Abstract
Intravenous infection of C57BL/6 and C57BL/10 mice with low doses of a highly virulent strain of Mycobacterium avium (ATCC 25291) led to the development of granulomas that underwent necrosis. In contrast, neither BALB/c nor DBA/1 mice developed granuloma necrosis after such infection despite a similar course of mycobacterial proliferation. Studies with C57BL/10 mice congenic for the Hc locus revealed that an intact complement C5 gene is required for granuloma necrosis. On the other hand, genetic disruption of the interleukin-10 gene in BALB/c mice made this strain susceptible to granuloma necrosis.
Keywords: caseous necrosis, cell-mediated immunity, macrophages, Mycobacteria
Introduction
Mycobacterial infections are characterized by the formation of granulomas that, in certain cases, may contain the infection. Both T-cell-dependent and -independent pathways of granuloma assembly have been described.1,2 The exact role of such lesions and their relation to the mechanisms involved in protective immunity are, however, not yet clear.2,3 Frequently, these granulomas undergo necrosis leading to their caseation, i.e. the necrosis of the centre of the granuloma which then exhibits an amorphous aspect under the microscope and, macroscopically, acquires a cheesy appearance (caseum being the Latin for cheese).4 The mechanisms underlying caseous necrosis are still being debated with two schools of thought defending opposite views. On one side, caseous necrosis is viewed as the consequence of inadequate protective mechanisms5 and is associated with innate immunity,6 whereas the other argument considers it to be the consequence of the activity of a highly stimulated immune system.7–9 Some genetically engineered mouse strains with disrupted immune genes such as those coding for interferon-γ (IFN-γ), type 1 tumour necrosis factor (TNF) receptor and β2-microglobulin develop necrosis of the granuloma when infected by Mycobacterium tuberculosis,10–12 suggesting that necrosis is a direct consequence of inadequate protection. However, human patients coinfected with M. tuberculosis and human immunodeficiency virus (HIV) show different types of necrotic lesions with less caseation as the CD4+ T-cell counts decrease.13 As a corollary, less cavitation is found among HIV-positive tuberculosis patients.13 We have studied an intravenous infection model using low doses of a highly virulent strain of M. avium, an infection that leads to the development of caseating lesions in 3–4 months.14 In this model, necrosis was abrogated by interfering with the expression of CD4+ T cells, with the cytokine IFN-γ, or with its inducers interleukin (IL)-12p40, IL-6 and CD40. Thus, the mediators of the necrotic phenomena14 were found to be the same as those that are required for effective protective immunity,15–18 with the exception of TNF which, although pivotal in protection, was not required for granuloma necrosis.19,20 Our results are similar to those reported by Ehlers et al.21 who used an aerogenic infection model with the same mycobacterium.
Inbred strains of mice often exhibit different immune responses to infection. In some cases, such differences may lead to distinct immunopathological outcomes in what regards the development of granulomas. For example, the Nramp1/Slc11A1 gene may affect the development of granulomas both to mycobacteria and to inert materials.22 Also, non-histocompatibility-2 (H2) traits influenced granulomatous responses to M. tuberculosis.23 The type of immune response that develops against a microbe can dictate the type of tissue reaction. Using a transgenic model of M. tuberculosis infection, Wangoo et al.24 have determined that a T helper type 2 (Th2) response leads to a much stronger fibrotic reaction within granulomas whereas Th1 responses were more protective in terms of bacillary loads. Similar findings were obtained with a schistosomal model, in which Th2 responses led to much higher collagen deposition than Th1 responses.25 Therefore, we analysed four strains of mice, permissive to M. avium growth, for the histopathology induced by the virulent strain of M. avium, previously used to study granuloma necrosis. We found that different strains differ in their ability to develop necrotic granulomas and identified one locus that was involved in determining such differences, namely the Hc locus, and the role of IL-10 in suppressing the development of necrosis in BALB/c animals.
