Abstract
Oral tolerance is the systemic unresponsiveness induced by orally administered proteins. To explore the roles of natural killer T (NKT) cells in oral tolerance, we induced oral tolerance to ovalbumin (OVA) in NKT cell-deficient mice. In CD1d–/– mice, the induction of tolerance to orally administered high- or low-dose OVA was impaired. Dendritic cells (DCs) in the Peyer's patches (PPs) of CD1d–/– mice fed OVA showed high expression of major histocompatibility complex (MHC) class II and B7 molecules, whereas DCs of control mice fed OVA expressed low levels of these molecules. The adoptive transfer of NKT cells restored oral tolerance and induction of tolerogenic DCs in the PPs and spleens of CD1d–/– mice. Moreover, interleukin (IL)-10 and transforming growth factor (TGF)-β1 production in vitro were reduced in cells from the spleen and PPs of CD1d–/– mice compared with those of control mice fed OVA. The numbers of OVA-specific CD4+ KJ1-26+ T cells were significantly reduced in the PPs and spleens of DO11·10 mice fed OVA. In contrast, OVA-specific CD4+ KJ1-26+ T cells were not deleted in the PPs or spleens of DO11·10 CD1d–/– mice. In conclusion, NKT cells were found to play an indispensable role in oral tolerance by inducing regulatory T cells, and clonally deleting antigen-specific CD4+ T cells.
Keywords: clonal deletion, natural killer T cells, oral tolerance, regulatory T cells
Introduction
Natural killer T (NKT) cells are now well established as a subset distinct from conventional αβ T cells and characterized by coexpressing surface markers of both conventional αβ T cells and NKT cells.1 NKT cells of mice express a single invariant Vα14Jα18 T-cell receptor (TCR)2 (Vα14i TCR NKT cells) which recognizes glycolipid antigens presented by non-polymorphic major histocompatibility complex (MHC) class I-like protein CD1d.3 α-galactosyl ceramide (α-GalCer) binds to CD1d molecules, and these complexes are recognized by TCRs of NKT cells.4,5 Upon activation, NKT cells rapidly produce large amounts of interleukin (IL)-4 and interferon (IFN)-γ.6 These cytokines have been demonstrated to play critical roles in the regulation of innate and adaptive immune responses by NKT cells.1 Thus, it has been established that NKT cells regulate immune responses by modulating T helper type 1/type 2 (Th1/Th2) balance in vivo. Although NKT cells exist as a minor population of T cells, they play critical roles in regulating various immune responses in vivo, including the maintenance of self-tolerance, autoimmune diseases,7–9 tumour rejection10 and response to various infectious agents.11–13
Immune responses generated by orally administered proteins result in a state of local and systemic unresponsiveness to the same antigens.14 This phenomenon has been termed oral tolerance15 or mucosally induced tolerance.16 Moreover, it has been demonstrated that during the inductive phase of oral tolerance anergy is preceded by antigen-specific T-cell activation and proliferation,17 and that CD4+ T cells play important roles in the induction of oral tolerance.18,19 However, the contribution of NKT cells to the induction of oral tolerance remains controversial.
Trop et al. reported that hepatic NK1·1+ liver-associated lymphocytes played a critical role in the induction of oral tolerance in an experimental colitis model.20 However, they could not clearly demonstrate the functional roles of NKT cells in the induction of oral tolerance because NK1·1+ liver-associated lymphocytes, including NK cells and NKT cells expressing NK1·1 protein in B6 mice, were depleted by injecting anti-NK1·1 monoclonal antibody (mAb). Watanabe et al. reported that hepatic CD4+ FasL+ regulatory cells secreting IL-4 play essential roles in systemic hyporesponsiveness induced by the administration of a high dose of antigen.21 However, hepatic CD4+ FasL+ regulatory cells do not seem to include hepatic NKT cells as the proportions of KJ1-26+ Vα14+ T cells and KJ1-26+ CD122+ T cells were unchanged in the livers of mice fed OVA and mice fed phosphate-buffered saline (PBS). Thus, NKT cells are not likely to play a crucial role in the experimental system of Watanabe et al.21 A recent report demonstrated that oral tolerance to a high dose of OVA (25 mg) was normally induced in Jα18–/– mice and in wild-type mice, suggesting that NKT cells do not contribute to the induction of oral tolerance.22 However, the abrogation of oral tolerance by polyethylene glycol 1 (PEG1) was not evident in Jα18–/– mice, whereas this phenomenon was clearly evident in wild-type mice. These findings suggest that NKT cells are dispensable for the induction of oral tolerance but indispensable in the abrogation of oral tolerance by PEG1.22 Moreover, several recent reports have suggested that NKT cells play critical roles in the induction of oral tolerance by modulating dendritic cells (DCs).23,24 These findings suggested that NKT cells play a role in induction of oral tolerance, depending on the antigens or types of in vivo systems used. Nevertheless, the mechanisms by which NKT cells contribute to the induction of oral tolerance remain unclear.
In the present study, we explored the mechanisms by which NKT cells exert functional effects in the regulation of systemic unresponsiveness in response to orally administered antigens. Our results show that NKT cells play critical roles in the induction of oral tolerance by inducing regulatory T cells and by clonally deleting antigen-specific T cells.
