Abstract
B cells bifurcating along ‘type 1’ or ‘type 2’ pathways under the influence of polarizing cytokines can, in turn, influence the direction of an immune response. Here, we compare the capacity of human B cells residing within naïve and memory compartments to participate in type 1 polarizing responses. B-cell receptor (BCR) engagement provided the main signal for interleukin (IL)-12Rβ1 expression in the two subsets: this was potentiated by CD154 together with interferon-γ (IFN-γ) but inhibited by IL-12. IL-12Rβ2 could be induced on a minority of B cells by the same signals, and also by IFN-γ alone. WSX-1, a receptor for IL-27, was expressed in both subsets with no evidence for its regulation by the signals studied. While neither subset was capable of secreting much IL-12 p70, memory B cells could produce a small amount of IL-12 p40 on CD40 ligation. Memory B cells also, exclusively, expressed IL-23 p19 mRNA on BCR triggering. Importantly, products of appropriately stimulated memory – but not naive – B cells were shown to promote the synthesis of IFN-γ in uncommitted T-helper cells. The data indicate an equal capacity for naïve and memory B cells to respond within a type 1 polarizing environment. Although poorly equipped for initiating type 1 responses, B cells – by virtue of the memory subset – reveal a capacity for their maintenance and amplification following T-dependent signalling.
Keywords: IL-12, IL-23, IL-27, B cells, immune polarization
Introduction
Professional antigen-presenting cells (APC), i.e. dendritic cells (DC) – play a critical role in initiating T-cell mediated immune responses.1 B cells can also serve as APC but are much less efficient than DC unless they meet already activated T cells.2 It is now appreciated that, analogous to T helper cells, B cells can be polarized to differentiate along ‘type 1’ or ‘type 2’ pathways and correspondingly – in their role as APC – impact upon the direction of an immune response.3 How commitment to and deviation to these pathways are regulated, particularly for human naïve and memory B cell subsets confronted with T-cell dependent signals, remains unclear. Some studies suggest a role for B cells in the induction of interleukin (IL)-4 gene expression by T lymphocytes.4,5 While B-cell deficient mice show normal priming of T cells on DC during the early stages of an immune response6 this may change as T cells further differentiate within follicles, as suggested by Moulin et al.7
Cross-talk between B cells and DC appears to be bidirectional with B cells regulating DC function possibly early in a response8 and DC and their products impacting, in turn, upon B-cell differentiation.9,10 The major initiating type 1 polarizing cytokine produced by DC upon interaction with T cells is IL-12.11 Whether B cells qualify as competent T helper (Th)1-promoting APC by producing IL-12 has been tested in a variety of models both in vivo and in vitro with a consensus indicating at least the potential for such a role.3,10,12–14
The capacity of a B cell to respond within an IL-12-rich polarizing environment appears to depend on the cell's activation status. IL-12 is considered to target a population via the heterodimeric IL-12R complex of IL-12Rβ1 and IL-12Rβ2,15 with regulation of the latter likely representing one of the major mechanisms through which IL-12 responsiveness is controlled. Human tonsillar B cells express transcripts for both receptor subunits as do freshly isolated human peripheral blood B cells; however, detailed analysis of surface expression of receptor subunits was performed for IL-12Rβ1 subunit only.16,17
Our own studies have demonstrated the importance of CD40 signals to human resting B cells developing IL-12-responsiveness.10 Airoldi et al. were the first to report IL-12Rβ2 expression in human B cells with Staphylococcus aureus Cowan (SAC) I strain, or rIL-12, selectively increasing transcripts for β2 chain: IL-4, interferon-γ (IFN-γ), anti-immunoglobulin light chain monoclonal antibodies (mAb), anti-CD40 alone or in combination with IL-4, were all ineffective at modulating the expression of either the IL-12R chain genes.16 Functional studies showed that IL-12 signals to B cells through the nuclear factor (NF)-κB pathway in naïve cells only, indicating possible post-transcriptional modifications of IL-12R subunits to explain this apparent discrepancy. In the case of possible tyrosine phosphorylation of signal transducer and activator of transcription (STAT)4, contradictory results have been reported.16,17
In the present study, we have generated highly enriched populations of naïve and memory B cells from human tonsils and followed their behaviour when confronted with signals that would be encountered during a T-dependent antigen response: primarily those delivered through the BCR and CD40. We investigated, for each compartment, both basal and regulated expression of not only the IL-12 receptor components but also the IL-27 receptor. IL-27 is a heterodimeric cytokine that consists of EBI3, an IL-12 p40-related protein, and p28, an IL-12 p35-related polypeptide. Similar to IL-12, IL-27 is involved in type 1 polarization of T cells but its actions seem to target exclusively naive and not memory T cells.18 In addition to establishing their potential to respond within a type 1 polarizing cytokine milieu, we assessed the capacity of the naive and memory compartments to produce the cytokines themselves and to impact type 1 polarization directly. The data generated strongly indicate an inability of B cells to act as frontline APC for initiating Th1 responses: they do, however, display the machinery necessary for participating in the maintenance and amplification of type 1 skewing of the response following secondary encounters with antigen.
Materials and methods
Reagents
The mAbs OKT3 (anti-CD3, immunoglobulin G1 (IgG1)), 61D3 (anti-CD14, IgG1), and OKT10 (anti-CD38, IgG1) were produced from hybridomas in the Medical Research Council Centre for Immune Regulation, University of Birmingham, UK, and purified by ion-exchange chromatography on DE52 (Whatman Ltd, Maidstone, UK). For phenotypic analysis on the flow cytometer we used fluorescein isothiocyanate (FITC)-conjugated mAbs to IgD, IgA, IgG, and CD23 (all from Dako Ltd, High Wycombe UK), CD11c, CD27, CD20 and CD19 (BD PharMingen, San Diego, CA), phycoerythrin (PE)-conjugated mAbs to CD14 and CD5 (Dako), CD27, IgD, IgM, CD3, IL-12Rβ1, and IL-12Rβ2 (BD PharMingen), and CyChrome-conjugated CD38 mAb (BD PharMingen). Human recombinant IL-12, recombinant IFN-γ and neutralizing antibodies to IL-12 and IFN-γ were purchased from R & D Systems Ltd. (Oxford, UK). IFN-α (Intron A) was obtained from Schering Plough (Brinny, Ireland).
