Abstract
Cytotoxic T-lymphocytes (CTL) play an important role in the control of human immunodeficiency virus (HIV) and of human cytomegalovirus (HCMV) infection. Following highly active antiretroviral therapy (HAART), most studies have demonstrated a decline in the frequency of HIV-specific CTL. We analysed the effect of HAART on the size, phenotype and function of individual HIV- and HCMV-specific CTL clones, using clonotypic oligonucleotide probing specific for the T-cell receptor (TCR) β-chain hypervariable sequence of defined immunodominant CTL clones specific for peptides of HIV or HCMV, and quantified the limiting dilution analysis frequencies of CTL precursors (CTLp) specific for the same viral peptides. We found that the clonal composition of CD8+ T cells specific for HIV gag and env epitopes was highly focused and did not change after HAART. Following HAART, there was progressive contraction of HIV-specific CD8+ clones, especially in the CD28– CD27– subpopulation – the remaining cells of contracting HIV-specific clones were predominantly CD28– CD27+ CD45ROhi. We observed maintenance of strong functional HIV-specific CD8+ T-cell responses in limiting dilution analysis following HAART, indicating preferential loss of HIV-specific cells that have reduced cloning efficiency in vitro. Following HAART, we also observed selective expansion of HCMV-specific CD8+ clones. Most HCMV-specific CD8+ clones were predominantly CD28– CD27+/– CD45RAhi following HAART. In one subject, a Vβ6.4+ clone specific for HCMV pp65 selectively expanded following HAART, without expansion of two other Vβ6.4+ clones, indicating that individual clonotypes specific for the same peptide can show different kinetics and phenotypes in response to antiretroviral therapy.
Keywords: cytotoxic, HIV, human, T cells, T-cell receptors
Introduction
In acute human immunodeficiency virus (HIV) infection there is a vigorous HIV-specific CD8+ cytotoxic T-lymphocyte (CTL) response that is temporally associated with partial control of viral replication.1–3 During chronic HIV infection high frequencies of HIV-specific CTL are maintained in the peripheral blood of asymptomatic HIV-infected subjects for years4–7 – in some but not all studies an inverse relationship between the frequency of HIV-specific CTL and plasma HIV viral load has been reported.3,8,9 In the majority of HIV-infected subjects HIV-specific CTL fail to prevent progressive immunodeficiency (reviewed in Lieberman et al.10). Previous quantitative cross-sectional and longitudinal studies of the frequency of CTL specific for HIV or for other viruses in subjects who have started highly active antiretroviral therapy (HAART) have yielded different results. Using peptide–major histocompatability complex (MHC) class I tetramers, Ogg et al. have reported that immediately following HAART the frequency of HIV-specific CTL shows early rapid fluctuations lasting 1–2 weeks followed by an exponential decline with a median half-life of 45 days.11 Using intracellular cytokine staining over a longer period of follow-up, Casazza et al. observed an initial rapid decline at initiation of HAART followed by a slower decrease in HIV-specific CTL frequency after the HIV viral load was undetectable, with median half-life of 38·8 weeks.12 Other investigators have reported a similar half-life for the rate of decay.13,14 In a proportion of HIV-infected subjects starting HAART, the frequency of CTL specific for Epstein–Barr virus15 or specific for human cytomegalovirus (HCMV)16 can increase in the period immediately following HAART.
It has been suggested that following encounter with antigen, CD28+ CD27+ CD8+ cells may undergo progressive differentiation through CD28– CD27+ to CD28– CD27– cells, which may have diminished clonogenic potential.17 Previous studies have found that HIV-specific CD8+ T cells are predominantly CD28– CD27+ CD45RO+ whereas HCMV-specific CD8+ T cells are predominantly CD28– CD27– CD45RO+/–.17–19 In a previous longitudinal study that identified HIV-specific CD8+ T cells by tetramer staining, CD27+ CD45RO+ tetramer+ cells maintained their phenotype in advancing infection.7 There have been few longitudinal studies of the phenotype of HIV-specific CD8+ T cells before and after HAART; there is some evidence that with efficient control of viral load by HAART there is a decline in CD27+ CD11ahi CD8+ T cells specific for HIV.16
There has been only one previous study in a single HIV-infected subject of the size of individual HIV-specific clones following HAART (which in this study was commenced shortly after HIV infection). The clone size of individual clonotypes specific for the A2-restricted peptide SLYNTVATL fluctuated in parallel with fluctuations in viral load resulting from non-adherence to therapy. This study did not measure the size of these clones before HAART.20
We previously analysed the clonal composition of CTL specific for defined peptides of HIV or HCMV by generating multiple independent peptide-specific CTL clones and sequencing the hypervariable variable/diversity/joining (VDJ) region of the T-cell receptor (TCR) β-chain of each clone. In each donor, we found that many of the independent CTL clones specific for a given peptide used the same TCR at the level of nucleotide sequence, indicating that the large circulating population of memory CTL specific for a given viral peptide contains individual CTL clones that had undergone extensive expansion in vivo. This clonal expansion was sufficient to permit molecular quantification of individual CTL clones directly ex vivo by the design of complementary oligonucleotide probes that were highly specific for the hypervariable TCR β-chain sequence of each immunodominant CTL clonotype.21,22 By purifying subpopulations of CD8+ T cells before clonotype probing, we have previously demonstrated phenotypic heterogeneity within the daughter cells of individual virus-specific CD8+ clonotypes.23
Therefore in this study for the first time, we quantified longitudinally the clone size and phenotype of dominant CTL clones specific for HIV or HCMV and the corresponding functional responses in limiting dilution analysis (LDA) for years before and after HAART.
Materials and methods
Donors and tissue typing
Five HIV-infected subjects attending Addenbrooke's Hospital, Cambridge, UK were studied. All donors were HCMV seropositive. Tissue types and dates of first HIV diagnosis were:
H0009 (A2 A24 B62 B27), February 1998;
H0018 (A2 A3 B7 B44), July 1989;
H0043 (A11 A24 B44 B55), July 1993;
H0045 (A11 A29 B8 B44), January 1991;
H0049 (A11 A24 B55 B56), September 1999.
H0049 commenced HAART with zidovudine, didanosine and nevirapine in October 1999 2 months after diagnosis of primary HIV infection (the nadir CD4 count was 360 cells/ml, and viral load prior to treatment was 34 000 copies/ml; following HAART, viral load was consistently < 50 copies/ml and CD4 T-cell counts remained stable at 550–760 cells/ml. Details of other patients' clinical status and antiretroviral regimens are contained in the legends to Figs 2, 3, 4 and 5.
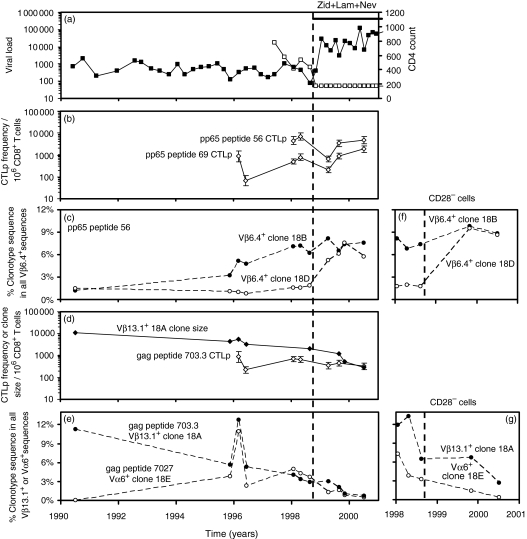
Figure 2.
