Abstract
CD4+ CD25+ regulatory T (Treg) cells play an important role in the control of the immune system by suppressing the proliferation of effector cells, thereby preventing autoreactive, unnecessary or inconvenient responses. Recently, it has been shown that the number of Treg cells increases during pregnancy, a period with high serum levels of female sex hormones. Oestrogen replacement therapy has been reported to alleviate the symptoms of autoimmune diseases, yet the cellular and molecular mechanisms involved are not fully understood. Here, we show that physiological doses of oestradiol (E2) found during pregnancy, combined with activation through CD3/CD28 engagement, promoted the proliferation of Treg cells without altering their suppressive phenotype. Enhanced suppression was detected when Treg cells were pretreated with the hormone as well as when both cell subpopulations (Treg and T effector) were exposed to E2 throughout the experiment. Together, these data suggest that when combined with an activating stimulus, E2 can modulate the function of human Treg cells by regulating their numbers, and highlight a potential use of E2, or its analogs, to manipulate Treg function.
Keywords: oestrogens, human Treg cells, pregnancy, proliferation
Introduction
Immune tolerance is mediated by mechanisms that control self- versus non-self-discrimination, leading to the deletion of autoreactive cells in the thymus during ontogeny and to the control of the immune response in the periphery. CD4+ CD25+ regulatory T (Treg) cells play an important role in peripheral tolerance.1 There are at least two populations of Treg cells (i.e. naturally occurring regulatory cells and induced regulatory cells) and these have different origins and effector mechanisms. Naturally occurring Treg cells constitutively express the intrleukin (IL)-2 receptor α chain (CD25), are generated in the thymus and act through cell contact-dependent mechanisms;1 they express the cytotoxic T-lymphocyte antigen 4 (CTLA-4),2,3 the glucocorticoid-induced tumour necrosis factor receptor (GITR)4 and the transcription factor FoxP3.5 Induced Treg cells can be generated in response to specific microenvironments, and one of the mechanisms through which they achieve suppression is dependent on the secretion of cytokines, such as IL-10 (Tr1) and transforming growth factor-β (TGF-β) (Th3).1 Based on their failure to proliferate in response to T-cell receptor (TCR) ligation, Treg cells have been considered to be anergic.2,4,6 However, recent studies challenge this view, demonstrating that Treg cells have a robust proliferative capacity.3,7,8
Gender and sex hormones influence humoral and cellular immune responses.9 The need for fetal tolerance during pregnancy, and the protective or positive role of 17β-oestradiol (E2) in autoimmunity models, such as experimental autoimmune encephalomyelitis (EAE)10–12 and collagen-induced arthritis,13,14 suggest that E2 influences peripheral tolerance mechanisms. In support of this hypothesis, TCR vaccination, in combination with supplemental doses of oestrogens, has been shown to induce T cells to produce IL-10 and TGF-β.10 Interestingly, in mice15 as well as in humans, 16–18 the number of Treg cells has been reported to increase during pregnancy, while low levels of CD4+ CD25bright T cells have been associated with spontaneous abortion.17 Moreover, the percentage of CD3+ CD4+ CD25+ T cells was reported to decrease significantly in term deciduas basalis and parietalis of spontaneous vaginal deliveries compared with elective Caesarean sections without labour.19
Given that the levels of E2 increase during pregnancy, we hypothesized that this sex hormone could regulate the number and/or the suppressive function of human Treg cells. We investigated whether the fluctuations of E2 levels that occur during the menstrual cycle of healthy women (low during the follicular phase and high during the luteal phase) modified the numbers of Treg cells. We found that the percentage of CD4+ CD25high Treg cells was comparable between women and men, and that there was no difference regarding the phases of the menstrual cycle. However, data from in vitro experiments revealed that physiological levels of E2 found during pregnancy promoted the proliferation of Treg cells in response to TCR/CD28 engagement, ultimately boosting Treg-mediated suppression. Together, these data suggest that E2-induced signals modulate the suppressive function of human Treg cells by driving their expansion after activation.
