Abstract
Stress-activated protein kinases (SAPKs) are activated in human inflammatory bowel disease (IBD). Recently it has been demonstrated that p38MAPK (mitogen-activated protein kinase) inhibition using SB203580 is effective in reducing disease in both dextran sulphate sodium (DSS)-induced and 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced murine colitides, underscoring the importance of this pathway in gastrointestinal inflammation. However, the contribution of c-Jun N-terminal kinase (JNK) in intestinal inflammation is unknown. Based on the known involvement of JNK in tumour necrosis factor-α (TNF-α) expression and in mediating the effects of oxidant stress, we hypothesized that JNK inhibition would also affect colitis. Our studies in mice with DSS-induced colitis treated with the JNK inhibitor SP600125, indicate that there is a significant reduction in wasting as well as a significant reduction in histological damage scores. Both total colonic and mesenteric lymphocyte CD3/CD28-stimulated TNF-α levels were dramatically reduced under the same circumstances. This was associated with a reduction in JNK protein expression and activity, as well as a reduction in AP-1 DNA binding with SP600125. Interestingly, there were no apparent changes in either p38MAPK or p42/44ERKs. Immunofluorescence of the colon for the active form of JNK revealed a prominent signal arising from the infiltrating inflammatory cells. SP600125 reduced this as well as, specifically, macrophage infiltration. Strikingly, we also demonstrate reduced epithelial cell apoptosis in response to treatment with SP600125. We conclude that specific inhibition of JNK is beneficial in the DSS model of colitis, and may be of value in human IBD.
Keywords: cytokines, dextran sulphate sodium, inflammatory bowel disease, mitogen-activated protein kinase, SP600125
Introduction
Efforts directed at the genetics of the inflammatory bowel diseases (IBDs) have led to the identification of mutations in the NOD2 gene in Crohn's disease (CD) but not ulcerative colitis (UC).1,2 This intracellular pathogen recognition receptor, which is analogous to the Toll-like receptors, is responsible for the sensing of microbial material within the gut (muramyl dipeptide)3,4 and consequently plays a role in maintaining the immunological homeostatic interface between the gut and the complex enteric flora. Whilst previous work indicated that NOD2 was redundant for nuclear factor-κB (NF-κB) activation in macrophages5 and that its genetic ablation did not lead to spontaneous intestinal inflammation,6 intriguing recent work using NOD2–/– mice reveals a role in increased tendency to infection with Listeria monocytogenes as well as a role in the elaboration of key intestinal antimicrobial chemicals known as cryptidins.7 Furthermore, mice bearing the most common of the human mutations with a C-terminal truncation (3020insC), exhibit enhanced NF-κB activation and colitis, together with increased interleukin-1β (IL-1β) processing.8 Hence, given the right genetic predisposition, breakdown of the intricate intestinal microbial/immunological balance has a direct bearing on inflammation. It is then perhaps not surprising that an array of genetic interventions leads to spontaneous IBD in animals.9 Observations from animal models which differ vastly in their molecular features (e.g. IL-10 and MDR1 knockouts) or tissue of origin (T-cell- or epithelial-cell-driven models), have provided useful insight into possible disease regulatory mechanisms.
The lack of a uniform underlying mechanism for IBDs makes it important to focus therapy upon targets that are common to a variety of pathways of immune activation, and in practice this works especially well at the milder end of the spectrum of disease activity. Thus the same drugs may be used in cases of CD and UC, best exemplified by 5-aminosalicylates, sulfasalazine and glucocorticoids. With more severe disease the situation becomes more complex. Hence, monoclonal anti-tumour necrosis factor-α (TNF-α) antibodies have considerably improved the outlook for patients with CD, but not convincingly so for UC.10 However such biological therapies have their drawbacks, notably their expense and sometimes their side-effects. In the search for newer agents it has been reported that the stress-activated protein kinase (SAPK) inhibitor CNI-1493 can attenuate human CD even in some cases in which anti-TNF-α antibody has failed.11 This work was the first to draw attention to these molecules in the context of IBD. Although concurrently it was reported that the specific p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 was not of much benefit in murine 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced colitis,12 more recently this observation has been challenged, and the same agent has been able to significantly impact on inflammation in models of both dextran sulphate sodium (DSS) and TNBS-induced disease.13,14
The SAPKs comprise two main classes of molecules, specifically the c-Jun N-terminal kinase (JNK) family and the p38 family. These are activated in response to numerous stimuli including cytokines and physiological stressors such as osmolarity, oxidant stress and UV irradiation.15 The JNK class of enzymes comprises three main types simply called JNK1, JNK2 and JNK3. The first two are more ubiquitously distributed whereas the third is confined to the central nervous system and cardiac myocytes. They are regulated by Map/Erk kinase4 (MEK4) and MEK7, which are dual specificity kinases mediating phosphorylation on the ‘TPY’ motif which is the hallmark of these kinases. The JNKs subsequently phosphorylate c-Jun and enable the activation of the activator protein 1 (AP-1) transcription factor that is known to be involved in the expression of many inflammatory genes.16 While there is accumulating information on the role of these proteins in inflammatory disorders such as arthritis,17 their role in IBD is not as well understood. However, there is recent documentation of their activation in IBD tissue.18 Here we report that SP600125, a more specific inhibitor of the JNK pathway, also modifies disease activity in the acute DSS-induced colitis model.