Materials and methods
Mice
Female C57BL/6J, BALB/c, C57BL/10, and DBA-1 mice were purchased from Harlan Iberica (Barcelona, Spain). IL-4-deficient BALB/c.IL4–/– mice and the congenic strains C57BL/10.Hc1 and C5-deficient C57BL/10.Hc0 were purchased from the Jackson Laboratories (Bar Harbor, ME). BALB/c.IL10–/– were bred in our facilities from a breeding pair kindly provided by Dr A. O'Garra (National Institute for Medical Research, London, UK). Mice were kept in high-efficiency particulate air (HEPA)-filter-bearing cages and fed autoclaved chow and water.
Bacteria
Mycobacterium avium strain 25291, exhibiting a smooth transparent morphotype, was obtained from the American Type Culture Collection (Manassas, VA). Mycobacteria were grown in Middlebrook 7H9 medium (Difco, Detroit, MI) containing 0·04% Tween-80 (Sigma, St Louis, MO) at 37° until the mid-log phase of growth. Bacteria were harvested by centrifugation and resuspended in a small volume of saline containing 0·04% Tween-80. The bacterial suspension was briefly sonicated with a Branson sonifier (Danbury, CT) to disrupt bacterial clumps, diluted and stored in aliquots at −70° until use. Before inoculation, bacterial aliquots were thawed at 37° and diluted in saline to the desired concentration.
In vivo infection
Mice were infected intravenously through the lateral tail vein with approximately 100 colony-forming units (CFU) of M. avium strain 25291. Infected mice were killed at different times during infection and the livers, spleens and lungs were aseptically collected and homogenized in a 0·04% Tween-80 solution in distilled water. The number of CFU of M. avium in the organs of the infected mice was determined by serial dilution and plating of the tissue homogenates into Middlebrook 7H10 agar medium (Difco) supplemented with oleic acid-albumin-dextrose-catalase (OADC). Bacterial colonies were counted after culture for 2 weeks at 37°.
In vitro stimulation of splenic cells
Single-cell suspensions from spleens of each of the infected mice were prepared by teasing portions of the spleen with forceps in Dulbecco's modified Eagle's tissue culture medium (DMEM; Life Technologies, Paisley, UK) supplemented with 10% fetal calf serum (FCS; Life Technologies). Erythrocytes were lysed by incubation of the cell suspensions with haemolytic buffer (155 mm NH4Cl, 10 mm KHCO3, pH 7·2) for 5 min at room temperature. The cell suspensions were then thoroughly washed with Hanks' balanced salt solution (Life Technologies) and resuspended in DMEM with 10% FCS. Cells were cultivated at a density of 2 × 105 cells/well in a U-bottomed 96-well microtitre plate. Cells were incubated in triplicate in DMEM with 10% FCS with no further stimulus or in the presence of mycobacterial culture filtrate proteins (4 μg/ml) or concanavalin A (4 μg/ml, Sigma). Supernatants from the cultures were collected after 72 hr of culture and the IFN-γ produced was quantified by a two-site sandwich enzyme-linked immunosorbent assay (ELISA) method using anti-IFN-γ-specific affinity-purified monoclonal antibodies (R4-6A2 as capture and biotinylated AN-18 as detecting antibody). IL-10 was detected by ELISA using the antibody pairs JES5–2A5 and biotinylated SXC-1. Standard curves were generated with known amounts of recombinant cytokines purchased from Genzyme (Cambridge, CA).
An ELISpot assay was used to determine the frequency of IL-4-producing cells. Microtitre plates were coated with 0·25 μg/well of monoclonal anti-mouse IL-4 (cell line BVD4–1D11) and after overnight incubation at 4° plates were emptied and blocked for 2 hr with phosphate-buffered saline (PBS) containing 3% bovine serum albumin and 0·05% Tween-20 and washed four times with PBS/Tween-20. Cells were cultured in duplicate in the presence of 4 μg/ml mycobacterial culture filtrate proteins for 24 hr at 37° in 7% CO2 atmosphere. For each cell sample, six serial twofold dilutions were performed from a starting concentration of 2 × 106 cells/ml. Cells were removed by washing the plates and cytokine secretion was detected using 0·25 μg/well of biotin-labelled rat anti-mouse IL-4 monoclonal antibody (cell line BVD6–24G2) and 0·1 μg/well of phosphatase-conjugated streptavidin. The enzyme reaction was developed with a solution of 0·9 mg of 5-bromo-4-chloro-3-indolylphosphate per ml of substrate buffer (0·74 mm MgCl2, 0·1% Triton-X405, 9·6% 2-amino-2-methyl-1-propanol, pH 10·25) containing 0·6% agarose. Blue spots were counted microscopically, and the relationship between the number of spots developed per well and the number of input cells was determined.
