Abstract
We have previously demonstrated that CD4+ CD25+ natural regulatory T cells (Treg cells) induce down-modulation of CD80 and CD86 (B7) molecules on dendritic cells (DCs) in vitro. In this report we show that the extent of down-modulation is functionally significant because Treg-cell conditioned DCs induced poor T-cell proliferation responses. Further, we report that down-modulation was induced rapidly and was inhibited by blocking cytotoxic T lymphocyte antigen-4 (CTLA-4), which is constitutively expressed by the Treg cells. Even though Treg cells have previously been reported to kill antigen-presenting cells, the down-modulation was not due to selective killing of DCs expressing high level of the costimulatory molecules. We propose that Treg cells down-modulate B7-molecules on DCs in a CTLA-4-dependent way, thereby enhancing suppression of T-cell activity.
Keywords: co-stimulation/costimulatory molecule, dendritic cells, regulatory T cells
Introduction
CD4+ CD25+ natural regulatory T cells (Treg cells) were first described by Sakaguchi and coworkers.1 These cells have during the last few years been extensively studied both in the mouse and in man. Natural Treg cells were initially described as anergic cells that suppress T-cell proliferation in vitro. Recent evidence has shown, however, that natural Treg cells proliferate extensively in vivo (reviewed in 2) and also in vitro provided that interleukin-2 (IL-2) is present in culture.3 The effector function of natural Treg cells is cell-contact dependent because these cells fail to suppress their target T cells when physically separated.3,4 The observation that natural Treg cells fail to suppress B7-deficient T cells provides further evidence for the cell-contact dependency of suppression.5
The effector mechanism of natural Treg cells has remained elusive. Some data suggest that the regulatory function in vitro is transforming growth factor-β (TGF-β) dependent,6 although this issue is controversial.7 Another proposed function of the Treg cells is competition for IL-2,8,9 which would be consistent both with their constitutive expression of the IL-2 receptor and the contact-dependence of their mechanism of suppression. However, the observation that Treg unresponsive to IL-2 effectively suppress T-cell proliferation indicates that competition for IL-2 is not essential for suppression.10
Early studies suggested that antigen-presenting cells (APC) would play only a minor role in suppression in vitro, because APCs could be fixed with aldehyde3,4 and also be replaced by antibody-coated plastic beads.11 However, more recent evidence indicates that natural Treg cells do communicate with APCs and in particular dendritic cells (DC). Thus, in vitro coculture of DC with natural Treg cells modulates DC function causing the induction of tolerogenic immune responses.12–14 During coculture, natural Treg cells induce the expression of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO) in DCs, generating an immunosuppressive milieu that induces abortive immune responses.12 The induction of IDO was shown to be cytotoxic T lymphocyte antigen-4 (CTLA-4) dependent12 and may involve an interaction between CTLA-4 on the Treg cells and CD80-molecules on the DCs.15 Further, DCs have been implicated in the expansion of natural Treg cells.16,17 Recently it was shown that Treg-mediated suppression in vivo involves prolonged Treg/DC interactions but only transient Treg/T-cell interactions.18 Studies performed in a mouse model for asthma also provided evidence suggesting an in vivo role of Treg as regulators of DC activation.19 Thus, interaction with DCs is an emerging function of Treg in suppression in vivo.
Our previous work provided evidence that Treg cells may interfere with DC function.20 We showed that natural Treg cells induced down-modulation of B7-molecules on cocultured DCs. In this report we have further investigated this mechanism. Our data show that down-modulation occurs rapidly, within the first hrs of coculture. Importantly, we show that this mechanism is CTLA-4-dependent indicating that the CTLA-4/B7 interaction between natural Treg cells and DCs apart from inducing IDO-activity also influences the costimulatory capacity of the DCs.
Materials and methods
Mice
BALB/c and C57BL/6 female mice were obtained from M & B, Ry, Denmark and were used for experiments at 6–12 weeks of age. Cd28−/− mice on C57BL/6 background were used for experiments at 6–8 weeks of age while Ctla-4−/− and wild type control mice on C57BL/6 background were used for experiments at 7–12 days of age.
Enrichment of cell populations
Single cell suspensions were prepared from pooled spleens and lymph nodes, the erythrocytes lysed and B cells were removed by panning on anti-mouse immunoglobulin-coated plates.20 Two different protocols were used to enrich for CD4+ CD25− and CD4+ CD25+ T cells. 1) Total CD4+ T cells were labelled with anti-CD4-fluoroscein isothiocyanate (FITC; Pharmingen, San Diego, CA) and were thereafter positively isolated using an anti-FITC Multisort Kit (magnetic-activated cell sorting; Miltenyi Biotec, Bergisch Gladbach, Germany). This procedure yielded >95% pure CD4+ T cells. To further divide CD4+ T cells into CD4+ CD25− and CD4+ CD25+ T cells, the isolated CD4+ T cells were released from the beads. Subsequently the cells were incubated with biotin-conjugated CD25 antibodies (Pharmingen) followed by SA-microbeads (Miltenyi Biotec) and separated into CD4+ CD25− and CD4+ CD25+ T cells, yielding >95% pure CD4+ CD25+ T cells. 2) Alternatively, the pooled spleen and lymph node cells were directly incubated with biotin-conjugated CD25 antibodies (Pharmingen) followed by SA-microbeads (Miltenyi Biotec) and separated into CD25− cells and CD4+ CD25+ T cells as CD25+ cells are almost exclusively found among CD4+ T cells. CD4+ CD25− T cells were subsequently isolated from the CD25− fraction using anti-CD4-conjugated magnetic microbeads (Miltenyi Biotec). We observed no functional differences between T cells prepared with these two protocols. To obtain more potent regulatory T cells purified CD4+ CD25+ T cells were cultured (1 × 106/ml) in 24-well plates (Falcon, BD) and stimulated by 10 µg/ml plate bound anti-CD3 (145.2C11) and 200 ng/ml recombinant mouse IL-2 (R & D Systems Inc., Minneapolis, MN) for 3 days.
