Abstract
Cytotoxic T-lymphocyte antigen 4 immunoglobulin (CTLA4-Ig) and interleukin (IL)-10 are immunomodulatory molecules which target CD28 costimulation by acting either directly or indirectly on the CD80/86 receptors on dendritic cells (DCs). This study examined the effect of combined treatment with CTLA4-Ig and IL-10 on T-cell responsiveness in a dendritic cell–mixed lymphocyte reaction (DC-MLR). T cells derived from nylon wool enrichment (NWT cells) demonstrated 15% (P = 0·006) and 10% (P = 0·0015) inhibition of proliferation with suboptimal doses of IL-10 (5 ng/ml) and CTLA4-Ig (20 ng/ml), respectively. Combined treatment with both agents resulted in 38% inhibition (P = 0·004) of the MLR response compared with untreated controls. In contrast to NWT cells, which consisted of CD4+, CD8+ and CD56+ (NK) cells, purified CD4+ T cells were less responsive to immunomodulation by CTLA4-Ig and IL-10. Repletion of the CD4+ T cells with NK cells restored IL-10 and CTLA4-Ig mediated immunomodulation, suggesting a role for NK cells in the regulation of DC–T-cell interactions. The specific effect of NK cells on DC activation was demonstrated by CD80 up-regulation on DCs in the absence of T cells. However, in the absence of DCs, NK cells augmented the proliferation of autologous CD4+ T cells stimulated by anti-CD3 monoclonal antibody (mAb), which was blocked by CTLA4-Ig. It is proposed that, in the MLR, immunomodulation by suboptimal CTLA4-Ig and IL-10 is influenced by cellular interactions of NK cells with DCs and T cells involving DC lysis and costimulation. Thus, NK cells prime both DCs and T cells to low doses of CTLA4-Ig and IL-10 during alloimmune responses, providing evidence for the potential interaction between innate and adaptive immunity.
Keywords: costimulation, cytotoxic T-lymphocyte antigen 4, dendritic cells, interleukin-10, mixed lymphocyte reaction, natural killer cells
Introduction
Dendritic cells (DCs) are potent activators of naïve T cells, and the specific blockade of costimulatory signals from the interactions between these cells can result in T-cell hyporesponsiveness or anergy. However, in the immature state DCs are less potent stimulators of T-cell proliferation, because of their intrinsically lower expression of the costimulatory molecules CD80/86 and CD40 compared with mature DCs.1 In addition, DCs rendered deficient in costimulation [major histocompatibility complex (MHC) class II+ CD80dim CD86–] by culturing progenitor cells in granulocyte–macrophage colony-stimulating factor (GM-CSF) alone are capable of inducing alloantigen-specific T-cell anergy2 and prolonging the survival of cardiac and islet allografts in murine models.3,4 However, in these studies, transplant rejection eventually occurred as a consequence of in vivo maturation of the DCs in secondary lymphoid tissues.3 In particular, DC progenitors treated with interleukin (IL)-10 prior to differentiation demonstrate down-regulation of CD80/86 and CD40 expression, low IL-12 secretion, and induction of anergy in T-cell allogeneic responders.5–9
T-cell hyporesponsiveness may also be induced with cytotoxic T-lymphocyte antigen 4 immunoglobulin (CTLA4-Ig) which binds to CD80 and CD86 with higher affinity than CD28 and consequently blocks T-cell activation mediated by these molecules.10–13 Furthermore, the observed in vitro immunomodulatory effect of CTLA4-Ig was corroborated by the observation of prolongation of allograft survival when the agent was administered in experimental models.14–16 Importantly, long-term graft survival was not achieved unless treatment was combined with anti-CD40 monoclonal antibody (mAb)17 or antisense nuclear factor (NF)-κB oligonucleotides.18 As redundancy in costimulation is expected for CTLA4-Ig monotherapy, we examined the effects of combining CTLA4-Ig with IL-10 in the dendritic cell–mixed lymphocyte reaction (DC-MLR).
We hypothesized that the combined treatment of the DC-MLR with CTLA4-Ig and IL-10 will augment the inhibition of alloreactive T-cell proliferation. To test this hypothesis, suboptimal concentrations of IL-10 and CTLA4-Ig were added singly or in combination to the DC-MLR using nylon wool enriched T (NWT) cells or negatively selected CD4+ T cells as the responder population. Surprisingly, in contrast to the NWT cells, suboptimal doses of CTLA4-Ig and IL-10 were not as effective in inhibiting CD4+ T-cell proliferation in the DC–T-cell MLR and, furthermore, repletion with autologous natural killer (NK) cells restored high responsiveness to the agents. Our data also show that NK cells individually were capable of priming DC activation and CD4+ T-cell proliferation. The observation that DCs precultured with NK cells are capable of mediating the inhibition of CD4+ T-cell proliferation when CTLA4-Ig and IL-10 are added to the MLR in the absence of NK cells suggests a plausible role for NK cells in modifying DC function in the MLR. These findings highlight the role of NK cells in promoting alloimmune responses in a three-way interaction involving allogeneic DCs and autologous T cells.