Isolation of naïve and memory B cells from human tonsils
Total human B cells (>95% CD19+) were isolated from human tonsils and subsequently separated in naïve and memory B cells by negative depletion on a magnetic cell separator (MidiMACS, Miltenyi Biotec GmbH, Bergish Gladbach, Germany) as previously described.10,19 Briefly, total resting B cells were incubated with a cocktail of antibodies specific to CD3 (OKT3), CD14 (61D3) and CD38 (OKT10), followed by goat anti-mouse IgG Microbeads® (Miltenyi Biotec).
Magnetically unlabelled resting B cells were collected and their purity was assessed by immunofluorescence labelling with mAb to CD3, CD19 or CD20, CD27, CD38, CD11c, IgA, IgG, IgD and IgM on a flow cytometer (FACScan or FACSCalibur, Becton Dickinson, San Jose, CA). In preparations used in this study less than 2% of T cells, CD14+, CD11c+ and CD38+ cells remained. Resting B cells were further divided for isolation of memory and naive B cells, based on the percentage of CD27+, and IgD+ IgM+ cells, respectively.19 For the isolation of memory B cells, resting B cells were first incubated with anti-IgD (Sigma, Poole, UK) and then with goat anti-mouse IgG Microbeads® (Miltenyi Biotec). For the isolation of naive B cells, we first depleted CD27- and IgA-expressing B cells (both mAbs were from BD PharMingen). IgG-expressing B cells were then removed with mouse anti-human IgG Microbeads® (Miltenyi Biotec). The purity of isolated naive and memory B cells was assessed by immunofluorescence labelling with CD27, CD38, IgA, IgD, IgG and IgM. In some experiments additional direct phenotypic analysis with fluorochrome-coupled mAbs specific for CD23 and CD5 was performed on freshly isolated cells.
Isolation of T cells
Untouched CD3+ CD4+ T cells were prepared from peripheral blood mononuclear cells (PBMC) of healthy blood donors using the CD4+ T Cell Isolation kit from Miltenyi Biotec. In a first step, non-T cells were depleted from PBMC of adult healthy blood donors using hapten-coupled antibodies to CD8, CD11b, CD16, CD19, CD36, and CD56, and antihapten magnetic beads. Preparations were always >97% CD3+ CD4+ as assessed by immunophenotypic analysis.
Culture of naive and memory B cells
Naive and memory B cells were cultured in flat-bottom 24-well microtitre plates at a concentration of 106 cells/ml in RPMI-1640 with 10% fetal calf serum (FCS; Dipro, Wiener Neudorf, Austria) and 50 µg/l gentamicin (complete medium, CM), in a total volume of 1 ml, in a humidified incubator at 37° and 5% CO2. Where indicated, B cells were cocultured with mouse L cells transfected with human CD32 (CD32-L cells), cotransfected with CD32 and CD40L (CD32/CD40L-L cells) or non-transfected mouse L cells. Adherent mouse L cells were cultured in CM and were recovered with 0·002% disodium ethylenediaminetetra-acetic acid (EDTA), resuspended in CM and γ-irradiated (70 Gy) before addition to B cells at the ratio 1 : 10, L cells : B cells. To stimulate B cells via their BCR 0·5 µg/ml of anti-κ and anti-λ mAb were added to the wells with CD32-L cells or CD32/CD40L-L cells, where indicated. IL-12 (10 ng/ml), IFN-γ (1000 U/ml) or IFN-α (5000 U/ml) were added at the beginning of the incubation, where indicated.
Flow cytometric analysis
The cells were recovered at different time points of culture by brief incubation with 0·02% disodium EDTA to disperse aggregates and resuspended in PBS with 5% FCS and 0·1% sodium azide (Sigma Chemical Co, St. Louis, MO). In some experiments supernatants were first collected and stored at −20° for subsequent enzyme-linked immunosorbent assay (ELISA). Cell suspensions were stained using standard direct two- or three-colour immunofluorescence staining methods as already described.10 Briefly, after harvesting, cells were washed in phosphate-buffered saline (PBS) supplemented with 5% fetal calf serum and 0·1% sodium azide and then incubated using at least 2 × 105 cells per sample with previously determined optimal concentrations of mAbs conjugated to different fluorochromes (FITC, PE and CyChrome) for 15 min in the dark at room temperature. Cells were then washed and subsequently resuspended in 0·5% formaldehyde (Sigma) in PBS containing 5% fetal calf serum and 0·1% sodium azide and analysed within 24 hr of staining on a flow cytometer.
Measures of IL-12 production in B cells
ELISA. Released IL-12 p70 and IL-12 p40 were measured in duplicate supernatants of cultured cells as indicated in the results using Human IL-12 Quantikine HS ELISA Kit® and Quantikine Human IL-12 p40 Kit®, respectively (R & D Systems) following the manufacturer's instructions. The detection limit of the IL-12 p70 kit was 0·5 pg/ml and of IL-12 p40 was 15 pg/ml.
Intracellular staining of IL-12. Intracellular IL-12 was detected by flow cytometry using a PE-labelled anti-IL-12 mAb that reacts with p40 monomer and p70 heterodimer, but not with p35 monomer (clone C11.5; PharMingen). B cells or plastic adherent fraction of human peripheral blood mononuclear cells were cultured as described previously.10 An inhibitor of protein secretion, monensin (2 µm; Sigma), was added during the last 6–8 hr of culture. Harvested cells were washed with PBS and then processed for surface and intracellular staining as previously described.10 Briefly, B cells or monocytes were first stained with FITC-conjugated anti-CD19 or anti-CD14 antibody, respectively, for 15 min in the dark at room temperature, washed and fixed for 20 min at 4° with 4% paraformaldehyde in PBS. Cells were then permeabilized with 0·1% saponin in PBS for 10 min prior to addition of PE-conjugated anti-IL-12 antibody or isotypic control (0·5 µg/106 cells; PharMingen) and then incubated for a further 30 min at 4°. In experiments when cells were stained for intracellular IFN-γ or IL-4, PE- or allophycocyanin-conjugated anti-IFN-γ or PE-conjugated IL-4 were added (both from BD PharMingen). Cells were then washed in PBS containing 0·1% saponin to wash out unbound antibody, resuspended in PBS, and analysed immediately on a flow cytometer. To ascertain the specificity of IFN-γ staining, unlabelled anti-IFN-γ (BD PharMingen) was added first for 30 min. Cells were washed again and incubated with fluorochrome-labelled anti-IFN-γ antibody.
