046/j.1365-3083.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 25.Mustafa T, Phyu S, Nilsen R, Bjune GRJ. Increased expression of Fas ligand on Mycobacterium tuberculosis infected macrophages: a potential novel mechanism of immune evasion by Mycobacterium tuberculosis? Inflammation. 1999;23:507–21. doi: 10.1023/a:1020286305950. [DOI] [PubMed] [Google Scholar]
- 26.Mustafa T, Bjune TG, Jonsson R, Pando RH, Nilsen R. Increased expression of fas ligand in human tuberculosis and leprosy lesions: a potential novel mechanism of immune evasion in mycobacterial infection. Scand J Immunol. 2001;54:630–9. doi: 10.1046/j.1365-3083.2001.01020.x. [DOI] [PubMed] [Google Scholar]
- 27.Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol. 2003;170:430–7. doi: 10.4049/jimmunol.170.1.430. [DOI] [PubMed] [Google Scholar]
- 28.Bingisser R, Stey C, Weller M, Groscurth P, Russi E, Frei K. Apoptosis in human alveolar macrophages is induced by endotoxin and is modulated by cytokines. Am J Respir Cell Mol Biol. 1996;15:64–70. doi: 10.1165/ajrcmb.15.1.8679223. [DOI] [PubMed] [Google Scholar]
- 29.Renis M, Calabrese V, Russo A, Calderone A, Barcellona ML, Rizza V. Nuclear DNA strand breaks during ethanol-induced oxidative stress in rat brain. FEBS Lett. 1996;390:153–6. doi: 10.1016/0014-5793(96)00647-3. [DOI] [PubMed] [Google Scholar]
- 30.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 31.Sarih M, Souvannavong V, Adam A. Nitric oxide synthase induces macrophage death by apoptosis. Biochem Biophys Res Commun. 1993;191:503–8. doi: 10.1006/bbrc.1993.1246. [DOI] [PubMed] [Google Scholar]
- 32.Messmer UK, Reed UK, Brune B. Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J Biol Chem. 1996;271:20192–7. doi: 10.1074/jbc.271.33.20192. [DOI] [PubMed] [Google Scholar]
- 33.Nicotera P, Bonfoco E, Brune B. Mechanisms for nitric oxide-induced cell death: involvement of apoptosis. Adv Neuroimmunol. 1995;5:411–20. doi: 10.1016/0960-5428(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 34.Cohen SB, Crawley JB, Kahan MC, Feldmann M, Foxwell BM. Interleukin-10 rescues T cells from apoptotic cell death. association with an upregulation of Bcl-2. Immunology. 1997;92:1–5. doi: 10.1046/j.1365-2567.1997.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–8. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mfinanga SG, Morkve O, Kazwala RR, Cleaveland S, Sharp JM, Shirima G, Nilsen R. Tribal differences in perception of tuberculosis: a possible role in tuberculosis control in Arusha, Tanzania. Int J Tuberc Lung Dis. 2003;7:933–41. [PubMed] [Google Scholar]
- 37.Mfinanga SG, Morkve O, Kazwala RR, Cleaveland S, Sharp JM, Shirima G, Nilsen R. The role of livestock keeping in tuberculosis trends in Arusha, Tanzania. Int J Tuberc Lung Dis. 2003;7:695–704. [PubMed] [Google Scholar]
- 38.Byrd TF. Multinucleated giant cell formation induced by IFN-gamma/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocyte monolayers. Cell Immunol. 1998;188:89–96. doi: 10.1006/cimm.1998.1352. [DOI] [PubMed] [Google Scholar]
- 39.Hagimoto N, Kuwano K, Inoshima I, Yoshimi M, Nakamura N, Fujita M, Maeyama T, Hara N. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol. 2002;168:6470–8. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 40.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 41.Mustafa T, Phyu S, Nilsen R, Jonsson R, Bjune G. A mouse model for slowly progressive primary tuberculosis. Scand J Immunol. 1999;50:127–36. doi: 10.1046/j.1365-3083.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- 42.Mustafa T, Phyu S, Nilsen R, Jonsson R, Bjune G. In situ expression of cytokines and cellular phenotypes in the lungs of mice with slowly progressive primary tuberculosis. Scand J Immunol. 2000;51:548–56. doi: 10.1046/j.1365-3083.2000.00721.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25:1375–82. [PubMed] [Google Scholar]
- 44.Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51:306–11. doi: 10.1136/thx.51.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price NM, Gilman RH, Uddin J, Recavarren S, Friedland JS. Unopposed matrix metalloproteinase-9 expression in human tuberculous granuloma and the role of TNF-alpha-dependent monocyte networks. J Immunol. 2003;171:5579–86. doi: 10.4049/jimmunol.171.10.5579. [DOI] [PubMed] [Google Scholar]
- 46.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 48.Kim R, Emi M, Tanabe K, Uchida Y, Toge T. The role of fas ligand and transforming growth factor beta in tumor progression – molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy. Cancer. 2004;100:2281–91. doi: 10.1002/cncr.20270. [DOI] [PubMed] [Google Scholar]
- 49.Oyaizu N, Mccloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules up-regulates Fas antigen expression in lymphocytes by inducing interferon-gamma and tumor-necrosis-factor-alpha secretion. Blood. 1994;84:2622–31. [PubMed] [Google Scholar]
- 50.Mustafa T, Mogga SJ, Mfinanga SGM, Mørkve O, Sviland L. Significance of Fas and Fas ligand in tuberculous lymphadenitis. Immunology. 2005;114:255–62. doi: 10.1111/j.1365-2567.2004.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fayyazi A, Eichmeyer B, Soruri A, Schweyer S, Herms J, Schwarz P, Radzun HJ. Apoptosis of macrophages and T cells in tuberculosis associated caseous necrosis. J Pathol. 2000;191:417–25. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH664>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 52.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–53. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 53.Manfredi AA, Heltai S, Rovere P, et al. Mycobacterium tuberculosis exploits the CD95/CD95 ligand system of gammadelta T cells to cause apoptosis. Eur J Immunol. 1998;28:1798–806. doi: 10.1002/(SICI)1521-4141(199806)28:06<1798::AID-IMMU1798>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 54.Genestier L, Kasibhatla S, Brunner K, Green DR. Transforming growth factor beta 1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–9. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Connell J, Bennett MW, Gc O, Collins JK, Shanahan F. Resistance to Fas (APO-1/CD95)-mediated apoptosis and expression of Fas ligand in esophageal cancer: the Fas counterattack. Dis Esophagus. 1999;12:83–9. doi: 10.1046/j.1442-2050.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- 56.Ungefroren H, Voss M, Bernstorff WV, Schmid A, Kremer B, Kalthoff H. Immunological escape mechanisms in pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:243–51. doi: 10.1111/j.1749-6632.1999.tb09529.x. [DOI] [PubMed] [Google Scholar]