Materials and methods
Mice
C57BL/6 and BALB/c mice were purchased from Orient (Seoul, Korea). CD1d–/– (C57BL/6 background) mice were a generous gift from Dr Hua Gu (Columbia University, New York, NY). Jα18–/– (C57BL/6 background) and RAG–/– Vα14tg Vβ8·2tg mice were a generous gift from Dr M. Taniguchi (Chiba University, Chiba, Japan). DO11·10 transgenic and CD1d–/– (BALB/c background) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice 6–8 weeks old were used in all experiments. Mice were bred and maintained under specific pathogen-free (SPF) conditions in the Clinical Research Institute of Seoul National University Hospital. All animal experiments were performed after receiving approval from the Institutional Animal Care and Use Committee (IACUC) of the Clinical Research Institute of Seoul National University Hospital.
Antibodies
The following antibodies and reagents used for flow cytometry were purchased from BD Pharmingen (San Diego, CA): CyChrome-conjugated anti-CD4, phycoerythrin (PE)-conjugated anti-CD25, fluorescein isothiocyanate (FITC)-conjugated anti-IL-10, biotinylated anti-TGF-β, PE-conjugated anti-CD80, PE-conjugated anti-I-Ad, FITC-conjugated anti-CD11c mAb, and FITC-conjugated streptavidin. FITC-conjugated KJ1-26 mAb was purchased from Caltag (Burlingame, CA).
Induction of oral tolerance to OVA
BALB/c or BALB/c CD1d–/– mice were adoptively transferred with splenocytes from DO11·10 mice (2·5 × 106 CD4+ KJ1-26+ T cells per recipient mouse) as previously described.25 Mice were given a single feed of 250 or 1 mg of OVA or PBS as a control 2 days after transfer. Mice were immunized intraperitoneally (i.p.) with 20 µg of OVA (grade V; Sigma, St Louis, MO) emulsified in complete Freund's adjuvant (CFA) (Sigma) 4 days after oral feeding with OVA. Eight days after immunization, OVA-specific immunoglobulin G (IgG) in serum was measured, and the spleen and Peyer's patch (PP) were removed for the assessment of proliferation and for cytokine analysis.
For inducing oral tolerance in B6 mice, mice were fed with 10 or 25 mg of OVA or PBS as a control. One week after feeding, the mice were immunized i.p. with 20 µg of OVA (grade V; Sigma) emulsified in CFA (Sigma). OVA-specific IgG in serum was measured and the spleens were removed for in vitro studies 14 days after immunization.
Enzyme-linked immunosorbent assay (ELISA) for OVA-specific IgG
Serially diluted serum was then added to immulon II 96-well ELISA plates (Dynatech Laboratories, Chantilly, VA) coated with OVA (5 µg/ml) in PBS (pH 7·4) and incubated for 2 hr at room temperature. Alkaline phosphatase-conjugated goat anti-mouse IgG (Pierce, Rockford, IL) diluted in PBS/0·1% bovine serum albumin (BSA)/Tween 20 (1 : 5000) was then added and the plates were incubated for 1 hr at room temperature. The plates were then washed, and 100 µl of phosphatase substrate (p-nitrophenyl-phosphate in a carbonate buffer, pH 9·6) was added to each well. Absorbance was read at 405 nm using SOFTmaxPro version 4·3LS (Molecular Devices, Berkeley, CA).
Proliferation assay
Splenocytes (5 × 105 cells/well) were cultured in 96-well U-bottom tissue culture plates for 72 hr. [3H]thymidine (0·5 µCi/well; New England Nuclear, Boston, MA) was added for the final 18 hr of incubation. 3H incorporation into DNA was determined using a scintillation counter (Wallac, Gaithersburg, MD). Alternatively, an MTS (Promega Corp., Madison, WI) assay was performed to measure OVA-specific immune cell proliferation.
Measurement of cytokines
Supernatants were harvested 48 hr after culturing cells (2 × 106 cells/ml) in 12-well plates stimulated with medium alone, or with OVA (100 μg/ml in serum-free media: X-Vivo; BioWhittaker, Walkersville, MD). PP and spleen cells were cultured seperately. Capture ELISAs were conducted to detect TGF-β, IL-4, IFN-γ and IL-10 according to the manufacturer's recommendations (BD Pharmingen).
Obtaining NKT cells and the adoptive transfer experiment
After they had been killed, the livers of C57BL/6 (B6) mice were homogenized and resuspended in loading buffer [PBS containing 10% fetal bovine serum (FBS) and 1 mm ethylenediaminetetraacetic acid (EDTA)], and overlaid onto lympholyte-M (Cedarlane, Ontario, Canada). After centrifugation at 900 g for 20 min at 25°, liver mononuclear cells (MNCs) were isolated at the interface and stained with PE-conjugated anti-NK1·1 (BD Pharmingen) and Cy-conjugated anti-TCR-β (clone H57-597; BD Pharmingen). NK1·1+ TCR-β+ NKT cells were then sorted using FACStar and CellQuest software (BD Biosciences, San Jose, CA). Sorted NKT cells (1 × 105 cells per recipient) from B6 mice were adoptively transferred into CD1d–/– mice 1 day before the oral administration of OVA.
Splenocyte suspensions of RAG–/– Vα14tg Vβ8·2tg mice or Jα18–/– mice were prepared and depleted of red blood cells with RBC lysis buffer (Sigma-Aldrich, St. Louis MO). 1 × 107 splenocytes were adoptively transferred by intravenously injection.
Statistics
Statistical significance was determined using the prism 3·0 program. Student's t-tests were used to determine the P-values of two-group comparisons. P-values of < 0·05 were considered significant. Data are expressed as means ± standard error of the mean.