Materials and methods
Animal studies
We used the DSS murine model of colitis, which has been previously validated as a reliable model for investigation of colitis.19 Eight-week-old C57BL/6 mice were obtained from Dr A. Mui (Department of Medicine, Vancouver, BC). Five animals were used in each limb of the study and the experiments were repeated twice. Mice were given 2·5% DSS in their drinking water and were killed on day 7 for evaluation of colitis. After death, several parameters were determined in the inflamed mucosa, namely: evaluation of macroscopic damage scores; histological evaluation of inflammation by haematoxylin and eosin staining; processing of tissue for Western blot analysis and electromobility shift assays. The tissue used for these determinations was processed as previously described.20 Macroscopic assessment of disease activity was scored as follows: 0–4 points for weight loss (0, no weight loss; 2, up to 10% loss; and 4, greater than 10% loss); 0–4 points for blood loss (0, no blood loss; 2, occult loss; and 4, gross bleeding); 0–4 points for stool consistency (0, normal stool; 2, semiformed but not adherent to anus; 4, liquid and adherent). After removal from the animal, colons were fixed in 4% formalin and embedded in paraffin before being cut into 4-mm thick sections. They were then stained with haematoxylin and eosin and scored by two different pathologists who were blinded to the treatment. Microscopic scoring was performed according to a modification of the system reported by Dieleman et al.21 This score grades the severity of the lesion from 0 to 16 based on the severity of inflammation (0–3), the extent of inflammation (0–3, depending on mucosal → transmural inflammation), ulceration (0–3), crypt damage (0–3, depending on extent), and percentage involvement (0–4, from 0 to 100% involvement).
Immunofluorescence
Paraffin-embedded colonic tissue samples were de-waxed in xylene twice for 5 min each time, rehydrated in an ethanol series (100–70%) for 3 min each followed by rehydration in phosphate-buffered saline (PBS) for 30 min. After rehydration, the endogenous peroxidase was blocked with 0·3% hydrogen peroxide followed by antigen retrieval by microwaving sections in citrate buffer pH 6·0 (10 mm sodium citrate). Following antigen retrieval, the sections were washed three times with PBS, blocked in 4% skimmed milk for 1 hr, and then stained using the kit mentioned below according to the manufacturer's recommendations but with the following modifications. Sections were incubated with the primary antibody at 4° overnight. The following antibodies were used at the indicated dilutions: PpJNK (1 : 100, NEB, Pickering, CAN), anti-rat F4/80 (1 : 100). Sections were stained using a Vectastain ABC elite kit and biotinylated ant-rabbit (or anti-rat) antibody, and avidin D–fluorescein isothiocyanate used for immunofluorescence (Vector Laboratories, Burlingame, CA). Each section had its own control using the secondary antibody only. Preimmune serum was initially used to ensure specificity of the signal with each of the antibodies. The sections were counterstained with Hoechst 342 (1 μg/ml).
Western blot analysis
Tissue was placed in homogenization buffer (20 mm MOPS, 150 mm NaCl, 50 mm β-glycerophosphate, 5 mm ethyleneglycoltetraacetic acid, 50 mm NaF, 1 mm dithiothreitol, 1 mm sodium vanadate, and 1 mm phenylmethylsulphonyl fluoride) and sonicated for 15 seconds (twice) and centrifuged at 13 000 g for 15 min. The protein concentration in the supernatant was determined by the Bradford assay (Bio-Rad, Mississauga, ON, Canada). From each sample, 25 μg protein was resolved using 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis before transferring to nitrocellulose membranes (Bio-Rad). The blots were blocked in 5% skimmed milk in TBST (20 mm Tris–HCl pH 7·4, 250 mm NaCl, 0·05% Tween-20) for 1 hr before probing for 2 hr using the appropriate primary antibody. The blots were washed with TBST for 10 min three times, before being incubated with the appropriate secondary antibody for 1 hr. Following three further washes in TBST, they were developed using the enhanced chemiluminescence detection system (ECL, Amersham, Montreal, QC, Canada). In all the figures the prefix ‘p’ denotes the protein form of the kinase (e.g. pJNK), and the prefix ‘P’ indicates the phosphorylated form.