Materials and methods
Monocyte-derived DCs
Buffy coats were prepared from heparinized peripheral blood obtained from healthy donors (Red Cross Blood Service, Adelaide, Australia) and peripheral blood mononuclear cells (PBMCs) were isolated by differential centrifugation through a Ficoll-Hypaque density gradient (Amersham Biosciences, Uppsala, Sweden). Monocytes were selected by adherence to plastic. Briefly, 5 × 107 PBMCs were panned for 1 hr at 37° in 10 ml of RPMI plus 1% fetal calf serum (FCS) in 75-cm2 plastic tissue culture flasks (Corning, Corning, NY, USA). Non-adherent cells were removed and the remaining adherent cells were cultured in complete medium supplemented with 400 U/ml IL-4 (Peprotech, Rocky Hill, NJ, USA) and 800 U/ml GM-CSF (Schering-Plough, Kenilworth, USA) for 5 days to generate immature DCs (iDCs). The addition of 10 ng/ml tumour necrosis factor (TNF)-α (Genzyme Corporation, Cambridge, MA, USA) to the iDCs for a further 2 days generated mature DCs (mDCs).
Enrichment of cell populations
Following the removal of monocytes by adherence, NWT cells were obtained by applying the non-adherent cells to nylon wool columns equilibrated with RPMI. The non-adherent cells were incubated in the columns for 30 min at 37° to adsorb B cells and the enriched NWT cells were obtained by elution with RPMI plus 10% FCS. CD4+ T cells were negatively selected using a human T-cell isolation kit (Miltenyi Biotech, Bergisch Gladback, Germany) by staining NWT cells with a biotin-labelled antibody cocktail against other cellular populations, followed by incubation with antibiotin microbeads and immunomagnetic separation with AutoMACS (Miltenyi Biotech). Additionally, NK cells were positively selected from NWT by staining with an anti-CD56-fluorescein isothiocyanate (FITC) conjugated antibody followed by incubation with anti-FITC microbeads and immunomagnetic selection.
Monoclonal antibodies and flow cytometry
The cell surface phenotypes of DCs, NK cells and T cells were determined by flow cytometry. Essentially, cells were washed in phosphate-buffered saline (PBS) containing 1% FCS and 0·1% sodium azide [fluorescence-activated cell sorter (FACS) buffer] and incubated, for 30 min on ice, with the primary mAb. For unconjugated primary mAb, cells were washed with FACS buffer and then incubated for 30 min on ice with an isotype-specific, FITC-conjugated anti-mouse secondary antibody and fixed in FACS lysing solution (BD Biosciences, San Jose, CA, USA). Isotype-matched control antibodies were used to determine background staining. Monoclonal antibodies used were: anti-CD4-phycoerythrin (PE) (BD Biosciences), anti-CD56-FITC (BD Biosciences), anti-CD80 (Immunotech, Marseille, France), anti-CD86 (Serotec, Oxford, UK) and anti-CD83-PE (Immunotech). Flow cytometric analysis was performed using a Becton Dickinson (San Jose, CA, USA) FACScan.
Proliferation assays
The MLR was performed by co-culturing DCs (1 × 103) with allogeneic T cells (1 × 105), which represents a stimulator:responder (S:R) ratio of 1 : 100. Cells were cultured in RPMI + 10% FCS for 96 hr and then pulsed with 1 µCi of [3H] thymidine (Amersham Biosciences) for a further 20 hr at 37°. CTLA4-Ig (R & D Systems, Minneapolis, MN, USA) or IL-10 (Bender MedSystems, Vienna, Austria) was added to the cultured cells in the proliferation assays. The [3H] thymidine-pulsed cells were harvested onto glassfibre filters and the incorporated radioactivity was determined by liquid scintillation counting in a Wallac Microbeta Counter (Wallace Oy, Turku, Finland).
For T-cell proliferation assays in the absence of DCs, T cells were activated by addition to a round-bottom 96-well plate precoated with 10 µg/ml anti-CD3mAb (OKT3). NK cells were added to T cells in some wells at a concentration of 10 or 30% to assess the ability of NK cells to augment T-cell proliferation. Triplicate determinations are expressed as mean counts per minute (c.p.m.) ± standard deviation (SD) in all experiments.
5 (and 6)-Carboxyflourescein diacetate succinimidyl ester–mixed lymphocyte reaction (CFSE-MLR)
Proliferation of T-cell subsets was also investigated in a CFSE-MLR. In order to determine the specific proliferation of the T-cell subset, NWT cells were labelled with CFSE in PBS by incubation for 10 min at 37°. Stained cells were washed three times with PBS and cultured with allogeneic DCs at a S:R ratio of 1 : 100. After 5 days of culture, cells were further labelled with either CD4-PE or CD8-PE and proliferation of each population was determined by the dilution of the CFSE signal in the FL1 channel by flow cytometry.