Results
Natural killer T cell-deficient mice showed impaired induction of oral tolerance to OVA
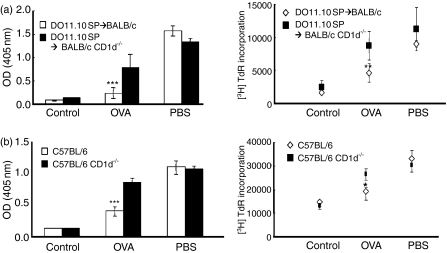
To determine the specific roles of NKT cells in the induction of oral tolerance, we induced oral tolerance to OVA in control and CD1d–/– mice lacking CD1d-dependent NKT cells. B6 and B6 CD1d–/– mice were orally administered OVA or PBS. These mice were immunized with OVA and CFA as adjuvant 7 days after feeding, and examined for OVA-specific serum IgG and T-cell proliferation 14 days after immunization with OVA. B6 mice fed OVA showed significantly reduced serum OVA-specific IgG and T-cell proliferation compared with PBS-fed mice, indicating that OVA-specific oral tolerance was induced in control mice. In contrast, the reduction of OVA-specific IgG in serum and T-cell proliferation was not significant (Fig. 1a) in CD1d–/– mice fed OVA. These findings suggest that induction of oral tolerance to OVA was impaired in NKT-deficient mice.
Figure 1.
Natural killer T (NKT) cell-deficient mice showed impairment in the induction of oral tolerance to ovalbumin (OVA). (a) B6 and B6 CD1d–/– mice were fed OVA or phosphate-buffered saline (PBS) and immunized with OVA. OVA-specific immunoglobulin G (IgG) in serum and T-cell proliferation were measured 14 days after immunization. (b) Spleen cells (2 × 106) from DO11·10 mice were adoptively transferred into BALB/c or BALB/c CD1d–/– mice, which were then fed OVA or PBS and immunized with OVA. OVA-specific IgG in serum and immune cell proliferation were measured 8 days after immunization. Data are the mean ± standard error of the mean for three mice in each group. These results are taken from a representative experiment of three repeated experiments (***P < 0·0001). OD, optical density; TdR, Thymidine uptake rate.
To demonstrate the impaired induction of oral tolerance in NKT cell-deficient mice using another oral tolerance model system and genetic background, we induced oral tolerance to OVA in a DO11·10 T-cell transfer model with a BALB/c background (Fig. 1b). As shown in the non-transfer model of B6 mice, oral tolerance to OVA was also impaired in CD1d–/– mice in the DO11·10 T-cell transfer model with a BALB/c background. These results suggest that NKT cells play critical roles in the induction of OVA-specific oral tolerance, and that their effect is independent of the model system or strain of NKT cell-deficient mice used.
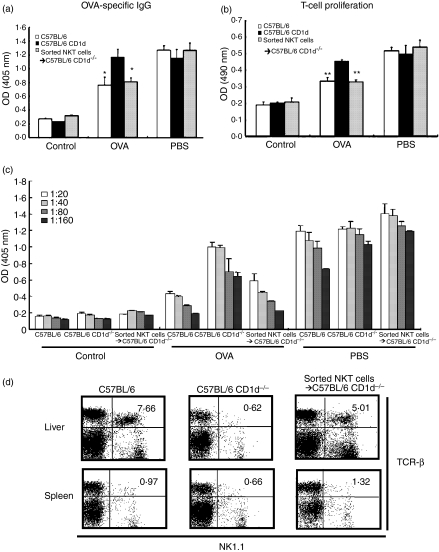
To confirm that the lack of NKT cells specifically caused the failure to induce oral tolerance to OVA in CD1d–/– mice, we adoptively transferred NKT cells sorted from B6 mice (Figs 2a, b and c) into CD1d–/– mice and then induced oral tolerance to OVA. In CD1d–/– mice administered NKT cells, OVA-specific serum IgG (Fig. 2a) and T-cell proliferation (Fig. 2b) were reduced as in control mice. Moreover, the levels of OVA-specific IgG in the variously diluted sera in these mice were similar to those in mice with a single diluted serum (Fig. 2c). In addition, the administered NKT cells were detected in the spleen and liver 7 days after the adoptive transfer into recipient CD1d–/– mice, suggesting that adoptively transferred NKT cells survived in CD1d–/– mice (Fig. 2d). These data indicate that adoptively transferred NKT cells restored the induction of oral tolerance to OVA in CD1d–/– mice. Taken together, these findings indicate that NKT cells are a critical subset of T cells for the induction of oral tolerance to OVA in this in vivo system.
Figure 2.
Adoptive transfer of natural killer T (NKT) cells into CD1d–/– mice restored oral tolerance to ovalbumin (OVA). Sorted NKT cells from B6 mice were adoptively transferred into CD1d–/– mice 1 day before the oral administration of OVA (100 mg per mouse). (a) OVA-specific immunoglobulin (IgG) in sera was evaluated using an enzyme-linked immunosorbent assay (ELISA) and (b) T-cell proliferation was measured by MTS assays. (c) OVA-specific IgG in variously diluted sera was measured on day 28. Data are the mean ± standard error of the mean for three mice in each group. These results are taken from a representative experiment of three repeated experiments. Statistical analysis was performed using the prism 3·0 program (**P < 0·001; *P < 0·05). (d) Flow cytometric analysis was performed using the mononuclear cells from the liver and spleen in B6, B6 CD1d–/– or B6 CD1d–/– mice to which NKT cells were administered 7 days after the adoptive transfer of NKT cells. OD, optical density; PBS, phosphate-buffered saline; TCR, T-cell receptor.