Materials and methods
Subjects and cell preparation
Healthy female volunteers completed a self-administered questionnaire regarding their menstrual status, reproductive history and use of oral contraceptives. Women with regular cycles of 28 ± 2 days were included in the study and subjects taking hormone-containing medication were excluded. The mean age of the female participants was 27·4 ± 4·3 years (range 22–33; n = 8), while that of the male participants was 28·7 ± 4·6 years (range 23–37; n = 7). After volunteers gave informed consent, peripheral blood was withdrawn by venepuncture in the morning, and peripheral blood mononuclear cells (PBMCs) were isolated from the blood samples by Ficoll density-gradient centrifugation, as described previously.20 The local Bioethics Committee approved this study.
Fluorescence-activated cell sorter (FACS) staining
Total T lymphocytes (> 98% CD3+), isolated magnetically from donors, were stained with the following antibodies: Tricolor-labelled anti-CD4, phycoerythrin (PE)-labelled anti-CD25 and fluorescein isothiocyanate (FITC)-labelled anti-CD69 (Caltag, Burlingame, CA) or biotin-labelled anti-GITR (R & D, Minneapolis, MN), followed by FITC-labelled streptavidin (Molecular Probes, Eugene, OR). Phospho-Akt was detected in cells permeabilized with 90% (v/v) ethanol, using anti-pAKT, an antibody that recognizes the phosphorylated Ser473 of AKT (Cell Signalling Technology, Beverly, MA) followed by incubation with PE-labelled anti-rabbit immunoglobulin (Caltag). After activating for 24 hr with E2, Treg cells were stained for CD25, CTLA-4 (Ancell, Bayport, MN) or GITR, followed by an FITC-labelled second-step reagent. IL-10 was detected by intracellular staining after the addition of 3 µm monensin to the wells for the final 4 hr of stimulation; cells were fixed, permeabilized with 3% saponin and stained with anti-IL-10 mAb (R & D) or a corresponding isotype control. All isotype controls were from Caltag.
Treg suppression assay
Male T cells were isolated from healthy blood bank donors, as described previously;20 CD4+ CD25+ cells were purified by a round of negative magnetic selection with the anti-CD8 monoclonal antibody (mAb) OKT8, followed by positive selection with CD25 microbeads (Miltenyi Biotec, Auburn, CA) (> 95% purity of CD4+ CD25+). Suppression assays were performed in a final volume of 200 µl/well of RPMI (Hyclone, Logan, UT) supplemented with 5% (v/v) fetal calf serum (FCS) (Hyclone) + 5% bovine calf serum (BCS) (Hyclone). The same batches of FCS and BCS were used throughout the study; none contained a significant concentration of E2, as determined by radioimmune analysis. Different concentrations of E2 (Sigma-Aldrich, Saint Louis, MO) or the same volume of solvent (EtOH, 1% final concentration) were added at the start of the experiment. Alternatively, CD4+ CD25+ cells were preincubated with E2 or solvent for 2 hr at 37° and 5% CO2, washed twice, mixed with 105 autologous CD4+ CD25– cells per well at the indicated CD4+ CD25– : CD4+ CD25+ ratios and plated on anti-CD3- (0·1 µg; OKT3; ATCC, Manassas, VA)/anti-CD28- (0·05 µg; ANC28.1/5D10; Ancell) coated wells of 96-well flat-bottom plates (Nunclon, Rochester NY). After 132 hr of incubation at 37° and 5% CO2, proliferation was measured by using a modification of the MTT assay.21 The number of cells in each well was calculated with reference to a standard curve generated using increasing cell numbers. For each well, the percentage proliferation (% p) was calculated as follows:
Alternatively, cells were labelled with 2 µm carboxiflourescein diacetate succimidylester (CFSE) (Molecular Probes) for 10 min at 37° prior to assay set-up. After 132 hr, cells were harvested and analysed using a FACScan and the cellquest software (BD Biosciences, San Jose, CA). For some assays, phenol red-free supplemented RPMI (Invitrogen, Gaithersbourg, MD) was used in parallel with standard RPMI; results were comparable to those obtained with standard RPMI.
Immunoblotting analysis
Purified CD4+ CD25+ or CD4+ CD25– cells were lysed in RIPA buffer (150 mm NaCl, 1% NP40, 0.5% Na+DOC, 0.1% SDS, 50 mm Tris pH8). Total cell lysates (≈ 1·5 × 106 cell equivalents per lane) were submitted to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Resolved proteins were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA), and oestrogen receptor α (ERα) was detected using anti-ERα mAb (Santa Cruz Biotechnologies, Santa Cruz, CA) and chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Sex hormone levels
Hormone levels from the same blood sample used to determine the proportion of T-cell subsets were measured by radioimmune analysis, using commercially available kits (DPC, Los Angeles, CA).