Results
Combined treatment with IL-10 and CTLA4-Ig in the DC-MLR induces T-cell hyporesponsiveness
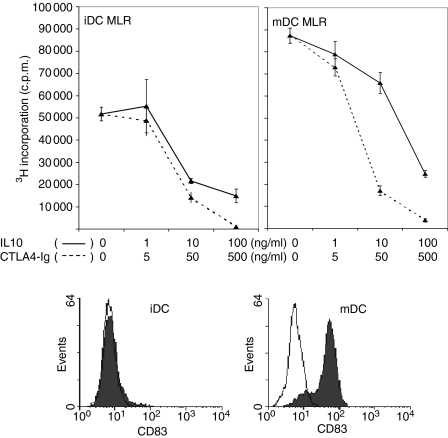
Dose–response analyses of the inhibition of allogeneic T-cell stimulation in the MLR by IL-10 and CTLA4-Ig were performed to determine effective suboptimal doses of each agent to be used in the combination studies. The DC-MLR was assessed with both iDCs and mDCs as stimulators and the maturation status of the DCs was confirmed by the expression of CD83 measured by flow cytometry (Fig. 1). Inhibition of NWT-cell proliferation in the MLR was observed in a wide concentration range of >1 ng/ml to 100 ng/ml for IL-10 and >5 ng/ml to 500 ng/ml for CTLA4-Ig (Fig. 1). The high concentrations of 100 ng/ml for IL-10 and 500 ng/ml for CTLA4-Ig demonstrated maximal inhibition of 70–95% of responder-cell proliferation in the MLR for both iDCs and mDCs as stimulators. However, IL-10 at a concentration of 5 ng/ml and CTLA4-Ig at 20 ng/ml consistently yielded suboptimal inhibition of the MLR (data not shown) and thus these doses were deemed to be suitable to evaluate the effects of combined treatment in the proliferation assay.
Figure 1.
Dose–response effects of interleukin (IL)-10 and CTLA4-Ig in the DC-MLR. IL-10 (solid line) and CTLA4-Ig (dashed line) were titrated with 10-fold dilutions in the DC-MLR. Nylon wool T cells were used as the responder population at a stimulator:responder (S:R) ratio of 1 : 100. All samples were run in triplicate and proliferation was measured by [3H] thymidine incorporation, with results presented as counts per minute (c.p.m.) ± standard deviation (SD). The maturation status of the DCs was demonstrated by flow cytometric analysis by direct staining with anti-CD83-phycoerythrin (PE) (shaded) and with the negative control, isotype-matched PE-conjugated monoclonal antibody (unshaded). iDC, immature dendritic cell; mDC, mature dendritic cell.
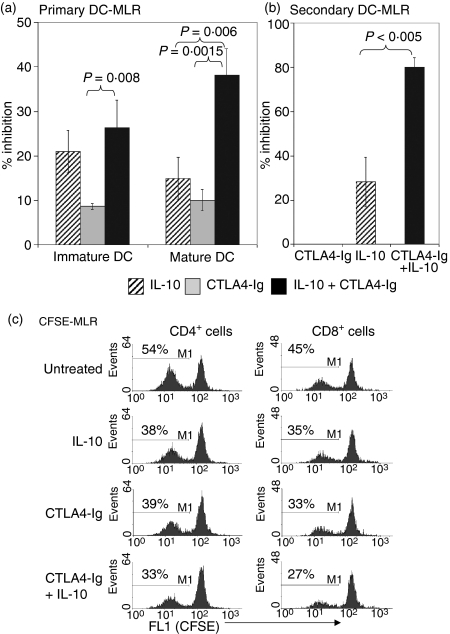
While CTLA4-Ig alone generated an 8–10% inhibition of T-cell proliferation, the combined treatment with IL-10 produced a further increase in inhibition to 25% (P = 0·008) and 38% (P = 0·0015) in the MLR stimulated by iDCs and mDCs, respectively (Fig. 2a). A significant difference in T-cell proliferation was observed between IL-10 alone and IL-10 in combination with CTLA4-Ig for mDCs as stimulators (P = 0·006). T cells from the primary MLR were used for restimulation in a secondary MLR in the absence of both immunomodulatory agents. The data (Fig. 2b) show that T-cell proliferation in the secondary MLR was 80% inhibited (P = 0·005 compared with IL-10) for those T cells that were subject to prior treatments with the combination of agents compared with those cells that received CTLA4-Ig or IL-10 alone in the primary MLR (0 and 28% inhibition, respectively) as shown in Fig. 2(b).
Figure 2.