Electromobility shift assay
This was performed as previously described.20 Briefly, 5 μg tissue lysate was preincubated in binding buffer (20 mm HEPES pH 7·9, 100 mm KCl, 10% glycerol, 1 mm dithiothreitol) and 1 mg of poly(dIdC) (Amersham, Montreal, QC, Canada), for 15 min; 20 000 counts per min of probe was then added, and the reaction mixture was incubated at room temperature for 30 min, and then resolved on a 5% non-denaturing polyacrylamide gel in 0·25 × Tris-Borate-EDTA (TBE) at 200 V for 1·5 hr. The gel was subsequently dried for 45 min before phospho-imaging analysis using a Bio-Rad molecular imager FX (or alternatively the gel was exposed to film overnight at − 80°C and then developed). For supershift or cold competitor reactions, the nuclear extract was preincubated with 1 μg anti-c-Jun antibody (Calbiochem, San Diego, CA), or 100-fold excess of unlabelled probe with binding buffer and poly(dIdC) for 30 min before adding the radiolabelled probe. The sequence of the probe (obtained from Santa Cruz, CA) was 5′-CGC TTG ATG ACT CAG CCG GAA-3′.
Mesenteric lymphocyte isolation
Mesenteric lymph nodes were identified, removed and processed as previously reported.22 After gentle grinding the suspension was passed through a 40-μm mesh. The cells were treated with ACK buffer (0·15 m NH4Cl, 10 mm KHCO3, 0·1 mm ethylenediaminetetraacetic acid) for 10 min followed by centrifugation at 200 g for 10 min. The cells were suspended in RPMI-1640 containing 10% serum-conditioned media containing 2 mm mercaptoethanol. Subsequently, cells were plated out at a concentration of 1 × 106/ml, into the wells of a 96-well plate coated with anti-CD3 (5 μg/ml) and costimulated with CD28 (1 μg/ml). The plates were incubated for 1–3 days at 37° in 5% CO2. The supernatants were collected and stored at −70° until analysis using commercially available enzyme-linked immunosorbent assay kits for TNF-α, interferon-γ (IFN-γ) and IL-12p40 (BD Pharmingen, Missisauga, CAN).
Detection of apoptosis using Apostain
Paraffin-embedded colon samples were de-waxed in xylene twice for 5 min each time and then rehydrated in graded ethanol (100–70%) three times, followed by rehydration in PBS for 30 min. The sections were then treated with PBS containing 0·2 mg/ml saponin and 20 μg/ml pronase K for 15–20 min, and washed in PBS three times for 5 min each time. The slides were then immersed in a Coplin jar containing 50% formamide at 56° for 30 min and then immediately transferred into ice-cold PBS. After blocking in 3% skimmed milk in PBS for 30 min, slides were stained with Mab F7-26 (Chemicon, Temecula, CA) 10 μg/ml in PBS with 1% skimmed milk, biotinylated secondary anti-mouse immunoglobulin M (Dako, Mississauga, CAN) for 20 min and finally with Avidin D–horseradish peroxidase for 20 min. The slides were developed using DAB (Vector Laboratories) for 5–10 min and were counterstained with Harris's haematoxylin.
Statistical analysis
The results are expressed as mean ± SD, with P < 0·05 being considered significant using Student's t-test. Band densities (for the Western immunoblots) were measured by scanning the film using the Bio-Rad gel-doc apparatus into a TIFF format file. The band densities and the results are expressed as mean ± SD.
Results
Effects of SP600125 on wasting and colonic inflammation
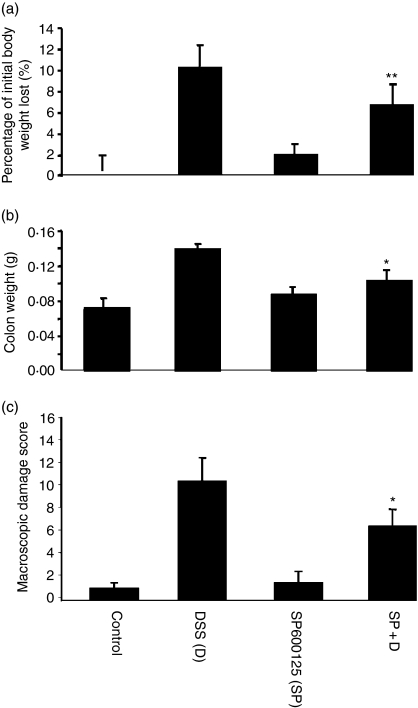
DSS-induced colitis is attended by weight reduction usually in association with reduced food intake, as reported in a recent study where it was shown that SB203580 could modulate colitis, and that it could attenuate wasting.13 We monitored the animals carefully in respect of this and feeding (food and water intake) behaviours. Mice given SP600125 alone or with DSS exhibited no untoward reactions. We observed that treatment with SP600125 led to a small but significant attenuation of weight loss that was observed at both concentrations of the inhibitor used (5·9% in the treated animals versus 9·5% in those with DSS intake alone, n = 10 animals/group). Their macroscopic damage scores were significantly reduced and accordingly their colonic weights were also lower (Fig. 1a–c). These findings indicate that the inhibition of JNK was associated with a beneficial effect on inflammation in this model.
Figure 1.