Statistical analysis
Comparisons were made by the two-tailed Student's t-test. P-values of < 0.05 were considered statistically significant.
Results
E2 signals contribute to the proliferation of Treg cells
Treg cell numbers have been found to be elevated during pregnancy,15–18 a period where E2 levels are particularly high.9 Considering that the serum levels of E2 are higher during the luteal phase of the menstrual cycle than during the follicular phase,9 we investigated whether the E2 concentration during the luteal phase was associated with higher numbers of Treg cells. We measured the percentage of CD4+ CD25high T cells, considered as human Treg cells,22 during the follicular (days 6–9) and the luteal (days 20–24) phases of the menstrual cycle of healthy women. Independently of whether the analysis was performed on the total T-cell population or on the CD4+ population (data not shown), no significant association was found between the E2 levels and the percentage of CD4+ CD25high T cells, or between the frequencies of activated cells (CD4+ CD25+ GITR+, CD4+ CD25+ CD69+). Comparison with values obtained from male donors showed no significant difference in the percentage of CD4+ CD25high cells between women and men (Table 1). As previously reported,23 we found that women have a higher proportion of CD4+ T cells than men (P < 0.01; Table 1).
Table 1.
17β-Oestradiol serum levels and percentages of T-cell subsets in total T lymphocytes1
| Women (n = 8) | ||||
|---|---|---|---|---|
| Low E2 | High E2 | Men (n = 7) | P-Value | |
| E2 (nm) | 0.21 ± 0.1 | 0.53 ± 0.2 | 0.11 ± 0.1 | <0.052,3 |
| % CD4+ | 55.5 ± 7.6 | 57.4 ± 6.0 | 42.8 ± 10.5 | <0.013 |
| % CD4+ CD25high | 1.2 ± 0.4 | 1.3 ± 0.5 | 1.0 ± 0.4 | NS |
| % CD4+ CD25+ GITR+ | 5.2 ± 1.1 | 7.4 ± 4.0 | 6.4 ± 3.2 | NS |
| % CD4+ CD25+ CD69+ | 4.2 ± 2.8 | 5.9 ± 4.0 | 5.7 ± 2.3 | NS |
Total T cells were purified from men and from women in the follicular and luteal phases of the menstrual cycle. Immediately after purification, three-colour cytometry analysis was carried out and the percentage of the different subsets of cells in the total T-cell lymphocytes was evaluated. For each serum sample, the concentration of 17β-oestradiol (E2) was determined.
Mean ± standard deviation; CD4+ CD25high cells were defined as cells with CD25 fluorescence intensity ≥ 102.
High E2 versus low E2.
Women versus men.
GITR, glucocorticoid-induced tumour necrosis factor receptor; NS, not significant.
These data suggested that the levels of E2 found in the serum of healthy individuals do not modulate the number of Treg cells. However, considering that a fetus is a semiallograft which is tolerated despite the presence of non-self major histocompatibility complex (MHC) proteins derived from the father, and that during pregnancy E2 levels are up to 100-fold higher than during the menstrual cycle, we reasoned that those non-self proteins, in combination with high levels of E2, might provide activation signals leading to the proliferation of the maternal Treg cells. To test this possibility, we evaluated whether E2 could promote the proliferation of TCR/CD28-activated Treg cells. In order to work with cells that had not been pre-exposed to high E2 levels, we purified CD4+ CD25+ T cells from the peripheral blood of healthy male donors. The purity of these cells (>95% CD4+ CD25+, <5% CD69+) and their ability to suppress the proliferation of autologous CD4+ CD25– effector T cells (Teff) in response to TCR/CD28 engagement, is shown in Fig. 1. To assess the direct effect of E2 on the Treg cell population, CFSE-labelled CD4+ CD25+ T cells were preincubated for 2 hr with concentrations of E2 similar to those found during human pregnancy (10 or 100 nm) prior to plating on anti-CD3/anti-CD28-coated wells, with or without CD4+ CD25– autologous cells. Cell proliferation was measured by cytometry after 5 days. Unstimulated Treg cells, seeded on uncoated wells, proliferated slightly in response to E2 (Fig. 2a); however, when activated by CD3/CD28 engagement, cell proliferation increased in an E2 dose-dependent manner (Fig. 2b). Moreover, when cocultured with autologous effector cells on CD3/CD28-coated plates, Treg cells proliferated vigorously as a function of E2 concentration (Fig. 2c). Under our experimental conditions, Treg cells exposed to E2 preserved their phenotype: they retained the ability to produce IL-10 following activation, and remained CD25+, CTLA-4+ and GITR+ (Fig. 2e–h).