Combined treatment of interleukin (IL)-10 and CTLA4-Ig inhibited the DC-MLR. Nylon wool T cells were used in the DC-MLR at a stimulator:responder (S:R) ratio of 1 : 100. (a) The percentage inhibition of IL-10 (5 ng/ml) and CTLA4 (20 ng/ml) alone or in combination in the immature or mature DC-MLR. (b) The percentage inhibition in a secondary MLR. T cells from the primary MLR were isolated and used for restimulation in a secondary MLR in the absence of both immunomodulatory agents. Proliferation in (a) and (b) was measured by [3H] thymidine incorporation, with results presented as the percentage inhibition compared with untreated controls. P-values were determined by unpaired Student's t-test. All samples were run in triplicate, and data shown for (a) and (b) are representative of 12 and three independent experiments, respectively. (c) Inhibition of CD4+ and CD8+ T-cell proliferation by IL-10 and CTLA4-Ig in a CFSE-MLR. Nylon wool T cells were stained with CFSE and added to DC stimulators. After 5 days in culture, cells were stained with anti-CD4-phycoerythrin (PE) or anti-CD8-PE and analysed by flow cytometric analysis to determine CFSE dilution in comparison to nonactivated nylon wool T cells. Histograms represent the percentage of proliferating CD4+ and CD8+ T cells based on CFSE dilution and are representative of four independent experiments.
CD4+ and CD8+ T-cell proliferation is inhibited by IL-10 and CTLA4-Ig in the CFSE-MLR
In our analysis, NWT cells typically had a composition of 65–80% CD4+ T cells, 15–30% CD8+ T cells and 5–15% CD56+ NK cells in normal healthy individuals (n = 4). CFSE was used to label NWT cells to determine the specific effects of IL-10 and CTLA4-Ig treatment on T-cell subset proliferation in the CFSE-MLR. After 5 days in culture, the CFSE-labelled cells were stained with either CD4-PE or CD8-PE to determine the proliferative capacity of each cell population by CFSE dilution in the FL-1 channel. Proliferating cells in the untreated CFSE-MLR represented 54% of the total CD4+ T-cell population with DC stimulators (Fig. 2c). Treatment with IL-10 or CTLA4-Ig reduced the percentage of proliferating cells to 38 and 39%, respectively, while the combined treatment reduced proliferating cells to 33%. A similar trend was observed for CD8+ T cells in the untreated CFSE-MLR with a proliferating population of 45% of the total CD8+ cells which was reduced to 35 and 33% with IL-10 and CTLA4-Ig treatment alone, and to 27% with the combination.
NK cells are required for the inhibition of T-cell proliferation by suboptimal doses of IL-10 and CTLA4-Ig
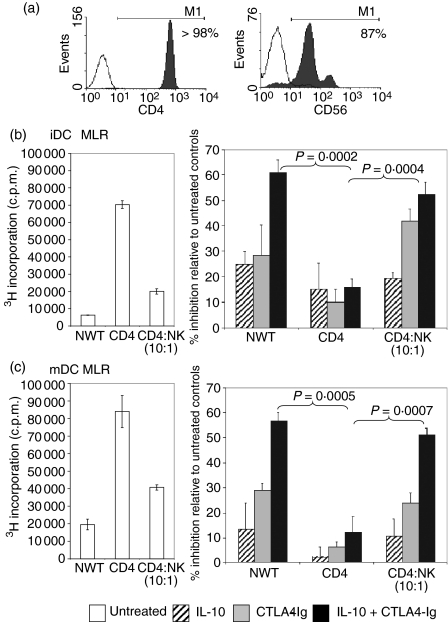
Immunomagnetic separation was used to enrich the CD4+ T-cell population by negative selection from NWT cells, resulting in greater than 98% purity (Fig. 3a). In comparison with NWT cells, purified CD4+ T cells used as responders in the DC-MLR showed a high proliferative response to both iDC and mDC stimulators in the MLR (Figs 3b and c). The reconstitution of the purified CD4+ T cells with 10% NK cells (87% purity, as shown in Fig. 3a) to match the NWT cell composition resulted in a reduction in T-cell proliferation for both types of DC stimulator.
Figure 3.
Natural killer (NK) cells restored the capacity of CTLA4-Igand interleukin (IL)-10 to inhibit CD4+ responder cells in the mixed lymphocyte reaction (MLR). (a) Miltenyi Microbead separation was used to purify CD4+ T cells and CD56+ NK cells from nylon wool T (NWT) cells. CD4+ T cells were isolated by negative selection and stained with anti-CD4-phycoerythrin (PE) (shaded) or negative control PE-conjugated monoclonal antibody (mAb) (unshaded). NK cells were positively selected with anti-CD56-fluorescein isothiocyanate (FITC)-conjugated mAb and captured by anti-FITC microbeads. The histogram shows the overlay of the positive fraction (shaded) against the negative fraction (unshaded). (b, c) NWT cells, CD4+ T cells or CD4+ T cells + 10% NK cells as the responder populations were added to either allogeneic immature dendritic cell (iDC) or mature dendritic cell (mDC) stimulators. A stimulator:responder (S:R) ratio of 1 : 100 was used. IL-10 (5 ng/ml) and CTLA4-Ig (20 ng/ml) were added alone or in combination to the MLR. Proliferation was measured by [3H] thymidine incorporation and results are expressed as the percentage inhibition of proliferation in comparison with untreated controls. P-values were determined by unpaired Student's t-test. Data shown are representative of three independent experiments.