DC were obtained by culturing total bone marrow (BM) cells in medium containing recombinant granulocyte–macrophage colony-stimulating factor, as previously described in detail.20 DC were subsequently isolated with anti-CD11c conjugated magnetic microbeads (Miltenyi Biotec) yielding approximately 95% of CD11c+ MHC II+ (major histocompatibility complex II) cells, when analysed by flow cytometry. Alternatively, DCs were directly isolated from collagenase type IV (Sigma-Aldrich Inc., Sweden AB; 1·6 mg/ml, 30 min, 37°) treated spleens using anti-CD11c-conjugated microbeads (Miltenyi Biotec) and then resuspended in medium and left to adhere on plastic for 2 hr and after removal of non-adherent cells cultured for an additional 12 hr in medium or where indicated in the presence of 0·5 µg/ml of lipopolysaccharide (LPS) (Difco, Detroit, MI) to induce maturation. Alternatively, DCs were matured by 5 hr preculture in vitro. DCs that had been cocultured with either CD4+ CD25− or CD4+ CD25+ T cells were purified using anti-CD11c-conjugated microbeads and then used for further cell cultures.
Cell cultures
Proliferation assays were performed in 200 µl cultures in round bottom 96-well plates (Costar, Cambridge, MA). T cells were polyclonally stimulated by the addition of 1 µg/ml of anti-CD3 antibodies (145·2C11) or 2·5 µg/ml concanavalin A (Con A; Amersham Pharmacia, Uppsala, Sweden) to the cultures. Cells were cultured in RPMI medium (Gibco BRL, Grand Island, NY) supplemented with 50 µm 2-mercaptoethanol, antibiotics, 10% fetal calf serum, 1 mm sodium pyruvate and 10 mm HEPES buffer (all supplements from Gibco) at 37° and 5% CO2. Thymidine incorporation was measured on day 3 of culture after a 4 hr pulse with 1 µCi [3H]-thymidine (Amersham, Biosciences, Uppsala, Sweden). DCs were fixed with 1% paraformaldehyde. For flow cytometric analyses, DCs were cultured for the time indicated either in medium alone, or cocultured with anti-CD3 (1 µg/ml) stimulated CD4+ CD25− and/or CD4+ CD25+ T cells, at the indicated ratios. IDO and inducible nitric oxide synthase (iNOS) activity was inhibited using 300 µm 1-methyl-d,l-tryptophan (1-MT; Sigma-Aldrich) and 300 µm L-N6-(1-iminoethyl)lysine hydrochloride (L-NIL; Sigma-Aldrich), respectively.
Conjugation of fusion-proteins to latex beads
Latex beads (Interfacial Dynamics, Portland, OR) were conjugated with anti-CD3 (145.2C11) and CD80-Fc or CD86-Fc (both bought from R & D Systems Inc., Abingdon, UK) as previously described.11 The beads were counted under a microscope before use in in vitro cell cultures.
Antibodies and flow cytometry
The following antibodies and fluorochrome-conjugated reagents were used for flow cytometry experiments: phycoerythrin (PE)-conjugated anti-CD86 (clone GL1, BD Biosciences, Pharmingen), biotin-conjugated anti-CD80 (clone 1610-A, Pharmingen) and PE-conjugated immunglobulin G1 (IgG1; Pharmingen), biotin-conjugated IgG1 (Pharmingen) as isotype controls, peridinin chlorophyll protein (PerCP)-conjugated anti-CD4 (clone RM4-5, Pharmingen), PE-conjugated anti-CD4 (clone L3T4, Pharmingen), Annexin V FITC (Molecular Probes, Leiden, Holland), 2 µg/ml 7-amino-actinomycin D (7AAD) (Sigma-Aldrich), FITC-conjugated anti-CD8α (clone YTS 169-41), Cy-5 conjugated anti-CD11c (clone N418), Cy-2 conjugated anti-CD4 (clone GK1.5), biotin-conjugated anti-Dd (clone HB102) (all prepared in our laboratory). Binding of biotin-conjugated CD80 was revealed in a second step using streptavidin (SA) Alexa Fluor 488 (Molecular Probes), SA-Cy5 (prepared in our laboratory), SA-Red 613 (Gibco) or SA-PE (Pharmingen). Intracellular staining of CTLA-4 was performed using fixation and permeabilization buffers, Cytofix/Cytoperm and Perm/Wash (Pharmingen), PE-conjugated anti-CTLA-4 (clone UC10-4F10-11, Pharmingen) and PE-conjugated IgG1 (Pharmingen) as isotype control. Stained cells were analysed with a FACSCalibur flow cytometer (Becton Dickinson, San José, CA). For cell culture experiments the following antibodies were used: anti-CD3 (145.2C11), anti-CD28 (37.51), anti-CD80 (1610-A), anti-CD86 (GL1) and 2.4G2 and all prepared in our laboratory. Purified anti-CTLA-4 (UC10-4F10-11) was purchased from Pharmingen.