NKT cell-deficient mice showed impaired induction of oral tolerance to high- and low-dose OVA
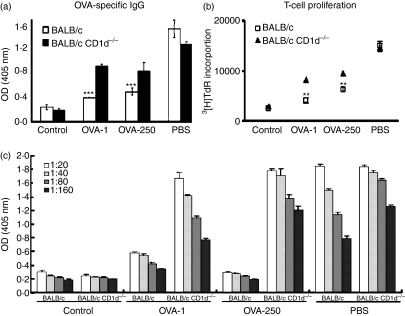
Oral tolerance occurs after either the administration of a single high dose of antigen (> 20 mg) or after repeated exposure to low doses (< 1 mg) in mice.26 These two forms of tolerance, termed high- and low-dose tolerance, are mediated by distinct mechanisms. A high dose of oral antigens induces anergy and/or the clonal deletion of antigen-specific T cells, whereas low-dose oral tolerance is mediated by the induction of active regulatory T cells.26–28 Thus, to explore the mechanism by which NKT cells contribute to the induction of oral tolerance, we designed an experiment to examine whether the dose of antigens administered orally affected the impairment of oral tolerance induction in NKT cell-deficient mice using a DO11·10 splenocyte adoptive transfer model. OVA-specific serum IgG and T-cell proliferation were down-regulated in wild-type mice after feeding with low-dose (1 mg) or high-dose (250 mg) OVA. The impairment of oral tolerance to OVA was found in CD1d–/– mice fed low- or high-dose OVA, as determined by measuring OVA-specific serum IgG and T-cell proliferation on day 14 (Figs 3a and b). Moreover, the levels of OVA-specific IgG in the variously diluted sera in these mice were similar to those in mice with a single diluted serum (Fig. 3c). The impairment of oral tolerance induction to OVA in CD1d–/– mice in terms of OVA-specific T-cell proliferation was more severe after low-dose administration. Taken together, these findings indicated that NKT cells play critical roles in induction of oral tolerance to both high and low doses of OVA.
Figure 3.
Natural killer T (NKT) cell-deficient mice showed impairment in the induction of oral tolerance to high- and low-dose ovalbumin (OVA). Splenocytes from DO11·10 mice were transferred to BALB/c and BALB/c CD1d–/– mice who were fed high (250 mg) or low (1 mg) doses of OVA and immunized with OVA. OVA-specific immunoglobulin G (IgG) in serum (a) and T-cell proliferation (b) in BALB/c and BALB/c CD1d–/– mice fed 250 or 1 mg OVA were measured on day 14. (c) OVA-specific IgG in variously diluted sera was measured on day 21. Data are the mean ± standard error of the mean for three mice in each group. These results are taken from a representative experiment of three repeated experiments. Statistical analysis was performed using the prism 3·0 program (***P < 0·0001; **P < 0·001).
NKT cells induced tolerogenic DCs during the induction of oral tolerance
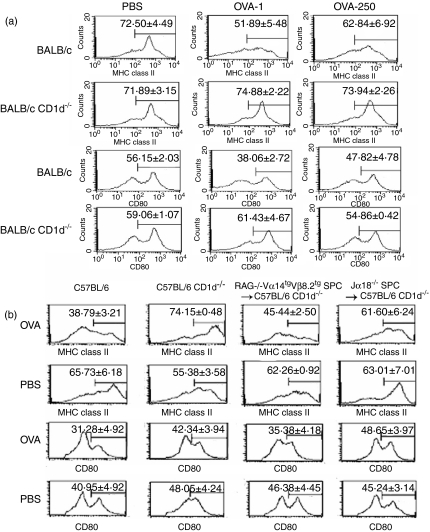
Mucosal immune responses are initiated following antigen uptake, processing and presentation by antigen-presenting cells (APCs).29 An intriguing possibility is that oral tolerance results from interactions between T cells and APCs, so that tolerant T cells or APCs prime T cell to deliver a tolerogenic signal to the next T cell.26 Recently, several reports have suggested that DCs play critical roles in oral tolerance induced by NKT cells.23,24 We therefore examined the possibility that the impairment of oral tolerance induction in CD1d–/– mice is a result of a failure in the induction of tolerogenic DCs in PPs, which express low levels of MHC class II molecules and costimulatory molecules such as CD80 compared with immunogenic DCs.
In the PPs of BALB/c (1 or 250 mg per mouse) and B6 mice (100 mg per mouse) fed OVA, CD11c+ DCs were found to express lower levels of I-Ad and CD80 molecules compared with those of PBS-fed mice (Figs 4a and b). In contrast, CD11c+ DCs of PPs in CD1d–/– mice with a B6 and BALB/c background fed OVA or PBS expressed high levels of I-Ad and CD80, suggesting that NKT cell-deficient mice are defective in terms of the induction of toleragenic DCs in PPs during the induction of oral tolerance.
Figure 4.
Natural killer T (NKT) cells induced tolerogenic dendritic cells (DCs) in the Peyer's patch (PP) during the induction of oral tolerance. (a) Spleen cells (2 × 106) from DO11·10 mice were adoptively transferred into BALB/c or BALB/c CD1d–/– mice, which were then fed 250 or 1 mg ovalbumin (OVA; OVA-250 and OVA-1, respectively) or phosphate-buffered saline (PBS) and immunized with OVA. The cells were taken from the PPs of these mice 14 days after the adoptive transfer of DO11·10 mouse splenocytes, and the expression of I-Ad and CD80 on these cells was evaluated among gated CD11c+ cells by flow cytometry. (b) Splenocytes (1 × 107 cells/mouse) from RAG–/– Vα14tg Vβ8·2tg mice or Jα18–/– mice were adoptively transferred into B6 CD1d–/– mice 1 day before the oral administration of OVA or PBS. Cells were taken from the PPs of B6, B6 CD1d–/– and B6 CD1d–/– mice 21 days after the adoptive transfer of splenocytes, and then the expression of I-Ad and CD80 on these cells was evaluated among gated CD11c+ cells by flow cytometry. Data are the mean ± standard error of the mean for three mice in each group, and taken from a representative experiment of three repeated experiments.