Real-time reverse transcription–polymerase chain reaction (RT–PCR)
To quantitate RNA levels total RNA was isolated from cell cultures using RNAzol B (Biogenesis, Poole, UK) and semiquantitative real time PCR was done as described.6 Beta-actin specific primers were as in Brown et al.20 Primers for genes to quantified were IL-12 p35 (probe 5′-AGGCCAGACAAACTCTAGAATTTTACCCTTGCA, primers: forward AGGGCCGTCAGCAACATG, reverse ATGGTAAACAGGCCTCCACTGT), IL-12 p40 (probe 5′-ACCCAGCAGCTTCTTCATCAGGGACATCA, primers: forward GCCGTTCACAAGCTCAAGTATG, reverse TCTTGGGTGGGTCAGGTTTG), IL-23 p19 (probe 5′-TGACCCCCAAGGACTCAGGGACAAC, primers: forward GTGGGACATGGATCTAAGAGAAG, reverse TGGATCCTTTGCAAGCAGAAC), WSX-1/TCCR (probe 5′-TGGAACCGGAGCTGAAGACCATACCC, primers: forward AAGTTCTGATCTGCCAGTTCCACTA, reverse GCTCCAAATCTTGGATCTCAACA). The data are presented as fold increase in comparison to unstimulated B cells. Mouse fibroblasts (l-cells) did not express mRNA for either of the tested transcripts so there was no need for their removal prior to mRNA extraction.
IFN-γ production by CD3+ CD4+ T cells
Human purified CD3+ CD4+ T cells were stimulated with phorbol myristate acetate (PMA) (10 ng/ml; Sigma Chemical Co.) and 5 µl of BD FastImmune CD28/CD49d costimulatory reagent (clones L293 and L25, respectively) at 106 cells/ml in 100 µl total volume/well in 96-well plates in triplicates. B cell-derived supernatants were generated by incubating naïve and memory B cells for 3 days with mouse L cells cotransfected with CD32 and CD40L (CD32/CD40L-L cells) or non-transfected mouse L cells. B cell-derived supernatant was added to the T cell culture at a volume of 1 : 1. After 3 days, supernatants from the T cell cultures were analysed for IFN-γ by Human IFN-γ Quantikine ELISA Kit® (R & D Systems; detection limit of the kit was 15·6 pg/ml). Where indicated, neutralizing antibodies for IL-12 (20 µg/ml; R & D Systems) were added from the beginning of the culture.
Statistics
Data were analysed by anova using STATISTICA v. 6 (StatSoft, Inc., Tulsa, OK). P < 0·05 was considered significant.
Results
Basal and regulated expression of IL-12R components and IL-27R on naïve and memory B-cell subsets via BCR and CD40
Previous analysis of freshly isolated normal human tonsil B-cell subsets focused on gene expression and showed the presence of transcripts for both the IL-12 receptor subunits.16,17,21 Because IL-12 is biologically active on resting B cells once they have been stimulated through BCR and/or CD4010 – critical early signals in a T-dependent antigen response – we asked how these signals act to influence the surface expression of IL-12Rβ1 and β2 on non-activated B cells: the total population as well as those negatively sorted into naïve and memory compartments. B cells were cultured up to 120 hr either on mouse fibroblast L cells alone (‘control’), with anti-κ and anti-λ antibodies held on CD32-transfected L cells (‘BCR signal’), or on L cells dually transfected with CD32 and CD40L (CD154) either without or with the anti-light chain antibodies (‘CD40 signal’ and ‘BCR/CD40 signal’, respectively). Similar to T cells and natural killer (NK) cells, we found that resting B cells failed to express constitutively either of the receptor subunits for IL-12 whether analysing the total population or enriched naïve and memory compartments (Fig. 1 and data not shown). BCR cross-linking was a major signal for the up-regulation of IL-12Rβ1 chain on resting B cells with a plateau being reached at 72 hr of culture (Fig. 1a). CD40-engagement induced only small levels of IL-12Rβ1 chain on resting B cells and did not substantially alter the BCR-dependent induction of this receptor subunit. Figure 1(b) shows that, although absent on each of the subsets basally, IL-12Rβ1 chain was induced in both the naïve and memory compartments on BCR-signalling to levels similar to those observed on otherwise unfractionated resting B cells.
Figure 1.
BCR-plus CD40-ligation triggers IL-12Rβ1 expression. B cells isolated from tonsils were cultured in the presence of non-transfected L cells (Control), CD32-transfected L cells with anti-κ and anti-λ antibodies (BCR), and dual CD32/CD40L-transfected L cells without (CD40) and with anti-κ and anti-λ antibodies (BCR + CD40). Expression of IL-12Rβ1 was measured on resting B cells after 1, 3 and 5 days (a) and on naïve and memory B cells (b) after 3 days of culture by flow cytometry. Data, expressed as the percentage positive cells, are the mean ± SD for three separate experiments. * Indicates significant increase in IL-12Rβ1 expression in comparison to unstimulated cells (P < 0·05).
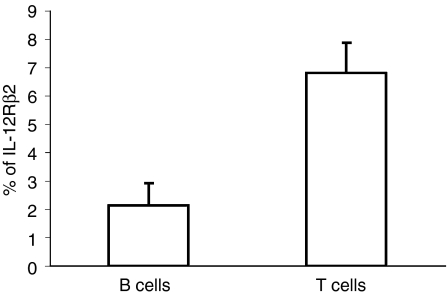
IL12Rβ2 is induced only with a combination of signals through both the BCR and CD40 (see later). Resting B cells were stimulated with BCR plus CD40 and analysed for IL-12Rβ2 expression. After 72 hr of treatment, only up to 3% of resting B cells expressed IL-12Rβ2 (Fig. 2); this was lost by day 5 of treatment (data not shown). In comparison, 8% of peripheral blood CD3+ T cells express IL-12Rβ2 after stimulation with phytohaemagglutinin (PHA; Fig. 2). In naïve and memory B cells only a minor fraction were found to express IL-12Rβ2 chain following coengagement of BCR and CD40 with no significant difference between the two B-cell subsets seen in this regard (data not shown).
Figure 2.
Effect of BCR- and CD40-triggering on IL-12Rβ2 expression on resting B cells. Resting tonsillar B cells were jointly stimulated through BCR and CD40 for 3 days and then analysed for IL-12Rβ2 chain expression by flow cytometry. IL-12Rβ2 expression on peripheral blood CD3+ T cells was determined following 3 day culture with PHA (10 µg/ml). Data, expressed as the percentage positive cells, are the mean ± SD for three separate experiments.
IL-12 drives distinct phenotypic change (e.g. down-regulation of CD23 and up-regulation of CD38) in a subset of unfractionated resting B cells triggered through BCR and CD40: we have shown this to be a consequence of IL-12-induced, endogenous IFN-γ production.10 We used this property as a marker to assess functional IL-12R expression within the naïve and memory compartments. Figure 3 shows that following BCR/CD40 costimulation, a similarly small proportion of naïve and memory cells produce IFN-γ and no IL-4 in response to IL-12. This staining was inhibited by unlabelled anti-IFN-γ antibody added to the cells prior to PE-conjugated IFN-γ monoclonal antibody (data not shown).