SP600125 partially attenuates wasting in murine colitis. (a) Animals were weighed daily after administration of 2·5% DSS in their drinking water (data shown are for experiments using 20 mg/kg of SP600125, 10 animals in each group). There was a reduction in the amount of weight loss in the animals that were treated with SP600125 (mean of 5·9% versus 9·6% for those treated with DSS alone, P < 0·01). (b) The colons were removed and weighed from the same group in (a). There was a reduction in the weights (0·14 g versus 0·10 g, P < 0·05). (c) Assessment of colonic damage was as indicated in the Materials and methods section. This parameter was also reduced in the SP600125 treated group. ‘SP’ refers to SP600125 at 20 mg/kg in all the figures (unless otherwise indicated). *P < 0·05, **P < 0·01.
SP600125 reduces histological damage
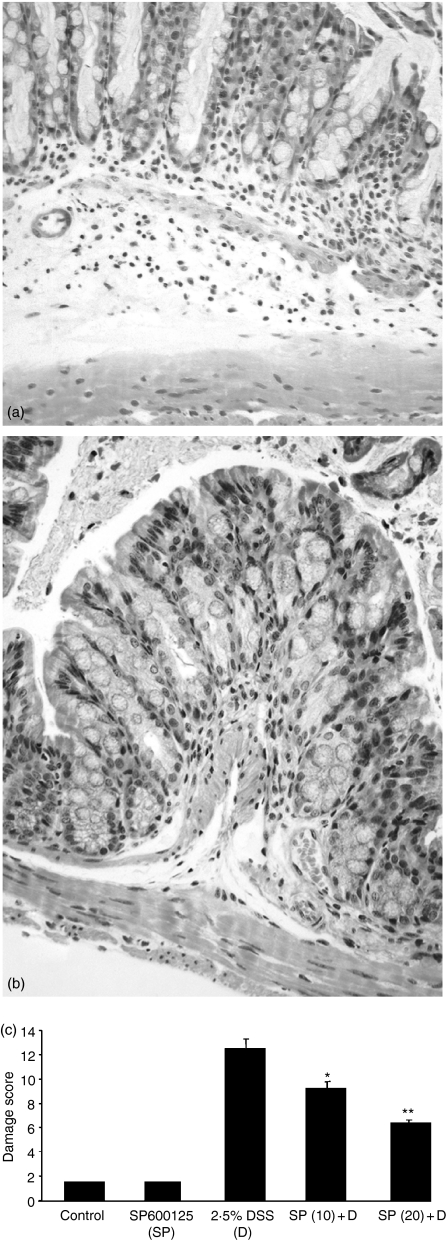
Our next aim was to evaluate the colonic microscopic changes consequent upon JNK inhibition in a setting of DSS-induced colitis. There was uniform distal inflammation in the group treated with DSS alone, manifested by oedema, crypt destruction and an impressive cellular infiltrate, together with a variable extent of surface epithelial cell destruction. Mice in the SP600125-treated group had significantly reduced inflammation and there were no deaths within either group using 2·5% DSS. The data indicate that there was a beneficial effect on inflammation-related damage and that this was more prominent at the higher dose of inhibitor (20 mg/kg), as can be seen in Fig. 2(c). Comparing Fig. 2(a) and 2(b), it can be seen that the inflammatory cell infiltrate was dramatically reduced in the SP600125-treated samples. This finding is compatible with the notion that JNK is involved in proinflammatory signalling in the intestine, and it is in accordance with the reduction observed in the macroscopic damage scores.
Figure 2.
SP600126 positively influences histological damage scores in DSS-induced colitis. Following treatment of animals, the colons were harvested and histological evaluation was carried out after staining with haematoxylin and eosin by two pathologists blinded to the origin of the samples. The sections demonstrate the colonic wall in transverse section format. On inspection of (a) (DSS treatment alone), there is an obvious inflammatory cell infiltrate, crypt destruction and submucosal oedema, that is attenuated in the SP600125 section (b). The data are represented graphically in (c), where it can be seen that there appears to be a dose-dependent reduction in the histological damage scores consequent upon SP600125 use, such that at 20 mg/kg of the inhibitor, there is a 50% reduction in this parameter. *P < 0·05, **P < 0·01.
SP600125-associated cytokine changes
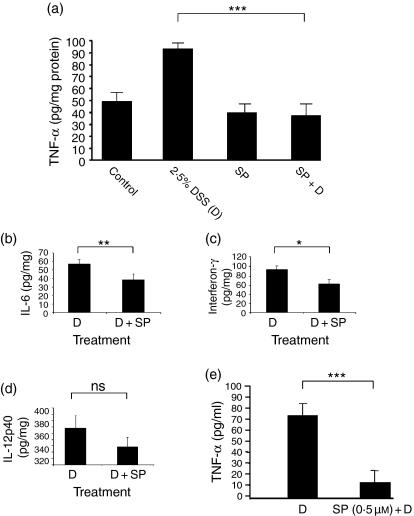
Previous work has detailed the role of JNK in TNF-α expression and revealed an effect at the level of translation.23 Furthermore, it is now established that TNF-α is an important mediator of inflammation in this model and in human disease,24,25 therefore we chose to focus on changes in this molecule, together with other key T helper type 1 (Th1) cytokines such as IFN-γ, IL-6 and IL-12. Initially we determined their protein concentrations in colonic homogenates. In keeping with the preceding data and an anti-inflammatory action for SP600125, we observed a significant reduction of the levels of TNF-α in the murine colon (Fig. 3a). IL-6 and IFN-γ also decreased in response to SP600125 (Fig. 3b,c). However, the data for IL-12 did not achieve statistical significance (Fig. 3d). We next characterized the effects of SP600125 upon CD3/CD28-activated mesenteric lymphocytes and demonstrated that it was capable of inhibiting TNF-α production (Fig. 3e).