To establish a functional link between NKT cells and induction of tolerogenic DCs in PPs during oral tolerance, NKT cells of RAG–/– Vα14tg Vβ8·2tg mice were adoptively transferred into CD1d–/– mice. Splenocytes from RAG–/– Vα14tg Vβ8·2tg mice contain a large number of Vα14i TCR NKT cells, whereas splenocytes from Jα18–/– mice are deficient in Vα14i TCR NKT cells.1 In CD1d–/– mice into which splenocytes from RAG–/– Vα14tg Vβ8·2tg mice were transferred, the expression levels of I-Ad and CD80 molecules on CD11c+ DCs of PPs were reduced to the control levels, whereas in CD1d–/– mice into which splenocytes from NKT cell-deficient Jα18–/– mice were transferred, high levels of I-Ad and CD80 expression were found on CD11c+ DCs of PPs (Fig. 4b). These results indicate that the reconstitution of NKT cells in CD1d–/– mice restores the induction of tolerogenic DCs in PPs during the induction of oral tolerance. Thus, it is proposed that NKT cells play indispensable roles in the induction of tolerogenic DCs in PPs during oral tolerance induction to high and low doses of OVA.
NKT cells induced regulatory T cells producing TGF-β1 and IL-10 during oral tolerance
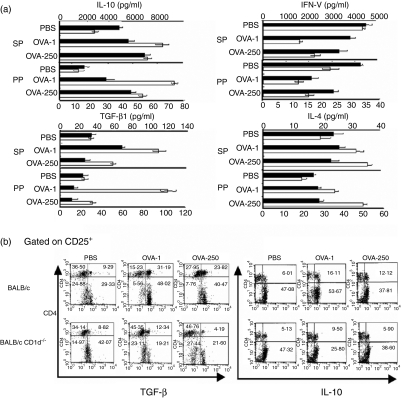
To explore the mechanism by which NKT cells contribute to the induction of oral tolerance, we measured the amounts of IL-4, IL-10, IFN-γ and TGF-β1 secreted by immune cells of the spleen and PP taken from CD1d–/– and control mice fed either OVA (1 or 250 mg per mouse) or PBS. After in vitro stimulation by OVA, immune cells from PPs and spleens of BALB/c mice secreted larger amounts of IL-4, IL-10 and TGF-β than those of CD1d–/– mice. In contrast, the amount of IFN-γ secreted by immune cells was higher in CD1d–/– mice than in BALB/c mice (Fig. 5a). These results suggest that IL-10- and TGF-β1-producing cells may be critical for the induction of NKT cell-mediated oral tolerance. Regulatory T cells are known to mediate immune suppression in oral tolerance via the production of inhibitory cytokines such as IL-10 and TGF-β1.26 Thus, we investigated how many CD4+ CD25+ regulatory T cells producing IL-10 or TGF-β are induced by the oral administration of OVA in CD1d–/– and control mice. Cells were taken from the PPs of these mice after the oral administration of OVA (1 or 250 mg per mouse), and then stimulated with OVA in vitro. The total numbers of immune cells in the PP and spleen in CD1d–/– mice fed OVA or PBS were quite similar to those in BALB/c mice fed OVA or PBS (data not shown). The percentages of CD4+ CD25+ regulatory T cells producing IL-10 or TGF-β were determined by intracellular staining and flow cytometric analysis. In the PPs of BALB/c mice, the percentage of CD4+ CD25+ regulatory T cells producing TGF-β or IL-10 was higher than in those of CD1d–/– mice, indicating that NKT cell-deficient mice are defective in the induction of CD4+ CD25+ regulatory T cells producing TGF-β or IL-10 in PPs during the induction of oral tolerance (Fig. 5b).
Figure 5.
Natural killer T (NKT) cells contributed to the induction of regulatory T cells producing interleukin (IL)-10 and transforming growth factor (TGF)-β1 in Peyer's patches (PPs). Spleen cells (2 × 106) from DO11·10 mice were adoptively transferred into BALB/c or BALB/c CD1d–/– mice, which were then fed ovalbumin (OVA) or phosphate-buffered saline (PBS). Immune cells were taken from the PPs and spleens of BALB/c and BALB/c CD1d–/– mice fed 250 mg (OVA-250) or 1 mg OVA (OVA-1) or PBS. (a) The production of IL-10, TGF-β, interferon (IFN)-γ and IL-4 was measured using enzyme-linked immunosorbent assay (ELISA) after stimulating immune cells (2 × 106 cells/ml) with OVA (100 μg/ml) from BALB/c mice (white bars) or BALB/c CD1d–/– mice (black bars). (b) The intracellular expression of TGF-β and IL-10 was evaluated in gated CD25+ cells by flow cytometry after stimulating immune cells with OVA. These results are taken from a representative experiment of five repeated experiments. SP, spleen.