Figure 3.
Similar IL-12-dependent IFN-γ production from naïve and memory B cells. Naïve and memory B cells were cultured for 3 days in the presence of dual CD32/CD40L-transfected L cells and with anti-κ and anti-λ antibodies in the presence of IL-12 (10 ng/ml). Monensin was added during the last 6–8 hr of culture. Cells were harvested and stained for surface CD19, intracellular IFN-γ and IL-4. Histograms show staining for IFN-γ (x-axis) and IL-4 (y-axis) in naïve (a) and memory (b) B cells gated upon the expression of CD19. The quadrants were drawn on the basis of control cells stained with isotypic antibody of irrelevant specificity. The percentage of positive cells is indicated. Results are representative of three independent experiments.
The recently described type 1 cytokine IL-27 is an early product of activated APC and strongly synergizes with IL-12 to trigger IFN-γ production in naive but not memory CD4+ T cells.18 We analysed the expression of its receptor WSX-1/TCCR by RT-PCR to ask whether naïve and memory B cells might similarly differ in their potential to respond to IL-27. No significant difference in mRNA levels for WSX-1/TCCR was found between the naive and memory B cell compartments before stimulation. Moreover, there was no significant change of WSX-1/TCCR expression in either of the two B-cell subsets following any of the stimuli investigated (i.e. BCR-cross-linking with or without CD40 triggering, Fig. 4), or in the absence or presence of IFN-γ (data not shown). These findings corroborate with findings of Yoshimoto et al. who recently showed that primary murine spleen B cells expressed WSX-1 mRNA even without the stimulation by anti-CD40 and was not greatly increased by the stimulation.22
Figure 4.
Comparison of WSX-1/TCCR mRNA expression in naïve and memory B cells in response to BCR and/or CD40 engagement. Naive (empty bars) and memory (filled bars) B cells were cultured in the presence of non-transfected L cells (Control), CD32-transfected L cells with anti-κ and anti-λ antibodies (BCR), and dual CD32/CD40L-transfected L cells without (CD40) and with anti-κ and anti-λ antibodies (BCR + CD40) for 24 hr. Expression of WSX-1/TCCR was measured by real-time PCR. Data, expressed as the fold change in comparison to unstimulated naïve and memory B cells are the mean ± SD for three separate experiments.
Cytokine regulation of IL12R subunit expression in naïve and memory B-cell compartments
We next asked whether the expression of the IL-12R subunits within the naïve and memory compartments might be affected by cytokines implicated in their regulation on either unfractionated B cells or other cell types. For example, expression of IL-12Rβ2 appears to be differentially regulated by type I and II interferons in human and mouse cells, respectively.23 IFN-α, as well as IFN-γ, is able to induce IL-12Rβ2 chain in mitogen-stimulated human T cells.24 Unfractionated resting B cells activated either by SAC, IFN-γ or IL-12 show a strong up-regulation of IL-12Rβ2 gene transcripts.16,17 Because most of the biological responses to IL-12 are mediated via induction of IFN-γ synthesis, we first determined whether IFN-γ might encourage further expression of IL-12Rβ1 and β2 subunits. A small percentage of both naïve and memory B cells were induced to express IL-12Rβ1 chain in response to IFN-γ alone (Fig. 5a). However, the effect of exogenous IFN-γ was most striking upon triggering through BCR either alone or together with CD40 on both naïve and memory cells such that up to 30% of cells became positive. IFN-γ induced more cells to express the IL-12Rβ2 subunit irrespective of the stimulation received although the number of positive cells remained small (Fig. 5b). IFN-α had no effect on IL-12R expression on human B cells (data not shown), which is similar to mitogen-activated human NK cells.25
Figure 5.
Effect of IFN-γ on IL-12Rβ1 (a) and IL-12Rβ2 (b) expression on naïve and memory B cells. Naive and memory B cells were cultured in the presence of non-transfected L cells (Control), CD32-transfected L cells with anti-κ and anti-λ antibodies (BCR), and dual CD32/CD40L-transfected L cells without (CD40) and with anti-κ and anti-λ antibodies (BCR + CD40) alone (empty bars) or in the presence of IFN-γ (1000 U/ml; filled bars) for 3 days. Expression of IL-12Rβ1 (a) and IL-12Rβ2 (b) was determined by flow cytometry. Data, expressed as percentages are the mean ± SD for three separate experiments. * Indicates significant effect of IFN-γ on the expression of IL-12Rβ1 and IL-12Rβ2 in comparison to the cultures without IFN-γ (P < 0·05).
It has been shown that IL-12 up-regulates the expression of IL-12Rβ1 and IL-12Rβ2 mRNA in mouse B cells and IL-12Rβ2 mRNA in human B cells.16,17,26 We tested whether IL-12 differentially modulates the expression of the two IL-12R subunits at the surface of naïve and memory cells. Figure 6 shows that IL-12 did not induce significant up-regulation of the IL-12Rβ1 subunit on either non-activated or BCR-stimulated naïve or memory cells. However, the effect of simultaneous engagement of BCR and CD40 on IL-12Rβ1 induction was significantly reduced in the presence of IL-12 (Fig. 6). This effect is not simply a consequence of IL-12-provoked IFN-γ production: addition of exogenous IFN-γ actually induces IL-12Rβ1 expression on BCR-stimulated as well as on BCR/CD40-stimulated naïve and memory cells (Fig. 5). IL-12 had no effect on either basal or induced levels of IL-12Rβ2 (data not detailed).
Figure 6.
Effect of IL-12 on IL-12Rβ1 expression on naïve and memory B cells. Naive and memory B cells were cultured in the presence of non-transfected L cells (Control), CD32-transfected L cells with anti-κ and anti-λ antibodies (BCR), and dual CD32/CD40L-transfected L cells without (CD40) and with anti-κ and anti-λ antibodies (BCR ± CD40) alone (empty bars) or in the presence of IL-12 (10 ng/ml; filled bars) for 3 days. Expression of IL-12Rβ1 was determined by flow cytometry. Data, expressed as percentages, are the mean ± SD for three separate experiments. * Indicates the IL-12-mediated down-regulation of IL-12Rβ1 following coligation of CD40 and BCR in comparison to the cultures without IL-12 (P ≤ 0·05).