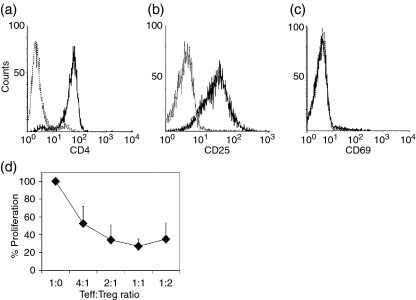
Figure 1.
Purified human CD4+ CD25+ cells show a CD4+ CD25+ regulatory T cell (Treg) phenotype. CD4+ CD25+ T cells purified from male donors were stained for CD4 (a), CD25 (b) and CD69 (c) surface antigens. (d) To evaluate the suppressive capacity, different numbers of isolated CD4+ CD25+ cells were tested for their ability to suppress the proliferation of 105 autologous CD4+ CD25– effector T cells (Teff) in response to plate-bound anti-CD3/anti-CD28 monoclonal antibodies (mAbs). After incubation for 132 hr, cell proliferation was measured and the percentage proliferation was calculated. Data shown are representative of three independent experiments. Teff : Treg ratio, ratio of T-effector cells to CD4+ CD25+ regulatory T cells.
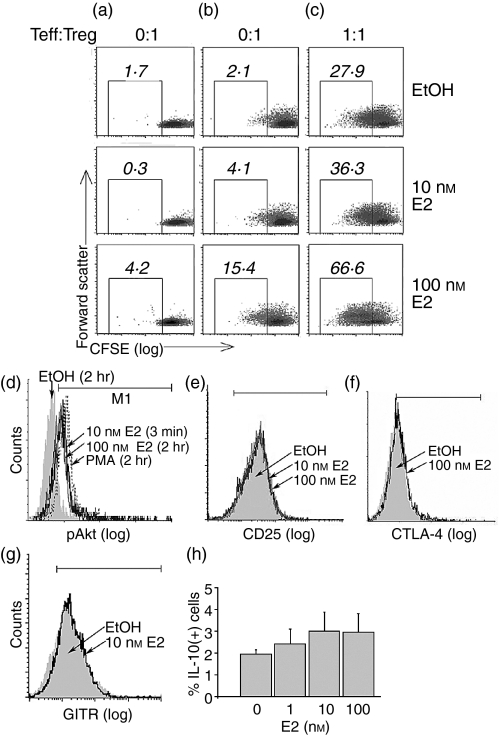
Figure 2.
17β-Oestradiol (E2) contributes to the proliferation of CD4+ CD25+ regulatory T cells (Treg). Purified male CD4+ CD25+ T cells were labelled with CFSE, pretreated for 2 hr with E2 or solvent and seeded onto a nonsensitized plate (a), a plate sensitized with anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) (b), or mixed with autologous CD4+ CD25– effector T (Teff) cells in a CD3/CD28 sensitized plate (c). After 132 hr, Treg cell proliferation was measured based on the dilution of the fluorescence of CFSE. Numbers shown on the top of each square represent the percentage of cells with decreased fluorescence as compared to non-proliferating cells. (d) Purified CD4+ CD25+ T cells were exposed to E2 or ethanol for the indicated times and stained for pAkt. (e–h) CD4+ CD25+ T cells preincubated with the indicated concentrations of E2 for 2 hr and activated with plate-bound anti-CD3/CD28 mAbs for 24 hr, were stained for CD25, cytotoxic T-lymphocyte antigen (CTLA-4), the glucocorticoid-induced tumour necrosis factor receptor (GITR) and interleukin-10 (IL-10). Data shown are representative of three independent experiments. PMA, phorbol 12-myristate 13-acetate; Teff : Treg ratio, ratio of CD4+ CD25– T-effector cells to CD4+ CD25+ regulatory T cells.