Measurement of iNOS and IDO activity
NO production was measured as nitrite concentration using the Griess assay. Griess reagent was added (v/v) to cell culture supernatants or serial dilutions of standard (NaNO2) in microplates. Plates were incubated for 10 min at room temperature and absorbance measured at 550 nm in an enzyme-linked immunosorbent assay reader. IDO activity was measured by quantifying tryptophan consumption and kynurenine production in tissue culture supernatants using a previously described method.21
Results
Rapid down-modulation of CD80 and CD86
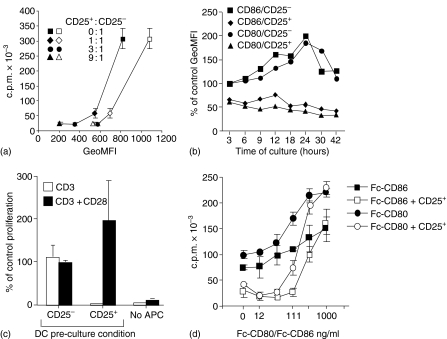
We have previously shown that ex vivo Treg cells down-modulate the expression levels of both CD80 and CD86 on cocultured BM-derived DCs.20 Similar down-modulation of CD80 and CD86 is also seen on splenic DCs in suppression cultures, where Treg cells in parallel inhibited the proliferation of CD4+CD25− responder T cells (Fig. 1a). We next studied the kinetics of down-modulation by analysing the expression of B7-molecules on DCs at various time points of coculture with natural Treg cells. Down-modulation occurred within the first few hours of coculture and the reduced levels were maintained throughout the culture period (Fig. 1b). In contrast, B7-molecules were up-regulated on DCs cocultured with conventional CD4+ CD25− T cells.
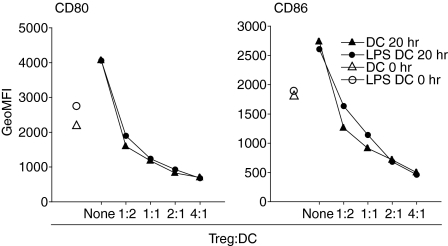
Figure 1.
Correlation between suppression and down-modulation. (a) Splenic DCs were precultured for 5 hr in vitro in medium before re-culture (2·5 × 104/ml) with anti-CD3 and CD4+ CD25− T cells (1 × 105/ml) in the absence or in the presence of CD4+ CD25+ T cells at the various ratios as indicated. T-cell proliferation was analysed after 72 hr and CD80 (open symbols) or CD86 (closed symbols) expression by CD11c+ cells was analysed in parallel cultures after 12 hr. Rapid down-modulation of CD80 and CD86 on DCs. (b) BM-derived DCs (5 × 105/ml) were cultured either with CD4+ CD25− T cells (1 × 106/ml) or CD4+ CD25+ T cells (2 × 106/ml) and stimulated with anti-CD3. The cocultures were harvested and the DCs analysed for cell surface expression of CD80 and CD86 at various time points as indicated. The results are presented as the percentage of the geometric mean fluorescence activity (GeoMFI; CD80 = 247 and CD86 = 816) obtained from analysis of DCs at the start of the experiment. Regulatory T cell-induced down-modulation of B7-molecules is functionally important. (c) BM-derived DCs (0·5 × 106/ml) were cultured for 48 hr in medium alone, or cocultured with anti-CD3-stimulated CD4+ CD25− (1 × 106/ml) or CD4+ CD25+ T cells (2 × 106/ml). The DCs were thereafter isolated from the cultures, fixed with 1% paraformaldehyde and used as APCs (1 × 104/well) in triplicate cultures with fresh responder CD4+ CD25− T cells (2·5 × 104/well) stimulated with anti-CD3 or with anti-CD3 + anti-CD28 (5 µg/ml). Proliferation was assayed after 72 hr of culture and the data are presented as the percentage of responder T cell proliferation induced by DCs precultured without T cells. Costimulation counteracts suppression. (d) CD4+ CD25− T cells (2·5 × 105/ml) were cultured alone or in the presence of CD4+ CD25+ T cells (8·3 × 104/ml) in cultures containing an equal number (2·5 × 105/ml) of latex beads coated with various concentrations of Fc-CD80 or Fc-CD86 and anti-CD3 (0·5 µg/ml) as indicated. Proliferation was assayed after 72 hr. Results from one experiment out of four (c) three (a and d) or two (b) with similar results are shown.
DCs conditioned by Treg cells are poor APC
Next we asked whether Treg cells influenced the functional capacity of DCs. To address this question, DCs were cocultured with natural Treg cells, purified and tested for their ability to induce proliferation of CD4+ CD25− T cells in secondary cultures. However, when re-cultured with CD4+ CD25− T cells in the absence of Treg cells the conditioned DCs regained their expression of the CD80 and CD86 molecules and effectively induced responder T-cell proliferation (data not shown). This may explain why Chai et al. failed to see any functional defect in DCs preconditioned with Treg cells in vitro.22 Therefore, to preserve their ‘conditioned’ state we fixed the precultured DCs with paraformaldehyde before testing their ability to support anti-CD3 induced CD4+ T-cell proliferation.
The fixed Treg-cell conditioned DCs induced poor CD4+ T-cell proliferation (Fig. 1c). Importantly, addition of anti-CD28 antibodies restored the proliferation, suggesting that suboptimal CD28-signalling might be the cause for the poor proliferation. Co-culture with CD4+ CD25− T cells increased the level of CD80 and CD86 on DCs (Fig. 1b) and DCs conditioned by these T cells, even after fixation, induced a robust proliferation response. These data demonstrate that DCs conditioned by Treg cells and CD4+ CD25− T cells on a per cell basis are distinctly different in their ability to induce responder T-cell proliferation.