Capacity for IL-12 and IL-23 production within naïve and memory B-cell compartments
IL-12-dependent IL-12Rβ1 chain down-regulation is seen only in B cells stimulated through their BCR and CD40. In vivo this would likely take place as B cells encounter antigen-specific T cells primed by DC. While DC are clearly established as the major source of IL-12 during immune responses, production of IL-12 by human B cells has been reported for a CD40-activated ‘non-germinal centre’ tonsillar fraction.13 IL-12 p40 functions in several forms: as a heterodimer with p35 in IL-12 p70, as p40 homodimers that (like IL-12 p70) bind to IL-12Rβ1, and as heterodimers with p19 polypeptide to generate the cytokine IL-23.27 Therefore, an evaluation of the physiological role for B cells in type 1 responses needs to consider their ability to produce the different forms of p40-containing cytokines.28 We attempted to establish whether the source of IL-12 might be contained within the naïve and/or memory B-cell compartment. Staining of freshly isolated tonsil B-cell subsets with CD14 and CD11c mAbs did not detect cells such as macrophages or DC capable of expressing IL-12 (data not shown).
Quantitative PCR showed that mRNA levels for IL-12 p35 failed to increase and, indeed, could even decrease in either the naïve or memory compartment in response to activation through BCR and/or CD40 (Fig. 7a). Though absolute levels of expression among different tonsil samples varied widely, CD40-activated memory B cells revealed a consistent up-regulation of IL-12 p40 mRNA reaching up to eight times that found in unstimulated counterparts. Interestingly, coligating BCR with CD40 on memory B cells did not produce a significant up-regulation of IL-12 p40 mRNA, while the CD40 stimulus alone did although substantial variations among experiments were observed (P < 0·05). This effect on memory B cells appears quite different to that of CD40 ligation on either dendritic cells or monocytes where T-independent signals, such as lipopolysaccharide stimulation, up-regulates IL-12 p40, whereas p70 production (for which p35 is considered the limiting factor) is T-dependent.27 In parallel experiments levels of mRNA expression for IL-12 p35 and IL-12 p40 were determined in PBMC stimulated with CD40L and rIFN-γ. Interestingly, these stimulated monocytes had a 10-fold increase in mRNA p40 levels compared to stimulated memory B cells while no significant modulation of p35 mRNA was observed in either of the cell types tested (data not shown).
Figure 7.
Comparison of the capacity of naïve and memory B cells for IL-12 expression. Naive (empty bars) and memory (filled bars) B cells were cultured in the presence of non-transfected L cells (Control), CD32-transfected L cells with anti-κ and anti-λ antibodies (BCR), and dual CD32/CD40L-transfected L cells without (CD40) and with anti-κ and anti-λ antibodies (BCR + CD40). (a) Expression of IL-12 p35 and IL-12 p40 mRNA was measured after 24 hr of culture by real-time PCR. Data, expressed as the fold change in comparison to unstimulated cells are the mean ± SD for three separate experiments. (b) IL-12 p40 and IL-12 p70 were determined by ELISA in supernatants obtained following the culture of naïve and memory B cells in the presence of non-transfected L cells (Control), CD32-transfected L cells with anti-κ and anti-λ antibodies (BCR), and dual CD32/CD40L-transfected L cells without (CD40) and with anti-κ and anti-λ antibodies (BCR + CD40) for 3 days. Data, expressed as percentages are the mean ± SD for three separate experiments. * Indicates significantly higher expression of IL-12 p40 in CD40 alone and BCR and CD40 stimulated memory B cells (P < 0·05). (c) Intracellular cytokine staining for IL-12 in naïve, memory and PBMC following the culture of cells in the presence of dual CD32/CD40L-transfected L cells and rIFN-γ (1000 U/ml) for 2 days. A representative experiment out of 4 is shown.
We next analysed levels of secreted p40 and p70 by ELISA. CD40-engagement (with or without BCR stimulation) increased the amount of IL-12 p40 released, particularly in memory B cells. It is secreted in 10–15-fold excess over free p70 (Fig. 7b). This is similar to DC and monocytes where p35 and p40 production are also found in non-stochiometric amounts. Although there is no increase of p40 mRNA in memory B cells after 8 hr (data not shown) and 24 hr stimulation with BCR and CD40, strong secretion of p40 protein was found by ELISA after 3 days. This difference may represent different kinetics of p40 mRNA expression or different regulation of p40 biosynthesis at the post-transcriptional level.
In order to establish rigorously whether the IL-12 detected by ELISA was the product of B cells rather than that of a minor non-B-cell contaminant flow cytometric analysis was determined the content of IL-12 in CD19+ cells specifically (Fig. 7c). The monoclonal antibody used to detect intracellular IL-12 binds to both IL-12 p40 and IL-12 p70 and therefore does not distinguish between the two molecules. We found that engaging BCR and CD40 (or CD40 alone), even in the presence of rIFN-γ, induces only a very small number (always <1%) of CD19+ cells in either the naïve or memory subset to express intracellular IL-12. At the same time, CD40 cross-linking could be seen to induce intracellular IL-12 in up to 10% of monocytes from peripheral blood indicating that the method and sensitivity of detection was reliable and sufficient.
Exogenous IL-12 inhibits the induction of CD23 expression on resting B cells.10 Here, we used this observation in a surrogate bioassay for endogenous IL-12 activity. Neutralizing antibody to IL-12 and/or IFN-γ (both 10 µg/l) was added at the beginning of culture to CD40-activated naïve or memory B cells and the resultant CD23 expression measured. As previously established, the two compartments differ in their basal expression of CD23 with it being either low or completely absent from memory B cells.19 CD40 signals up-regulate CD23 expression on both memory and naïve cells although the level of expression on activated memory B cells remained lower than on naïve equivalents (Table 1). Neutralizing anti-IL-12 produced a significant increase in CD23 expression after CD40 stimulation of memory B cells. Because neutralizing anti-IFN-γ had the same effect, this most likely reflected the blocking of endogenous IL-12-dependent IFN-γ production. In contrast, neutralizing antibodies did not significantly alter the expression of CD23 on CD40-stimulated naïve B cells, indicating the absence of significant, functional IL-12 production by this compartment.
Table 1.