Mycobacterial culture filtrate proteins were prepared from M. avium strain 25291 grown in Sauton medium. The supernatant of the culture was concentrated by ultrafiltration, precipitated with ammonium sulphate and extensively dialysed against PBS.
Flow cytometry
For the immunofluorescence staining, 106 cells were incubated in a 96-well microtitre plate with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 antibody (dilution 1 : 100) and phycoerythrin (PE)-conjugated anti-CD8 antibody (dilution 1 : 100) or FITC-conjugated anti-CD19 antibody (dilution 1 : 100) and PE-conjugated anti-CD11b antibody (dilution 1 : 100) in PBS containing 3% FCS and 0·1% sodium azide. All antibodies were purchased from BD Pharmingen (San Diego, CA). The cells were washed twice with PBS containing 3% FCS and propidium iodide (Sigma) was added to the cells at a final concentration of 1 μg/ml to allow the exclusion of dead cells. The analysis of the cell populations was based on the acquisition of 10 000 events in a Becton Dickinson FACSort equipped with CellQuest software.
Histology
Portions of the organs of the infected mice were fixed in buffered formaldehyde and embedded in paraffin. Sections were stained with haematoxylin and eosin or stained for acid-fast bacteria by the Ziehl–Neelsen method and counterstained with methylene blue.
Fibrosis was detected using trichrome staining.
Genomic polymerase chain reaction
Genomic polymerase chain reaction (PCR) was performed to determine the Nramp1 allele expressed by B10.Hc0 mice according to the methods and using the primers described in ref. 26. Genomic DNA samples were obtained from each mouse by treating a portion of an ear with proteinase K. The amplification of the Nramp1 gene was performed using Taq DNA polymerase (Ampligene-Oncor, Gaithersburg, MD) and primers from the Nramp1 gene, one oligonucleotide being common to both alleles and the other being specific for either the R or S allele as described elsewhere.26 The amplification was performed in a Gene Amp PCR System 9600 (Perkin Elmer-Roche, Branchblurg, NJ).
Statistical analysis
Statistical analysis was performed using Student's t-test for comparing pairs of data or the analysis of variance (anova) test for larger groups of data.
Results
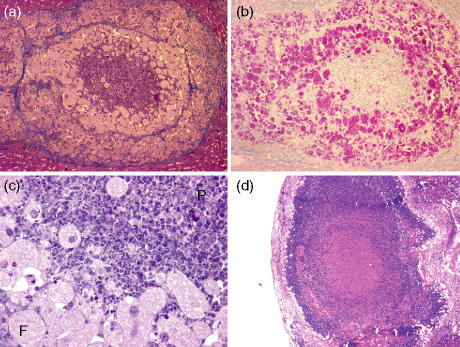
Four mouse strains were selected based on their natural susceptibility to M. avium. C57BL/6, C57BL/10, BALB/c and DBA-1 mice were intravenously infected with around 100 CFU of M. avium ATCC 25291. At 120 and 180 days of infection, mice were killed and the liver was studied for the development of lesions. Macroscopic inspection of the organs of mice at 120 days of infection revealed macroscopic white lesions 1–2 mm in diameter in the livers of both C57BL/6 and C57BL/10 mice. Livers from BALB/c mice showed punctate white lesions whereas the livers of DBA-1 mice were devoid of detectable macroscopic lesions. As shown in Fig. 1, the two strains on a black background developed exuberant necrotic granulomas which enlarged further at 180 days of infection. The caseating lesions were enveloped by a fibrotic capsule as revealed by the trichrome staining. Lesions in BALB/c mice exhibited no necrosis; instead, they showed a core of inflammatory cells composed mostly of polymorphs surrounded by a crown of foamy macrophages heavily parasitized by M. avium(Figs 1 and 2). Finally, no necrosis was detectable in the granulomas of DBA-1 mice (Fig. 1). Similar data were observed in the spleens of infected animals (data not shown) although granulomas were more difficult to delineate from the surrounding tissue given its lymphoid nature. As shown in Fig. 3(a) M. avium proliferated extensively in all four mouse strains with a tendency for increased proliferation in the DBA-1 strain.