Subject H0018. (a) HIV viral load (copies/ml plasma, □) and CD4+ T-cell count (per microlitre of whole blood, ▪). H0018 was asymptomatic until HSV retinitis immediately before commencing HAART with stavudine, lamivudine and nevirapine on 25/9/98, then changed stavudine for zidovudine on 25/10/00 because of a mild neuropathy. (b) LDA frequencies of CD8+ CTLp specific for pp65 peptides 69 and 56. (c, f) Selective expansion in the proportion of pp65 peptide 56-specific clonotype 18D sequence (○) within all Vβ6.4+ sequences in PBMC and in CD28– PBMC following HAART, with stable maintenance of clonotype 18B (•) (d) Progressive contraction in the clone size (expressed as cells of the clone/106 CD8+ T cells) of gag peptide 703.3 specific clonotype 18A following HAART (⋄). The calculated half-life of decline was 51 weeks. The LDA frequency of CTLp specific for this peptide remained stable (◊). (e, g) Progressive contraction in the proportion of clonotype 18A sequences within all Vβ13.1+ sequences in PBMC and in CD28– PBMC following HAART (•; used to calculate clone size data in d). Contraction in the proportion of gag peptide 7027-specific clonotype 18E sequences within all Vα6+ sequences in PBMC and in CD28– PBMC following HAART (○).
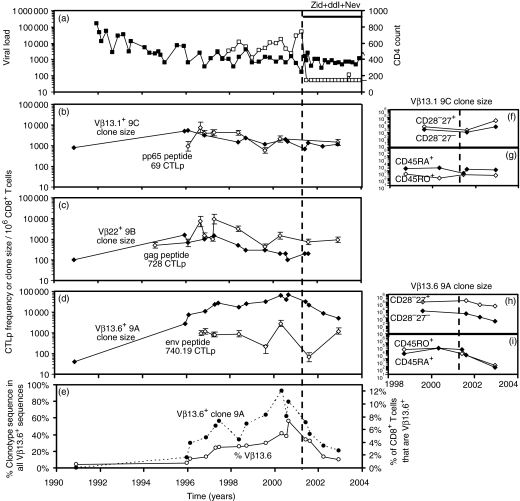
Figure 3.
Subject H0009. (a) HIV viral load (copies/ml plasma, □) and CD4+ T-cell count (per microlitre of whole blood, ▪). H0009 was asymptomatic until commencing HAART with zidovudine, didanosine and nevirapine on 14/05/01. The clone size of pp65 peptide 69-specific clonotype 9C was stable over 12 years (⋄). The frequency of CD8+ CTLp specific to peptide 69 was high and maintained at a stable level at multiple time-points over 7 years (◊). Clone size of clonotype 9B (⋄) and frequency of CD8+ CTLp specific for gag peptide 728 (◊). (d) Progressive expansion in the clone size of env peptide 740.19 specific clonotype 9A then contraction following HAART (⋄) – the calculated half-life of decline was 40 weeks. LDA frequency of CTLp specific for this peptide (◊). (e) Progressive expansion then contraction in the proportion of clonotype 9A sequences within all Vβ13.6+ sequences (•) and the proportion of all CD8+ cells that were Vβ13.6+ by flow cytometry (○). (f, h) Clone size per 106 CD8+ T cells of each subpopulation of clonotypes 9C and 9A within CD28– CD27– (◊) and CD28– CD27+ subpopulations (⋄). (g, i) Clone sizes of clonotypes 9C and 9A within CD45RA+ (⋄) and CD45RO+ (◊) subpopulations.
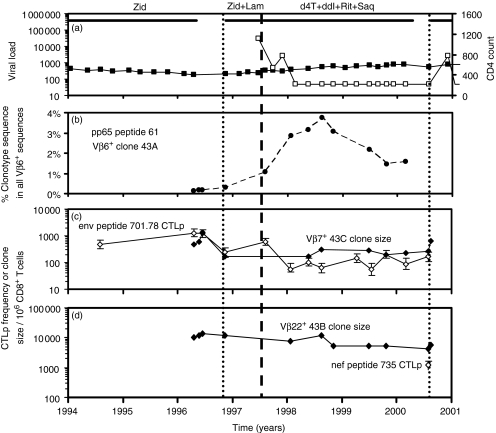
Figure 4.
Subject H0043. (a) HIV viral load (copies/ml plasma, □) and CD4+ T-cell count (per microlitre of whole blood, ▪). H0043 commenced zidovudine monotherapy on 30/9/93, interrupted therapy for 6 months from 3/5/96 when receiving chemotherapy for lymphoma; began zidovudine and lamivudine dual therapy on 13/11/96, then began quadruple therapy on 23/7/1997. He had a four-month break in therapy from 15/04/00 to 12/08/00. (b) Expansion in the proportion of pp65 peptide 61 specific clonotype 43A sequence within all Vβ6 sequences in PBMC following HAART. (c) Clone size of clonotype 43C (⋄) and LDA frequency of CD8+ CTLp specific for env peptide 701.78 (◊). (d) Progressive contraction in the clone size of clonotype 43B following HAART (⋄) – the calculated half-life of decline was 103 weeks. LDA frequency of CD8+ CTLp specific for nef peptide 735 (◊, only measured on one occasion).
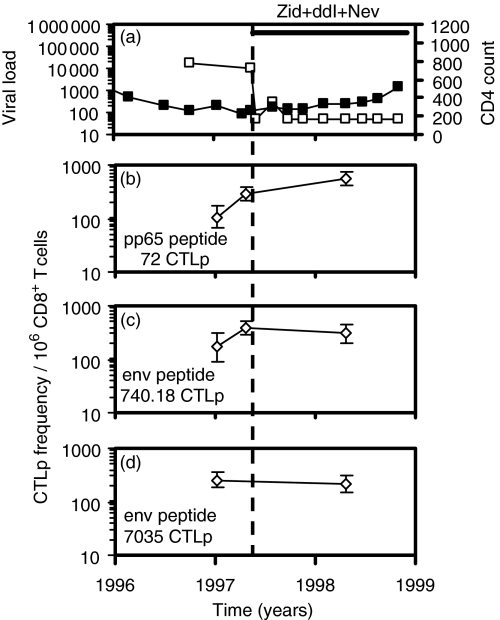
Figure 5.
Subject H0045. (a) HIV viral load (copies/ml plasma, □) and CD4+ T-cell count (per microlitre of whole blood, ▪). H0045 was asymptomatic until commencing dual therapy with zidovudine and didanosine on 21/5/97, then additionally started taking nevirapine on 13/5/98. (b–d) LDA frequencies of CTLp specific for pp65 peptide 72 and env peptides 740.18 and 7035.
Synthetic peptides
In some of the subjects in this study we previously identified allele-specific viral peptides from HCMV and HIV (Table 1).21,24 In each of subjects H0018 and H0049 we identified one further HIV gag allele-specific peptide by testing published gag epitopes from a database25 (Table 1). The MRC AIDS Reagent Project, UK, provided all HIV peptides.
Table 1.