Effect of cytokine neutralizing antibodies on the percentage of CD23 expressing B cells and CD23 MFI (brackets)1
| Control | CD40 | CD40 + anti-IL-12 | CD40 + anti-IFN-γ | CD40 + anti-IL-12 + anti-IFN-γ | |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Naive | 2 (31) | 63 (156) | 63 (142) | 68 (126) | 61 (103) |
| Memory | 2 (32) | 7 (53) | 21 (95) | 15 (76) | 34 (114) |
| Experiment 2 | |||||
| Naive | 1 (48) | 51 (137) | 57 (145) | 54 (130) | 61 (136) |
| Memory | 0 (45) | 14 (60) | 19 (64) | 19 (66) | 20 (76) |
| Experiment 3 | |||||
| Naive | 4 (42) | 73 (260) | 72 (262) | 74 (245) | 72 (215) |
| Memory | 2 (49) | 7 (65) | 15 (84) | 13 (84) | 17 (92) |
Neutralizing antibodies to IL-12 (10 µg/ml) and/or IFN-γ (10 µg/ml) were added at the beginning of culture to CD40-activated naïve or memory B cells and the resultant CD23 expression was measured after 72 hr.
Control, B cells cultured in the presence of non-transfected L cells.
MFI, mean fluorescence intensity.
In addition, we measured the capacity of stimulated naïve and memory B cells to induce secretion of IFN-γ by CD3+ CD4+ T cells. For this purpose supernatants of CD40-activated naïve and memory B cells collected after 3 days of culture were added to PMA and anti-CD28/CD49d stimulated peripheral blood CD3+ CD4+ T cells and the level of IFN-γ was determined after 3 days. Figure 8 shows the increase in IFN-γ in supernatants of activated helper T cells cultured in the presence of supernatants of CD40-activated memory B cells only. Addition of neutralizing anti-IL-12 antibody (20 µg/ml) to cultured T cells reduced IFN-γ secretion by these cells.
Figure 8.
IL-12 derived from CD40-activated memory B cells induce T cells to produce IFN-γ. CD3+ CD4+ T cells were stimulated with PMA (10 ng/ml) and 5 µl of CD28/CD49d costimulatory antibodies with or without supernatants from naïve or memory B cells cultured for 3 days on dual CD32/CD40L-transfected L cells. After 3 days, supernatants were harvested and analysed for IFN- production by ELISA. Where indicated, neutralizing anti-IL-12 mAb (20 µg/ml) was added. Data are the mean ± SD for three separate experiments.
The high level secretion of IL-12 p40 by memory B cells prompted us to analyse whether these cells are capable of producing p19 to form IL-23. IL-23 has been shown to induce proliferation and promote IFN-γ secretion in human memory T cells. Quantitative PCR demonstrates that BCR stimulation is capable of inducing IL-23 p19 mRNA in memory – but not naïve – B cells (P < 0·05, Fig. 9). This induction is, similar to that of IL12Rβ1, independent of CD40 signalling.
Figure 9.
Comparison of IL-23 p19 mRNA expression in naïve and memory B cells in response to BCR and/or CD40 engagement. Naive (empty bars) and memory (filled bars) B cells were cultured in the presence of non-transfected L cells (Control), CD32-transfected L cells with anti-κ and anti-λ antibodies (BCR), and dual CD32/CD40L-transfected L cells without (CD40) and with anti-κ and anti-λ antibodies (BCR + CD40) for 24 hr. Expression of IL-23 p19 mRNA was measured by real-time PCR. Data, expressed as the fold change in comparison to unstimulated cells are the mean ± SD for three separate experiments.
Discussion
A role for B cells as APC for primed T cells is well established. The degree to which, and the manner whereby, they might contribute to the polarization of an immune response is less clear however, particularly with regard to possible differences between those residing within naïve and memory compartments. The present study has focused on the potential of the two B subsets both to express receptors for and to produce type 1 polarizing cytokines with a focus on the initiating type 1 cytokine, IL-12.
There is some evidence to suggest that IL-12 may act directly on B cells. For example, in B-cell deficient mice, IFN-γ production in response to systemic IL-12 administration is diminished relative to wild-type controls.4 Human B cells respond to IL-12 only after activation. IL-12 can potentiate growth of highly purified human B cells after polyclonal activation with SAC.29 In these activated B cells, IL-12 plus IL-2 results in enhanced immunoglobulin secretion.29 In B cells triggered via BCR and CD40, IL-12 can induce IFN-γ secretion.10 Most of the studies regarding IL-12 responsiveness of human B cells lack information as to the surface expression of the IL-12R chains, thus making it difficult to establish whether reported effects are always direct – being mediated through an IL-12R that is functional and capable of signal transduction – or indirect, via paracrine interactions. We have defined the activation signals required and shown, for the first time, that expression of the different IL-12 receptor subunits is regulated similarly within the naïve and memory B-cell compartments. That BCR and CD40 engagement is essential for the induction of a complete IL-12R would explain why earlier studies not incorporating such a signal failed to detect IL-12R using radiolabelled IL-12.30,31
A number of reports have demonstrated that whereas the IL-12Rβ1 subunit is constitutively up-regulated in CD4+ T cells after T-cell stimulation, expression of IL-12Rβ2 is influenced by the presence of key cytokines and accessory molecules at the time of stimulation. In our hands, IFN-γ had a positive regulatory effect on IL-12Rβ2 surface expression on human B cells. Conversely, while IL-12 and type I interferon (IFN-α) induces IL-12Rβ2 expression on T cells25 neither of these cytokines had an effect on the BCR/CD40-mediated up-regulation of IL-12Rβ2 on B cells in our study.
IL-12Rβ1 is critical for IL-12 responsiveness in vitro and in vivo.32 In DC and T cells, IL-12 up-regulates its own receptor: this has been interpreted to imply a potential autocrine pathway of regulation that could lead to an amplification of Th1 responses. We have shown here that for B cells engaged in signalling via BCR and CD40, the presence of IL-12 leads to a reduced expression of surface IL-12Rβ1. A recent study analysing IL-12Rβ1 mRNA failed to detect any significant variation in IL-12Rβ1 expression in human tonsillar B cells activated with either SAC, IL-12, IFN-γ or anti-CD40.16 This may be explained by the fact that levels of IL-12Rβ1 mRNA do not necessarily correspond with IL-12Rβ1 protein expression at the cell surface. Thus, down-regulation of IL-12Rβ1 by IL-12 in human B cells could act as a negative feedback mechanism controlling the size of the IL-12-responsive B-cell pool participating in type 1-polarizing responses.