An increase in costimulatory signals circumvents suppression.3,4,11,23 Consequently, reduced expression of costimulatory molecules on DCs would be expected to promote suppression. Fixation of the DCs prevents membrane movements but allows costimulation-dependent, albeit less efficient, induction of T-cell proliferation (data not shown). To mimic this situation, latex beads were coated with anti-CD3 and various concentrations of recombinant CD80 or CD86 proteins and used as surrogate APCs in suppression cultures.11 As shown in Fig. 1(d), increased concentration of CD80 or CD86 resulted in increased responder T-cell proliferation. In suppression cultures, however, Treg cells inhibited responder T-cell proliferation most efficiently when stimulated with beads conjugated either with anti-CD3 alone or with anti-CD3 and low concentrations of CD80 or CD86. These data support the hypothesis that a reduction in B7-expression on Treg cell conditioned DCs is the cause for their poor capacity to stimulate T-cell proliferation (Fig. 1d).
Taken together, the present and the previous data3,4,11 suggest some common denominators of live APCs, fixed APCs and antibody-coated beads. First, they provide a surface where regulatory and responder T cells can interact and receive T-cell receptor (via anti-CD3) and costimulatory signals (via CD80/CD86). Second, in all these cases suppression is more efficient when the responder T cells are exposed to a low level of costimulatory signals. In fact as suggested by previous reports3,4,11 and supported by the data here (Fig. 1d), increasing the costimulatory signals inhibits the suppression, which in the effector phase involves direct regulatory/responder T-cell interactions.24 We propose therefore that the main functional impact of down-modulation of the B7-molecules on APCs is to enhance suppression mediated by direct Treg cell/responder T-cell interactions.
Down-modulation is CTLA-4-dependent
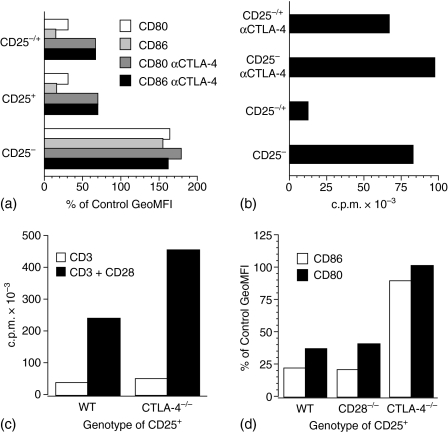
Natural Treg cells, but not CD4+ CD25− T cells, express CTLA-4 constitutively25–27 and CTLA-4 has previously been implicated in the function of Treg cells.26–28 Further, Treg cells induce IDO activity in DCs through a CTLA-4-dependent mechanism, implicating this molecule in Treg cell/DC interactions.12 CTLA-4 therefore appeared as a likely candidate for a molecule mediating the rapid down-modulation of B7-molecules on the APCs.
To address this possibility we performed antibody-blocking experiments. As shown in Fig. 2(a), natural Treg cells either when cultured with DCs alone or when cocultured with responder T cells and DCs, decreased the expression of CD80 and CD86 on DCs. When a high concentration of anti-CTLA-4 antibody was added into such cultures, the down-modulation of both CD80 and CD86 was reversed. To avoid cross-linking by anti-CTLA-4, a saturating concentration of anti-Fc receptor antibody was added into the cultures.29 Importantly, CTLA-4-blocking also interfered with suppression of proliferation, because the proliferation of responder T cells recovered to almost normal level in cocultures (Fig. 2b), confirming previous results.27,28 It should be noted that CTLA-4-blocking did not significantly influence responder T-cell proliferation in the absence of natural Treg cells, indicating that the blocking indeed inhibited the performance of the Treg cells in the cocultures.
Figure 2.
Down-modulation of B7-molecules and suppression of T-cell proliferation is CTLA-4-dependent. (a) BM-derived DCs (0·5 × 106/ml) were cultured either in medium alone, or with wild type CD4+ CD25+ T cells (2 × 106/ml) (CD25+), CD4+ CD25− T cells (CD25−), or a 1 : 1 mixture of T cells (CD25+/−) (2 × 106/ml) and Con A (2·5 µg/ml). Where indicated, anti-CTLA-4 (100 µg/ml) together with Fc-receptor blocking antibody 2.4G2 (2·5 µg/ml) were included in the cultures. The cultures were harvested and the DCs analysed for cell surface expression of CD80 and CD86 after 48 hr of culture. The results are presented as percentage of the GeoMFI (CD80 = 534 and CD86 = 581) obtained from analysis of DCs grown in the absence of T cells. (b) Parallel cultures to those in (a) were assayed for proliferation after 48 hr of culture. Ctla-4-deficient CD4+ CD25+ T cells are anergic but do not down-modulate B7-molecules on DCs. (c) CD4+ CD25+ T cells (2·5 × 104/well) from Ctla-4-deficient and normal (wild type) mice were cultured with DCs (1 × 104/well) and anti-CD3 (1 µg/ml) or anti-CD3 + anti-CD28 (5 µg/ml) and proliferation assayed after 72 hr (d) DCs (0·5 × 106/ml) were cultured for 48 hr either in medium alone or with anti-CD3 stimulated (1 µg/ml) CD4+ CD25+ T cells (2 × 106/ml) either from wild type, Cd28-deficient or Ctla-4-deficient mice. Cells were harvested, stained and CD11c+ cells were analysed for CD80 and CD86 cell surface expression by flow cytometry. The results are presented as percentage of the GeoMFI (CD80 = 402 and CD86 = 2184) obtained from analysis of DCs grown in the absence of T cells. In (d) only age-matched wild type controls for the Ctla-4-deficient mice are shown, because the adult controls for the Cd28-deficient mice gave similar results. The data shown are representative of two to four independent experiments.