In a given subject, multiple independent CD8+ formal CTL clones or clonal microcultures specific for a defined HCMV or HIV peptide have identical TCR β-chain nucleotide sequences
| Donor | HIV or HCMV protein | Peptide number* | Amino acid sequence | Position in protein | Restricting HLA allele | Vβ or Vα | No. of identical clones or cultures | Clonotype code |
|---|---|---|---|---|---|---|---|---|
| H0018 | pp65 | 56 | TPRVTGGGAM | aa 417–426 | B7 | Vβ6.4 | each 1/3† | 18B,C, D¶ |
| gag p17 | 703.3 | LRPGGKKKYKLKHIV | aa 21–35 | A3 | Vβ13.1 | 5/6‡ | 18A | |
| gag p17 | 7027 | SLYNTVATL | aa 77–85 | A2 | Vα6 | 8/8 | 18E | |
| H0009 | pp65 | 69 | NLVPMVATV | aa 495–503 | A2 | Vβ13.1 | 4/6† | 9C |
| gag p24 | 728 | KRWILGLNKIVRMY | aa 265–279 | B27 | Vβ22 | 2/2‡ | 9B | |
| env gp120 | 740.19 | PIPIHYCAPAGFAILKCNNK | aa 183–202 | A2 | Vβ13.6 | 6/6‡ | 9A | |
| H0043 | pp65 | 61 | ACTSGVMTRGRLKAE | aa445–459 | A11 | Vβ6.2 | 3/3 | 43A |
| nef | 735 | PQVPLRPMTYK | aa73–82 | A11 | Vβ22 | 4/6 | 43B | |
| env gp41 | 701.78 | ALIWEDLRSLCLFSY | aa 761–775 | B55 | Vβ7 | 2/11§ | 43C | |
| H0045 | pp65 | 72 | EFFWDANDIY | aa 511–525 | B44 | ND | 1/1‡ | ND |
| env gp120 | 7035.1 | AAEQLWVTVYYGVPVWKEAT | aa 30–49 | A11 | Vβ6 | 4/6‡ | ND | |
| env gp120 | 740.18 | TQACPKVSFEPIPIHYCAPA | aa 173–192 | ND | Vβ13.1 | 3/5‡ | ND | |
| H0049 | pp65 | 61 | ACTSGVMTRGRLKAE | aa445–459 | A11 | Vβ23 | 2/5 | 49A |
| gag | 788.9 | DRVHPVHAGPIAPGQMREPR | aa213–231 | B55 | Vβ6.2 | 4/4 | 49B |
Numbering as in MRC AIDS reagent project and ref. 26. Where available, the minimum epitope for each peptide is underlined.
A total of seven different clonotypes derived over 3 years recognized env peptide 701.78.
Independently derived peptide-specific clones all using Vβ6.4 but with different hypervariable DNA sequence.
Preparation of defined subsets of CD8+ cells for LDA and oligonucleotide probing
Peripheral blood mononuclear cells (PBMC) were prepared from fresh heparinized venous blood samples and aliquots of PBMC or CD16-depleted PBMC were sorted into subpopulations using magnetic antibody cell sorting microbeads as previously described.26
To determine the proportion of CD8+ T cells that possessed the relevant TCR Vβ segment within each purified population, three or four colour flow cytometry measurements were performed on aliquots of PBMC.21 The methodology of LDA used was as described elsewhere.21
Sequencing of the TCR α-chain and β-chain of single-cell clones and clonally derived T-cell microcultures
We identified individual LDA microcultures that showed strong peptide-specific cytotoxicity and subcultured residual cells at 0·5 cells/well to generate formal single-cell clones.21 Polymerase chain reaction (PCR) amplification and sequencing was performed using a panel of TCR Vβ primers and a Cβ primer or a panel of TCR Vα primers and a Cα primer.21
Quantitative clonotypic analysis
From the TCR α-chain or β-chain hypervariable sequence of each immunodominant peptide-specific CD8+ T-cell clone, we designed a highly specific complementary 15–20-mer oligonucleotide probe. We used these probes and a TCR β-chain constant region probe that detects all amplified TCR sequences21,27 to calculate the relative abundance of the clonotype sequence as a proportion of all amplified TCR sequences of the same Vβ family.27
The absolute clone size within CD8+ cells of each subpopulation was then estimated from the proportion of clonotype sequence within all amplified sequences multiplied by the proportion of the CD8+ T cells that stained with the Vβ-specific monoclonal antibody within each subpopulation.21
Results
Characterization of MHC class I restricted HIV and HCMV peptides
In subjects H0043 and H0049 using a panel of overlapping peptides that spanned the whole of pp65 as described previously26 we identified a novel pp65 allele-specific peptide, peptide 61 (Table 1). By testing peptide 61-specific CD8+ T-cell clones against autologous and partially matched target cells we demonstrated that peptide 61 is restricted by HLA-A11 (data not shown). Peptide 61 contains the 9-mer GVMTRGRLK, which has favourable amino acids at positions 2 and 9 for binding to HLA-A11.28 We previously identified six CD8+ T-cell epitopes in HIV env or gag and three CD8+ T-cell epitopes from HCMV pp65 and determined their MHC restriction21,24 (Table 1).
The clonal composition of CD8+ T cells specific for HIV gag and env and HCMV pp65 epitopes was highly focused and did not change after HAART
In subjects H0009, H0018 and H0045 we have previously determined the TCR β-chain sequences of dominant clonotypes specific for peptides of pp65, gag or env (Table 1).21,22
In our present study, we derived further formal CTL clones and clonal CTL microcultures to identify immunodominant clonotypes specific for other HCMV or HIV peptides. For HIV gag peptide 7027-specific CTL in subject H0018, two of two clonal CTL microcultures derived in June 1999 (06/99) and two of two formal CTL clones derived in 09/99 had the same Vβ16+ TCR β-chain nucleotide sequence. We also found that each of these four clones or cultures had identical Vα6+ TCR α-chain nucleotide sequences. Four of six further independent peptide 7027-specific clonal CTL microcultures derived in 07/00 had the same Vα6+ TCR α-chain nucleotide sequences (clonotype 18E, Table 1).
For subject H0043, all three independent pp65 peptide 61-specific formal clones derived at one time-point had identical Vβ6.2+ TCR β-chain nucleotide sequences (clonotype 43A, Table 1). Four of six independent nef peptide 735-specific clonal CTL microcultures derived at one time-point had the same Vβ22+ TCR β-chain nucleotide sequence (clonotype 43B, Table 1). Two of the three independent env peptide 701.78-specific formal clones derived at one time-point had identical Vβ7+ TCR β-chain nucleotide sequences (clonotype 43C, Table 1), although at three other time-points we derived eight further formal clones or CTL microcultures, also specific for env peptide 701.78 that had different TCR β-chain nucleotide sequences. Two of these eight clones used Vβ7, but had TCR β-chain nucleotide sequences different from that of clonotype 43C.
For subject H0049, two of five independent pp65 peptide 61-specific formal clones derived at one time-point had identical Vβ23+ TCR β-chain nucleotide sequences (clonotype 49A, Table 1). All four independent gag peptide 788.9-specific formal clones derived at one time-point had identical Vβ6.2+ TCR β-chain nucleotide sequences (clonotype 49B, Table 1).
These new data confirm our previous observation of clonal focusing of CD8+ T cells specific for peptides of HIV and HCMV.21,22
In subjects H0009 and H0018 we also analysed the clonal composition of multiple independent env or gag peptide-specific CTL microcultures following HAART (Table 2). Before HAART in subject H0009, all six formal single-cell CTL clones or clonal microcultures specific for env peptide 740.19 at two time-points had the same TCR β-chain nucleotide sequence (Table 1). Following HAART, all three clonal microcultures specific for peptide 740.19 analysed at two time-points also had the same TCR β-chain nucleotide sequence. Similarly in subject H0018 the same gag peptide 703.3-specific clonotype was immunodominant both before and after HAART (Table 2).