Figure 1.
Characteristic granulomas in the indicated mouse strains at 120 (top and middle rows) and 180 (lower row) days after intravenous infection with 100 CFU of Mycobacterium avium 25291. Representative images are presented for groups of five mice (magnification 15×). Sections were stained with H&E (top and lower rows) or by the trichrome stain (middle row).
Figure 2.
Detailed analysis of pathology in BALB/c mice. Typical granuloma at 120 days of infection stained with H&E (a, 100×) or stained for acid-fast bacteria (b, 100×). A detail (magnification 200×) of the granuloma is shown in (c) lower left to indicate the presence of foamy macrophages (F) and polymorphonuclear cell infiltration (P). In (d), a lesion representative of those present in BALB/c.IL10–/– mice is shown (magnification 17×).
Figure 3.
Mycobacterial loads in the indicated organs of the four strains of mice studied (a) as well as the analysis of the immune response as regards IFN-γ secretion in the culture of spleen cells stimulated with Mycobacterium avium antigen and the frequency of IL-4-producing cells as evaluated by ELISPOT assay (b). The cellularity of the spleens in the mice is shown in (c) for the populations of cells staining with antibodies specific for CD4, CD8, CD19 and CD11b. In all panels data for C57BL/6 (black columns), C57BL/10 (dark grey columns), BALB/c (white columns), and DBA-1 (light grey columns) mice are represented as means ± one standard deviation for groups of five mice.
To identify the mechanisms determining the different responses among the four strains, we analysed both the cytokine response and the cellular response in the spleen. Given that Th1 cytokines are essential for necrosis,14,21 we looked at the in vitro production of IFN-γ by splenocytes as well as the number of IL-4-producing cells. Increased IFN-γ responses at 4 months were found in the C57BL/6 (P < 0·01 versus BALB/c and DBA-1) and C57BL/10 (P < 0·01 versus BALB/c and P < 0·05 versus DBA-1) mice although these differences were rather small (Fig. 3b). At 6 months, when infection was massive, IFN-γ secretion was drastically reduced, as is typically observed in mice during infection with this strain of M. avium when bacterial loads are high.27 No correlation between the frequency of IL-4-producing cells and pathology was identified (at 4 months of infection the only statistically significant difference was between C57BL/10 and DBA-1, with P < 0·05, and at 6 months C57BL/6 was significantly different from all other strains, with P < 0·01 versus C57BL/10 and DBA-1 and P < 0·05 versus BALB/c). In an independent experiment, IFN-γ secretion at 2 and 3 months of infection was superior in C57BL/6 mice as compared to BALB/c mice but the number of IL4-secreting cells was equivalent in both strains (data not shown). The spleen cell populations differed among the four strains (Fig. 3c). In BALB/c mice, increased survival or expansion of CD4+ T cells (statistically significant at 4 months versus DBA-1, with P < 0·01, and at 6 months versus all other strains, with P < 0·01 versus C57BL/6 and C57BL/10 and P < 0·05 versus DBA-1) and CD19+ B cells (statistically significant at 4 months versus DBA-1, with P < 0·01, and at 6 months versus all other strains, with P < 0·01) was found as compared to the other three strains. Also, DBA-1 mice showed much less accumulation of CD11b+ phagocytic cells, namely at the later time-point of infection (statistically significant at 4 months with P < 0·05 versus C57BL/10 and P < 0·01 versus BALB/c, and at 6 months with P < 0·01 versus C57BL/6 and C57BL/10 and P < 0·05 versus BALB/c).