To address the involvement of CTLA-4 in the down-modulation in an independent way, we used CD4+ CD25+ T cells from Ctla-4-deficient mice. Because these mice develop a lymphoproliferative disease at about 2 weeks of age30–32 we used cells from 7–12 day-old mice. Even though the CD4+ CD25+ T cells from Ctla-4-deficient mice express Foxp328 and were anergic (Fig. 2c), similar to Treg cells from age-matched control mice, they consistently failed to down-modulate CD80 and CD86 on cocultured APCs (Fig. 2d). This further supports the observation that down-modulation is CTLA-4 dependent. Natural Treg cells from Cd28-deficient and normal mice down-modulated B7-molecules to a similar extent.
As previously reported27 we found that Cd28-deficient Treg cells also suppressed responder T-cell proliferation (data not shown). In our hands, the ability of Ctla-4-deficient CD4+ CD25+ T cells to suppress responder T-cell proliferation28,33 was variable. In some experiments these cells inhibited T-cell proliferation similarly to normal control Treg cells, while in other experiments suppression was bimodal with initial inhibition at low CD4+ CD25+/responder T-cell ratios followed by recovery of proliferation at high ratios (data not shown). Taken together, the results from both these approaches indicate that down-modulation of B7-molecules on DC is CTLA-4 dependent.
Activated Treg cells potently down-modulate B7-expression but do not reduce DC survival
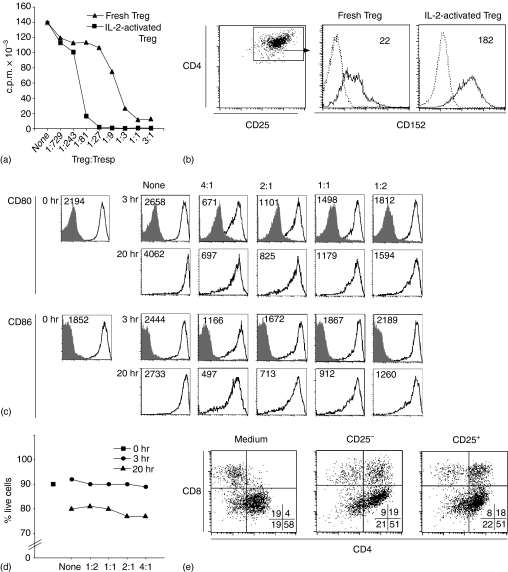
Natural Treg cells were initially described as anergic cells, but divide extensively in vitro when activated in the presence of IL-2.8,34 Such in vitro activated Treg cells efficiently suppressed T-cell proliferation in secondary cultures (Fig. 3a) and expressed an increased level of CTLA-4 (Fig. 3b). Furthermore, the activated cells efficiently down–modulated B7-expression on DCs (Fig. 3c).
Figure 3.
Activated Treg cells express elevated levels of CTLA-4 and are potent suppressor cells.(a) CD4+ CD25− responder T cells were cultured (2·5 × 105/ml) either in medium alone (None), or at various ratios with either ex vivo, or IL-2-activated Treg cells, splenic DCs (2·5 × 104/ml) and anti-CD3 (1 µg/ml). Proliferation was assayed after 72 hr of culture. (b) Ex vivo or IL-2-activated Treg cells were stained with anti-CD4 and anti-CD25 and with anti-CTLA-4 (filled line) or isotype control antibody (dotted line) after permeabilizing the cells. GeoMFI of CTLA-4 is indicated in the histograms. Down-modulation of B7-molecules does not depend on apoptosis of, or selection for DC subpopulations. (c) Splenic DCs were cultured (2·5 × 105/ml) either in medium alone (None), or in the presence of activated Treg cells at the indicated ratios. Cells were stained with anti-CD11c and anti-CD80, anti-CD86 (or isotype controls for anti-CD80 and anti-CD86) and 7AAD and Annexin V both at the start of culture (0 hr) and at 3 hr and 20 hr of culture, respectively. CD80 and CD86 expression (open histograms; Geo MFI is indicated) or isotype controls (filled histograms) on CD11c+ cells and (d) the percentage of 7AAD– Annexin V–, i.e. live cells among CD11c+ cells was determined by fluorescence-activated cell sorting (FACS). Data from one of four experiments with similar results are shown. (e) Splenic DCs were cultured (2·5 × 105/ml) for 12 hr, either in medium alone, or with ex vivo CD4+ CD25− T cells or IL-2-activated Treg cells and anti-CD3 (1 µg/ml), at 1 : 2 ratio between DCs and T cells. Thereafter cells were stained with anti-CD11c, anti-CD4 and anti-CD8 and analysed by FACS. Subset composition of CD11c+ cells is indicated in percent in the plots. Data from one of two experiments with similar results are shown.