Table 2.
In subjects H0018 and H0009, before and after antiretroviral therapy multiple independent CD8+ formal CTL clones or clonal microcultures specific for a defined HIV peptide have identical TCR β-chain nucleotide sequences
| Donor | Peptide | Date | Clone or culture code | V | CDR3† | J | C | Clono-type | No. of identical clones or cultures | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H0018 | gag 703.3 | 06/96 | Clone 4.6 | Vβ5.2 | C | A | S | T | S | G | W | G | S | Y | E | Q | Y | Jβ2.7 | Cβ2 | 1/1 | |
| 01/98 | Clone 2 | Vβ13.1 | C | A | S | N | L | A | P | Y | E | Q | Y | Jβ2.7 | Cβ2 | 18A | 3/3 | ||||
| 04/98 | Culture 3 | Vβ13.1 | C | A | S | N | L | A | P | Y | E | Q | Y | Jβ2.7 | Cβ2 | ” | 2/2 | ||||
| 07/00 | Culture 1 | Vβ13.1 | C | A | S | N | L | A | P | Y | E | Q | Y | Jβ2.7 | Cβ2 | ” | 2/2 | ||||
| H0009 | env 740.19 | 09/96 | Clone 3.2 | Vβ13.6 | C | A | S | S | Y | T | L | D | N | Q | P | Q | H | Jβ1.5 | Cβ1 | 9A | 1/1 |
| 04/97 | Culture 1 | Vβ13.6 | C | A | S | S | Y | T | L | D | N | Q | P | Q | H | Jβ1.5 | Cβ1 | ” | 5/5 | ||
| 06/01 | Culture 1 | Vβ13.6 | C | A | S | S | Y | T | L | D | N | Q | P | Q | H | Jβ1.5 | Cβ1 | ” | 1/1 | ||
| 08/01 | Culture 1 | Vβ13.6 | C | A | S | S | Y | T | L | D | N | Q | P | Q | H | Jβ1.5 | Cβ1 | ” | 2/2 | ||
Bold type indicates cultures derived before starting antiretroviral therapy.
See Table 1 footnote.
Progressive contraction of HIV-specific CD8+ clones and selective expansion of HCMV-specific CD8+ clones with maintenance of strong functional HIV and HCMV-specific CD8+ T-cell responses following HAART
For subjects H0009, H0018 and H0043 we used quantitative oligonucleotide clonotype probing to measure the sizes of individual immunodominant CTL clones over a period of 5–12 years, during which each subject commenced HAART.
From the relative abundance of clonotype within cells of the same TCR Vβ family, and the proportion of Vβ+ cells in the CD8+ population determined by flow cytometry, the number of cells of the clone in 106 CD8+ T cells could be estimated. Because Vβ6.4 and Vα6-specific monoclonal antibodies were not available, it was not possible to determine the clone sizes of Vβ6.4+ or Vα6+ CTL clonotypes.
In subject H0018, following HAART we observed contraction of two HIV-specific CD8+ clones 18 A and 18E (Figs 1 and 2d,e) and selective expansion of one of three Vβ6.4+ HCMV-specific clones specific for the same pp65 peptide 56. The proportion of pp65 peptide 56-specific clonotype 18D sequences within all Vβ6.4+ sequences before HAART was 1·6–1·8%; following HAART, this clonotype increased to 5·2–7·6% (Fig. 2c). In contrast, over a 6-year period before and after HAART, two further pp65 peptide 56-specific clonotypes were stable. The proportion of clonotype 18B sequences within all Vβ6.4+ sequences was stable at 6·2–8·1% (Fig. 2c), and the proportion of clonotype 18C sequences within all Vβ6.4+ sequences was also stable at 0·6–2·8% (data not shown).
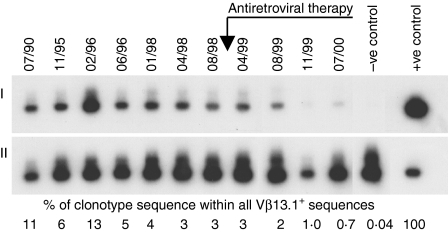
Figure 1.
Progressive contraction of HIV-specific CD8+ clonotype 18A following HAART in subject H0018 (I) Amplified TCR sequences were blotted onto a filter, which was first probed using the labelled clonotypic probe. (II) By stripping the filter and reprobing with a conserved constant region-specific probe that detects all amplified TCRs, we calculated the relative abundance of clonotype sequence as a proportion of all TCR sequences of the Vβ13.1 family.
Prior to HAART in the same subject, the clone size of gag peptide 703.3-specific clonotype 18A was 2000–4500 cells/106 CD8+ T cells. Following HAART, clonotype 18A gradually contracted in size, reaching 280 cells/106 CD8+ T cells 20 months after HAART commenced (Fig. 2d,e). We observed a similar decline in Vα6+ gag peptide 7027-specific clonotype 18E following HAART (Fig. 2e). Over a period of 3–5 years spanning the introduction of HAART despite some variability in the absolute frequency of CTL precursors (CTLp), the LDA frequencies of CTLp specific for two pp65 and one gag peptide were relatively maintained (Fig. 2b,d). Following HAART the proportion of peptide 7027-specific clonotype 18E sequences in all Vα6+ sequences decreased (Fig. 2e), however, the LDA frequency of CTLp specific for gag peptide 7027 was stable on three occasions after HAART (660–1000 CTLp per 106 CD8+ T cells; the LDA frequency for this peptide was not measured prior to HAART).
In subject H0009, env peptide 740.19-specific clonotype 9A expanded over the space of 6 years, from 0·9% of clonotype sequence within all Vβ13.6+ sequences to 33–64% of clonotype sequence within all Vβ13.6+ sequences (Fig. 3e, black circles). This was accompanied by a progressive expansion in Vβ13.6+ CD8+ T cells (Fig. 3e, white circles) causing a progressive 40-fold increase in clone size (Fig. 3d, black diamonds). Following HAART, the clone size progressively decreased from 33 000 to 67 000 cells/106 CD8+ T cells to 5000 cells/106 CD8+ T cells (Fig. 3d,e).
In the same subject, the clone sizes of pp65 peptide 69-specific clonotype 9C and gag peptide 728-specific clonotype 9B were stable over a period of up to 12 years spanning the commencement of HAART (Fig. 3b,c). The frequencies of CTLp specific for pp65 peptide 69 and gag peptide 728 were relatively stable over 6–8 years (Fig. 3b,c), and were unaffected by HAART. The frequency of CTLp specific for env peptide 740.19 was more variable, but 2 years after starting HAART the CTLp frequency was similar to that during the 3–4 years prior to HAART (Fig. 3d).
In subject H0043 we similarly observed contraction of two HIV-specific clones and expansion of an HCMV-specific clone following HAART. The clone size of env peptide 701.78-specific clonotype 43C decreased shortly after starting therapy with only two antiretroviral agents (Fig. 4c), and the clone size of nef peptide 735-specific clonotype 43B decreased gradually after commencement of HAART (Fig. 4d). The pp65 peptide 61-specific clonotype 43A expanded during the first 2 years of HAART – before commencement of HAART, the proportion of clonotype 43A sequences in all Vβ6+ sequences was 0·1–0·2%. Following HAART this clonotype expanded, up to 3·7% of all Vβ6+ sequences (Fig. 4b).