Figure 3.
SP600125 reduces tissue and lymphocyte-activated cytokine production. (a) – (d) Colonic homogenates were used for evaluation of tissue levels of the cytokines TNF-α, IL-6, IFN-γ and IL-12. The data are for 10 animals per group (five animals, repeated once). There appears to be a normalization of the TNF-α levels in those animals given SP600125. No obvious effect was observed using the inhibitor alone. Significant reductions are observed for both IFN-γ and IL-6 in the treated group. (e) Lymphocytes were obtained from mesenteric lymph nodes and stimulated using anti-CD3/28 antibodies, with or without the SP compound (500 nm). After incubation for 3 days the supernatant was collected and used for performing TNF-α estimations using a commercially available enzyme-linked immunosorbent assay kit (BD Pharmingen). There is a marked reduction in the amount of TNF-α secreted. *P < 0·05, **P < 0·01, ***P < 0·001.
Signalling events in the intestine
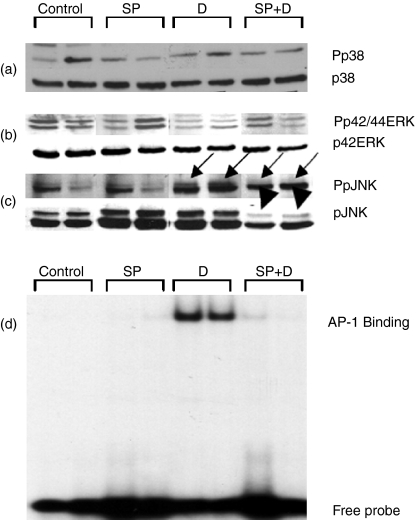
The MAPK family members were then addressed both in terms of the level of expression of the individual members and of their levels of activation (Fig. 4a–c). The representative blot shows that there was no change in either p38 or p42ERK at the 7-day time-point, particularly in the two comparison groups of interest (SP600125 + DSS versus DSS alone). There was no significant change in the intensity of the Pp42/44Erk signal in the treated group compared with the DSS-alone group. This contrasts with the changes in the JNK. Surprisingly, there was a reduction in the expression of the protein in response to intervention with SP600125. Expression was reduced to a mean of 65% of that for DSS alone, for the p54JNK isoform, and 77% of that for DSS alone for the p45JNK isoform (P < 0·05, both comparisons, n = 6 per comparison group, three paired samples, repeated once). There was no significant change in the expression of the JNK isoforms in response to SP treatment, in comparison with control animals. The signal intensity for the SP-treated group for PpJNK was 74% of the DSS group (P < 0·05). We next directed attention to the AP-1 transcription factor, which is the target of the JNK signalling pathway and, as mentioned earlier, is a pivotal transcription factor involved in the expression of proinflammatory genes. The data clearly indicate an impressive attenuation of the DSS-induced activation of this signal (Fig. 4d). Collectively these findings indicate that the JNK signalling pathway is activated in DSS-induced colitis and is modified by a specific inhibitor.
Figure 4.
Signalling effects of SP600125. We performed Western blot analysis on tissue lysates obtained from each group of animals. After resolution on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis the subsequent steps are as described in the Materials and methods. Phosphospecific antibodies (Pp38, Pp42/44ERK and PpJNK) were obtained from Cell Signaling, and the remaining antibodies were from Santa Cruz (p42ERK and pJNK), and p38 was from Stressgen Biotechnology Corporation (Stressgen, Victoria). The data are representative of the experiment performed three times. (a,b) p38 and p42Erk; the arrows indicate changes induced by inflammation in the phosphosignal for JNK (c, solid arrows) and reduction of the signal with SP600125 treatment (dashed arrows). The arrowheads indicate that there is a reduction in the expression of the slower migrating JNK (p54) band using this inhibitor. In (d) a representative electromobility shift assay autoradiogram is shown. This shows that there is a marked induction of AP-1 DNA binding with DSS treatment that is attenuated with SP600125.