Consistent with the first set of experiments (Fig. 2a), the NWT cells demonstrated strong hyporesponsiveness to CTLA4-Ig and IL-10 treatments. However, compared with NWT cells, the combined doses of IL-10 (5 ng/ml) and CTLA4-Ig (20 ng/ml) in the DC-MLR were not as potent (61% for NWT versus 16% for CD4 T; P = 0·0002) for iDC and (57% for NWT versus 12% for CD4+ T; P = 0·0005) for mDC stimulators, as shown in Figs 3(b) and (c). Repletion of the purified CD4+ T cells with 10% NK cells restored the inhibition of T-cell proliferation mediated by the agents for both the iDC (52 ± 4·8%) and mDC (51 ± 2·7%) MLRs. However, repletion of the CD4+ T cells with CD8+ cells did not restore the inhibitory effect of these agents (data not shown).
DC function is modified by contact with NK cells
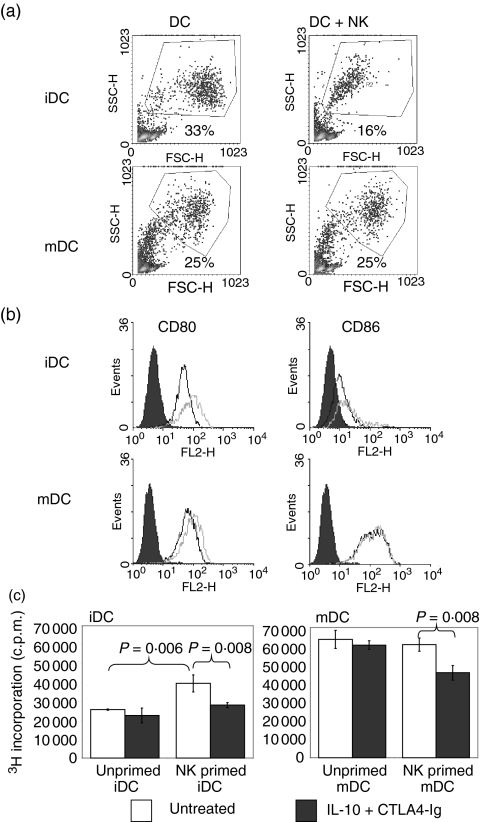
The effect on DC phenotype and function was examined by co-culturing NK cells with allogeneic DCs for 4 days at a NK:DC ratio of 1 : 5. Forward/side-scatter density plots (Fig. 4a) demonstrated a significant reduction in the numbers of gated iDCs in cells co-cultured with NK cells in comparison with untreated cells. In contrast, the profile for mDCs was unaffected by co-incubation with NK cells. While the expression of CD80 on gated DCs showed up-regulation on the iDC population, only minor changes were noted in the mDC population (Fig. 4b). CD86 expression on either iDCs or mDCs showed no changes. NK cells were excluded from the analysis based on forward/side-scatter profiles and antibody staining patterns for CD56.
Figure 4.
Natural killer (NK) cells modified allogeneic dendritic cell (DC) function. Immature and mature DCs (iDCs and mDCs, respectively) were co-cultured with allogeneic NK cells at a ratio of 5 : 1 for 3 days. (a) Gated histograms represent the forward side-scatter profile of DCs and reveal a significant decrease in iDC numbers after culture with NK cells. (b) Flow cytometric analysis was performed on gated DCs using monoclonal antibodies (mAbs) directed against CD80 and CD86. The expression of CD80 and CD86 on DCs cultured alone (black line) or DCs co-cultured with NK cells (grey line) is shown in the histogram and demonstrates up-regulation of CD80 on iDCs. The shaded histogram represents isotype-matched control mAb staining. NK cells were distinguished from DCs and excluded from analysis by gating based on their forward/side-scatter profiles. Data shown are representative of three independent experiments. (c) After 3 days of culture with allogeneic DCs, NK cells were removed by aspiration and density gradient separation. Viable DCs were counted and used as stimulators of allogeneic CD4+ T cells in the absence of NK cells. MLR stimulated by DCs, which were precultured with NK cells, was sensitive to the inhibitory effects of suboptimal CTLA4-Ig/interleukin (IL)-10. Proliferation was measured by [3H] thymidine incorporation and results are expressed as the percentage inhibition of proliferation in comparison with untreated controls. P-values were determined by unpaired Student's t-test.