Following HAART in the same subject there was a fivefold decline in the frequency of CTLp specific for env peptide 701.78 that was stable over the next 2·5 years (Fig. 4c). The LDA frequency of CTLp specific for pp65 peptide 61 was not measured prior to HAART – after starting HAART the LDA frequency of CTLp specific for pp65 peptide 61 was 190–730 CTLp per 106 CD8+ T cells over five time-points from 04/99 to 07/00 (data not shown).
In subject H0045, the frequencies of CTLp specific for pp65 peptide 72, env peptide 740·18 and env peptide 7035 were stable at three time-points over 1·3 years, and were maintained following HAART (Fig. 5).
Distribution of virus-specific CD8+ clonotypes in the CD28– CD27– and CD28– CD27+ CD8+ subpopulations
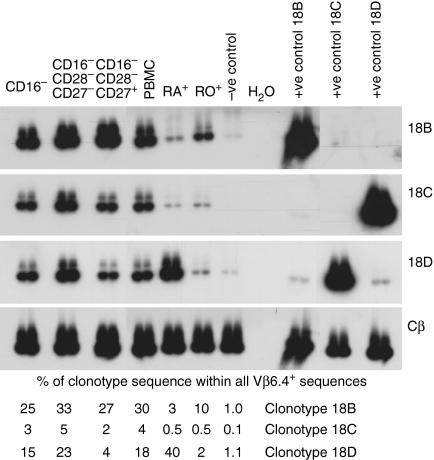
We examined the distribution of individual CD8+ clonotypes in the CD28– CD27– and CD28– CD27+ subpopulations of CD8+ T cells using clonotype probing (Table 3, Figs 3 and 6). Virus-specific clonotype sequences often accounted for a large proportion of all TCR sequences of the same Vβ family. For most CD8+ clones, cells of the clone were distributed in both the CD28– CD27– and CD28– CD27+ subpopulations (Table 3). In subject H0018, after starting HAART, gag peptide 703.3 specific clonotype 18A was almost entirely partitioned in the CD28– CD27+ population – the abundance of this clonotype in CD28– CD27– Vβ13.1+ sequences was very low. Gag peptide 7027-specific clonotype 18E was also relatively more abundant in CD28– CD27+ cells in this subject (Table 3). Clonotypes 18A and 18E contracted following HAART within purified CD28– cells (Fig. 2g). We also studied three HCMV-specific Vβ6.4+ clonotypes each of which was specific for pp65 peptide 56. At three time-points after starting HAART we found that clonotype 18B was distributed evenly between CD28– CD27– and CD28– CD27+ cells, whereas clonotype 18D was relatively more abundant in CD28– CD27– cells (Fig. 6, Table 3). Clonotype 18D expanded following HAART within purified CD28– cells, whereas clonotype 18B remained stable (Fig. 2f).
Table 3.
(a) The proportion of clonotype sequence within all sequences of the same Vβ family in different subpopulations determined by oligonucleotide probing. (b) Clone size of individual clonotypes in different CD8+ subpopulations
| (a) % of clonotype sequence within all Vβ+ sequences | (b) Clone size in 106 CD8+ cells of each phenotype‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sorted cells | Sorted cells | ||||||||||
| Donor | Peptide | Clonotype | Vβ | Date of sample | Total CD8+ cells* | CD28– CD27– | CD28+ CD27+ | Background† | Total CD8+ cells* | CD28– CD27– | CD28+ CD27+ |
| H0018 | pp65 56 | 18B§ | 6.4 | 08/99 | 25% | 33% | 27% | 1.0% | ND | ||
| 11/99 | 13% | 17% | ND | 1.0% | |||||||
| 07/00 | 11% | 16% | 16% | 1.0% | |||||||
| 18C | 6.4 | 08/99 | 3% | 5% | 2% | 0.1% | ND | ||||
| 11/99 | 1% | 2% | ND | 0.0% | |||||||
| 07/00 | 0.6% | 1.4% | 0.7% | 0.0% | |||||||
| 18D | 6.4 | 08/99 | 15% | 23% | 4% | 1.1% | ND | ||||
| 11/99 | 29% | 43% | ND | 1.4% | |||||||
| 07/00 | 21% | 44% | 12% | 1.4% | |||||||
| gag 703.3 | 18A | 13.1 | 08/99 | 6% | 0.8% | 45% | 0.5% | 3100 | 160 | 9300 | |
| 11/99 | 1.2% | 0.8% | >43% | 0.2% | 700 | 140 | >700 | ||||
| 07/00 | 1.3% | 0.2% | 6.4% | 0.2% | 1000 | 40 | 1700 | ||||
| gag 7027 | 18E | Vα6 | 08/99 | 1.8% | 0.8% | 5% | 0.03% | ND | |||
| H0009 | pp65 69 | 9C | 13.1 | 08/99 | 13% | 3% | 0.2% | 2200 | 5100 | ||
| 08/01 | 54% | 15% | 0.2% | 1100 | 1800 | ||||||
| 03/02 | 63% | 53% | 0.2% | ND | ND | ||||||
| 12/02 | 59% | 47% | 0.2% | 5400 | 34900 | ||||||
| gag 728 | 9B | 22 | 08/99 | 0.5% | 0.4% | 0.3% | 0.1% | 260 | 40 | 80 | |
| 08/01 | 0.3% | 0.2% | 0.3% | 0.1% | 100 | 20 | 80 | ||||
| env 740.19 | 9A | 13.6 | 08/99 | 47% | 86% | 0.1% | 7100 | 108000 | |||
| 08/01 | 60% | 77% | 0.1% | 3600 | 166000 | ||||||
| 03/02 | 17% | 70% | 0.1% | 1400 | 50000 | ||||||
| 12/02 | 26% | 91% | 0.1% | 400 | 33700 | ||||||
Bold, samples derived prior to starting antiretroviral therapy.
Either derived from whole PBMC or CD16− depleted PBMC.
Background indicates the magnitude of non-specific binding of the clonotype-specific probe to amplified negative control cDNA.
Calculated from percentage clonotype [shown in (a)] and percentage of CD8+ cells that are Vβ+ (see methods).
Cells of clonotypes 18A-D weere previously also found in both CD28−CD57− and CD28−CD57+ populations (23).
Figure 6.
In subject H0018 three independent Vβ6.4+ CD8+ clonotypes specific for the same HCMV peptide have different phenotypes. Following HAART, clonotype 18B was distributed evenly between CD28– CD27– and CD28– CD27+ cells and was predominantly CD45ROhi whereas clonotype 18D was more abundant in CD28– CD27– cells than CD28–CD27+ cells and was almost exclusively CD45RAhi.
In subject H0009 before HAART the clone sizes of HIV-specific clonotypes 9B and 9A and HCMV-specific clonotype 9C in 106 cells of each subpopulation was greater in the CD28– CD27+ subpopulation compared to the CD28– CD27– subpopulation. Following HAART, the clone size of env peptide 740.19-specific clonotype 9A contracted in the CD28– CD27– and especially in the CD28– CD27+ subpopulation consistent with the contraction in the clone size of this clonotype in whole CD8+ PBMC. The clone size of pp65 peptide 69-specific clonotype 9C expanded in both of these subpopulations (Table 3, Fig. 3f,h).
Distribution of virus-specific CD8+ clonotypes in the CD45RAhi and CD45ROhi CD8+ subpopulations
The cell purification method that we used, purified either CD45RAhi or CD45ROhi cells. For most CD8+ clones, cells of the clone were distributed in both the CD45RAhi and CD45ROhi subpopulations (Table 4, Figs 3 and 6). In subject H0018 after starting HAART, gag-specific clonotypes 18A and 18E, both of which contracted, were predominantly CD45ROhi (Table 4). The pp65 peptide 56-specific clonotype 18B, which did not expand, was predominantly CD45ROhi whereas clonotype 18D, which expanded, was almost exclusively CD45RAhi (Fig. 6, Table 4).