The participation of the phosphatildyinositol 3-kinase (PI3K)/Akt axis in cell survival and proliferation is well documented,24 and a recent publication has reported that the PI3K/Akt axis also participates in Treg cell proliferation.25 In addition, it has been recently shown that the E2-mediated signals activate this pathway.26,27 Under our experimental conditions, incubating Treg cells with E2 resulted in enhanced phosphorylation of Akt at Ser473 (pAkt) within 3 min and for the following time-period, up to 2 hr (Fig. 2d). Together, these results suggest that E2-mediated signals contribute to the proliferation of Treg cells.
E2 treatment boosts the suppressive function of Treg cells
Treg cells modulate immune responses by suppressing the proliferation of CD4+ CD25– effector cells as a function of the Teff : Treg ratio.1 As our data pointed out that E2-mediated signals augment the TCR/CD28-induced proliferation of Treg cells, we evaluated whether the suppressive function of these cells was modified by E2. To investigate this, CD4+ CD25– cells were labelled with CFSE in order to monitor their proliferation. Proliferation of the CFSE-labelled CD4+ CD25– effector T cells seeded on anti-CD3/anti-CD28-coated wells was inhibited by 55% in the presence of autologous Treg cells at a 1 : 1 ratio. Remarkably, when Treg cells were preincubated with E2 for 2 hr, their ability to suppress proliferation was significantly enhanced, reaching 80% inhibition when Treg cells were treated with 100 nm E2 (Fig. 3). Similar results were obtained when total cell proliferation was measured by MTT reduction (data not shown).
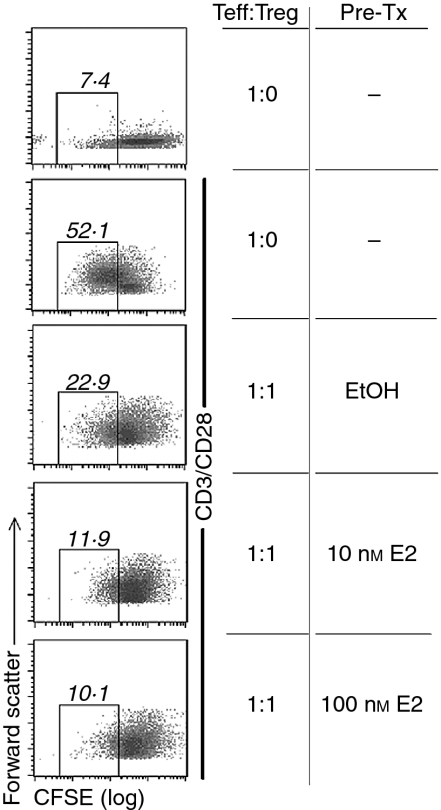
Figure 3.
17β-Oestradiol (E2)-treated CD4+ CD25+ regulatory T cells (Treg) suppress more efficiently the proliferation of effector cells. Purified male CD4+ CD25+ T cells were pretreated for 2 hr with E2 or solvent. To ensure that no traces of E2 remained, cells were washed twice with 5 ml of RPMI before combining with autologous CFSE-labelled CD4+ CD25– cells at a 1 : 1 ratio of Teff cells to Treg cells (Teff : Treg). After 132 hr, proliferation was evaluated by assessing the fluorescence of the CD4+ CD25– T cells. Numbers shown on the top of each square represent the percentage of cells with decreased fluorescence compared to non-proliferating cells. Data shown are representative of three independent experiments.