NKT cells were also involved in the clonal deletion of OVA-specific CD4+ T cells during oral tolerance induction
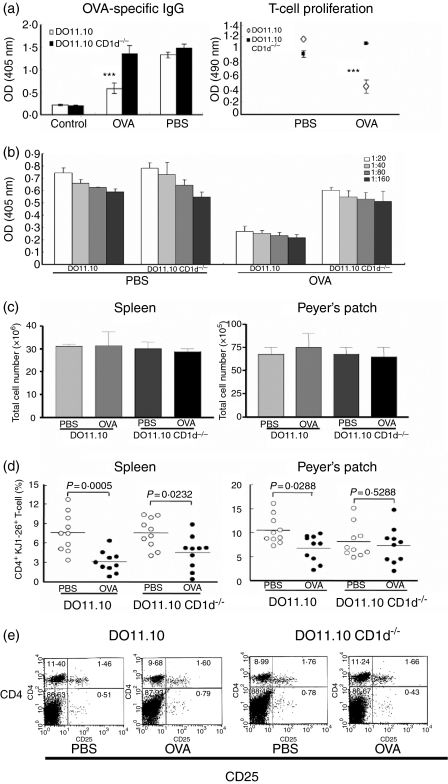
To explore whether NKT cells induce the clonal deletion of OVA-specific T cells during the induction of oral tolerance to OVA, we generated DO11·10 CD1d–/– mice by crossing DO11·10 and CD1d–/– mice. In DO11·10 CD1d–/– mice, the induction of oral tolerance to OVA was impaired, whereas OVA-specific serum IgG and immune cell proliferation were significantly reduced in DO11·10 mice orally fed OVA (100 mg per mouse for 5 days) on day 9 (Fig. 6a). Moreover, the levels of OVA-specific IgG in the variously diluted sera in DO11·10 and DO11·10 CD1d–/– mice fed OVA were similar to those in mice with a single diluted serum (Fig. 6b). The total cell numbers in PPs and spleens in DO11·10 mice fed OVA or PBS were similar to those in DO11·10 CD1d–/– mice fed OVA or PBS (Fig. 6c). We analysed the numbers of OVA-specific T cells in PPs and spleens, as detected by clonotypic KJ1-26 mAb, in DO11·10 and DO11·10 CD1d–/– mice fed OVA (Fig. 6d). In DO11·10 mice fed OVA, the percentages of OVA-specific CD4+ T cells in PPs and spleens were significantly reduced compared with control mice fed PBS. In contrast, OVA-specific CD4+ T cells were not deleted in PPs and spleens of NKT cell-deficient DO11·10 mice fed OVA or PBS. In addition, there was no significant difference in the percentages of CD4+ CD25+ cells between DO11·10 and DO11·10 CD1d–/– mice fed OVA (Fig. 5e). These findings suggest that, during the induction of oral tolerance, NKT cells contribute to the clonal deletion of OVA-specific CD4+ T cells.
Figure 6.
Natural killer T (NKT) cells contributed to the deletion of ovalbumin (OVA)-specific CD4+ T cells during the induction of oral tolerance. DO11·10 and DO11·10 CD1d–/– mice were fed OVA (100 mg per mouse for 5 days) and then immunized with OVA. (a) OVA-specific immunoglobulin G (IgG) in serum and T-cell proliferation in DO11·10 and DO11·10 CD1d–/– mice were measured by enzyme-linked immunosorbent assay (ELISA) and MTS assay, respectively. (b) OVA-specific IgG in variously diluted sera was measured on day 9. (c) The total numbers of immune cells were counted in the spleens and Peyer's patches (PPs) from DO11·10 and DO11·10 CD1d–/– mice fed phosphate-buffered saline (PBS) or OVA. (d) The percentages of OVA-specific CD4+ T cells were measured using clotypic monoclonal antibody (mAb) KJ1-26 in the PPs and spleens of DO11·10 and DO11·10 CD1d–/– mice. (e) The percentages of CD4+ CD25+ cells were measured in the PPs of DO11·10 and DO11·10 CD1d–/– mice. Statistical analysis was performed using the prism 3·0 program.
Discussion
The present study demonstrates that the induction of tolerance to orally administered high- and low-dose OVA is impaired in CD1d–/– mice compared with the wild type. The impairment of oral tolerance induction in NKT cell-deficient mice was observed in both a B6 (Fig. 1a) and a BALB/c background. Moreover, we also found impaired oral tolerance induction in two different independent models of BALB/c mice, namely, an adoptive transfer model using DO11·10 T cells and a non-adoptive transfer model (data not shown). Thus, the present study confirms that oral tolerance to OVA is impaired in NKT cell-deficient mice, and that this is independent of OVA concentration, strain of mice, and type of mouse oral tolerance model.
Although the mechanism by which orally administered antigen induces the activation of NKT cells is unknown, it is unlikely that NKT cells are OVA-specifically activated by interaction with APCs presenting peptide derived from OVA. Instead, it has been speculated that cellular glycolipid provided by apoptotic cells during oral tolerance is presented by CD1d-expressing cells to NKT cells in vivo. Several studies have demonstrated that CD1d molecules are able to present cellular glycolipid to activate NKT cells, supporting this speculation.30,31 However, APCs in CD1d–/– mice did not provide CD1d molecules for NKT cells during the interaction between NKT cells and APCs in vivo. Therefore, NKT cells appear to be activated independently of TCR signals in this in vivo system. During the sorting of NKT cells from hepatic MNCs by flow cytometry, TCR-β and NK1·1 on NKT cells were engaged by mAbs before adoptively transferring NKT cells into CD1d–/– mice. It is likely that TCR-β engagement on NKT cells by mAb could provide enough TCR signal for activation prior to adoptively transferring NKT cells into CD1d–/– mice. A recent study supported this suggestion by demonstrating that sorted NKT cells attenuate lung fibrosis in CD1d–/– mice by adoptive transfer in bleomycin-pulmonary fibrosis.32 Alternatively, various membrane proteins expressed on NKT cells could be engaged by their ligands on APCs in the absence of CD1d, and these molecules could sufficiently activate NKT cells rather than TCRs. However, this possibility is less likely in vivo, because no activating molecule is known to provide signals as potent as TCRs with respect to NKT-cell activation. In the case of adoptive transfer of total splenocytes from RAG–/– Vα14tg Vβ8·2tg mice into CD1d–/– mice, splenocytes of these mice contain a large number of APCs expressing CD1d molecules, which might contribute to activating Vα14i TCR NKT cells in a CD1d-dependent manner in the in vivo system.