Table 4.
(a) The proportion of clonotype sequence within all sequences of the same Vβ family in different subpopulations determined by oligonucleotide probing. (b) Clone size of individual clonotypes in different CD8+ subpopulations
| (a) % of clonotype sequence within all Vβ+ sequences | (b) Clone size in 106 CD8+ cells of each phenotype§ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sorted cells | Sorted cells | ||||||||||
| Donor | Peptide | Clonotype | Vβ | Date of sample | Total CD8+ cells* | CD45RA+ | CD45RO+ | Background† | Total CD8+ cells* | CD45RA+ | CD45RO+ |
| H0018 | pp65 56 | 18B | 6.4 | 04/99 | 30% | 3% | 10% | 1.0% | ND | ||
| 18C | 6.4 | 4% | 0.5% | 0.5% | 0.1% | ND | |||||
| 18D | 6.4 | 18% | 40% | 2% | 1.1% | ND | |||||
| gag 703.3 | 18A | 13.1 | 04/99 | 7% | 0.9% | 5.0% | 0.5% | 3900 | 510 | 3900 | |
| gag 7027 | 18E | Va6 | 04/99 | 1.5% | 0.5% | 0.7% | 0.03% | ||||
| H0009 | pp65 69 | 9C | 13.1 | 09/98 | 3.5% | 0.5% | 0.3% | 1900 | 350 | ||
| 04/00 | 6.5% | 0.2% | 0.3% | 2200 | 100 | ||||||
| 06/01 | 1.7% | 0.3% | 0.3% | 430 | 300 | ||||||
| 08/01 | 6.3% | ND | 0.3% | 1600 | ND | ||||||
| 03/02 | 10.8% | 0.3% | 0.3% | ND | ND | ||||||
| 12/02 | 3.7% | 0.2% | 0.3% | 1200 | 250 | ||||||
| gag 728 | 9B | 22 | 09/98 | 0.2% | 0.9% | 0.1% | 60 | 400 | |||
| 04/00 | 0.2% | 0.3% | 0.1% | 90 | 230 | ||||||
| 06/01 | 0.1% | 0.2% | 0.1% | 30 | 200 | ||||||
| 08/01 | 0.2% | 0.2% | 0.1% | 30 | 200 | ||||||
| env 740.19 | 9A | 13.6 | 09/98 | 17% | 30% | 0.2% | 3600 | 12 200 | |||
| 04/00 | 72% | 36% | 0.2% | 19 900 | 20 700 | ||||||
| 06/01 | 56% | 10% | 0.2% | 11 900 | 3600 | ||||||
| 08/01 | 12% | ND | 0.2% | 2500 | ND | ||||||
| 03/02 | 1.1% | 1.3% | 0.2% | ND | ND | ||||||
| 12/02 | 0.7% | 0.5% | 0.2% | 80 | 120 | ||||||
| H0043 | pp65 61 | 43A | 6.2 | 11/98 | 1.9% | 0.8% | 0.2% | 0.2% | ND | ||
| 07/99 | 1.8% | 0.5% | 0.3% | 0.2% | ND | ||||||
| env 701.78 | 43C | 7 | 11/98 | 0.2% | 0.1% | 0.7% | 0.1% | ND | |||
| 07/99 | 0.2% | 0.1% | 0.4% | 0.1% | 110 | 30 | 110 | ||||
| H0049 | pp65 61 | 49A | 23 | 03/00 | 20% | 0.6% | ND | ||||
| 07/00 | 20% | 36% | 1.6% | 0.6% | 3200 | ||||||
| gag 788.9 | 49B | 6.2 | 03/00 | 2% | 0.1% | ND | |||||
| 07/00 | 2% | 0.2% | 0.7% | 0.1% | ND | ||||||
Bold, samples derived prior to starting antiretroviral therapy.
Either derived from whole PBMC or CD16− depleted PBMC.
Background indicates the magnitude of non-specific binding of the clonotype-specific probe to amplified negative control cDNA.
Calculated from percentage clonotype [shown in (a)] and percentage of CD8+ cells that are Vβ+ (see methods).
Cells of clonotypes 18A-D weere previously also found in both CD28−CD57− and CD28−CD57+ populations (23).
In subject H0009, env 740.19-specific clonotype 9A was distributed in both CD45RAhi and CD45ROhi cells and remained so during contraction following HAART (Table 4, Fig. 3i). After HAART on 12/2002, we also analysed cells of intermediate CD45RA/RO coexpression that contained large clone sizes of this clonotype (3300 cells per 106 CD8+ cells of this population). The pp65 peptide 69-specific clonotype 9C was predominantly CD45RAhi and remained so following HAART (Table 4, Fig. 3g).
In subjects H0043 and H0049 after starting HAART, HIV-specific clonotypes 43C and 49B were predominantly CD45ROhi, whereas pp65 specific clonotypes 43A and 49A were predominantly CD45RAhi.
Discussion
We found that the clonal composition of CD8+ T cells specific for HIV gag and env epitopes was highly focused and did not change after HAART. Following HAART, there was progressive contraction in the size of dominant HIV-specific CD8+ clones with relative preservation of the functional memory response in vitro. In longitudinal analysis this clonal contraction occurred in each of the phenotypic subpopulations studied and in one case was most marked in CD28– CD27– cells. In the same subjects individual HCMV-specific clones had different kinetics and phenotypes – in two-thirds of subjects, individual HCMV-specific clonotypes progressively expanded following HAART.