SP600125 targets macrophages
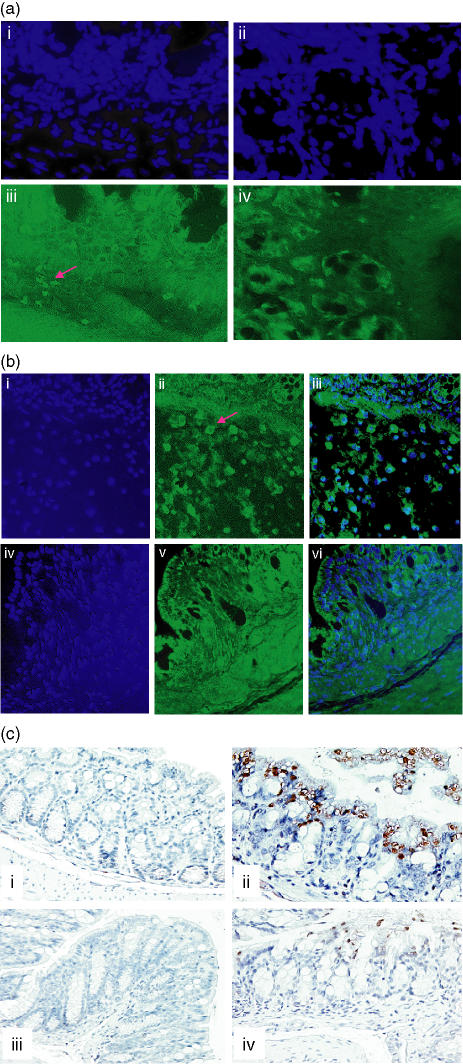
We wanted to investigate the origin of the enhanced JNK signal from the inflamed intestine and so used immunofluorescence to examine this further. As the figure indicates (Fig. 5a(iii)), there was increased PpJNK signal intensity within parts of the intestinal wall as well as from the inflammatory cells located there. This signal was attenuated in response to SP600125 treatment (Fig. 5a(iv)). To determine if macrophages formed a significant component of this response we made use of the F4/80 antibody. The data indicate (Fig. 5b(ii)) that macrophage numbers were increased in the DSS-treated animals. This effect was markedly attenuated in the SP600125-treated animal shown (Fig. 5b(v)). These findings are in keeping with previous observations identifying the macrophage as an important component of the inflammatory response in this model.9
Figure 5.
(a) Site-specific modulation of JNK activity. The PpJNK antibody was used in immunofluorescence studies to determine the site of change in JNK activity. The specimens had also been counterstained with Hoechst 342 at 1 μg/ml (panels i and ii are the controls for iii and iv, respectively). Panel (iii) shows that the PpJNK signal for the DSS-treated animals arises from several sites including the crypts as well as from the infiltrating immune cells. A representative section from an animal treated with SP600125 (iv) reveals that there is an attenuation of this signal in both the crypts but it is also associated with a reduction in the number of positively staining immune cells (experiment repeated in five other paired DSS versus DSS + SP600125 samples). (b) Macrophages are targeted by SP600125. Colonic sections were then probed with the F4/80 antibody to address the relative numbers of macrophages within the DSS-treated and SP600125-treated samples. Panels (i) and (iv) indicate the Hoechst 342 controls for the two conditions. (ii) and (iii) depict positively staining cells, which are clearly reduced in the SP600125 treated sample (v, vi). Panels (iii) and (vi) are the overlayed images for the two stains. These data clearly indicate a reduction in macrophage numbers within the colons of animals where JNK has been inhibited. (c) Inhibition of JNK is associated with a reduction in epithelial cell apoptosis. Colonic sections were probed with the monoclonal antibody F7-26 (Chemicon), and after incubation with a biotinylated secondary antibody and avidin-conjugated horseradish peroxidase, colour was developed using DAB. Control section reveals very little spontaneously occurring apoptosis (i), whereas there is a clear increase in the DSS treated animals (ii). The inhibitor alone does not lead to any increase in the extent of spontaneously occurring apoptosis (iii). Panel (iv) however, reveals that the numbers of apoptotic cells are dramatically reduced in the SP600125 treated animal shown (data are representative for the entire group, n = 5 per analysis group at 20 mg/kg dose).
SP600125 targets intestinal epithelial cell (IEC) apoptosis
Ultimately the brunt of the inflammatory response falls on the epithelial cell lining of the intestine, resulting in mucosal ulceration, which is probably responsible for the symptoms associated with the IBDs. As we observed a beneficial response with SP600125, we were keen to explore the extent of the protection exerted by suppression of JNK signalling on the integrity of the IECs. For this we used ‘Apostain’, which is a marker for apoptosis. Using immunohistochemistry we found that there was significantly increased apoptosis in the IECs of animals with DSS-induced colitis (Fig. 5c(ii)). This was apparent in all parts of the colon, and was clearly reduced in the SP600125-treated animals (Fig. 5c(iv)). In conjunction with the above data, this supports the notion that macrophage-derived cytokines are important determinants of inflammatory damage in the intestine.