To investigate whether NK cells precultured with DCs prime T-cell responsiveness to IL-10/CTLA4-Ig, viable DCs were separated from NK cells after a period of 3 days of co-culture by metrizamide density gradient centrifugation and used as stimulators in the DC–T-cell MLR with purified CD4 T cells. The separated DCs were confirmed by flow cytometry to contain <1% residual NK cells. As shown in Fig. 4(c), iDCs primed with NK cells produced a significant increase in T-cell proliferation (35%) compared with unprimed iDCs (P = 0·006); however, primed mDCs did not induce changes. Moreover, while IL-10/CTLA4-Ig demonstrated significant inhibition (P < 0·01) of the MLR for primed iDCs (29%) and mDCs (25%), neither the unprimed iDCs nor mDCs were responsive to IL-10/CTLA4-Ig in the MLR.
NK cells prime CD4+ T-cell proliferation
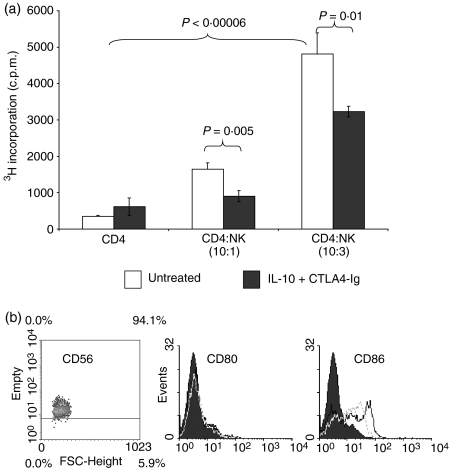
NK cells were co-cultured for 4 days with OKT3 (anti-CD3 mAb)-stimulated autologous CD4+ T cells to investigate the effects of NK cells in the absence of DC stimulators. The data in Fig. 5(a) show that the baseline CD4+ T-cell proliferation increased in a dose-dependent manner with NK cell additions at ratios of CD4+ T:NK cells of between 10 : 1 and 10 : 3 (P = 0·00006) as determined by [3H] thymidine incorporation. The addition of CTLA4-Ig at a concentration of 20 ng/ml to the CD4+ T:NK co-cultures demonstrated significant inhibition of CD4+ T-cell proliferation (P < 0·005) compared with untreated controls (Fig. 5a). NK cells cultured in OKT3-coated plates showed negligible counts (data not shown).
Figure 5.
Natural killer (NK) cells primed CD4 T-cell proliferation and costimulatory molecule expression on dendritic cells (DCs).(a) CD4+ T cells were cultured in anti-CD3-coated plates (10 µg/ml) in the presence or absence of CD56+ NK cells. CTLA4-Ig was added to the co-cultures at 20 ng/ml (black bar). Samples were set up in triplicate, and proliferation was measured by [3H] thymidine incorporation and expressed as counts per minute (c.p.m.) ± standard deviation. P-values were determined by unpaired Student's t-test. The data are representative of three independent experiments. (b) Effect of interleukin (IL)-2 on CD80 and CD86 expression on NK cells. From flow cytometric analysis, histograms of CD80 or CD86 staining of NK cells cultured in the presence (black line) or absence (grey line) of 200 U/ml IL-2 were obtained. Shaded histograms represent isotype-matched control monoclonal antibody staining. Data are representative of two independent experiments.
In order to determine the expression of CD80 or CD86 on NK cells, NK cells were cultured for 4 days in the presence or absence of 200 U/ml IL-2. Flow cytometric analysis showed moderate expression of CD86, which was up-regulated with the addition of IL-2 to the culture. There was no observed expression of CD80 irrespective of the addition of IL-2 to the culture (Fig. 5b).