Previous studies that have examined the clonal composition of CD8+ CTL specific to HIV have observed a comparable degree of clonal focusing,20,29 although some subjects have a more polyclonal response to individual peptides20 as we observed for CD8+ clonotypes recognizing env peptide 701.78 in subject H0043 (Table 1). In this study we used clonotype probing to study CTL responses specific for 13 viral peptides restricted by six different MHC alleles for many of which tetramers are not available. The clonotype probing technique provides a higher level of resolution by allowing the study of the sizes of individual peptide-specific CTL clones over time and the distribution of individual clones into different phenotypic subpopulations. In one subject who had three different Vβ6.4 clonotypes specific for the same HCMV pp65 peptide, one clonotype selectively expanded following HAART, without expansion of the other two clonotypes. We have also previously reported differential kinetics of clonotypes specific for the same HCMV peptide with delayed emergence of different HCMV-specific clonotypes in recipients following allogeneic haematopoietic stem cell transplantation.30
For a period of 7–11 years following the first HIV seropositive test in subjects H0009 and H0018, we observed progressive selective expansion of HIV-specific clonotypes 9A and 18E while HCMV-specific clonotypes remained stable (Figs 2 and 3). Individual expanded virus-specific clonotypes could be very large, accounting for up to 6·7% of all CD8+ T cells (Clonotype 9A, Fig. 3). We have previously demonstrated similarly large sizes of virus-specific CTL clones in healthy HCMV carriers and HIV-infected subjects;22 by tetramer staining up to 2·7% of CD8+ T cells were specific for individual HIV peptides.11 Because both HIV and HCMV are persistent viruses characterized by sustained or intermittent viral reactivation and antigen expression, repeated exposure of virus-specific memory CTL to viral antigen in vivo over time leads to selective expansion and maintenance of large CD8+ clones that express certain high-affinity TCR with characteristic Vβ framework and hypervariable VDJ amino acid sequences.21,31
Following HAART, we show for the first time progressive contraction of five individual HIV-specific clonotypes in three subjects (Figs 2–4). The half lives of decline of clonotypes 9A, 18A and 43B were between 40 and 103 weeks (calculated starting from first undetectable viral load), which is in agreement with data from tetramer studies of Casazza and Kalams (20–68 weeks and 35–48 weeks, respectively)12,13 but is longer than the half life of 45 days or less measured over a short period immediately after initiation of HAART.11
In subject H0009, before HAART, HIV-specific clonotype 9A was distributed in the CD45ROhi and CD45RAhi subpopulations and in the CD28– CD27– and CD28– CD27+ subpopulations. Following HAART, the clone size of clonotype 9A progressively contracted but remained detectable in each of these subpopulations (Fig. 3) – it was most enriched in the CD28– CD27+ subpopulation. After 1·5 years of contraction following HAART this clonotype was predominantly CD45RA/ROintermediate. After HAART in the other three subjects, the remaining cells of contracting HIV-specific clones were predominantly CD28– CD27+ and CD45ROhi (Tables 3 and 4). This phenotype resembles that reported for HIV tetramer+ cells in untreated HIV infection.17,18,32
In three-quarters of subjects, strong functional HIV-specific CD8+ CTLp frequencies in LDA were maintained following HAART (Figs 2–5). In subject H0018, the dominant gag peptide 703.3-specific clone 18A was almost exclusively CD28– CD27+ (Table 4) and we observed high frequencies of functional gag peptide 703.3-specific CTLp as we have previously reported within CD28-depleted PBMC.22 In the fourth subject following HAART there was a five-fold decline in the frequency of CTLp specific for env peptide 701.78 that was stable over the next 2·5 years (Fig. 4c). In three other studies before and after HAART, in the majority of subjects there was relative maintenance of the frequency of CTLp specific for gag, env, nef or RT (assessed using targets infected with recombinant vaccinia viruses); in a few subjects, HIV-specific CTLp decreased in frequency following HAART. In some individuals there was a transient increase in CTLp frequency immediately following HAART, which we did not observe.13,33,34
There is growing evidence that in untreated HIV infection the function/maturation of HIV-specific CTL may be impaired – 80–85% of HIV-specific CD8+ T cells identified by tetramer staining do not secrete interferon-γ35 and HIV-specific CD8+ T cells may show reduced perforin expression.36 In three HIV-infected subjects prior to HAART the sizes of individual HIV-specific clonotypes were greater than the corresponding CTLp frequencies, indicating that a proportion of these large clones failed to proliferate efficiently in vitro and/or to express cytotoxicity in vitro.36 During longitudinal analysis following HAART for HIV-specific CD8+ T cells the clone size and CTLp frequency tended to converge, implying that cells of the clone which had reduced in vitro cloning efficiency were preferentially lost following HAART. Exposure to high levels of HIV antigen in untreated infection may favour differentiation to CD28– CD27– cells, which may have diminished clonogenic potential. The fact that there was relative maintenance of HIV-specific clones in CD28– CD27+ cells compared to CD28– CD27– cells following HAART suggests that the CD28– CD27+ cells may have relatively preserved clonogenic potential. Despite sustained suppression of HIV viral replication below 50 copies/ml in plasma there may nevertheless be sufficient antigen in lymphoid tissue as a result of persistent viral transcription37 to maintain a high frequency of HIV-specific CTLp – the quantity of viral antigen needed to maintain a virus-specific memory CTLp response is unknown.38 Redistribution of HIV-specific CTL from peripheral blood to lymph nodes following HAART appears unlikely to be the main explanation for the observed clonal contraction in peripheral blood – similar frequencies of HIV-specific CD8+ T cells have been demonstrated in blood and lymph node before and after HAART.39,40
Most HCMV-specific clonotypes were stably maintained for years before HAART, with stable maintenance of the frequency of pp65-specific CTLp (Figs 2–5). In this study, as in our previous work,22 before HAART the estimated cloning efficiency (the LDA response as a proportion of peptide-specific CD8+ T-cell clone size) for HCMV-specific clones was higher than the cloning efficiency of HIV-specific clones (for example in subject H0009 the size of pp65-specific clonotype 9C and the frequency of pp65 peptide 69-specific CTLp were similar). In two-thirds of subjects, we observed progressive expansion of individual HCMV-specific clonotypes within total CD8+ cells over a period of a year following HAART (Figs 2c and 4b), consistent with previous studies demonstrating increased frequencies of CTL specific for pp65 peptide 69 in a proportion of subjects following HAART.12,16 HCMV-specific clonotypes are predominantly CD28– CD45RAhi in HIV-uninfected individuals; individual clones may be predominantly CD28– CD27+ or CD28– CD27–.41 We show here that in untreated HIV infection, HCMV-specific clonotype 9C was also predominantly CD28– CD45RAhi, and its clone size and phenotype did not change following HAART. In subject H0018 the expanding Vβ6.4+ clonotype 18D was predominantly CD28– CD27– CD45RAhi. Vβ6.4+ clonotypes 18B and 18C, which were specific for the same pp65 peptide but did not expand, were distributed in both CD28– CD27– and CD28– CD27+ subpopulations; 18B was predominantly CD45ROhi. The expansion of HCMV-specific clonotypes following HAART is probably related to improved function in vivo of both CD4+ T cells and antigen presenting cells. We have previously reported that in subject H0043 the frequency of HCMV-specific CD4+ T cells was maintained before and after HAART42 whereas HIV-specific CD4+ T cells decline during prolonged HAART.43 The fact that some clones expand whilst others remain unchanged suggests that there may be focal expression of HCMV antigen in specific anatomical locations through which some but not all clones traffic. The fact that expanded HCMV-specific clone 18D remained CD45RAhi may be related to re-expression of CD45RA following in vivo proliferation as we have previously reported during primary HCMV infection.27 Before HAART became available, AIDS patients who had HCMV disease required lifelong anti-HCMV drug treatment. Following HAART, expanded HCMV-specific CD8+ CTL clones appear better able to control HCMV reactivation and prevent HCMV disease without the need for long-term anti-HCMV drug therapy. Following HAART, a minority of patients who have had previous HCMV retinitis experience worsening ocular inflammation, which is likely to be mediated by enhanced virus-specific T-cell responses.
From our work in an albeit limited number of individuals, we conclude that following HAART the size of immunodominant HIV-specific CD8+ T-cell clones diminishes with relative preservation of functional memory responses. Individual HCMV-specific clones had different kinetics and phenotypes in the same subject, and could expand following HAART.
Acknowledgments
This work was supported by Medical Research Council Program Grant G9202171 (to J.G.P.S.) and by a Medical Research Council Cooperative Group Grant. A.J.C. is a Lister Institute Research Fellow. M.P.W. received funding from the Wellcome Trust. We thank Kim Mynard for her excellent technical support.