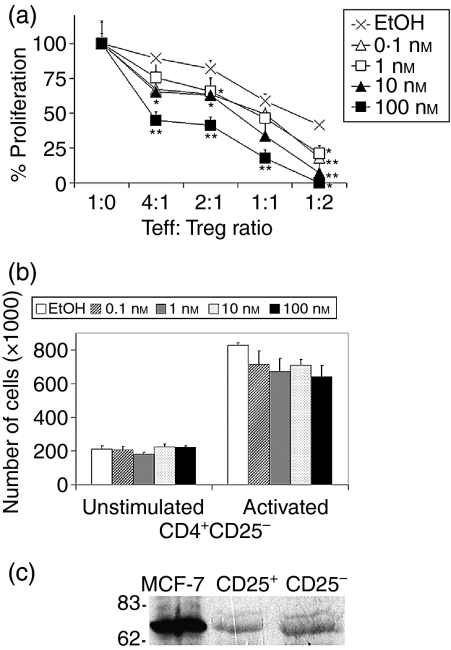
In vivo, at a given time of the menstrual cycle, CD4+ CD25– and CD4+ CD25+ T cells are continuously exposed to the same serum levels of E2. Therefore, we cocultured different numbers of male CD4+ CD25+ cells with autologous CD4+ CD25– cells on anti-CD3/anti-CD28 mAb-coated wells, with or without physiological concentrations of E2 (0·1–100 nm) throughout the experiment. Total cell proliferation, as measured by MTT reduction, revealed that when added at the onset of the assay, E2 also inhibited cell proliferation (Fig. 4a). For all Teff: Treg ratios tested, the highest E2 concentration (100 nm) reduced proliferation by ≈ 50% compared to cells incubated with ethanol; statistical analysis revealed a significant difference between these two conditions (P ≤ 0·01). Interestingly, at a Teff : Treg ratio of 1 : 2, a significant reduction in proliferation was observed, even at the lowest E2 concentration tested (0·1 nm). In the absence of Treg cells, the proliferation of CD4+ CD25– cells to CD3/CD28-activating signals was not significantly modified by E2 (Fig. 4b).
Figure 4.
17β-Oestradiol (E2) potentiates the suppression mediated by CD4+ CD25+ regulatory T cells (Treg). (a) Different numbers of CD4+ CD25+ cells of male volunteers were tested for their ability to suppress the proliferation of autologous CD4+ CD25– T effector (Teff) cells (105) in response to plate-bound anti-CD3/anti-CD28 monoclonal antibodies (mAbs) in the presence of different doses of E2 added from the onset of the assay. After incubation for 132 hr, proliferation was measured. (b) In parallel, CD4+ CD25– effector cells (105) of the same individual were activated on anti-CD3/CD28-coated wells in the presence of E2. After 132 hr of incubation, the cell number was measured by the MTT method. (c) Total cell lysates from 1·5 × 106 purified cells were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to a nitrocellulose membrane and probed with an anti-oestrogen receptor α (ERα) mAb. MCF-7 cells were used as a positive control. Results shown are representative of two (a and b) or three (c) independent experiments carried out in triplicate. *P ≤ 0.05, **P ≤ 0.01 versus solvent. Teff : Treg ratio, ratio of CD4+ CD25– T-effector cells to CD4+ CD25+ regulatory T cells.
Two structurally and functionally distinct oestrogen receptors (ERα and ERβ) have been characterized and found to be encoded by two separate genes.28 Both receptors have been identified on lymphoid cells;29 however, it has been shown that the protective effect of E2 on EAE is dependent on the expression of ERα, but not ERβ.30 As E2 was found to drive the expansion of the Treg compartment, and this effect was substantially lower in mice lacking ERα,12 we evaluated whether Treg cells expressed ERα. ERα was detected by immunoblot on resting human Treg cell lysates (Fig. 4c). Overall, these data show that E2-mediated signals, combined with TCR/CD28 activation, result in an improved suppressive function of the Treg cell population.
Discussion
Modulation of the Treg function by high concentrations of sex hormones, such as those found during pregnancy, has been proposed, suggesting a relationship between pregnancy, the number of Treg cells and fetal protection.15–18 We evaluated the effects of E2 on the function and frequency of human Treg cells. In healthy young women, with normal E2 serum concentrations (0·21–0·53 nm), we did not find an association between the percentages of CD4+ CD25high T cells and serum E2 levels during the two phases of the menstrual cycle (Table 1). These results, and the lack of association between the percentage of CD4+ CD25high cells and the levels of progesterone in women, as well as of testosterone, progesterone or E2 in male healthy volunteers (data not shown), suggests that under basal immune conditions, physiological levels of sex hormones do not substantially alter the number of Treg cells. We also found that women and men have similar percentages of CD4+ CD25high cells, supporting the idea that gender does not affect Treg activity.31
CD4+ CD25+ Treg cells can be generated in the thymus or induced in the periphery.1 The expansion of the Treg population during pregnancy could thus result from de novo generation in the thymus and/or in the periphery, or by proliferation of the existing CD4+ CD25+ Treg cells. The addition of E2 to CD4+ CD25– cells activated by CD3/CD28 cross-linking has been shown to augment the proportion of CD4+ CD25+ cells and the expression level of FoxP3 mRNA,12 probably reflecting the proliferation of the recently described CD4+ CD25– FoxP3+ regulatory T-cell population.32 Even though we cannot conclude whether the natural or the induced population of Treg cells is being activated, our data show that E2 enhances the proliferation of CD4+ CD25+ Treg cells in response to CD3/CD28 activation (Fig. 2), thus favouring the pregnancy-associated E2-induced Treg proliferation hypothesis. Interestingly, the expansion of the Treg cells was dependent on the presence of CD4+ CD25– cells (Fig. 2c), suggesting that activation-induced signals (such as IL-2) provided by effector cells contribute to proliferation of the Treg cell population. These results emphasize the important role that the activation/hormonal status plays in the modulation of the number of Treg cells during pregnancy. Although it has been reported that the expansion of the Treg cell pool during murine pregnancy is alloantigen-independent,15 suggesting that Treg cell proliferation does not require an activating stimulus, it should be noted that this assumption was based on the proportion of CD4+ CD25+ cells present in the CD4+ population. As the total number of CD4+ cells could be differentially affected after exposure to an allogeneic or a syngeneic mate, the absolute count of Treg cells in each condition is necessary to solve this discrepancy.
