Abstract
Regulatory T cells are proposed to play a central role in the maintenance of immunological tolerance in the periphery, and studies in many animal models demonstrate their capacity to inhibit inflammatory pathologies in vivo. At a recent meeting [Clinical Application of Regulatory T Cells, 7–8 April 2005, Horsham, UK, organized by the authors of this review, in collaboration with the British Society for Immunology and Novartis] evidence was discussed that certain human autoimmune, infectious and allergic diseases are associated with impaired regulatory T-cell function. In contrast, evidence from several human cancer studies and some infections indicates that regulatory T cells may impair the development of protective immunity. Importantly, certain therapies, both those that act non-specifically to reduce inflammation and antigen-specific immunotherapies, may induce or enhance regulatory T-cell function. The purpose of this review was to summarize current knowledge on regulatory T-cell function in human disease, and to assess critically how this can be tailored to suit the therapeutic manipulation of immunity.
Keywords: allergy, autoimmunity, immune regulation, tolerance, T lymphocytes
Immune regulation by regulatory T cells
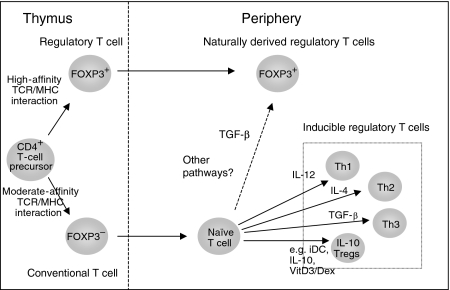
A number of mechanisms contribute to the capacity of the immune system to discriminate self from non-self, facilitating the maintenance of immunological tolerance to self-antigens and the induction of protective immunity to foreign antigens. Although the removal of immature self-reactive lymphocytes by negative selection in the thymus is considered pivotal to the former process, it is becoming increasingly clear that regulatory T cells (Tregs) are equally important in inducing and maintaining peripheral self-tolerance and thus preventing immune pathologies. Two broad categories of regulatory T cells have been described (Fig. 1). The first is the thymus-derived naturally occurring CD4+ CD25+ Treg subset that comprises 1–10% (estimates vary) of the CD4+ T-cell population in healthy adult humans and mice.1–3 In addition, inducible antigen-specific populations, generated following a variety of antigenic stimulatory regimes in vitro or in vivo, have been described.4–6 These secrete inhibitory cytokines such as interleukin (IL)-10 and transforming growth factor (TGF)-β.4–7 Together, these cells are thought to play a specialized role in controlling both innate and acquired immune responses. We will not go into the biology of these cells as this subject has been covered extensively in a number of recent excellent reviews, for example those by Sakaguchi2 and Hawrylowicz and O'Garra.5
Figure 1.
The development of CD4+ regulatory T cells (Tregs). The thymus naturally produces FOXP3 + CD4+ CD25+ Tregs, possibly by high-affinity engagement of the T-cell receptor (TCR) with self-ligand presented by the thymic stroma. In the periphery, some of the naïve CD4+ CD25– T cells may also differentiate into FOXP3 + CD4+ CD25+ Tregs. Several inducible Tregs have been identified, including T helper type 1 (Th1), Th2, Th3 and interleukin (IL)-10-producing Tregs. Th1 and Th2 cells are included here as signals that drive one of these lineages impair the development of the other (e.g. IL-12 and IL-4). Although IL-10 Tregs and Th3 cells are shown in this figure to originate following appropriate stimulation of naïve CD4+ T cells, the possibility that they derive from previously activated effector T cells exists. iDC, immature dendritic cell; MHC, major histocompatibility complex; TGF, transforming growth factor.