It has been reported that both human and mouse Treg cells can kill APCs such as DCs and B cells,35,36 suggesting that the down-modulation of B7-molecules could be the consequence of selective death of DCs expressing high level of these molecules. To address this possibility, we determined the fraction of apoptotic DCs in cocultures with activated Treg cells. Down-modulation of B7-molecules was detectable in DC cocultured with Treg cells for 3 hr (Fig. 3c), confirming the data obtained using BM-derived DCs and ex vivo Treg cells (Fig. 1b). As shown in Fig. 3(d), there was no increase in apoptotic DCs at 3 hr of culture as compared to DCs analysed at the start of the culture or compared to control DCs cultured in the absence of Treg cells. The down-modulation was more pronounced at 20 hr of coculture. The reduced survival observed in these DCs, was Treg-independent since it was also observed in DCs grown in their absence. Co-culture with the activated Treg cells did not change the composition of the DC population (Fig. 3e). The increased frequency of CD4+ CD8α+ DC observed in coculture with activated Treg cells as well as with CD4+ CD25− T cells may represent uptake of T-cell membrane by the CD8α+ DCs, as previously demonstrated.37 Thus, we could exclude that down-modulation was caused by selective survival of DCs expressing low level of B7-molecules.
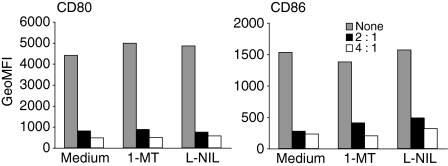
Maintenance of Treg cells has been reported to be dependent on CD40-L/CD40 interactions17,38 and activated CD4+ T cells induce expression of iNOS in DCs via CD40-L/CD40 interactions.39 Further, Treg cells induce IDO expression in DCs via CTLA-4/B7 interactions. We therefore investigated whether these pathways might be involved in the Treg cell-mediated down-modulation of B7 molecules on DCs. Addition of pharmacological inhibitors of these pathways to cocultures of activated Treg cells and DCs did not reduce the down-modulation (Fig. 4). Control experiments confirmed that the inhibitors inhibited LPS-induced NO-production by peritoneal cells and kynurenine production by LPS + interferon-γ-activated spleen-derived DCs (data not shown). We conclude therefore that the iNOS and IDO pathways are not involved in the down-modulation mechanism.
Figure 4.
Down-modulation of B7-molecules is independent of IDO activity and NO production. (a) Splenic DCs were cultured (2·5 × 105/ml) for 20 hr in medium alone (None) or at the indicated ratios with IL-2-activated Treg cells and anti-CD3 (1 µg/ml), either in the presence of the IDO inhibitor 1-MT (300 µm) or of the NO inhibitor L-NIL (300 µm). Cells were thereafter stained with anti-CD11c, anti-CD80 and anti-CD86 and the GeoMFI of CD80 and CD86 on CD11c+ cells was determined by FACS. Data from one of three experiments with similar results are shown.
The above data demonstrated that Treg cells down-modulated B7-molecules on both bone marrow-derived DCs and splenic DCs. It is well established that activation of DCs with bacterial components induces both functional and phenotypic changes in these cells. However, activated Treg cells down-modulated B7-expression equally efficiently on DCs matured in vitro in the presence or absence of LPS (Fig. 5) further supporting the generality of the mechanism.
Figure 5.
LPS does not render DCs refractory to modulation by regulatory T cells. Splenic DCs were precultured for 12 hr (2 × 106/ml) in medium or in the presence of LPS (0·5 µg/ml) and re-cultured (2·5 × 105/ml) for 20 hr either in medium alone (None), or at the indicated ratios with IL-2-activated Treg cells and anti-CD3 (1 µg/ml). Cells were stained with anti-CD11c, anti-CD80 and anti-CD86 and the GeoMFI of CD80 and CD86 on CD11c+ cells was determined by FACS at the start of the second culture (0 hr) and at 20 hr of culture. Data from one of three experiments with similar results are shown.
Discussion
We have previously reported that ex vivo Treg cells down-modulate B7-molecules on cocultured DCs in a cell-contact dependent way.20 In this report we have addressed the functional significance and the mechanism responsible for the down-modulation. We show that the down-modulation occurred within the first few hours of in vitro culture and that it was functionally significant because Treg-cell conditioned DCs induced poor T-cell proliferation responses compared to DCs conditioned by conventional CD4+ T cells. We provide evidence supporting the view that reduced expression of B7-molecules causes that deficiency. The mechanism is selective for some membrane molecules as MHC II molecules were not down-modulated in the same way.40 Certain anergic Treg cells on the other hand can by an unknown mechanism down-modulate both B7- and MHC II molecules.41
Activation of Treg cells in the presence of exogenous IL-2 increases their regulatory function.42 Our previous data demonstrated that in vivo superantigen-activated Treg cells, as compared to ex vivo Treg cells, were more efficient both in down-modulating B7-molecules on DCs and in inhibiting T cell proliferation.40 We show here that in vitro activated Treg cells did not selectively kill DCs expressing high level of B7-molecules nor did they detectably select for certain DC populations. Thus, the down-modulation is most likely caused by a direct effect on the cocultured DCs and not caused by changing the composition of the DC population.
The in vivo superantigen-activated Treg cells in our previous report40 and the in vitro-activated Treg cells used in the present report, both expressed high level of CTLA-4 as compared to ex vivo Treg cells. This protein has been implicated in Treg cell function both in vivo and in vitro,12,26,27 although its role in Treg cell-mediated suppression of T-cell proliferation in vitro still is controversial.27,28,33,42 It has been shown that Ctla-4-deficient Treg cells can suppress T-cell proliferation in vitro.28,33 However, this suppression was partially TGF-β1 dependent and might represent a compensatory mechanism, as suppression by Ctla-4-sufficient Treg cells was shown to be TGF-β1 independent.28
In this report we present data indicating that the down-modulation of B7-molecules by Treg cells is CTLA-4-dependent. Addition of anti-CTLA-4 antibodies to in vitro cocultures of Treg cells and DCs significantly reduced the down-modulation of B7-molecules and also inhibited suppression of T-cell proliferation. Treg cells from Ctla-4-deficient mice did not down-modulate the B7-molecules of cocultured DCs, consistent with the antibody blocking data. In contrast to previous reports28,33 Ctla-4-deficient Treg cells in our laboratory did not suppress T-cell proliferation as reproducibly as Treg cells from normal mice. Even though we used young mice in these experiments we believe that the variability might be caused by contamination of the Treg cell population by activated conventional CD4+ CD25+ T cells. Such cells might produce IL-2 and could therefore interfere with the suppression when present in sufficiently high numbers.