To understand the immunological basis of the lack of necrosis in BALB/c and DBA-1 mice we tested the involvement of likely candidates. Thus, data from other infection models, such as Leishmania major infection, have shown BALB/c mice to be prone to a type 2 response whereas C57BL/6 animals mount vigorous type 1 responses during such infections. Although we failed to observe a pronounced IL-4 response to M. avium in any of the strains studied here, we studied IL-10 production by spleen cells stimulated with a mitogen, concanavalin A, during the infection of C57BL/6 and BALB/c mice with M. avium. As shown in Fig. 4, splenocytes from BALB/c mice produced higher levels of IL-10 than those of C57BL/6 mice either before infection or at 60, 90 and 120 days of infection (P < 0·01 at day 0 or 90). At late stages of the infection, high levels of IL-10 were produced by either strain with large variability in C57BL/6 animals. Despite the observed differences in IL-10 secretion in response to concanavalin A, no differences in IL-10 secretion in response to M. avium antigen were observed (data not shown).
Figure 4.
Production of IL-10 by spleen cells from C57BL/6 or BALB/c mice infected with Mycobacterium avium 25291. Cells were stimulated with 4 μg/ml concanavalin A for 72 hr and the concentration of IL-10 was determined by ELISA. Statistically significant differences according to the Student's t-test are labelled ** for P < 0·01.
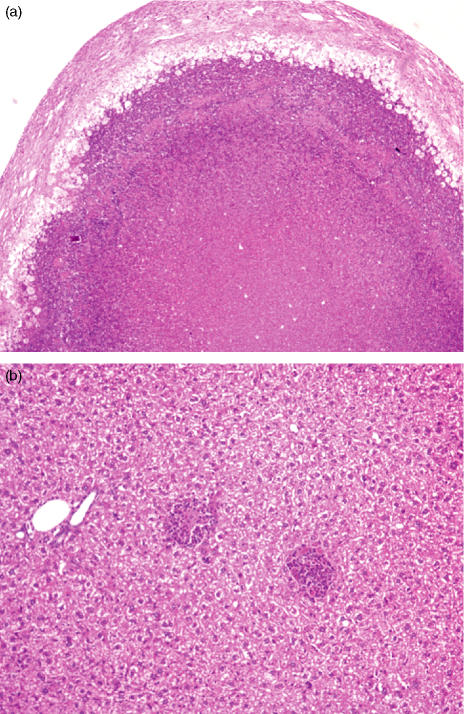
We then tested whether the absence of IL-4 or IL-10 would make BALB/c mice susceptible to the development of granuloma necrosis. Experiments using BALB/c mice with disrupted IL-4 genes failed to show any effects on the development of necrosis (data not shown). However, when BALB/c.IL-10–/– mice were infected they showed a transient increase in resistance to infection (Table 1) and granuloma necrosis was observed in most animals at 180 days of infection (Fig. 2). We then focused on the mechanism responsible for the lack of necrosis in the DBA-1 strain. Given that the DBA mice have a mutation that renders them deficient in the C5 component of complement, we addressed the role of the locus controlling C5 production (the Hc locus) in determining the resistance to necrosis. We thus infected congenic mice on a B10 background with M. avium 25291 and studied them at 180 days of infection. As shown in Fig. 5, B10.Hc1 mice (C5-sufficient) reproduced the data obtained previously with the C57BL/10 mice in so far as exuberant caseating lesions were found in the livers of the infected animals. In contrast, C5-deficient B10.Hc0 mice had minimal sized granulomas with no evidence of necrosis. Although mice in the latter strain had lower bacterial loads in their organs (Table 2), the number of bacteria detected was similar to that previously found to be sufficient to induce necrosis in other strains prone to this type of pathology14 but necrosis was never observed in B10.Hc0 mice. No statistically significant differences in IFN-γ production were found between the two strains but C5-deficient animals had significantly lower phagocyte numbers in their spleens as compared to control mice. We also confirmed that the congenic C5-deficient mouse strain still expressed the Nramp1 allele of the C57BL/10 strain because this is a major determinant of resistance to M. avium. As shown in Fig. 6, B10.Hc0 mice expressed the D169 mutation (responsible for susceptibility) as any of the other strains used in this study.
Table 1.
Mycobacterial growth in BALB/c and BALB/c.IL10–/– mice infected with Mycobacterium avium 25291 150 or 180 days earlier
| Mycobacterial loads (log10 CFU/organ) | ||
|---|---|---|
| BALB/c. | BALB/c.IL10–/– | |
| At 150 days of infection | ||
| Spleen | 10·46 ± 0·39 | 9·36 ± 0·111 |
| Liver | 10·15 ± 0·31 | 9·12 ± 0·231 |
| Lung | 9·87 ± 0·43 | 8·29 ± 0·421 |
| At 180 days of infection | ||
| Spleen | 10·86 ± 0·14 | 10·44 ± 0·10 |
| Liver | 10·49 ± 0·12 | 10·37 ± 0·15 |
| Lung | 10·39 ± 0·32 | 10·27 ± 0·17 |
P < 0·05 according to Student's t-test.