Probably the greatest barrier to fully understanding the function of naturally occurring CD4+ CD25+ Tregs in humans is the lack of specific markers that define these cells and distinguish them from activated effector T-cell populations and other Treg populations. However, the forkhead (winged helix) transcription factor forkhead box P3 (FOXP3) has been suggested to represent a reliable intracellular marker for naturally occurring Tregs.8 Alongside animal studies, a fascinating recent observation highlights the importance of Tregs in controlling human immunity. Patients carrying rare loss-of-function mutations in the Foxp3 gene develop a range of autoimmune and inflammatory disorders referred to as immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome [IPEX; also known as X-linked autoimmunity and allergic dysregulation (XLAAD) syndrome]. This includes type I diabetes, autoimmune thyroiditis, eczema, bleeding abnormalities and chronic wasting.9,10 These individuals also demonstrate an increased susceptibility to infection and an elevated incidence of allergic-type symptoms, including severe eczema, increased serum immunoglobulin E (IgE), eosinophilia and food allergy. The subsequent finding that FOXP3 is a transcription factor selectively expressed in and essential for the development of the CD4+ CD25+ Treg lineage suggests that a defect in the naturally occurring Treg population underlies the clinical manifestations in IPEX patients. More importantly, it implies that the presence and efficient function of Tregs is required to maintain health. However, although FOXP3 in mice seems to be exclusively expressed by CD4+ CD25bright T cells with regulatory function, in humans the situation is less clear, as FOXP3 appears also to be up-regulated upon activation of naïve CD4+ CD25– T cells11,12 and CD8+ T cells.12 Be that as it may, most studies on CD4+ regulatory T cells use a combination of CD25, cytotoxic T-lymphocyte-associated antigen (CTLA)-4, FOXP3, IL-10 and/or TGF-β to define Treg populations. Ultimately only the demonstration of actual suppressive function confirms the presence of Tregs. In this review we discuss the evidence for defective (lack of or over-exuberant) naturally occurring and inducible Treg activity in human disease and strategies that aim to manipulate Treg function for therapeutic benefit.
Alterations in regulatory T-cell function in human disease
Autoimmune disease
Recent studies have investigated whether human autoimmune diseases are associated with defective numbers or function of CD4+ CD25+ Tregs. In patients with multiple sclerosis, purified CD4+ CD25bright Tregs from peripheral blood were found to have a reduced capacity to suppress T-cell proliferation and interferon (IFN)-γ production ex vivo.13 Similar defects in the capacity of peripheral CD4+ CD25+ Tregs to inhibit T-cell proliferation were described for patients with autoimmune polyglandular syndrome type II,14 type I diabetes,15 psoriasis16 and myasthenia gravis.17 The percentage of CD4+ CD25+ or CD4+ CD25bright Tregs in the peripheral blood of these patients was unaltered compared with healthy controls, suggesting that a defect in Treg function rather than number contributes to disease. Alternatively this may reflect (i) an inability to distinguish between increased numbers of activated CD4+ CD25+ effector T cells and CD4+ CD25+ Tregs in the periphery, and/or (ii) an increased migration of CD4+ CD25+ Tregs to the tissues, and/or (iii) refractoriness of effector T cells and/or antigen-presenting cells (APCs) to regulation, all potentially resulting from disease-induced activation.
In rheumatoid arthritis (RA), a recent study suggested that CD4+ CD25bright Tregs in the peripheral blood of patients were normal in number and ability to suppress T-cell proliferation but deficient in the ability to inhibit T-cell and monocyte-derived cytokines, i.e. IFN-γ and tumour necrosis factor (TNF)-α.18 Interestingly, in patients that received anti-TNF-α treatment this defect appeared to be restored, and an increase in peripheral Treg numbers was observed, suggesting that anti-TNF-α therapy might lead to immunomodulation of Treg function. However, other studies on patients with RA or juvenile idiopathic arthritis (JIA) showed that at the site of inflammation (i.e. in the synovial fluid) the percentage of CD4+ CD25+ Tregs was significantly increased compared with the percentage in peripheral blood.19–22 Moreover, these synovial Tregs were able to inhibit TNF-α production19,21 and displayed an enhanced capacity to suppress T-cell proliferation compared with their peripheral blood counterparts.19,20 These results thus might suggest recruitment/migration of Tregs from the blood to the inflammatory site, rather than defective function.
A recent study has extended the observations in arthritis to suggest that CD27 distinguishes CD4+ CD25+ Tregs from other effector populations in the synovial fluid of patients with JIA, and that these cells demonstrate potent suppressive function ex vivo, but that their inhibitory function may be impaired in situ by high levels of IL-7 and IL-15.23 As the proportions of CD4+ CD25+ T cells are unchanged or increased at the site of the autoimmune lesions, for example the rheumatoid joint, therapeutic intervention on the basis of Treg manipulation should be concentrated on the use of mediators that enhance suppressive function of existing populations rather than simply increasing regulatory cell numbers. A crucial question in this regard is whether any enhancement of Treg function that may be achieved by appropriate therapy is sustained once therapy is terminated.