Exogenous IL-12 induces IFN-γ in B cells activated via their BCR and CD40. IL-12-driven down-regulation of IL-12Rβ1 was, however, deemed to be IFN-γ independent as exogenous IFN-γ actually increased IL-12Rβ1 expression on activated B cells. For human T cells, several negative regulators of IL-12Rβ1 expression have been identified. These include cytokines such as transforming growth factor-β, IL-4, and IL-10; the biochemical mediator prostanglandin E; the hormone dexamethasone; and cholera toxin. However, none of these appear to exert such an effect on B cells.33–36
It has recently been suggested that p40 could combine with subunits other than p35 and p19.37 IL-12Rβ1 is also a part of the receptor termed IL-23R.38 IL-23R mRNA is expressed by human Th1 and Th2 clones, several NK cell lines and clones, as well as at lower levels by Epstein–Barr virus-transformed B cells, a similar expression pattern to that observed for IL-12Rβ2.28 Studies on the expression of IL-23R in normal B-cell subsets are clearly warranted.
More recently, IL-27 was identified as a type 1-driving cytokine that participates in the initiation but not in the maintenance of Th1 responses.20 In naive CD4+ T cells it synergizes with IL-12 for IFN-γ production. Our analysis so far suggests that naïve and memory B subsets do not substantially differ in their quantitative expression of mRNA for its receptor, WSX-1/TCCR: neither did activation via BCR, CD40, and/or IFN-γ appreciably modulate basal levels of WSX-1 expression in either of the B cell compartments. Naïve CD4+ T cells appear to down-regulate WSX-1 expression upon their differentiation.39 Once IL-27R-specific antibodies become available it will be important to ascertain whether the number of transcripts for IL-27R is also reflected at both the protein and functional level.
We have shown that BCR engagement up-regulates not only IL-12Rβ1 surface protein expression but also, and exclusively in memory B cells, mRNA for IL-23 p19. IL-23 has similar effects to IL-12, and among T cells these are manifested predominantly within the memory compartment.40 Taken together, these observations identify IL-23 production by memory B cells as a good candidate for enhancing the polarization of secondary type 1 responses.
One earlier study reported IL-12 synthesis in human ‘non-germinal centre’ tonsillar B cells following their stimulation via CD40.13 More recently however, Duddy et al. were unsuccessful in detecting IL-12 production from BCR and CD40 stimulated peripheral blood B cells.41 We similarly failed to establish IL-12 production as a clear phenotype of resting B cells whether using a highly sensitive ELISA, intracellular cytokine staining, or RT–PCR for IL-12 p35 and IL-12 p40. Analysis of mRNA levels of the two IL-12 subunits, p35 and p40, by quantitative PCR showed that neither BCR nor CD40 signals, alone or together, induced significant p35 expression. The earlier study13 failed to take into account the expression of CD27 when defining memory B-cell subsets. In our hands, memory B cells had the capacity to express increased p40 mRNA, a finding confirmed by ELISA. A bioassay for IL-12 action making use of our previous observation that IL-12 down-regulates CD40-dependent up-regulation of CD2310 revealed that neutralizing IL-12 resulted in increased amounts of CD23 on CD40-stimulation: but only within the memory, and not the naive, B-cell compartment. In addition, we also demonstrated that IL-12 secreted by CD40-activated memory B cells was capable of promoting low level IFN-γ synthesis in activated helper CD3+ CD4+ T cells. This result does indicate, that at least among memory B cells, CD40 signals for the production of biologically active IL-12. Although produced in seemingly small quantity, this may accumulate to more significant amounts over time. While Schultze et al. have shown that cord blood T cells skewed towards a Th1 pathway maintained their cytokine expression pattern when restimulated with allogeneic resting B cells13 this has not been addressed for isolated naïve and memory B cells.
In summary, our results indicate that the limited ability of naïve B cells to produce the IL-12 p70 heterodimer would compromise their capacity as frontline antigen presenting cells for the initiation of Th1 responses. Restriction of responsiveness of naïve and memory B cells to IL-12 via down-regulation of IL-12Rβ1 corroborates previous findings that these cells might regulate the magnitude of type 1 responses as observed in B-cell deprived animals. Additional mechanisms that may work in concert to this end have been described: namely, the delivery of IL-12 by DC during B-cell activation resulting in IL-10 release with a subsequent dampening of further IL-12 secretion.7,42 Notably, our establishing of the memory B-cell population as a source of IL-12 and IL-23, together with an ability to produce IFN-γ, offers this B-cell compartment the capacity both to maintain and amplify a type 1-direction of skewing during the course of an immune response or following subsequent encounter with the antigen. However, it is not clear whether memory B cells that have already undergone a class switch would be employed to extend or amplify a Th1 response. A tendency for isotype stabilization would ensure that a secondary response to the same antigen generates those isotypes which have been instructed by signals during a primary response. However, it is known that sequential switch can occur;43 the question is whether this happens frequently and does so for all isotypes. In human B cells, in particular a sequential switch to IgE has been reported.44,45 We found only a small subset of memory B cells secreting IL-12 and promoting Th1-like T cells which corroborate with recent data suggesting that human memory B cells are heterogeneous. Further investigations are therefore necessary to address these issues in details.
Acknowledgments
This work was supported by The Wellcome Research Development Award (A.G.), grant no. 0021004 from Ministry of science, education and sports of the Republic of Croatia (A.G.) and a Medical Research Council Programme Grant (J.G., K.-M.T., G.G.).