Figure 5.
Influence of the locus Hc on the development of pathology. Congenic C57BL/10 mice (a) expressing (Hc1, 35×) or (b) not expressing (Hc0, 70×) a functional C5 gene were infected with Mycobacterium avium and the lesions were studied by histology at 180 days of infection.
Table 2.
Comparison of mycobacterial growth and host response in Hc congenic mice infected with Mycobacterium avium 25291 180 days earlier
| C57BL/10.Hc1 | C57BL/10.Hc0 | |
|---|---|---|
| Mycobacterial loads (log10 CFU/organ) | ||
| Spleen | 9·16 ± 0·76 | 7·77 ± 0·281 |
| Liver | 9·01 ± 1·14 | 7·06 ± 0·841 |
| Lung | 8·29 ± 0·62 | 7·37 ± 0·45 |
| In vitro IFN-γ response2 | 860·1 ± 787·6 | 2457·0 ± 1582·9 |
| Spleen cell numbers (× 10−6) | ||
| CD4+ cells | 7·0 ± 2·8 | 6·6 ± 1·2 |
| CD8+ cells | 3·1 ± 2·0 | 3·0 ± 0·3 |
| CD19+ cells | 68·3 ± 36·9 | 70·5 ± 6·6 |
| CD11b+ cells | 21·9 ± 7·2 | 8·7 ± 1·21 |
P < 0·05 according to Student's t-test.
Amounts of IFN-γ in pg/ml of supernatants from spleen cell cultures stimulated in vitro with M. avium culture filtrate proteins. The amounts of IFN-γ in supernatants of nonstimulated cells were below detection levels.
Figure 6.
Genomic PCR of DNA from B10.Hc0 mice as compared to two well-known strains: BALB/c expresses the D169 allele and the BALB/c congenic C.D2 strain expresses the G169 allele.
Discussion
Although many of the cellular and molecular mechanisms underlying the structuring of a granuloma have been identified, very little is known about what causes these granulomas to undergo necrosis in mycobacterial infections. Previous work from our group focused on the cells and cytokines involved.14,20 Here we show that the phenomenon of granuloma necrosis is genetically determined. Among four strains of mice, only two exhibited extensive necrosis during infection with low doses of a highly virulent strain of M. avium whereas no necrosis was found in the first 6 months of infection in BALB/c and DBA/1 mice. Our studies were limited to those first 6 months of infection and cannot therefore exclude the possibility of delayed granuloma necrosis. We provide evidence that the complement component C5 is required for the development of this type of immunopathology and that strains deficient in C5 fail to exhibit granuloma necrosis in the time frame of our study. Furthermore, the anti-inflammatory cytokine IL-10 was responsible for the inhibition of granuloma necrosis in BALB/c mice.
Granuloma necrosis requires a strong type 1 cellular immune response. IFN-γ-producing CD4+ T cells and the immune components required for their differentiation are absolute requirements for the development of this pathology.14 Although type 2 immune responses have been incriminated in other models where necrosis of inflammatory sites was induced28 and an association was found between an enhanced Th2 response in human tuberculosis patients and cavitary disease,29 we failed to find a correlation between IL-4 production and pathology. Furthermore, studies using IL-4-gene-disrupted BALB/c mice failed to explain the lower degree of pathology found in this strain through an inhibitory role of this cytokine or an immune deviation that might be associated with infection in this strain. However, this strain is a higher producer of IL-10 in response to infection by M. avium than C57BL/6 mice and this may decrease their susceptibility to necrosis although at the expense of increased susceptibility to infection. Also, attempts to immunize BALB/c mice with M. avium proteins and type 1 response-inducing adjuvants led to the subsequent development of necrosis in a fraction of the immunized mice upon challenge with the virulent mycobacterium (unpublished data). Overall, the immune response to M. avium may vary among strains with some, such as the BALB/c mice, exhibiting less polarized responses, lower pathology and increased susceptibility to mycobacterial proliferation. The source of IL-10 is still not clear. The fact that differences in production can be already observed in cells from uninfected healthy animals upon stimulation with a mitogen raises the interesting possibility that regulatory T cells might be the producers of this cytokine. This issue is currently under investigation.