Allergic disease
Studies from at least three separate groups have provided evidence for impaired naturally occurring CD4+ CD25+ Treg-mediated inhibition of allergen-specific T helper type 2 (Th2) responses in allergic patients during active hayfever season24,25 or in individuals who mount vigorous Th2 responses to allergen.26 Furthermore, depletion of CD4+ CD25+ T cells from the peripheral blood of healthy individuals reveals enhanced proliferative and Th2 cytokine responses to various allergens including milk, nickel and grass,24,27,28 implying that naturally occurring CD4+ CD25+ Tregs play an active role in suppressing allergen-specific Th2 responses in healthy subjects.
Recent evidence also suggests an increased frequency or ratio of CD4+ CD25+ IL-10-secreting T cells in healthy individuals compared with individuals with allergic or asthmatic disease.29,30 It is unclear whether these cells represent naturally occurring CD4+ CD25+ Tregs or IL-10 Treg that may have been induced to increase CD25 expression upon activation in culture, highlighting the lack of appropriate markers to distinguish the different Tregs in humans. Furthermore, the induction of IL-10-secreting Tregs is impaired in patients with severe asthma who do not show clinical improvement upon steroid treatment (glucocorticoid resistant).31 Finally, in situations where tolerance is ‘naturally’ induced, for example in children who grow out of their allergy to cow's milk or in bee keepers who receive multiple stings, associated increases in IL-10-producing and CD4+ CD25+ Tregs have been reported.32,33 These studies suggest both naturally occurring CD4+ CD25+ Tregs and IL-10-secreting Treg populations actively control immune responses to allergen in healthy individuals and that their function might be impaired in disease, particularly during chronic antigen exposure,34 suggesting that novel therapeutic strategies may need to target both Treg populations.
Infectious disease
The immune response to infection represents a complex balance between the successful induction of proinflammatory antipathogen responses and anti-inflammatory responses required to limit damage to host tissues. Tregs undoubtedly play an important role in controlling this balance during infection, and the results can range from highly detrimental to the host to highly beneficial to both host and pathogen. The role of both naturally occurring CD4+ CD25+ Treg and IL-10-secreting Treg in infection has been the subject of several excellent recent reviews35–37 and we highlight here a few examples from studies in humans.
Recent work on Helicobacter pylori-infected individuals suggests that CD4+ CD25+ Tregs might contribute to chronic infection by suppressing appropriate memory T-cell responses to H. pylori.38 This is further supported by the demonstration that infected patients have increased frequencies of CD4+ CD25+ FOXP3+ T cells in the stomach and duodenal mucosa as compared with uninfected controls.39 Chronic exposure to pathogens might itself result in the induction of a strong immunoregulatory network, mediated by IL-10,40 and antigen-specific IL-10-producing Treg cells have indeed been isolated from helminth-infected patients.41
CD4+ CD25+ Tregs also appear to be involved in chronic virus infection. In patients with chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, increases in peripheral CD4+ CD25+ Treg numbers have been described.42–44 Moreover, depletion of CD4+ CD25+ Tregs from peripheral blood from virally infected patients results in increased T-cell responses to HBV, HCV, cytomegalovirus and human immunodeficiency virus (HIV) antigens, implying that the presence of CD4+ CD25+ Tregs prevents effective antiviral immunity.42–46 A recent study on HIV-infected patients demonstrated that decreased Treg numbers were associated with immune hyperactivation in these patients.47 Immune hyperactivation, however, is associated with disease progression, indicating that the presence of Tregs might have some protective effect in HIV infection. Indeed, a recent study showed that in asymptomatic HIV-infected patients strong HIV-specific Treg function in vitro was correlated with lower levels of plasma viraemia and higher CD4+:CD8+ T-cell ratios.48 These findings suggest that, rather than being detrimental to immunity to infection, intact Treg activity may be beneficial to HIV-infected patients.