Consistent with other reports,3,7,8,33 our data also suggest that despite the break of anergy in response to E2 treatment, the Treg function of the CD4+ CD25+ cells prevailed because, when tested in a suppression assay, they inhibited the proliferation of effector cells as efficiently as that reported with Treg cells from pregnant women.16 In addition, under our experimental conditions, Treg cells exposed to E2 preserved their phenotype: they retained the ability to produce IL-10 following activation and remained CTLA-4+, GITR+ and CD25+ (Fig. 2). Moreover, these data highlight the potential use of the E2-expanded human Treg cells in clinical therapy, as it is severely limited by the small numbers of Treg cells (1–2% of CD4+ T cells) found in the circulation.22
Although Treg cells are hypoproliferative in response to either TCR2–4,6 or IL-2 stimulation,6 combining these signals,2,3,6,33 or a strong CD28 costimulation,3,8 restores their proliferative capacity. Under our experimental conditions, Treg cells were found to have augmented levels of pAkt following treatment with E2, suggesting crosstalk between the CD3/CD28 and the E2 signalling pathways, ultimately leading to Treg cell proliferation. The fact that we detected the ERα molecule on Treg cells (Fig. 4c) as well as an enhanced phosphorylation of Akt within the first 3 min of E2 treatment, is indicative of an E2 non-genomic activating signal mediated through a membrane/cytoplasmic ERα, as previously described on total T cells.34 However, the contribution of ERβ or of an as-yet-unknown ER receptor cannot be ruled out, and further experiments are required to identify the different signalling pathways activated through these receptors, and to understand their contribution to the suppressive function of Treg cells.
Consistent with the fact that activation of total T lymphocytes in the presence of E2 has been shown to inhibit CD25 and IL-2 expression,35 we found that E2 boosted the suppressive function of the CD4+ CD25+ population, ultimately resulting in a diminished proliferation of CD4+ CD25– cells. These data, and those of a previous report with murine Treg cells,11 underscore an important role for this hormone in modulating cell-mediated tolerance and are consistent with the fact that during pregnancy autoimmune disease is ameliorated, but often relapses after delivery, when hormonal levels decrease.14,36 Whether the combination of the cyclical fluctuations in the E2 levels that occur during the menstrual cycle with an activating stimulus, such as an infection, participates in the modulation of tolerance, favouring the expansion of autoreactive cells and contributing to enhanced incidence of autoimmunity in women,36 remains to be investigated.
In summary, data shown here provide evidence that E2 enhances the suppressive function of human Treg cells by augmenting their number following activation. These results also highlight a potential therapeutic use for E2 to drive the expansion of Treg cells in vitro.
Acknowledgments
The authors thank Drs G. Koretzky, G. Pedraza-Alva and R. Hernandez-Pando for critical reading of this manuscript, and the Blood Bank from the Hospital de Zona del IMSS, Cuernavaca, Morelos, and Norma Olivares-Zavaleta, Erika Melchy and Alma Valle for technical support. This work was supported by grants from the Dirección General de Apoyo al Personal Académico/UNAM and the Consejo Nacional de Ciencia y Tecnologia (CONACyT), Mexico;. G.A.P was a recipient of fellowships from CONACyT and from the Fundación Alberto y Dolores Andrade, Mexico.