The complement component C5 has been shown to be required for efficient organization of granulomas and protective immunity during an experimental infection with M. tuberculosis.30,31 In this work, the lack of C5 impacted negatively in the expression of a number of chemokines that were probaby involved in the recruitment of leucocytes into nascent granulomas. Here we confirm the important role of C5 in the development of granulomas during M. avium infection and extend these observations by showing that C5 is also a requisite for the later development of granuloma necrosis. We should point out that development of necrosis at very late time-points of infection was not formally excluded by our studies, which did not follow the pathology beyond the 6th month of infection, but we feel this is rather unlikely because the lesions observed in Hc0 animals remain very small and necrosis always appears in very large granulomas.
It is still unclear how C5 affects the evolution of the granulomas. It is possible that the reduced influx of phagocytes in the absence of C5 may limit the size of the granuloma such that it stays resistant to necrosis. On the other hand, we cannot exclude the possibility that elements of the complement cascade might interfere with the immune response that is responsible for the induction of necrosis in formed granulomas. Given the participation of C5 in chemokine and cytokine production30,32 and in the migration of dendritic cells33 required for the initiation of an immune response, any of these explanations remains possible. Our data show that it may be possible to manipulate the complement system to prevent damage in infected organs. In contrast with the work of Actor et al.30 the lack of C5 in our system did not impact negatively on the resistance of the congenic mouse strains to M. avium. Thus, it may be possible to pharmacologically interfere with C5 to prevent tissue damage while not hampering protective immunity. In conclusion, the data reported here provide evidence for a new model for the study of granuloma necrosis and the design of potential therapeutic approaches that might be useful in the management of this type of pathology.
Acknowledgments
This work was supported by grants POCTI/32629/99 and PSIDA/MGI/49647/2003 from the Fundação pare a Ciência ea Tecnologia (FCT, Portugal), and SDH.IC.I.01·15 from the Calouste Gulbenkian Foundation (Portugal). M. Flórido received a fellowship from the FCT. The authors are indebted to Dr A. O'Garra for her gift of IL-10-deficient BALB/c mice and to Alexandra Rêma, Fátima Faria and Célia Lopes for their technical help.
Abbreviations
- CFU
colony-forming units
- DMEM
Dulbecco's modified Eagle's medium
- FITC
fluorescein isothiocyanate
- IFN-γ
interferon-γ
- IL-1
interleukin-1
- PE
phycoerythrin
- TNF
tumour necrosis factor
References
- 1.Hänsch HCR, Smith DA, Mielke MEA, Hahn H, Bancroft GJ, Ehlers S. Mechanisms of granuloma formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Intern Immunol. 1996;8:1299–310. doi: 10.1093/intimm/8.8.1299. [DOI] [PubMed] [Google Scholar]
- 2.Saunders BM, Frank AA, Orme IM. Granuloma formation is required to contain bacillus growth and delay mortality in mice chronically infected with Mycobacterium tuberculosis. Immunology. 1999;98:324–8. doi: 10.1046/j.1365-2567.1999.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson CM, Cooper AM, Frank AA, Orme IM. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis-infected mice with a disruption in the intracellular adhesion molecule 1 gene. Infect Immun. 1998;66:1666–70. doi: 10.1128/iai.66.4.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GAW. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192:1201–10. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 5.Orme IM. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–7. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 6.Turner OC, Basaraba RJ, Orme IM. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect Immun. 2003;71:864–71. doi: 10.1128/IAI.71.2.864-871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannenberg AM. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–33. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg AM, Jr, Rook GAW. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses – dual mechanisms that control bacillary multiplication. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection, and Control. Washington: ASM Press; 1994. pp. 459–43. [Google Scholar]