Discussion
There is a continuing need for developing novel therapies in the management of inflammatory disorders such as IBD because it is obvious that none of these entities can be managed in a ‘one glove fits all’ manner. Furthermore, the current interest in biological therapies will probably be tempered not only by cost concerns but possibly also by increasing concerns regarding safety, especially long-term safety.26 Hence it is not surprising that cellular signalling pathways that play integral roles in inflammation continue to receive considerable attention. In the context of IBD, current information indicates that not only are these pathways (SAPKs) activated in diseased patients, but also their modulation can be associated with positive effects on outcome. This work was undertaken to determine the relative contribution of the JNK pathway, because previous work did not clarify this issue and also because this is an important determinant of TNF-α production. Herein we have described for the first time that a specific JNK inhibitor, SP600125, is able to favourably affect disease outcome at several levels. We have demonstrated that SP600125 inhibits JNK activation as well as AP-1 activation in murine colitis. As TNF-α-mediated AP-1 activation has been previously established to be JNK-dependent,27 this is a potential mechanism for our observations also. Furthermore, our data are compatible with SP600125 primarily reducing the extent of macrophage influx and activation, thereby reducing the levels of TNF-α. Another potential mechanism for the reduced macrophage numbers is enhanced apoptosis of these cells in the presence of the JNK inhibitor; however, we did not observe a significant difference in this parameter in the treated group compared to the group given DSS alone.
We noted that there was some discrepancy between the effects of SP600125 on TNF-α production (dramatic reduction) and an incomplete reversal of the DSS-mediated inflammatory insult. Although, we observed a reduction in the concentrations of other Th1 cytokines, principally IFN-γ and IL-12, it is conceivable that additional molecules such as chemokines and intracellular adhesion molecule-1 may play a significant role, as these are only weakly modulated by SP600125.28 Additionally, it is conceivable that there is a component of necrosis that is not readily interrupted by modulation of the JNK activation in this model, although recent work appears to indicate that reactive-oxygen-species-dependent necrosis is JNK-dependent.29 We cannot exclude other mechanisms for cell damage that are not dependent on reactive oxygen species, for example through enhanced protease activity.
Whilst it has been observed that TNF-α neutralization reduces IEC apoptosis, most of the reported observations have been directed at the small bowel.30,31 Our observations are the first to correlate the effects of JNK inhibition with a reduction in TNF-α expression and IEC apoptosis in the colon.
The question of whether TNF-α is involved in acute DSS-induced colitis has been addressed in the past with findings that either support a role in disease improvement25 or worsening of colitis, when TNF-α is inhibited.32,33 Myers and colleagues demonstrated a beneficial effect on colitis of using both an antibody against TNF-α as well as antisense oligonucleotide therapy to down-regulate TNF-α.25 Notably, they found that the amount of antibody used had a significant impact on the outcome; 25 μg/mouse was effective in ameliorating colitis but 50 μg/mouse was not. In contrast to these findings, Kojouharoff et al. observed that a more complete inhibition of TNF-α was deleterious (they used 100 μg/mouse) in their study.32 Accordingly, if it is proposed that TNF-α serves a protective function in acute colitis, genetic disruption would also be expected to show a similar outcome. This was the experience of Naito et al.33 who reported more severe disease in TNF-α knockout mice. The findings of these studies would appear to indicate that whilst a pathologically increased amount of TNF-α is capable of worsening colitis in the acute setting, a more regulated expression is required to mediate a protective response.
With respect to the role of other cytokine involvement, it has been reported that IL-6 knockout mice have less severe disease.34 Our observation that IL-6 is reduced by SP600125 is in keeping with this finding.
There is unlikely to be a direct effect on anoikis by the inhibitor as at least two previous studies have indicated that JNK is not directly involved in this physiological event. In the first an inverse effect upon apoptosis was correlated with activation of phosphatidylinositol 3-kinase, but inactivation of JNK failed to protect against detachment-induced cell death in Madin–Darby canine kidney (MDCK) cells.35 In the second a similar lack of association was observed with Fas-induced HT29 cellular apoptosis.36 However, this does not absolutely exclude a role for JNK in other forms of cell death as we have previously linked JNK with oxidant-induced cell death.37 Hence it is possible that the response to reactive oxygen species is being regulated at the level of IECs.
An important point is whether or not the dose or frequency of administration used is adequate. This is based upon the fact that two reports investigating SB203580 in the same model of colitis (TNBS-induced) achieved contradictory results.12,14 The more recent of the two questioned the dosing frequency of the first study, and was able to show a beneficial effect. We cannot discount the possibility that changing the frequency of SP600125 administration may improve our findings further. It should be noted that we used the same concentration as that used by Firestein's laboratory in the previously reported arthritis model.17 Undoubtedly a follow-up study will need to establish the optimum dosing schedule.
The model used in this work is generally taken to be one that resembles UC, albeit one that exhibits prominent expression of TNF-α. Previous work has indicated that it depends more on components of innate than adaptive immunity.38 (A role for the latter has been invoked for the chronic but not acute phase of DSS-induced colitis.) This has implications for this study also because JNK has previously been ascribed (demonstrated both genetically and using the SP600125 inhibitor) a role in T-cell differentiation but not T-cell activation.39 Whilst a similar effect can be expected to occur in Th1 models of colitis (as in animal models of arthritis) further work is necessary to address this issue.