Cancer
Evidence from cancer patients suggests that increased Treg activity may be associated with poor immune responses to tumour antigens and contribute to immune dysfunction. High numbers of CD4+ CD25+ Tregs have been found in lung, pancreatic, breast, liver and skin cancer patients, either in the blood or in the tumour itself.49–53 These Tregs were able to inhibit proliferation and IFN-γ production by CD4+ and CD8+ T cells, as well as natural killer (NK) cell-mediated cytotoxicity. A recent study on ovarian carcinoma patients elegantly demonstrated that the presence of CD4+ CD25+ FOXP3+ Tregs that suppress tumour-specific T-cell immunity inversely correlated with survival.54 These Tregs preferentially moved to and accumulated in the tumour and ascites, but not the draining lymph nodes, with evidence for a role of the chemokine CCL22 in directing Treg homing to the tumour.
In addition to CD4+ CD25+ Tregs, IL-10-producing Tregs may also contribute to ineffective anti-tumour responses in cancer patients. Both CD4+ CD25+ and IL-10-producing Tregs are found in Hodgkin lymphoma infiltrating lymphocytes, which suppress mitogen- and antigen-specific peripheral blood mononuclear cell responses.55 A recent study reported that internalization of primary myeloma cells by dendritic cells (DCs) resulted in IL-10 production, but no IL-12 production, and these DCs stimulated the generation of IL-10-producing T cells.56 Besides IL-10, TGF-β produced by various cell types, including Tregs, may inhibit the development of effective tumour immunity in vivo.7 Thus, for cancer immunotherapy, strategies that deplete Tregs, inhibit their function or block their migration, rather than enhance or restore their function, are likely to be advantageous (Table 1).
Table 1.
Targeting regulatory T cells (Treg) in immune-mediated diseases
| Diseases | Therapeutic strategies | Concerns |
|---|---|---|
| Cancer and infection | Depletion of Tregs | Induction of autoimmunity |
| Inhibition of Treg homing | ||
| Inhibition of Treg function | ||
| Autoimmune diseases, allergy, transplantation and infection | Induction of antigen-specific Tregs in vivo | Increased susceptibility to infection |
| Boosting of endogenous Tregs | ||
| Adoptive transfer of Tregs | Risk of tumour development |
Regulatory T cells (Tregs) contribute to many immune-mediated diseases either by hampering effective immunity (e.g. in cancer and infection) or by failing to control unwanted immune responses (e.g. in autoimmunity, allergy, certain infections and transplantation). Different therapeutic strategies could be employed to inhibit or boost Treg function; however, the risk of serious adverse side effects warrants careful consideration.
Clinical application – manipulation of regulatory T cells
Treg-inducing therapies: existing evidence
Data are emerging that certain immune-based therapies in humans, particularly in transplantation57,58 and allergy,5,34 may result in the induction of Tregs in vivo. An important early study demonstrated that CD4+ T-cell clones isolated from patients with severe combined immunodeficiency who had successfully undergone allogeneic bone marrow transplantation produced IL-10 and IFN-γ, but little IL-2, and that these cells were associated with a lack of graft-versus-host disease.59 Other examples have emerged from allergen desensitization immunotherapy (allergen-IT), which is essentially the oldest and only widely used antigen-specific immunomodulation currently in use in humans. It involves the injection of increasing concentrations of specific allergen into the patient to induce immunological tolerance and can be highly effective in selected patient groups, although the treatment itself is not without risk, and requires injections over several years for maximal efficacy. Initial studies suggested that successful allergen-IT was associated with immune deviation from a disease-promoting allergen-specific Th2 towards a Th1 phenotype. However, more recently two studies by Bellinghausen et al.60 and Akdis et al.32 were the first to provide evidence for allergen-IT-induced IL-10-producing Tregs, which are now being widely demonstrated in additional allergen-IT systems (reviewed by Robinson et al.34). However, allergen-IT appears to have little effect on naturally occurring CD4+ CD25+ Tregs.61 There remains a need for improved allergen-specific therapies with the capacity to provide safe, long-term relief from disease symptoms, and a number of novel approaches are currently being investigated in experimental studies in humans (reviewed by Hawrylowicz and O'Garra5).