Down-modulation might be the result of internalization of B7-molecules by the DCs or by the Treg cells themselves. Indeed, there is evidence that T cells can acquire membrane molecules from APCs.43 In either of these cases, CTLA-4 might either be directly involved in the internalization process or alternatively in transmitting signals leading to this event. Another possibility would be that CTLA-4 molecules, secreted or shed locally in the cell contact area, would block the costimulatory molecules. Putative soluble forms of the CTLA-4 molecule have been reported in both human and in the mouse.44,45 Whatever the role of CTLA-4 might be, its role in down-modulation is specific for Treg cells, because activated conventional CD4+ T cells, even though they up-regulate CTLA-4 expression upon activation46 fail to down-modulate B7-expression on DC (Fig. 1b). We propose that CTLA-4 may have several functional roles in suppression. First, as shown here, it mediates down-modulation of B7-molecules on the DC. Second, it is involved in the activation of the tryptophan metabolism, at least in DC12. Third, cross-linking of CTLA-4 induces production of the immunosuppressive cytokine TGF-β1.47
Previous studies from other laboratories have suggested that the APC has only a passive role in the in vitro suppression of T-cell proliferation by Treg cells.3,11,24,48 Further, antibody-coated latex beads can replace APCs in suppression.11 Our present results and those of Ermann et al.11 indicate that even when presented on a rigid matrix (latex beads) the level of B7-signals clearly influences suppression. Thus, at a given suppressor to responder T-cell ratio, suppression was only seen when the level of B7-signals was low11 or in the absence of deliberate B7-signals, as shown here. We therefore propose that the essential role,6 of both live and surrogate APCs in contact dependent suppression of CD4+ T cells, is to bring the Treg cells and responder T cells in close physical contact and to activate the T cells. In fact, in parallel experiments using the same latex beads and cell concentration, we observed suppression in round-bottom microplates and enhanced proliferation in flat bottom microplates (C. Oderup, unpublished data). The fact that surrogate APCs can replace live APCs in vitro does, however, not exclude an active role of live APCs in suppression. This conclusion is supported by a substantial number of reports that indicate that both natural Treg cells and anergic Treg cells functionally interact with APCs.12–14,41,49–51 Recent in vivo studies provided further evidence supporting that Treg may regulate DC activity in vivo.18,19
Originally natural Treg cells were described as anergic cells, exhibiting poor proliferation when activated in vitro. However, although these cells produce little IL-2, they proliferate extensively when exposed to exogenous IL-2 in culture.8,42 Recent reports have demonstrated that both responder T cells and natural Treg cells divide in suppression cultures.8,42 Treg cells efficiently bind and consume IL-2 and IL-2 production by the responder T cells therefore becomes a critical component of in vitro suppression. Induction of T-cell proliferation and IL-2 production are both costimulation dependent. Consequently, the down-modulation mechanism could potentially interfere with T-cell activation and IL-2 production by activated responder T cells in the suppression cultures. In addition, the natural Treg cells, which constitutively express CTLA-4, would be expected to more efficiently engage remaining B7-molecules than the responder T cells, therefore promoting suppression rather than T-cell proliferation.
In conclusion, we propose that the role of the APC in suppression is twofold. First, it is required to establish Treg cells to responder T-cell contact and activation. Second, it promotes suppression by decreasing its expression of costimulatory molecules and by producing suppressive molecules. Because the effector phase of suppression only requires direct interaction between the Treg cells and its T-cell target, one can replace the APCs with various substitutes in in vitro settings, which fulfil the requirement of both activating the regulatory T cell and bringing the two T cells together.
Acknowledgments
We would like to thank Dr Su-Ling Li and Dr Tak W. Mak for providing the Cd28-deficient and Ctla4-deficient mice, respectively. We thank Dr Anette Sundstedt for helpful comments on the manuscript. FI was supported by grants from The Swedish Research Council, project number K2005-06X-14184-04A, Anna Greta Crafoords Stiftelse för Reumatologisk forskning, Crafoordska Stiftelsen, Greta och Johan Kocks Stiftelser, Alfred Österlunds stiftelse and the Medical Faculty of Lund University. C.M.C. was supported by grants from Crafoordska Stiftelsen, Vetenskapligt Arbete inom Diabetologi and by a fellowship from the Juvenile Diabetes Foundation International. L.C., C.O. and A.M. were supported by the Medical Faculty of Lund University.