The findings from this study may facilitate understanding of several previous observations, by causally linking JNK to intestinal inflammation. These include the observation that JNK is activated in human IBD18 and a correlation has been proposed between the site of its activity and steroid responsiveness.40 More specifically in steroid-resistant disease a prominent signal was seen to arise from the IECs whilst in steroid-responsive cases it was seen in inflammatory cells. The fact that some cases of CD resistant to anti-TNF-α responded to CNI-1493 may be supportive of an additional IEC-dependent mechanism of IBD activity/persistence. In further support of a direct role for JNK in intestinal pathophysiology, PARP-1–/– mice were noted to have less severe TNBS-induced colitis in association with reduced JNK and AP-1 DNA binding activity.41
A role for SAPKs has not been directly established in NOD2-mediated events. However, in the two recent reports on the use of the SB203580 compound, an inhibitory effect on RICK, an important effector of NOD2 in NF-κB activation, was demonstrated.13,14 Nevertheless, the mechanisms resulting in chronic intestinal inflammation remain far from clear, but attention to the signalling pathways involved in microbial sensing and subsequent responses of the gut are going to be of continuing interest.
Acknowledgments
This study was supported by funds from the Crohn's and Colitis Foundation of Canada, the Canadian Society for Intestinal Research and the Geraldine Dow Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked, ‘advertisement’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 2.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 3.Inohara N, Ogura Y, Nunez G. Nods. A family of cytosolic proteins that regulate the host response to pathogens. Curr Opin Microbiol. 2002;5:76–80. doi: 10.1016/s1369-5274(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 4.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–12. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 5.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 6.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–9. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 8.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 9.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 10.Hanauer SB, Dassopoulos T. Evolving treatment strategies for inflammatory bowel disease. Annu Rev Med. 2001;52:299–318. doi: 10.1146/annurev.med.52.1.299. [DOI] [PubMed] [Google Scholar]
- 11.Hommes D, van den Blink B, Plasse T, et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology. 2002;122:7–14. doi: 10.1053/gast.2002.30770. [DOI] [PubMed] [Google Scholar]
- 12.ten Hove T, van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, van Deventer SJ. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50:507–12. doi: 10.1136/gut.50.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollenbach E, Neumann M, Vieth M, Roessner A, Malfertheiner P, Naumann M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. Faseb J. 2004;18:1550–2. doi: 10.1096/fj.04-1642fje. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-κB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280:14981–8. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- 15.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 16.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–65. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 17.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 Mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–51. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 19.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 20.Salh BS, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–G243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- 21.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera-Nieves J, Bamias G, Vidrich A, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–82. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 23.Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–82. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 25.Myers KJ, Murthy S, Flanigan A, et al. Antisense oligonucleotide blockade of tumor necrosis factor-alpha in two murine models of colitis. J Pharmacol Exp Ther. 2003;304:411–24. doi: 10.1124/jpet.102.040329. [DOI] [PubMed] [Google Scholar]
- 26.Colombel JF, Loftus EV, Jr, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, Zinsmeister AR, Sandborn WJ. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 27.Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH2-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23:2871–82. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–15. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piguet PF, Vesin C, Guo J, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499–505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Marini M, Bamias G, Rivera-Nieves J, Moskaluk CA, Hoang SB, Ross WG, Pizarro TT, Cominelli F. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci USA. 2003;100:8366–71. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Scholmerich J, Gross V, Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito Y, Takagi T, Handa O, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–9. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 34.Naito Y, Takagi T, Uchiyama K, et al. Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin-6-deficient mice. Int J Mol Med. 2004;14:191–6. [PubMed] [Google Scholar]
- 35.Khwaja A, Downward J. Lack of correlation between activation of Jun-NH2-terminal kinase and induction of apoptosis after detachment of epithelial cells. J Cell Biol. 1997;139:1017–23. doi: 10.1083/jcb.139.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abreu-Martin MT, Palladino AA, Faris M, Carramanzana NM, Nel AE, Targan SR. Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am J Physiol. 1999;276:G599–605. doi: 10.1152/ajpgi.1999.276.3.G599. [DOI] [PubMed] [Google Scholar]
- 37.Salh BS, Martens J, Hundal RS, Yoganathan N, Charest D, Mui A, Gomez-Munoz A. PD98059 attenuates hydrogen peroxide-induced cell death through inhibition of Jun N-terminal kinase in HT29 cells. Mol Cell Biol Res Commun. 2000;4:158–65. doi: 10.1006/mcbr.2001.0271. [DOI] [PubMed] [Google Scholar]
- 38.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 39.Rincon M, Pedraza-Alva G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol Rev. 2003;192:131–42. doi: 10.1034/j.1600-065x.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 40.Bantel H, Schmitz ML, Raible A, Gregor M, Schulze-Osthoff K. Critical role of NF-kappaB and stress-activated protein kinases in steroid unresponsiveness. Faseb J. 2002;16:1832–4. doi: 10.1096/fj.02-0223fje. [DOI] [PubMed] [Google Scholar]
- 41.Zingarelli B, Hake PW, Burroughs TJ, Piraino G, O'Connor M, Denenberg A. Activator protein-1 signalling pathway and apoptosis are modulated by poly (ADP-ribose) polymerase-1 in experimental colitis. Immunology. 2004;113:509–17. doi: 10.1111/j.1365-2567.2004.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]