A number of commonly used non-specific therapies using drugs with anti-inflammatory properties have been documented to induce anti-inflammatory cytokines and to modify Treg function (extensively referenced in Hawrylowicz and O'Garra5). One such example is anti-TNF-α therapy, as discussed above.18 Another, well-studied, example is that of glucocorticoids, which induce IL-10 both in vivo and in vitro.62,63 Evidence supporting a role for glucocorticoids and their beneficial effects on Tregs includes reports that in asthma patients poor clinical responsiveness to steroids correlates with poor induction of IL-10 in CD4+ T cells,31 that FOXP3 and IL-10 mRNA expression are significantly increased in CD4+ T cells from glucocorticoid-treated asthmatic patients,64 and in vitro evidence that glucocorticoids may promote IL-10 synthesis and regulatory function of CD4+ CD25+ Tregs.65 Furthermore, particular combinations of immunosuppressive drugs, such as 1α,25-dihydroxyvitamin D3 in combination with glucocorticoids66 or mycophenolate mofetil67 as well as glucocorticoids with β2-agonists,68 have been shown to induce Tregs and/or tolerogenic DCs in mice and humans and could be utilized to more efficiently derive Tregs in vivo. One example is a study of the administration of vitamin D3 to glucocorticoid-insensitive asthma patients, which reversed the defective induction of IL-10-secreting regulatory CD4+ T cells in response to glucocorticoids.69 Although the action of these drugs is non-specific, it may be possible to harness these effects to improve the efficacy and safety of other antigen-specific approaches.5
Treg-inducing therapies: experimental/translational protocols
A major therapeutic goal in autoimmune and allergic diseases is to provide inhibitory mechanisms with the capacity to suppress inappropriate immune activation upon disease-promoting antigen/allergen exposure, locally and specifically, with minimal risk and damage to the host. Two strategies which are the subject of active investigation are the adoptive transfer of ex vivo generated CD4+ CD25+ Tregs, which requires tailor-made therapies for each patient, and the induction of appropriate Treg populations in patients in vivo. Indeed, some studies have now reached the ‘proof of principle’ stage.
In vitro Treg expansion and transfer
Recent studies have examined the potential to isolate CD4+ CD25bright Tregs from peripheral blood and expand them in vitro for re-injection into patients under clinically controlled conditions. Efficient in vitro expansion of human Tregs (up to 200- or even 40 000-fold) was reported upon stimulation of CD4+ CD25+ T cells with anti-CD3/CD28 monoclonal antibody (mAb)-coated beads and high doses of IL-2.70,71 The expanded cells retained the expression of CD25, FOXP3 and lymph node homing receptors and, importantly, were more efficient in suppression assays than freshly isolated Tregs. A different study in rats reported on the use of an anti-CD28 superagonist which preferentially expanded CD4+ CD25+ Tregs over other T-cell subsets in vitro and in vivo.72 Combining these experimental approaches with protocols for the induction of antigen-specific Tregs, for example via the transfection with T-cell receptor (TCR) specific for the disease-promoting antigen73 or in vitro priming with alloantigen,74 could eventually lead to large-scale generation of tailor-made Tregs with appropriate antigen specificity. Potential problems with expanding pre-existing Tregs are that such a labour-intensive protocol needs to be adopted for each patient, the purity and antigen specificity of Tregs need to be extremely well controlled to avoid outgrowth of autoreactive T (effector) cells or Treg with inappropriate antigen specificity (e.g. to pathogen-derived antigens), and there is a risk of infection or transformation ex vivo.
Strategies to induce or boost Tregs in vivo
Recent studies have shown that non-regulatory CD4+ CD25– T cells can develop into suppressor T cells depending on the way in which they encounter antigen, for example as soluble or peptide antigen, or in the presence of immunosuppressive drugs. One strategy is to target the APC population. IL-10-producing Tregs are reportedly induced by repetitive in vitro stimulation of naïve T cells with antigen-presenting immature DCs, although these Tregs expand poorly in vivo.75 Proof of principle was provided in an elegant study by Steinman and coworkers where immunization of human volunteers with a single subcutaneous injection of influenza peptide-pulsed immature DCs resulted in a reduction in antigen-specific IFN-γ-producing CD8+ T cells with a concomitant increase in IL-10-producing cells relative to preimmunization.76 Importantly, such a strategy requires that the DCs remain ‘frozen’ in their immature state in order to prevent immune activation and potential autoimmune reactions, and this represents a major research focus.