Abbreviations
- CTL
cytotoxic T lymphocyte
- CTL
precursor (CTLp)
- ddI
didanosine
- HAART
highly active antiretroviral therapy
- HCMV
human cytomegalovirus
- HIV
human immunodeficiency virus
- Lam
lamivudine
- LDA
limiting dilution analysis
- MHC
major histocompatibility complex
- Nev
nevirapine
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- Rit
ritonavir
- Saq
saquinavir
- TCR
T-cell receptor
- VDJ
variable/diversity/joining
- Zid
zidovudine
References
- 1.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Wei X, et al. Antiviral pressure exerted by HIV-1 specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–11. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 3.Ogg G, Jin X, Bonhoeffer S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–6. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 4.Alter G, Merchant A, Tsoukas CM, et al. Human immunodeficiency virus (HIV)-specific effector CD8 T cell activity in patients with primary HIV infection. J Infect Dis. 2002;185:755–65. doi: 10.1086/339338. [DOI] [PubMed] [Google Scholar]
- 5.Lacabaratz-Porret C, Urrutia A, Doisne JM, et al. Impact of antiretroviral therapy and changes in virus load on human immunodeficiency virus (HIV) -specific T cell responses in primary HIV infection. J Infect Dis. 2003;187:748–57. doi: 10.1086/368333. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein–Barr virus in late disease. J Exp Med. 1993;177:249–56. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Baarle D, Kostense S, Hovenkamp E, et al. Lack of Epstein–Barr virus- and HIV-specific CD27– CD8+ T cells is associated with progression to viral disease in HIV-infection. AIDS. 2002;16:2001–11. doi: 10.1097/00002030-200210180-00004. [DOI] [PubMed] [Google Scholar]
- 8.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. Analysis of total human immunodeficiency virus (HIV) -specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–91. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gea-Banacloche JC, Migueles SA, Martino L, et al. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–92. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–77. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- 11.Ogg GS, Jin X, Bonhoeffer S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75:6508–16. doi: 10.1128/JVI.75.14.6508-6516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, Walker BD. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–8. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao J, McNevin J, Malhotra U, McElrath MJ. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J Immunol. 2003;171:3837–46. doi: 10.4049/jimmunol.171.7.3837. [DOI] [PubMed] [Google Scholar]
- 15.Dalod M, Dupuis M, Deschemin JC, et al. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein–Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–16. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollet L, Li TS, Samri A, et al. Dynamics of HIV-specific CD8+ T lymphocytes with changes in viral load. The RESTIM and COMET Study Groups. J Immunol. 2000;165:1692–704. doi: 10.4049/jimmunol.165.3.1692. [DOI] [PubMed] [Google Scholar]
- 17.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Shankar P, Lange C, Valdez H, Skolnik PR, Wu L, Manjunath N, Lieberman J. CD8 T cells specific for human immunodeficiency virus, Epstein–Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–64. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 19.Papagno L, Spina CA, Marchant A, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. Plos Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douek DC, Betts MR, Brenchley JM, et al. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J Immunol. 2002;168:3099–104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 21.Weekes MP, Wills MR, Mynard K, Carmichael AJ, Sissons JGP. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J Virol. 1999;73:2099–108. doi: 10.1128/jvi.73.3.2099-2108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weekes MP, Carmichael AJ, Wills MR, Mynard K, Sissons JGP. Human CD28– CD8+ T-cells contain greatly expanded functional virus-specific memory cytotoxic T-lymphocyte clones. J Immunol. 1999;162:7569–77. [PubMed] [Google Scholar]
- 23.Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JGP, Carmichael AJ. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28– CD8+ T-cell population. Immunology. 1999;98:443–9. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin X, Wills M, Sissons JGP, Carmichael A. Progressive loss of IL-2-expandable HIV-1-specific cytotoxic T lymphocytes during asymptomatic HIV infection. Eur J Immunol. 1998;28:3564–76. doi: 10.1002/(SICI)1521-4141(199811)28:11<3564::AID-IMMU3564>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo G, Graziosi C, Fauci AS. Virologic and immunologic events in primary HIV infection. Springer Semin Immunopathol. 1997;18:257–66. doi: 10.1007/BF00813497. [DOI] [PubMed] [Google Scholar]
- 26.Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65. Frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills MR, Carmichael AJ, Weekes MP, Mynard K, Okecha G, Hicks R, Sissons JGP. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhigh CD8+ T cells comprise both naive and memory cells. J Immunol. 1999;162:7080–7. [PubMed] [Google Scholar]
- 28.Zhang Q, Gavioli R, Klein G, Masucci MG. An HLA-A11-specific motif in nonamer peptides derived from viral and cellular proteins. PNAS. 1993;90:2217–21. doi: 10.1073/pnas.90.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalams SA, Johnson RP, Trocha AK, Dynan MJ, Ngo HS, D'Aquila RT, Kurnick JT, Walker BD. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J Exp Med. 1994;179:1261–71. doi: 10.1084/jem.179.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandhi MK, Wills MR, Okecha G, Day EK, Hicks R, Marcus RE, Sissons JG, Carmichael AJ. Late diversification in the clonal composition of human cytomegalovirus-specific CD8+ T cells following allogeneic hemopoietic stem cell transplantation. Blood. 2003;102:3427–38. doi: 10.1182/blood-2002-12-3689. [DOI] [PubMed] [Google Scholar]
- 31.Silins SL, Cross SM, Elliott SL, et al. Development of Epstein–Barr virus-specific memory T cell receptor clonotypes in acute infectious mononucleosis. J Exp Med. 1996;184:1815–24. doi: 10.1084/jem.184.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogg GS, Kostense S, Klein MR, Jurriaans S, Hamann D, McMichael AJ, Miedema F. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J Virol. 1999;73:9153–60. doi: 10.1128/jvi.73.11.9153-9160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinaldo CR, Jr, Huang XL, Fan Z, et al. Anti-human immunodeficiency virus type 1 (HIV-1) CD8(+) T-lymphocyte reactivity during combination antiretroviral therapy in HIV-1-infected patients with advanced immunodeficiency. J Virol. 2000;74:4127–38. doi: 10.1128/jvi.74.9.4127-4138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontesilli O, Kerkhof-Garde S, Notermans DW, et al. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Iglesias E, Samri A, Kamkamidze G, Decoville T, Carcelain G, Autran B. A systematic comparison of methods to measure HIV-1 specific CD8 T cells. J Immunol Meth. 2003;272:23–34. doi: 10.1016/s0022-1759(02)00328-9. [DOI] [PubMed] [Google Scholar]
- 36.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 38.Oehen S, Waldner H, Kundig TM, Hengartner H, Zinkernagel RM. Antivirally protective cytotoxic T-cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J Exp Med. 1992;176:1273–81. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altfeld M, van Lunzen J, Frahm N, et al. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J Clin Invest. 2002;109:837–43. doi: 10.1172/JCI14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oxenius A, Yerly S, Ramirez E, Phillips RE, Price DA, Perrin L. Distribution of functional HIV-specific CD8 T lymphocytes between blood and secondary lymphoid organs after 8–18 months of antiretroviral therapy in acutely infected patients. AIDS. 2001;15:1653–6. doi: 10.1097/00002030-200109070-00007. [DOI] [PubMed] [Google Scholar]
- 41.Wills MR, Okecha G, Weekes MP, Gandhi M, Hicks R, Carmichael AJ, Sissons JGP. Identification of naive or antigen-experienced human CD8+ T-cells by expression of co-stimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T-cell response. J Immunol. 2002;168:5455–64. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 42.Weekes MP, Wills MR, Sissons JGP, Carmichael AJ. Long-term stable expanded human CD4+ T cell clones specific for human cytomegalovirus are distributed in both CD45RAhigh and CD45ROhigh populations. J Immunol. 2004;173:5843–51. doi: 10.4049/jimmunol.173.9.5843. [DOI] [PubMed] [Google Scholar]
- 43.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral supression. Nat Med. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]