Discussion
In this study, we have demonstrated that IL-10 augmented CTLA4-Ig-mediated inhibition of T-cell proliferation in an allogeneic DC-MLR. In the first set of experiments, the DC-MLR was performed using NWT responder cells with either immature or mature DCs as stimulators. As shown in Fig. 1, iDCs were poor stimulators of the MLR compared with mDCs, and the dose–response studies identified optimal and suboptimal concentrations for CTLA4-Ig and IL-10 in these assays. At suboptimal concentrations, the combined dose of CTLA4-Ig and IL-10 produced 2- to 3-fold greater inhibition of NWT responder cell proliferation compared with either agent alone, irrespective of the maturation status of the DC stimulators. Of note was the finding that the responder cells derived from the primary MLR treated with the combination of agents were the least responsive to mDCs in a secondary MLR, suggesting the induction of T-cell anergy. Co-culture of CFSE-labelled NWT cells with DC stimulators was also performed to determine the individual responsiveness of both CD4+ and CD8+ T cells in the NWT preparation. Flow analysis of CFSE-labelled NWT cells, further stained with CD4+ and CD8+ T-cell phenotypic markers, demonstrated that both populations were inhibited by IL-10 and CTLA4-Ig. The augmentation of T-cell hyporesponsiveness found in the present study is probably attributable to the combined effects of CTLA4-Ig blockade of CD28 costimulation10 and the down-regulation of CD80/86 expression on DCs by IL-10.9 Moreover, apart from directly acting on T cells, IL-10 also inhibits the expression of CD40 and IL-12 production by DCs, which consequentially affects T-cell proliferation.5,7,19
In another set of DC-MLR experiments, purified CD4+ T cells, in contrast to the NWT cells in the DC-MLR, demonstrated low susceptibility to immunomodulation mediated by suboptimal doses of CTLA4-Ig and IL-10 added singly or in combination to the MLR (Fig. 3). This result suggested a potential role for either NK-cell or CD8+ T-cell populations in priming the DC-MLR. While repletion of the purified CD4+ T cells with CD8+ T cells did not produce any effect on the MLR (data not shown), the addition of NK cells at a concentration of 10% restored the higher inhibitory capacity of CTLA4-Ig and IL-10, indicating that NK cells can potentially interact with either DCs or T cells to modify the alloimmune response. Similarly, in another study it was shown that anti-CD3-stimulated PBMC cultures were responsive to IL-10-mediated inhibition while purified CD45RO T cells were unaffected.20 This suggests that accessory populations such as NK cells or monocytes within PBMCs may influence the susceptibility of T cells to IL-10-mediated inhibition.
Confirming the specific interactions between NK and T cells with respect to CD28 costimulation,21,22 our data demonstrate that NK cells cultured with IL-2 up-regulated the expression of CD86. Furthermore, our study has demonstrated that NK cells costimulated CD4+ T-cell proliferation and has confirmed the involvement of CD86, as CTLA4-Ig blockade was able to inhibit proliferation (Fig. 4). Other costimulatory pathways apart from CD28 have also previously been implicated in driving NK cell-mediated costimulation of T cells, including OX40–OX40L22 or 2B4–CD4823 interactions. Taken together with the findings of these studies, our observations (Fig. 4) suggest that the interactions between NK cells and T cells are sensitive and non-redundant, as blockade of either one of these pathways or of CD28 costimulation abrogated the effect of the NK cells.
NK cells were also shown to have a direct effect on DCs, which is consistent with other reports in that autologous NK cells were able to activate DCs by both cell-contact-dependent and cytokine-mediated effects.24,25 While NK cells have a propensity to lyse autologous iDCs at high effector:target ratios of 50 : 1 to 2 : 1,26,27 it is important to emphasize that our co-culture experiments involved ratios of 0·2 : 1, which are within levels reported to be incapable of inducing lysis of DCs by NK cells. However, we cannot exclude the possibility of NK cell-mediated lysis of the allogeneic DCs in the DC-MLR reported in the present study. Reduction in DC numbers as a result of lysis (Fig. 4a) may partially account for the increased sensitivity of T cells to IL-10- and CTLA4-Ig-mediated inhibition at suboptimal concentrations. However, the non-lysed viable iDCs after culture with NK cells showed up-regulation of CD80 and, when used as stimulators in the MLR, showed increased stimulatory capacity even after the removal of NK cells and in the MLR. In addition, both iDCs and mDCs precultured with NK cells showed sensitivity to suboptimal concentrations of IL-10 and CTLA4-Ig, while DCs not exposed to NK cells were non-responsive. Thus, the role of NK cells in mediating responsiveness to the suboptimal combination of CTLA4-Ig and IL-10 appears to involve direct interactions of NK cells with allogeneic DCs, which lead to the lysis of DCs, up-regulation of costimulation and the potential contribution of soluble factors. The effect of NK cells in the MLR is not limited to DCs, as T-cell costimulation may also occur by interaction with autologous NK cells via a CTLA4-Ig-sensitive mechanism, as discussed above.
While the primary role of NK cells in the innate immune system is in the killing of autologous tumour and pathogen-infected cells, their consequent effects on the adaptive immune response may increase the range of function of these cells in directly activating the alloimmune response.28,29 Recently, evidence has been accumulating for a fundamental role for NK cells in allograft rejection, as these cells promote cardiac allograft vasculopathy30 and cellular infiltration in the graft.31 In addition, studies have demonstrated that the depletion of NK cells ensures robust allograft acceptance in association with tolerance-inducing protocols.31,32
In summary, we report that the combined treatment of the allogeneic DC-MLR with suboptimal doses of IL-10 and CTLA4-Ig inhibited T-cell activation. Notably, NK cells are potentially able to render autologous T cells and allogeneic DCs susceptible to the inhibitory effects of IL-10 and CTLA4-Ig. Thus, therapeutic strategies that involve the combination of CTLA4-Ig and IL-10 may be effective in promoting allograft tolerance by targeting NK cells associated with DC–T-cell interactions.