Abbreviations
- APC
antigen-presenting cells
- DC
dendritic cells
- BCR
B-cell receptor
- IL
interleukin
- IFN
interferon
- Th
T helper
- STAT
signal transducer and activator of transcription
- SAC
Staphylococcus aureus Cowan I strain
- mAbs
monoclonal antibodies
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- ELISA
enzyme-linked immunosorbent assay
- PBMC
peripheral blood mononuclear cells
- FCS
fetal calf serum
- CM
complete medium
- PHA
phytohaemagglutinin
- PMA
phorbol myristate acetate
- NK
natural killer
References
- 1.Dubey C, Croft M. Accessory molecule regulation of naive CD4 T cell activation. Immunol Res. 1996;15:114–25. doi: 10.1007/BF02918501. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cell and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–82. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 4.Macaulay AE, DeKruyff RH, Umetsu DT. Antigen-primed T cells from B cell-deficient JHD mice fail to provide B cell help. J Immunol. 1998;160:1694–700. [PubMed] [Google Scholar]
- 5.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183:891–9. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham AF, Fallon PG, Khan M, Vacheron S, Acha-Orbea H, MacLennan IC, McKenzie AN, Toellner KM. Th2 activities induced during virgin T cell priming in the absence of IL-4, IL-13, and B cells. J Immunol. 2002;169:2900–6. doi: 10.4049/jimmunol.169.6.2900. [DOI] [PubMed] [Google Scholar]
- 7.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–82. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kushnir N, Liu L, MacPherson GG. Dendritic cells and resting B cells form clusters in vitro and in vivo: T cell independence, partial LFA-1 dependence, and regulation by cross-linking surface molecules. J Immunol. 1998;160:1774–81. [PubMed] [Google Scholar]
- 9.Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–31. [PubMed] [Google Scholar]
- 10.Gagro A, Gordon J. The interplay between T helper subset cytokines and IL-12 in directing human B lymphocyte differentiation. Eur J Immunol. 1999;29:3369–79. doi: 10.1002/(SICI)1521-4141(199910)29:10<3369::AID-IMMU3369>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri G. Interleukin-12. a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 12.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, Tamura G. B cells capturing antigen conjugated with CpG oligodeoxynucleotides induce Th1 cells by ellaborating IL-12. J Immunol. 2002;169:787–94. doi: 10.4049/jimmunol.169.2.787. [DOI] [PubMed] [Google Scholar]
- 13.Schultze JL, Michalak S, Lowne J, Wong A, Gilleece MH, Gribben JG, Nadler LM. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–12. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner M, Poeck H, Jahrsdoerfer B, et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:954–63. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 15.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu C-Y, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–7. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Airoldi I, Gri G, Marshall JD, Corcione A, Facchetti P, Guglielmino R, Trinchieri G, Pistoia V. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J Immunol. 2001;166:6880–8. doi: 10.4049/jimmunol.165.12.6880. [DOI] [PubMed] [Google Scholar]
- 17.Durali D, de Goer de Herve MG, Giron-Michel J, Azzarone B, Delfraissy JF, Taoufik Y. In human B cells, IL-12 triggers a cascade of molecular events similar to Th1 commitment. Blood. 2003;102:4084–9. doi: 10.1182/blood-2003-02-0518. [DOI] [PubMed] [Google Scholar]
- 18.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 19.Gagro A, Toellner KM, Grafton G, et al. Naive and memory B cells respond differentially to T-dependent signaling but display an equal potential for differentiation toward the centroblast-restricted CD77/globotriaosylceramide phenotype. Eur J Immunol. 2003;33:1889–98. doi: 10.1002/eji.200323357. [DOI] [PubMed] [Google Scholar]
- 20.Brown G, Drayson MT, Durham J, et al. HL60 cells halted in G1 or S phase differentiate normally. Exp Cell Res. 2002;281:28–38. doi: 10.1006/excr.2002.5654. [DOI] [PubMed] [Google Scholar]
- 21.Airoldi I, DiCarlo E, Banelli B, et al. The IL-12Rbeta2 gene functions as a tumor suppressor in human B cell malignancies. J Clin Inves. 2004;113:1651–9. doi: 10.1172/JCI20303. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Yoshimoto T, Okada K, Morishima N, et al. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–85. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 23.Sinigaglia F, D'Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev. 1999;170:65–72. doi: 10.1111/j.1600-065x.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu C-Y, Gadina M, Wang K, O'Shea J, Seder RA. Cytokine regulation of IL-12 receptor β2 expression: differential effects on human T and NK cells. Eur J Immunol. 2000;30:1364–74. doi: 10.1002/(SICI)1521-4141(200005)30:5<1364::AID-IMMU1364>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Thibodeaux DK, Hunter SE, Waldburger KE, et al. Autocrine regulation of IL-12 receptor expression is independent of secondary IFN-gamma secretion and not restricted to T and NK cells. J Immunol. 1999;163:5257–64. [PubMed] [Google Scholar]
- 26.Smeltz RB, Chen J, Ehrhardt R, Shevach EM. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J Immunol. 2002;168:6165–72. doi: 10.4049/jimmunol.168.12.6165. [DOI] [PubMed] [Google Scholar]
- 27.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 28.Abdi K. IL-12. the role of p40 versus p75. Scand J Immunol. 2002;56:1–11. doi: 10.1046/j.1365-3083.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 29.Jelinek DF, Braaten JK. Role of IL-12 in human B lymphocyte proliferation and differentiation. J Immunol. 1995;154:1606–13. [PubMed] [Google Scholar]
- 30.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–32. [PubMed] [Google Scholar]
- 31.Wu CY, Warrier RR, Carvajal DM, et al. Biological function and distribution of human interleukin-12 receptor beta chain. Eur J Immunol. 1996;26:345–50. doi: 10.1002/eji.1830260212. [DOI] [PubMed] [Google Scholar]
- 32.Wu CY, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–65. [PubMed] [Google Scholar]
- 33.Wu CY, Warrier RR, Wang X, Presky DH, Gately MK. Regulation of interleukin-12 receptor beta1 chain expression and interleukin-12 binding by human peripheral blood mononuclear cells. Eur J Immunol. 1997;27:147–54. doi: 10.1002/eji.1830270122. [DOI] [PubMed] [Google Scholar]
- 34.Gollob JA, Kawasaki H, Ritz J. Interferon-gamma and interleukin-4 regulate T cell interleukin-12 responsiveness through the differential modulation of high-affinity interleukin-12 receptor expression. Eur J Immunol. 1997;27:647–52. doi: 10.1002/eji.1830270311. [DOI] [PubMed] [Google Scholar]
- 35.Wu CY, Wang K, McDyer JF, Seder RA. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol. 1998;161:2723–30. [PubMed] [Google Scholar]
- 36.Braun MC, He J, Wu CY, Kelsall BL. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J Exp Med. 1999;189:541–52. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehmann J, Bellmann S, Werner C, Schroder R, Schutze N, Alber G. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J Immunol. 2001;167:5304–15. doi: 10.4049/jimmunol.167.9.5304. [DOI] [PubMed] [Google Scholar]
- 38.Parham C, Chirica M, Timas J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–20. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 40.Frucht DM. IL-23. A cytokine that acts on memory T cells. Sci STKE. 2002;114:PE1. doi: 10.1126/stke.2002.114.pe1. [DOI] [PubMed] [Google Scholar]
- 41.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–7. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 42.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 43.Snapper CM, Marcu KB, Zelazowski P. The immunoglobulin class switch: beyond ‘accessibility’. Immunity. 1997;6:217–360. doi: 10.1016/s1074-7613(00)80324-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Werner-Favre C, Tang H, Brouwers N, Bonnefoy JY, Zubler RH. IL-4-dependent IgE switch in membrane IgA-positive human B cells. J Immunol. 1991;147:3001–4. [PubMed] [Google Scholar]
- 45.Zhang K, Mills FC, Saxon A. Switch circles from IL-4-directed epsilon class switching from human B lymphocytes. Evidence for direct, sequential, and multiple step sequential switch from mu to epsilon Ig heavy chain gene. J Immunol. 1994;52:3427–35. [PubMed] [Google Scholar]