Extensive evidence of the induction of IL-10-producing Tregs following peptide administration in animal models of autoimmune and allergic disease exists (reviewed by Larche and Wraith77). However, use of self (glutamic acid decarboxylase) peptides in non-obese diabetic (NOD) mice and use of altered peptide-ligands in multiple sclerosis patients have both been associated with severe side effects.78,79 More encouragingly, in patients with recent-onset RA, oral administration of the bacterial heat-shock protein dnaJP1 – which contains sequence homology with the shared epitope – resulted in a decrease in antigen-induced IFN-γ, IL-2 and TNF-α, and an increase in IL-10 and IL-4 production and FOXP3 expression in peripheral CD4+ CD25+ Tregs.80 Also, recently, in support of the earlier findings in bee venom-specific IT by Akdis et al.,32 it was shown that peptide immunotherapy in cat-allergic asthmatic patients resulted in an increase in IL-10, a decrease in IL-5 and the induction of a CD4+ Treg population that actively suppressed allergen-specific T-cell proliferation.81 These and other ongoing studies are clearly just a prelude to the testing, by a number of groups, of antigen-specific therapies and induction of Tregs in clinical interventions in humans.
Two recent reports highlight the potential for anti-CD3 mAb therapy in patients with new-onset type I diabetes.82,83 Patients treated with humanized non-mitogenic anti-CD3 mAb (ChAglyCD3) early after disease onset better maintained residual beta cell function and required lower insulin doses at 18 months than the comparable placebo-treated group.83 This effect was most pronounced in those patients who had initial residual beta-cell function at or above the 50th percentile of the total patient group, suggesting that anti-CD3 mAb-based immune therapy works best when the autoimmune-mediated destruction is still ongoing. In the other study, early-onset patients were treated with a single course of hOKT3g1 (Ala-ala) which resulted in improved clinical parameters for at least 2 years in the absence of immunosuppressive medication.82 Although not proven in these clinical studies, previous work by these groups in NOD mice indicates that anti-CD3 mAb therapy might work via the induction of immunoregulatory mechanisms, including those involving TGF-β and CD4+ CD25+ Tregs.84 Other future candidates for mAb-based approaches to induce Tregs in vivo could include anti-CD45RO/RB mAb (chA6), which was shown to induce anergic and suppressive human antigen-specific CD4+ and CD8+ T cells upon stimulation in vitro.85
Treg-depleting therapies
In cancer, strategies that inhibit or deplete Tregs and boost anti-tumour immunity are under investigation. In mice, the removal of CD4+ CD25+ T cells with anti-CD25 depleting mAbs,86 albeit in combination with anti-CTLA-4 mAb,87 led to tumour rejection. However, a cautionary note comes from studies using CTLA-4 blockade along with tumour-specific peptide vaccinations in human melanoma patients, which resulted in tumour regression in some patients but also significant development of autoimmunity in six of 14 patients, thus emphasizing the delicate balance between tolerance and immunity.88 At present, no depleting anti-CD25 mAbs are licensed for use in humans, but eventually these findings might open up novel immunotherapeutic avenues.
Concluding remarks and questions for the future
The field of therapeutic application of Tregs in immune-mediated pathologies is at a very exciting crossroads. Clearly, many questions remain regarding optimization of strategies that target Tregs. We believe that some of the critical issues are optimization of protocols for Treg induction in vivo, a full understanding of the migration/homing capacity of the different Treg populations, their longevity and stability in vivo, especially upon therapeutic induction, and the natural ontogeny of Tregs in childhood. In adults, transplantation, perhaps uniquely, offers the opportunity to deliver antigen-specific Treg-directed therapies prior to disease induction. Crucially, there is a need to expand on existing experimental evidence that Tregs can reverse established disease89 rather than prevent induction of disease, in order to apply these cells in a clinical setting. A major concern to be resolved is the question of how great is the potential for spreading of immunosuppression and therefore how great is the threat of inducing other unrelated immune pathologies. These considerations notwithstanding, the enormous progress achieved in our understanding of fundamental Treg biology, and in experimental protocols to isolate Tregs and to generate them in vitro and in vivo, makes it likely that in the next few years the experimental data will lead to translational studies and even clinical trials in humans.