Although it remains unclear whether the interaction between NKT cells and DCs is direct or indirect, the current study demonstrates that the induction of tolerogenic DCs requires the presence of NKT cells in vivo during oral tolerance induction. DCs in the presence of NKT cells produce greater amounts of IL-10 and lose the ability to secrete IL-12, a phenotype that is consistent with a tolerogenic function.33,34 It has therefore been proposed that activated NKT cells potentially induce tolerogenic DCs and vice versa. Upon injecting α-GalCer into mice, activated NKT cells up-regulate the expression of the costimulatory molecules of splenic DCs, reflecting a more mature DC status, and suggesting that the activation of NKT cells promotes the maturation of DCs in vivo. Moreover, recently it was reported that the injection of α-GalCer into mice blocked the induction of oral tolerance by triggering DC maturation.23 These findings suggest that the activation of NKT cells in vivo induces the immune reaction to fed antigen by modulating the functions of DCs, resulting in the discontinuance of oral tolerance induction. However, this hypothesis appears to be inconsistent with our result that NKT cells contribute to the induction of oral tolerance by inducing tolerogenic DCs. Although α-GalCer is a potent activator of NKT cells by TCR engagement, α-GalCer is an artificial ligand of unclear physiological relevance in mouse and human systems.4,5 The activation of NKT cells by α-GalCer in vivo might not reflect the physiological activation process in NKT cells in terms of signal strength during the induction of oral tolerance. Therefore, functional defects in NKT cell-deficient mice in relation to the induction of oral tolerance provide evidence for a wider physiological NKT cell function as compared with the in vivo system using α-GalCer.
We undertook to explore whether NKT cells are involved in oral tolerance induction by the clonal deletion of OVA-specific CD4+ T cells and/or the induction of regulatory T cells producing TGF-β and IL-10. To address the issue concerning the deletion of OVA-specific CD4+ T cells in NKT cell-deficient mice, we induced oral tolerance in DO11·10 and DO11·10 CD1d–/– mice, and measured the percentage of KJ1-26+ CD4+ T cells. The induction of oral tolerance to OVA was impaired in DO11·10 CD1d–/– mice, whereas OVA-specific immune responses were significantly reduced in DO11·10 mice orally fed OVA. However, Simioni et al. reported that DO11·10 mice in a BALB/c background could not be induced to oral tolerization after feeding with OVA. In their experiments, they orally administrated 0·05, 0·5 or 5 mg of OVA by gavage every day for 14 days into the DO11·10 mice for oral tolerance induction, which is in stark contrast with the dose used in our experiments (100 mg per mouse for 5 days). This large discrepancy in the dose of OVA administrated is likely to have affected the induction of oral tolerance in DO11·10 mice in these two independent experiments. Moreover, consistent with our results, two independent reports demonstrated that oral tolerance was induced in DO11·10 mice in a BALB/c background after feeding with 100 mg OVA/day for 5 or 7 days.21,35 In DO11·10 CD1d–/– mice, the number of OVA-specific CD4+ T cells was not reduced, whereas in DO11·10 mice KJ1-26+ CD4+T cells were significantly deleted in the PPs and spleens after the induction of OVA-specific oral tolerance. These findings indicate that NKT cells are involved in the clonal deletion of OVA-specific CD4+ T cells during the induction of oral tolerance to OVA. In addition, the production of IL-10 and TGF-β by immune cells from the PPs of DO11·10 CD1d–/– mice fed OVA was also significantly reduced after OVA activation, compared with DO11·10 mice fed OVA (data not shown). These findings suggest that the clonal deletion of T cells and the induction of regulatory T cells are not mutually exclusive but synergistic with respect to the induction of oral tolerance. Moreover, in CD1d–/– mice, the induction of TGF-β- and IL-10-producing regulatory T cells was significantly impaired in the OVA-specific oral tolerance model compared with wild-type mice.
To summarize, NKT cells contribute to OVA-specific oral tolerance by inducing regulatory T cells producing TGF-β and IL-10 and by the clonal deletion of OVA-specific CD4+ T cells. Based on these findings that NKT cells play indispensable roles in the induction of tolerogenic DCs in PPs, it is proposed that the induction of tolerogenic DCs by NKT cells might be related to the induction of the clonal deletion of antigen-specific T cells and regulatory T cells producing IL-10 or TGF-β.
The oral administration of OVA to OVA-specific TCR transgenic mice activates CD25+ CD4+ regulatory T cells expressing cytotoxic T-lymphocyte antigen (CTLA)-4 in an antigen-specific manner.28 CD25+ CD4+ T cells from OVA-fed mice potently suppress the in vitro stimulation of CD25– CD4+ T cells, which are partially dependent on IL-10, TGF-β and CTLA-4.28,36–38 Although CD25+ CD4+ regulatory T cells appear to be required for the induction of oral tolerance, the precise mode of action of CD25+ CD4+ regulatory T cells during the induction of oral tolerance remains to be defined. Our experiments demonstrate that NKT cells are required for the induction of CD4+ CD25+ regulatory T cells producing IL-10 and TGF-β in PPs and spleens during oral tolerance. A recent report demonstrated that IL-4- and IL-10-producing CD4+ iNKT cells are required for the induction of oral nickel tolerance and for the infectious spread of tolerance from APCs to regulatory T cells.24 Thus, NKT cells may play a critical role in the induction of regulatory T cells in oral tolerance.