Acknowledgments
The authors wish to acknowledge the support of Kidney Health Australia, the Queen Elizabeth Hospital Research Foundation and the Muriel Gunn Foundation. AN is a recipient of the Kidney Health Australia Biomedical Research Scholarship. Dr Henry Betts is gratefully acknowledged for providing helpful suggestions in the preparation of the manuscript.
Abbreviations
- CFSE
5 (and 6)-carboxyflourescein diacetate succinimidyl ester
- DC
dendritic cell
- FCS
fetal calf serum
- mAb
monoclonal antibody
- IL
interleukin
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- NK
natural killer
- NWT
nylon wool T-cell
- PBMC
peripheral blood mononuclear cell
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Lu LMD, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2−) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539–45. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rastellini CLL, Ricordi C, Rao AS, Thomson AW. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60:1366–70. [PMC free article] [PubMed] [Google Scholar]
- 5.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 6.Caux C, Massacrier C, Vanbervliet B, Barthelemy C, Liu YJ, Banchereau J. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int Immunol. 1994;6:1177–85. doi: 10.1093/intimm/6.8.1177. [DOI] [PubMed] [Google Scholar]
- 7.Shurin MR, Yurkovetsky ZR, Tourkova IL, Balkir L, Shurin GV. Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10. Int J Cancer. 2002;101:61–8. doi: 10.1002/ijc.10576. [DOI] [PubMed] [Google Scholar]
- 8.Buelens C, Willems F, Delvaux A, Pierard G, Delville JP, Velu T, Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–72. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 9.Coates PT, Krishnan R, Kireta S, Johnston J, Russ GR. Human myeloid dendritic cells transduced with an adenoviral interleukin-10 gene construct inhibit human skin graft rejection in humanized NOD-scid chimeric mice. Gene Ther. 2001;8:1224–33. doi: 10.1038/sj.gt.3301513. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–40. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newland A, Kireta S, Russ G, Krishnan R. Ovine dendritic cells transduced with an adenoviral CTLA4eEGFP fusion protein construct induce hyporesponsiveness to allostimulation. Immunology. 2004;113:310–7. doi: 10.1111/j.1365-2567.2004.01966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayegh MH, Zheng XG, Magee C, Hancock WW, Turka LA. Donor antigen is necessary for the prevention of chronic rejection in CTLA4Ig-treated murine cardiac allograft recipients. Transplantation. 1997;64:1646–50. doi: 10.1097/00007890-199712270-00003. [DOI] [PubMed] [Google Scholar]
- 15.Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, Turka LA. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–6. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Rourke RW, Kang SM, Lower JA, Feng S, Ascher NL, Baekkeskov S, Stock PG. A dendritic cell line genetically modified to express CTLA4-IG as a means to prolong islet allograft survival. Transplantation. 2000;69:1440–6. doi: 10.1097/00007890-200004150-00039. [DOI] [PubMed] [Google Scholar]
- 17.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonham CA, Peng L, Liang X, et al. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169:3382–91. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- 19.Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation. 1998;66:1567–74. doi: 10.1097/00007890-199812270-00001. [DOI] [PubMed] [Google Scholar]
- 20.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000;14:1666–8. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 21.Hanna J, Fitchett J, Rowe T, et al. Proteomic analysis of human natural killer cells: insights on new potential NK immune functions. Mol Immunol. 2005;42:425–31. doi: 10.1016/j.molimm.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40–OX40 ligand interactions. J Immunol. 2004;173:3716–24. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 23.Assarsson E, Kambayashi T, Schatzle JD, Cramer SO, von Bonin A, Jensen PE, Ljunggren HG, Chambers BJ. NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J Immunol. 2004;173:174–80. doi: 10.4049/jimmunol.173.1.174. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 25.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–34. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 26.Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163:6365–70. [PubMed] [Google Scholar]
- 27.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biron C, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather T. Natural killer cells in antiviral defence: function and regulation by innate cytokines. Annu Rev Immunol. 1997;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 29.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–38. [PubMed] [Google Scholar]
- 30.Uehara S, Chase CM, Colvin RB, Russell PS, Madsen JC. Further evidence that NK cells may contribute to the development of cardiac allograft vasculopathy. Transplant Proc. 2005;37:70–1. doi: 10.1016/j.transproceed.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 31.Maier S, Tertilt C, Chambron N, Gerauer K, Huser N, Heidecke CD, Pfeffer K. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28–/– mice. Nat Med. 2001;7:557–62. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 32.Westerhuis G, Maas WG, Willemze R, Toes RE, Fibbe WE. Long-term mixed chimerism after immunological conditioning and MHC-mismatched stem cell transplantation is dependent on NK cell tolerance. Blood. 2005;106:2215–20. doi: 10.1182/blood-2005-04-1391. [DOI] [PubMed] [Google Scholar]