Abbreviations
- BCS
bovine calf serum
- CFSE
carboxiflourescein diacetate succimidylester
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- E2
17β-oestradiol
- EAE
experimental autoimmune encephalomyelitis
- ERα
oestrogen receptor α
- FACS
fluorescence-acivated cell sorter
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GITR
glucocorticoid-induced tumour necrosis factor receptor
- IL
interleukin
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide
- PE
phycoerythrin
- PMBC
peripheral blood mononuclear cells
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- TCR
T-cell receptor
- TGF-β
transforming growth factor-β
- Teff
CD4+ CD25− effector T cells
- Treg
CD4+ CD25+ regulatory T cells
References
- 1.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–8. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+) CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levings MK, Sangregorio R, Roncarolo MG. Human CD4+CD25+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–301. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+) CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 5.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 6.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin-2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–91. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker LS. CD4+ CD25+ Treg: divide and rule? Immunology. 2004;111:129–37. doi: 10.1111/j.0019-2805.2003.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuurs AH, Verheul HA. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990;35:157–72. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 10.Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J Clin Invest. 2000;105:1465–72. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offner H. Neuroimmunoprotective effects of estrogen and derivatives in experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis. J Neurosci Res. 2004;78:603–24. doi: 10.1002/jnr.20330. [DOI] [PubMed] [Google Scholar]
- 12.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–30. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 13.Latham KA, Zamora A, Drought H, Subramanian S, Matejuk A, Offner H, Rosloniec EF. Estradiol treatment redirects the isotype of the autoantibody response and prevents the development of autoimmune arthritis. J Immunol. 2003;171:5820–7. doi: 10.4049/jimmunol.171.11.5820. [DOI] [PubMed] [Google Scholar]
- 14.Mattsson R, Mattsson A, Holmdahl R, Whyte A, Rook GA. Maintained pregnancy levels of oestrogen afford complete protection from post-partum exacerbation of collagen-induced arthritis. Clin Exp Immunol. 1991;85:41–7. doi: 10.1111/j.1365-2249.1991.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 16.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 18.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136:373–8. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol. 2004;62:125–37. doi: 10.1016/j.jri.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Pedraza-Alva G, Merida LB, Burakoff SJ, Rosenstein Y. CD43-specific activation of T cells induces association of CD43 to Fyn kinase. J Biol Chem. 1996;271:27564–8. doi: 10.1074/jbc.271.44.27564. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Kang L, Lu H, et al. Normal values for CD4 and CD8 lymphocytes subsets in healthy chinese adults from Shanghai. Clin Diagn Lab Immunol. 2004;11:811–3. doi: 10.1128/CDLI.11.4.811-813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bensinger SJ, Walsh PT, Zhang J, et al. Distinct IL-2 receptor signalling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–96. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stirone C, Boroujerdi A, Duckles S, Krause D. Estrogen receptor activation of PI-3 kinase, Akt and nitric oxide signaling in cerebral blood vessels: rapid and chronic effects. Mol Pharmacol. 2004;67:105–13. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- 27.Simoncini T, Rabkin E, Liao JK. Molecular basis of cell membrane estrogen receptor interaction with phosphatidylinositol 3-kinase in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:198–203. doi: 10.1161/01.atv.0000053846.71621.93. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson S, Gustafsson JA. Estrogen receptor transcription and transactivation. Basic aspects of estrogen action. Breast Cancer Res. 2000;2:360–6. doi: 10.1186/bcr81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nancy P, Berrih-Aknin S. Differential estrogen receptor expression in autoimmune myasthenia gravis. Endocrinology. 2005;146:2345–53. doi: 10.1210/en.2004-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polanczyk M, Zamora A, Subramanian S, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163:1599–605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsaknaridis L, Spencer L, Culbertson N, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- 32.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the Forkhead transcription factor FoxP3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benten WP, Lieberherr M, Giese G, Wunderlich F. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Lett. 1998;422:349–53. doi: 10.1016/s0014-5793(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 35.McMurray RW, Ndebele K, Hardy KJ, Jenkins JK. 17-Beta-estradiol suppresses IL-2 and IL-2 receptor. Cytokine. 2001;14:324–33. doi: 10.1006/cyto.2001.0900. [DOI] [PubMed] [Google Scholar]
- 36.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]