In summary, the present study demonstrates that NKT cell-deficient mice show impaired induction of oral tolerance to high- and low-dose OVA. During the induction of oral tolerance, NKT cell-deficient mice showed a failure to induce regulatory T cells producing IL-10 and TGF-β, and a failure to delete antigen-specific CD4+ T cells. Our findings suggest that NKT cells play a critical functional role in the induction of oral tolerance by inducing regulatory T cells, and by clonally deleting antigen-specific T cells.
Acknowledgments
We are grateful to Dr Seong Hoe Park for his support of this project, and all members of our laboratory for their enthusiasm. We also thank all members of the Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital for animal management. This work was supported by a grant (R01-2001-000-00194-0) from the Korean Science & Engineering Foundation.
References
- 1.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 2.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8– T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac A. CD1: presenting unusual antigens to unusual T lymphocytes. Science. 1995;269:185–6. doi: 10.1126/science.7542402. [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 5.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–34. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–9. [PubMed] [Google Scholar]
- 7.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Wilson MT, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–11. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HY, Kim HJ, Min HS, Kim S, Park WS, Park SH, Chung DH. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor β1 production. J Exp Med. 2005;201:41–7. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 11.Dieli F, Taniguchi M, Kronenberg M, et al. An anti-inflammatory role for Vα14 NK T cells in Mycobacterium bovis bacillus Calmette-Guerin-infected mice. J Immunol. 2003;171:1961–8. doi: 10.4049/jimmunol.171.4.1961. [DOI] [PubMed] [Google Scholar]
- 12.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–30. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. α-galactosylceramide-activated Vα 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasi TB., Jr Oral tolerance. Transplantation. 1980;29:353–6. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kweon MN, Fujihashi K, VanCott JL, Higuchi K, Yamamoto M, McGhee JR, Kiyono H. Lack of orally induced systemic unresponsiveness in IFN-γ knockout mice. J Immunol. 1998;160:1687–93. [PubMed] [Google Scholar]
- 17.Sun J, Dirden-Kramer B, Ito K, Ernst PB, Van Houten N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J Immunol. 1999;162:5868–75. [PubMed] [Google Scholar]
- 18.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice. both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–6. [PubMed] [Google Scholar]
- 19.Hirahara K, Hisatsune T, Nishijima K, Kato H, Shiho O, Kaminogawa S. CD4+ T cells anergized by high dose feeding establish oral tolerance to antibody responses when transferred in SCID and nude mice. J Immunol. 1995;154:6238–45. [PubMed] [Google Scholar]
- 20.Trop S, Nagler A, Ilan Y. Role of NK1.1+ and AsGm-1+ cells in oral immunoregulation of experimental colitis. Inflamm Bowel Dis. 2003;9:75–86. doi: 10.1097/00054725-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T, Yoshida M, Shirai Y, et al. Administration of an antigen at a high dose generates regulatory CD4+ T cells expressing CD95 ligand and secreting IL-4 in the liver. J Immunol. 2002;168:2188–99. doi: 10.4049/jimmunol.168.5.2188. [DOI] [PubMed] [Google Scholar]
- 22.Ishimitsu R, Yajima T, Nishimura H, Kawauchi H, Yoshikai Y. NKT cells are dispensable in the induction of oral tolerance but are indispensable in the abrogation of oral tolerance by prostaglandin E. Eur J Immunol. 2003;33:183–93. doi: 10.1002/immu.200390021. [DOI] [PubMed] [Google Scholar]
- 23.Chung Y, Chang WS, Kim S, Kang CY. NKT cell ligand α-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol. 2004;34:2471–9. doi: 10.1002/eji.200425027. [DOI] [PubMed] [Google Scholar]
- 24.Roelofs-Haarhuis K, Wu X, Gleichmann E. Oral tolerance to nickel requires CD4+ invariant NKT cells for the infectious spread of tolerance and the induction of specific regulatory T cells. J Immunol. 2004;173:1043–50. doi: 10.4049/jimmunol.173.2.1043. [DOI] [PubMed] [Google Scholar]
- 25.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 26.Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407–19. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–80. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+ CD4+ regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 29.Williamson E, Westrich GM, Viney JL. Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol. 1999;163:3668–75. [PubMed] [Google Scholar]
- 30.Gumperz JE, Roy C, Makowska A, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–21. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 31.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH, Kim HY, Kim S, Chung JH, Park WS, Chung DH. Natural killer T (NKT) cells attenuate bleomycin-induced pulmonary fibrosis by producing interferon-γ. Am J Pathol. 2005;167:1231–41. doi: 10.1016/s0002-9440(10)61211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naumov YN, Bahjat KS, Gausling R, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci USA. 2001;98:13838–43. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–8. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 35.Ise W, Nakamura K, Shimizu N, Goto H, Fujimoto K, Kaminogawa S, Hachimura S. Orally tolerized T cells can form conjugates with APCs but are defective in immunological synapse formation. J Immunol. 2005;175:829–38. doi: 10.4049/jimmunol.175.2.829. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji NM, Mizumachi K, Kurisaki J. Antigen-specific, CD4+CD25+ regulatory T cell clones induced in Peyer's patches. Int Immunol. 2003;15:525–34. doi: 10.1093/intimm/dxg051. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 38.Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]