Abbreviations
- BM
bone marrow
- GeoMFI
geometric mean fluorescence intensity
- IDO
indoleamine 2,3-dioxygenase
- iNOS
inducible nitric oxide synthase
- L-Nil
L-N6-(1-iminoethyl)lysine hydrochloride
- 1-MT
1-methyl tryptophan
- SA
streptavidin
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 2.von Boehmer H. Dynamics of suppressor T cells: in vivo veritas. J Exp Med. 2003;198:845–9. doi: 10.1084/jem.20031358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 4.Thornton AM, Shevach EM. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398–403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4 (+) CD25 (+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4 (+) CD25 (+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–46. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+ CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–8. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 9.Scheffold A, Huhn J, Hofer T. Regulation of CD4 (+) CD25 (+) regulatory T cell activity: it takes (IL-) two to tango. Eur J Immunol. 2005;35:1336–41. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 11.Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, Lejon K. CD4 (+) CD25 (+) T cells facilitate the induction of T cell anergy. J Immunol. 2001;167:4271–5. doi: 10.4049/jimmunol.167.8.4271. [DOI] [PubMed] [Google Scholar]
- 12.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 13.Serra P, Amrani A, Yamanouchi J, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+ CD25+ T cells. Immunity. 2003;19:877–89. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 14.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge. human CD4+ CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–84. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 16.Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+ CD4+ regulatory cell activation and function by dendritic cells. Int Immunol. 2004;16:1769–80. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 17.Guiducci C, Valzasina B, Dislich H, Colombo MP. CD40/CD40L interaction regulates CD4+ CD25+ T reg homeostasis through dendritic cell-produced IL-2. Eur J Immunol. 2005;35:557–67. doi: 10.1002/eji.200425810. [DOI] [PubMed] [Google Scholar]
- 18.Tang Q, Adams JY, Tooley AJ, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewkowich IP, Herman NS, Schleifer KW, et al. CD4+ CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–61. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cederbom L, Hall H, Ivars F. CD4+ CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Laich A, Neurauter G, Widner B, Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem. 2002;48:579–81. [PubMed] [Google Scholar]
- 22.Chai JG, Tsang JY, Lechler R, Simpson E, Dyson J, Scott D. CD4+ CD25+ T cells as immunoregulatory T cells in vitro. Eur J Immunol. 2002;32:2365–75. doi: 10.1002/1521-4141(200208)32:8<2365::AID-IMMU2365>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.George TC, Bilsborough J, Viney JL, Norment AM. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro. Eur J Immunol. 2003;33:502–11. doi: 10.1002/immu.200310026. [DOI] [PubMed] [Google Scholar]
- 24.Piccirillo CA, Shevach EM. Cutting edge: control of CD8 (+) T cell activation by CD4 (+) CD25 (+) immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 25.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 26.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25 (+) CD4 (+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25 (+) CD4 (+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+ CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 29.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4 [see comments] Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 31.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 32.Chambers CA, Cado D, Truong T, Allison JP. Thymocyte development is normal in CTLA-4-deficient mice. Proc Natl Acad Sci USA. 1997;94:9296–301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataoka H, Takahashi S, Takase K, Yamasaki S, Yokosuka T, Koike T, Saito T. CD25 (+) CD4 (+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol. 2005;17:421–7. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 34.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge. IL-2 is critically required for the in vitro activation of CD4+ CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 35.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+ CD25+ T cells lyse antigen-presenting B cells by Fas–Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–12. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 36.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Nolte-′t Hoen EN, Wagenaar-Hilbers JP, Peters PJ, Gadella BM, van Eden W, Wauben MH. Uptake of membrane molecules from T cells endows antigen-presenting cells with novel functional properties. Eur J Immunol. 2004;34:3115–25. doi: 10.1002/eji.200324711. [DOI] [PubMed] [Google Scholar]
- 38.Kumanogoh A, Wang X, Lee I, et al. Increased T cell autoreactivity in the absence of CD40–CD40 ligand interactions: a role of CD40 in regulatory T cell development. J Immunol. 2001;166:353–60. doi: 10.4049/jimmunol.166.1.353. [DOI] [PubMed] [Google Scholar]
- 39.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]
- 40.Grundstrom S, Cederbom L, Sundstedt A, Scheipers P, Ivars F. Superantigen-induced regulatory T cells display different suppressive functions in the presence or absence of natural CD4+ CD25+ regulatory T cells in vivo. J Immunol. 2003;170:5008–17. doi: 10.4049/jimmunol.170.10.5008. [DOI] [PubMed] [Google Scholar]
- 41.Vendetti S, Chai JG, Dyson J, Simpson E, Lombardi G, Lechler R. Anergic T cells inhibit the antigen-presenting function of dendritic cells. J Immunol. 2000;165:1175–81. doi: 10.4049/jimmunol.165.3.1175. [DOI] [PubMed] [Google Scholar]
- 42.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+ CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–76. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 43.Hwang I, Huang JF, Kishimoto H, et al. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells [in process citation] J Exp Med. 2000;191:1137–48. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magistrelli G, Jeannin P, Herbault N, Benoit De Coignac A, Gauchat JF, Bonnefoy JY, Delneste Y. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29:3596–602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA-4. Cell Immunol. 2000;201:144–53. doi: 10.1006/cimm.2000.1649. [DOI] [PubMed] [Google Scholar]
- 46.Lindsten T, Lee KP, Harris ES, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–99. [PubMed] [Google Scholar]
- 47.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4 (+) T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornton AM, Shevach EM. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 49.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro[see comments] Science. 1994;264:1587–9. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 50.Frasca L, Carmichael P, Lechler R, Lombardi G. Anergic T cells effect linked suppression. Eur J Immunol. 1997;27:3191–7. doi: 10.1002/eji.1830271216. [DOI] [PubMed] [Google Scholar]
- 51.Taams LS, van Rensen AJ, Poelen MC, et al. Anergic T cells actively suppress T cell responses via the antigen-presenting cell. Eur J Immunol. 1998;28:2902–12. doi: 10.1002/(SICI)1521-4141(199809)28:09<2902::AID-IMMU2902>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]