Abbreviations
- APC
antigen-presenting cell
- CTLA-4
cytotoxic T-lymphocyte-associated antigen-4
- DC
dendritic cell
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- iDC
immature dendritic cell
- IFN
interferon
- IL
interleukin
- IT
immunotherapy
- JIA
juvenile idiopathic arthritis
- mAb
monoclonal antibody
- NK
natural killer
- NOD
non-obese diabetic
- RA
rheumatoid arthritis
- TCR
T-cell receptor
- TGF
transforming growth factor
- TNF
tumour necrosis factor
- Tregs
regulatory T cells
References
- 1.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nature Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 4.Roncarolo M-G, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 5.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10 secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 6.Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory T cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 7.Gorelik L, Flavell RA. Transforming growth factor-β in T cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 8.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–8. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 10.Wildin R, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 11.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan ME, van Bilsen JHM, Bakker AM, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in Autoimmune Polyglandular Syndrome Type II. J Exp Med. 2004;199:1285–91. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TIM. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: Mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4+CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–41. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNF-α therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Amelsfort JMR, Jacobs KMG, Bijlsma JWJ, Lafeber FPJG, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in presence, phenotype and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 20.de Kleer IM, Wedderburn LR, Taams LS, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of Juvenile Idiopathic Arthritis. J Immunol. 2004;172:6435–43. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 21.Cao D, Vollenhoven RV, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–7. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 25.Grindebacke H, Wing K, Andersson A-C, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4+CD25+ regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34:1364–72. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 26.Bellinghausen I, Klostermann B, Knop J, Saloga J. Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J Allergy Clin Immunol. 2003;111:862–8. doi: 10.1067/mai.2003.1412. [DOI] [PubMed] [Google Scholar]
- 27.Taams LS, Vukmanovic-Stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Cavani A, Nasorri F, Ottaviani C, Sebastiani S, De Pita O, Girolomoni G. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J Immunol. 2003;171:5760–8. doi: 10.4049/jimmunol.171.11.5760. [DOI] [PubMed] [Google Scholar]
- 29.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiemessen MM, Van Ieperen-Van Dijk AG, Bruijnzeel-Koomen CAFM, Garssen J, Knol EF, Van Hoffen E. Cow's milk-specific T-cell reactivity of children with and without persistent cow's milk allergy: Key role for IL-10. J Allergy Clin Immunol. 2004;113:932–9. doi: 10.1016/j.jaci.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Hawrylowicz C, Richards D, Loke TK, Corrigan C, Lee T. A defect in corticosteroid-induced IL-10 production in T lymphocytes from corticosteroid-resistant asthmatic patients. J Allergy Clin Immunol. 2002;109:369–70. doi: 10.1067/mai.2002.121455. [DOI] [PubMed] [Google Scholar]
- 32.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med. 2004;199:1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–97. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–8. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills KHG. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 38.Lundgren A, Suri-Payer E, Enarsson K, Svennerholm A-M, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755–62. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundgren A, Stromberg E, Sjoling A, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–31. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 41.Satoguina J, Mempel M, Larbi J, et al. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis) Microbes Infection. 2002;4:1291–300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 42.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+) CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 43.Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–8. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–48. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–56. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 47.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, Mugyenyi P, Cao H. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–14. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 48.Kinter AL, Hennessey M, Bell A, et al. CD25+CD4+ Regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–43. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 50.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 51.Liyanage UK, Moore TT, Joo H-G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 52.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–53. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 53.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–64. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 54.Curiel T, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 55.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 56.Fiore F, Nuschak B, Peola S, et al. Exposure to myeloma cell lysates affects the immune competence of dendritic cells and favors the induction of Tr1-like regulatory T cells. Eur J Immunol. 2005;35:1155–63. doi: 10.1002/eji.200425093. [DOI] [PubMed] [Google Scholar]