- 9.Grosset J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob Antig Chemother. 2003;47:833–6. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 12.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–17. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas S, Nelson AM. Pathogenesis of tuberculosis in human immunodeficiency virus-infected people. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection, and Control. Washington: ASM Press; 1994. pp. 503–13. [Google Scholar]
- 14.Flórido M, Cooper AM, Appelberg R. Immunological basis of the development of necrotic lesions following Mycobacterium avium infection. Immunology. 2002;106:590–601. doi: 10.1046/j.1365-2567.2002.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelberg R, Castro AG, Pedrosa J, Minóprio P. Role of interleukin 6 in the induction of protective T cells during mycobacterial infections in mice. Immunology. 1994;82:361–4. [PMC free article] [PubMed] [Google Scholar]
- 16.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–71. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro AG, Silva RA, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–19. [PubMed] [Google Scholar]
- 18.Silva RA, Pais TF, Appelberg R. Evaluation of IL-12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–85. [PubMed] [Google Scholar]
- 19.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel ET, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun. 1999;67:3571–9. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flórido M, Appelberg R. Granuloma necrosis during Mycobacterium avium infection does not require tumor necrosis factor. Infect Immun. 2004;72:6139–41. doi: 10.1128/IAI.72.10.6139-6141.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlers S, Benini J, Held HD, Roeck C, Alber G, Uhlig S. αβ T cell receptor-positive cells and interferon-γ, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J Exp Med. 2001;194:1847–59. doi: 10.1084/jem.194.12.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato IY, Kobayashi K, Kasama T, et al. Regulation of Mycobacterium bovis BCG and foreign body granulomas in mice by the Bcg gene. Infect Immun. 1990;58:1210–16. doi: 10.1128/iai.58.5.1210-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orrell JM, Brett SJ, Ivanyi J, Coghill G, Grant A, Beck JS. Morphometric analysis of Mycobacterium tuberculosis infection in mice suggests a genetic influence on the generation of the granulomatous inflammatory response. J Pathol. 1992;166:77–82. doi: 10.1002/path.1711660112. [DOI] [PubMed] [Google Scholar]
- 24.Wangoo A, Sparer T, Brown IN, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J Immunol. 2001;166:4342–9. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Boros DL. Polarization of the immune response to the single immunodominat epitope of p38, a major Schistosoma mansoni egg antigen, generates Th1- or Th2-type cytokines and granulomas. Infect Immun. 1999;67:4570–7. doi: 10.1128/iai.67.9.4570-4577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medina E, Rogerson BJ, North RJ. The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis. Immunology. 1996;88:479–81. doi: 10.1046/j.1365-2567.1996.d01-700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flórido M, Pearl JE, Solache A, Borges M, Haynes L, Cooper AM, Appelberg R. Gamma interferon-induced T-cell loss in virulent Mycobacterium avium infection. Infect Immun. 2005;73:3577–86. doi: 10.1128/IAI.73.6.3577-3586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez Pando R, Orozcoe H, Sampieri A, Pavon L, Velasquillo C, Larriva-Sahd J, Alcocer JM, Madrid MV. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzarella G, Bianco A, Perna F, D'Auria D, Grella E, Moscariello E, Sandussi A. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clin Exp Immunol. 2003;132:283–8. doi: 10.1046/j.1365-2249.2003.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Actor JK, Breij E, Wetsel RA, Hoffmann H, Hunter RL, Jr, Jagannath C. A role for complement C5 in organism containment and granulomatous response during murine tuberculosis. Scand J Immunol. 2001;53:464–74. doi: 10.1046/j.1365-3083.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 31.Jagannath C, Hoffmann H, Sepulveda E, Actor JK, Wetsel RA, Hunter RL. Hypersusceptibility of A/J mice to tuberculosis is in part due to a deficiency of the fifth complement component (C5) Scand J Immunol. 2000;52:369–79. doi: 10.1046/j.1365-3083.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- 32.Czermak BJ, Sarma V, Bless NM, Schmal H, Friedl HP, Ward PA. In vitro and in vivo dependency of chemokine generation on C5a and TNF-alpha. J Immunol. 1999;162:2321–5. [PubMed] [Google Scholar]
- 33.Sozzani S, Sallusto F, Luini W, et al. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155:3292–5. [PubMed] [Google Scholar]