- 57.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 58.Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Semin Immunol. 2004;16:119–26. doi: 10.1016/j.smim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Bacchetta R, Bigler M, Touraine J, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellinghausen I, Metz G, Enk AH, Christmann S, Knop J, Saloga J. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol. 1997;27:1131–9. doi: 10.1002/eji.1830270513. [DOI] [PubMed] [Google Scholar]
- 61.Smith TR, Alexander C, Kay AB, Larche M, Robinson DS. Cat allergen peptide immunotherapy reduces CD4(+) T cell responses to cat allergen but does not alter suppression by CD4(+) CD25(+) T cells: a double-blind placebo-controlled study. Allergy. 2004;59:1097–101. doi: 10.1111/j.1398-9995.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 62.Wan S, LeClerc J-L, Vincent J-L. Cytokine responses to cardiopulmonary bypass: Lessons learned from cardiac transplantation. Ann Thorac Surg. 1997;63:269–76. doi: 10.1016/s0003-4975(96)00931-9. [DOI] [PubMed] [Google Scholar]
- 63.Richards DF, Fernandez M, Caulfield J, Hawrylowicz CM. Glucocorticoids drive human CD8(+) T cell differentiation towards a phenotype with high IL-10 and reduced IL-4, IL-5 and IL-13 production. Eur J Immunol. 2000;30:2344–54. doi: 10.1002/1521-4141(2000)30:8<2344::AID-IMMU2344>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 64.Karagiannidis C, Akdis M, Holopainen P, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114:1425–33. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Dao Nguyen X, Robinson DS. Fluticasone propionate increases CD4CD25 T regulatory cell suppression of allergen-stimulated CD4CD25 T cells by an IL-10-dependent mechanism. J Allergy Clin Immunol. 2004;114:296–301. doi: 10.1016/j.jaci.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 66.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 68.Peek EJ, Richards DF, Faith A, Lavender P, Lee TH, Corrigan CJ, Hawrylowicz CM. Interleukin-10-secreting ‘regulatory’ T cells induced by glucocorticoids and β2-agonists. Am J Respir Cell Mol Biol. 2005;33:105–11. doi: 10.1165/rcmb.2005-0100OC. [DOI] [PubMed] [Google Scholar]
- 69.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55. doi: 10.1172/JCI21759. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 71.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. In vitro expanded human CD4+CD25+ T regulatory cells can markedly inhibit allogeneic dendritic cell stimulated MLR cultures. Blood. 2004;104:453–61. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 72.Lin C-H, Hünig T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003;33:626–38. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- 73.Fujio K, Okamoto A, Tahara H, Abe M, Jiang Y, Kitamura T, Hirose S, Yamamoto K. Nucleosome-specific regulatory T cells engineered by triple gene transfer suppress a systemic autoimmune disease. J Immunol. 2004;173:2118–25. doi: 10.4049/jimmunol.173.3.2118. [DOI] [PubMed] [Google Scholar]
- 74.Jiang S, Camara N, Lombardi G, Lechler RI. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex-vivo. Blood. 2003;101:2180–86. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- 75.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 76.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:569–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 78.Kappos L, Comi G, Panitch H, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. 2000;6:1176–82. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 79.Pedotti R, Sanna M, Tsai M, DeVoss J, Steinman L, McDevitt H, Galli S. Severe anaphylactic reactions to glutamic acid decarboxylase (GAD) self peptides in NOD mice that spontaneously develop autoimmune type 1 diabetes mellitus. BMC Immunol. 2003;4:2. doi: 10.1186/1471-2172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prakken BJ, Samodal R, Le TD, et al. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci USA. 2004;101:4228–33. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verhoef A, Alexander C, Kay AB, Larche M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Medicine. 2005;2:253–61. doi: 10.1371/journal.pmed.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1 (Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 84.Belghith M, Bluestone JA, Barriot S, Megret J, Bach J-F, Chatenoud L. TGF-β-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–8. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 85.Gregori S, Mangia P, Bacchetta R, et al. An anti-CD45RO/RB monoclonal antibody modulates T cell responses via induction of apoptosis and generation of regulatory T cells. J Exp Med. 2005;201:1293–305. doi: 10.1084/jem.20040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 87.Sutmuller RPM, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mottet C, Uhlig HH, Powrie F. Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]